Abstract
Oligodeoxyribonucleotides containing pseudorotationally locked sites derived from bicyclo[3.1.0]hexane pseudosugars have been synthesized using adenosine, thymidine and abasic versions of North- and South-methanocarba nucleosides. The reaction conditions for coupling and oxidation steps of oligonucleotide synthesis have been investigated and optimized to allow efficient and facile solid-phase synthesis using phosphoramidite chemistry. Our studies demonstrate that the use of iodine for P(III) to P(V) oxidation leads to strand cleavage at the sites where the pseudosugar is North. In contrast, the same cleavage reaction was not observed in the case of South pseudosugars. Iodine oxidation generates a 5′-phosphate oligonucleotide fragment on the resin and releases the North pseudosugar into the solution. This side reaction, which is responsible for the extremely low yields observed for the incorporation of the North pseudosugar analogs, has been studied in detail and can be easily overcome by replacing iodine with t-butylhydroperoxide as oxidant.
INTRODUCTION
The degree of flexibility of the sugar-phosphate backbone in free DNA depends on the base sequence, sugar pucker and other base pair features (1). When proteins bind to DNA, they often distort its conformation and the barrier to local deformability is more than compensated for by the energy of the resulting protein-DNA complex (2). Many of the structural adjustments made by the sugar-phosphate backbone in these complexes can be associated with the rearrangement of the furanose sugar pucker between the two known structural families of DNA, A-type and B-type (3). Interconversion between A-type and B-type also occurs as a result of changes in the degree of hydration, metal ion coordination and interactions with small molecules (1,4). In the A-form, sugar puckering is closely clustered around a 3′-endo (North) conformation, while the puckering distribution in the standard B-form is more diffuse around the C2′-endo conformation (5).
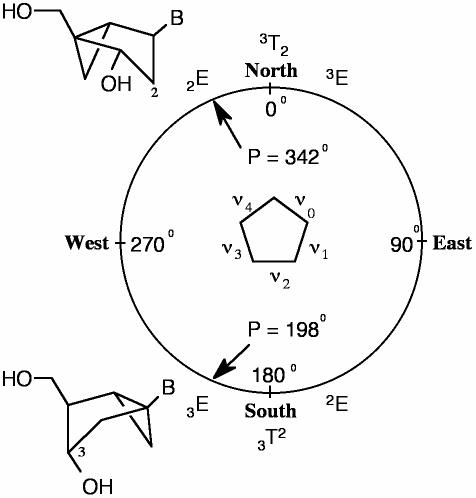
Herein, we report the synthesis of short oligodeoxynucleotides (ODNs), which incorporate at specific sites of the ODN nucleotide building blocks that are conformationally ‘locked’ into North or South units. Because the locking of the conformation is achieved with a rigid bicyclo[3.1.0]hexane pseudosugar, the embedded cyclopentane ring can be secured into either a 2′-exo (2E, North) or 3′-exo (3E, South) conformation within the range of the conventional North and South conformations depicted in the pseudorotational cycle (Figure 1). The introduction of locked 2′-exo (North) units into B-type DNA is expected to mimic an A-like microenvironment commonly associated with the induction of a bend (3). On the other hand, the introduction of locked 3′-exo (South) units should resist bending. Both antipodal effects on conformation should restrict, to a certain degree, the flexibility of the sugar-phosphate backbone and are expected to influence the biophysical and biological properties of the duplex. To date, an important biological study describing the consequences of these changes in short ODNs has been reported (6), and additional work on their biophysical and biological properties will be forthcoming.
Figure 1.
Fixed location of the bicyclo[3.1.0]hexane templates in the pseudorotational cycle.
Normally, one would expect the synthesis of these modified ODNs to proceed as efficiently as with conventional ODNs. Unfortunately, during early attempts to make modified ODNs containing locked 2′-exo (North) bicyclo[3.1.0]hexane units, some serious difficulties were reported with the phosphoramidite approach, and the extremely low coupling yields obtained prevented the synthesis of ODNs incorporating two or more 2′-exo (North) thymidine residues (7). These difficulties were not encountered with the hydrogen phosphonate protocol during the synthesis of a phosphorothioate 15mer ODN containing 10 modified 2′-exo (North) thymidine units (8). Surprisingly, the same phosphoramidite chemistry when used with locked 3′-exo (South) bicyclo[3.1.0]hexane units presented no such problems (9). This contrasting chemical behavior between locked 2′-exo and 3′-exo units was not only intriguing, but demanded an investigation on the ultimate goal of adjusting the standard coupling conditions to overcome the problem. We became aware that the altered structure and reactivity of the 2′-exo pseudosugar monomers compared to conventional phosphoramidites required some adjustments to the standard coupling and oxidation conditions in order to obtain oligonucleotides in high yields and quality. High coupling yields were sought using extended coupling time periods and macroporous polystyrene as the solid support. However, iodine-mediated oxidation of the internucleotide phosphite linkage to the corresponding phosphate triester involving 2′-exo (North) pseudosugars resulted in strand cleavage and the generation of 5′-phosphate-containing oligonucleotide fragments on the resin. More detailed studies revealed that this bond breaking reaction, which does not occur to a measurable extent with the 3′-exo (South) pseudosugars, was due to an intramolecular Mitsunobu-type reaction involving the nucleobase and the ensuing cleavage of the internucleotide linkage.
MATERIALS AND METHODS
General procedures
All chemical reagents were commercially available. Column chromatography was performed on silica gel 60, 230–400 mesh (E. Merck), and analytical thin layer chromatography (TLC) was performed using Analtech Uniplates silica gel GF. Routine 1H NMR and 31P NMR spectra were recorded under standard conditions at 400 and 121.42 MHz, respectively. For compounds 1b, 14 and 7, positive-ion fast-bombardment mass spectra (FAB–MS) were obtained on a VG 7070E mass spectrometer at an accelerating voltage of 6 kV and a resolution of 2000. Glycerol was used as the sample matrix, and ionization was effected by a beam of xenon atoms.
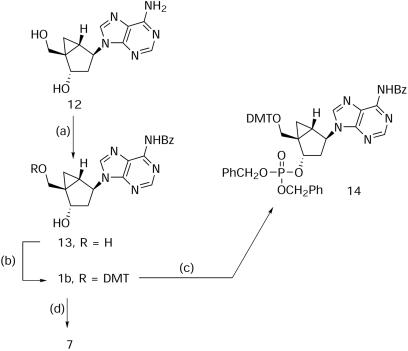
(1′S,2′S,4′S,5′R)-N-[9-(5-{[bis(4-Methoxyphenyl) phenylmethoxy]methyl}-4-hydroxybicyclo[3.1.0]hex-2-yl)purin-6-yl]benzamide (1b)
A suspension of 12 (8) (100 mg, 0.382 mmol) in dry pyridine (10 ml) was treated with chlorotrimethylsilane (0.37 ml, 2.91 mmol) and stirred at ambient temperature for 20 min. Benzoyl chloride (0.24 ml, 2.02 mmol) was added in one portion and the mixture was stirred for 2 h. After cooling to 0°C in an ice bath, water (2 ml) was added, followed by concentrated ammonium hydroxide (4 ml) 15 min later. After 1 h stirring, the temperature was allowed to reach ambient conditions and all volatiles were removed under vacuum. The residue was taken into water (20 ml) and washed twice with ether (10 ml). The aqueous layer was concentrated in vacuo and the solid residue was washed with ether and dried to give 13 (110 mg, 79%) as a white solid. A solution of 13 (75 mg, 0.205 mmol) and catalytic amounts of 4-dimethylaminopyridine (DMAP) in dry pyridine (8 ml) was treated with triethylamine (0.043 ml, 0.308 mmol) and 4,4′-dimethoxytrityl chloride (DMTCl; 84 mg, 0.248 mmol) at 0°C under argon. After stirring for 24 h at 50°C, additional DMTCl (84 mg, 0.248 mmol) was added. The next day, the reaction mixture was reduced to dryness in vacuo and the residue was taken up in EtOAc (30 ml), washed three times with water (10 ml) and dried (MgSO4). Removal of the solvent followed by silica gel column chromatography of the residue (EtOAc/MeOH/Et3N, 40:2:0.5) gave 1b (300 mg, 79%) as a pale yellow foam after trituration with ether: 1H NMR (CD3OD, δ) 8.72 (s, 1H, H-2), 8.64 (s, 1H, H-8), 8.04–7.53 (m, 5H, Ph), 7.34–6.82 (m, 13H, Ph), 5.07 (d, J = 7.3 Hz, 1H, H-2′), 4.94 (irregular t, J = 8.8, 7.3 Hz, 1H, H-4′), 4.76 (d, J = 6.6 Hz, OH, D2O exchangeable), 3.69 (2s, 6H, OCH3), 3.31 (d, J = 9.5 Hz, 1H, –CHHO–), 2.79 (d, J = 9.5 Hz, 1H, –CHHO–), 2.18 (dd, J = 14.7, 8.1 Hz, 1H, H-3a′), 1.86–1.78 (m, 1H, H-3b′), 1.59 (m, 1H, H-1′), 0.96 (t, J = 4.4 Hz, 1H, H-6a′), 0.61 (irregular t, J = 7.3, 5.8 Hz, 1H, H-6b′); FAB–MS (relative intensity) 668 (MH+, 35).
(1′R,2′S,4′S,5′S)-Phenylmethyl-1-{[bis (4-methoxyphenyl)phenylmethoxy]methyl}-4-[6-(phenylcarbonylamino)purin-9-yl]bicyclo[3.1.0]hexane-2-phosphate (14)
A solution of 1b (96 mg, 0.143 mmol) in dry THF (10 ml) was treated with dibenzyl diisopropylphosphoramidite (0.24 ml, 0.717 mmol) followed by tetrazole (50 mg, 0.717 mmol) and stirred for 4 h at ambient temperature. t-BuOOH (Luperox® 98%, 0.06 ml, 0.032 mmol) was added and after 30 min stirring all volatiles were removed in vacuo. The residue was partitioned between CH2Cl2 (10 ml) and 5% NaHCO3 (5 ml). The organic layer was washed with 5% NaHCO3 (5 ml) and dried (MgSO4). Removal of the solvent followed by silica gel column chromatography (EtOAc/MeOH/Et3N, 40:7:0.5) gave 14 (300 mg, 79%) as a white foam: 1H NMR (DMSO-d6, δ) 11.2 (s, 1H, NH), 8.64 (s, 1H, H-2), 8.58 (s, 1H, H-8), 8.06–7.54 (m, 15H, Ph), 7.32–6.77 (m, 13H, Ph), 5.72–5.67 (m, 1H, H-2′), 5.10 (d, J = 7.2, 8.0 Hz, 1H, H-4′), 4.98–4.87 (m, 4H, PhCH2O–), 3.66 (d, J = 10.2 Hz, 1H, –CHHO–), 3.65 (2 s, 6H, OCH3), 2.93 (d, J = 10.2 Hz, 1H, –CHHO–), 2.32 (dd, J = 15.4, 8.6 Hz, 1H, H-3a′), 2.12 (dd, J = 15.6, 8.0 Hz, 1H, H-3b′), 1.71 (dd, J = 8.6, 3.5 Hz, 1H, H-5′), 1.07 (irregular t, J = 5.0, 4.5 Hz, 1H, H-6a′), 0.82 (irregular t, J = 7.3, 5.8 Hz, 1H, H-6b′); FAB–MS (relative intensity) 928.5 (MH+, 2.2).
(1′S,2′S,4′R,5′R)-N-[9-(5-{[bis(4-Methoxyphenyl)phenylmethoxy]methyl}-4-hydroxybicyclo[3.1.0]hex-2-yl)purin-6-yl]benzamide (7)
A solution of 1b (87 mg, 0.13 mmol) in dry CH3CN (3 ml) was treated with dibenzyl diisopropylphosphoramidite (0.055 ml, 0.163 mmol) followed by tetrazole (12 mg, 0.165 mmol) and stirred for 4 h at ambient temperature. A 0.02 M solution (3.25 ml) of I2 in pyridine/H2O (75:25) was added and after 30 min stirring, all volatiles were removed in vacuo. The residue was extracted with CH2Cl2 (20 ml) and 1% sodium thiosulfate (10 ml). The organic layer was washed with brine solution (10 ml) and dried (MgSO4). Removal of the solvent followed by silica gel column chromatography (hexanes/EtOAc/pyridine, 10:30:0.5) gave 7 (20 mg, 23%) as a white foam: 1H NMR (DMSO-d6, δ) 10.38 (br d, 1H, J < 1 Hz, D2O exchangeable, NH), 9.69 (s, 1H, H-2), 8.67 (br d, 1H, J < 1 Hz, D2O exchangeable, OH), 8.00 (m, 2H, Ph), 7.80 (s, 1H, H-8), 7.50–6.62 (m, 16H, Ph), 5.33 (d, J = 3.3 Hz, 1H, H-2′), 4.86 (d, J < 1 Hz, 1H, H-4′), 3.65, 3.64 (2 s, 6H, OCH3), 3.27 (d, J = 9.9 Hz, 1H, –CHHO–), 3.07 (d, J = 9.9 Hz, 1H, –CHHO–), 2.06 (dm, J = 13 Hz, 1H, H-3a′), 1.81 (d, J = 13 Hz, 1 H, H-3b′), 1.72–1.71 (m, J = 5.1, 2.9 Hz, 1H, H-5′), 0.96 (dd, J = 5.8, 3.5 Hz, 1H, H-6a′), 0.44 (irregular t, J = 7.0, 6.6 Hz, 1H, H-6b′); FAB–MS (relative intensity) 668 (MH+, 11.3).
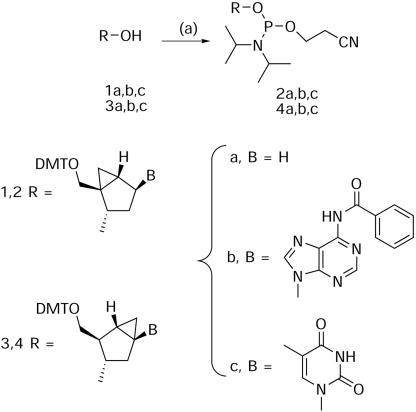
Phosphoramidite synthesis
The DMT-protected pseudosugar precursors were prepared as described previously (6,8) and converted into the corresponding phosphoramidites using 2-cyanoethyl bis(N,N-diisopropyl)phosphoramidite as the phosphitylating agent in the presence of 1H-tetrazole according to a previously published procedure for phosphoramidite synthesis (10). In brief, the pseudosugar precursor was co-evaporated three times with anhydrous pyridine and twice with acetonitrile and dried overnight in vacuo. The bis(N,N-diisopropyl)phosphoramidite (1.3 equiv.) was added to a 0.1 M solution of the pseudosugar or pseudonucleoside in acetonitrile under argon atmosphere with stirring. A 0.45 M solution of tetrazole in acetonitrile was then added. The reaction was monitored using TLC (5% triethylamine in ethyl acetate/hexanes, 1:1) and an excess of triethylamine was added to quench the reaction. After evaporation of the solvent, the remaining oil was dissolved in ethyl acetate, washed three times with a 5% aqueous solution of NaHCO3 and once with brine solution, and dried over Na2SO4. The crude products were purified by silica gel chromatography using 5% triethylamine in ethyl acetate/hexanes (1:1) as eluant. 31P NMR of the compounds: North abasic (2a): δ 148.67, 148.39; North dA (2b): δ 149.27; North T (2c): δ 149.30, 149.16; South abasic (4a): δ 146.87, 146.77; South dA (4b): δ 148.69, 148.42; South T (4c): δ 148.49, 148.15 (Scheme 1).
Scheme 1. Reagents and conditions: (a) 1.3 equiv. bis(N,N-diisopropyl)phosphoramidite, 0.7 equiv. tetrazole (0.45 M solution in acetonitrile), r.t., 2–6 h (60–89%).
Oligonucleotide synthesis and purification
Solid-phase synthesis of oligonucleotides containing carbocyclic pseudosugars was carried out at either a 2 or 20 μmol scale on an Applied Biosystems DNA/RNA synthesizer 380B using phosphoramidite chemistry and macroporous polystyrene (Primer Support, Pharmacia) as the solid support. The pseudosugar or pseudonucleoside phosphoramidites were coupled using an extended coupling time period of 5 min using 5–10 equiv. of monomer (0.1 M in acetonitrile) and tetrazole in acetonitrile (Applied Biosystems) as the activator. For the smaller scale syntheses, the coupling step was performed manually and in batch in a custom-fabricated glass column. After the coupling, the resin was transferred back into the continuous flow column used for automated synthesis on the synthesizer. P(III) to P(V) oxidation was performed either with a commercially available iodine-containing oxidizer solution (0.02 M iodine in water/pyridine/THF; Applied Biosystems), a solution of 0.2 M (1S)-(+)-(camphorsulfonyl)oxaziridine (CSO) in acetonitrile, or a solution of 10% t-butylhydroperoxide in acetonitrile/water (96:4) with reaction time periods of 60 s, 2 and 10 min, respectively. Phosphorothioate linkages were introduced by using a 0.1 M solution of 3H-1, 2-benzodithiol-3-one 1,1-dioxide (Beaucage Reagent) in acetonitrile. After cleaving the oligonucleotides from the solid support under standard conditions (conc. aq. NH3, 55°C, 6 h), they were analyzed and purified by reverse-phase high-perfomance liquid chromatography (HPLC; DMT-on). The HPLC conditions were as follows: column: Waters Deltapak C18 reverse phase (15 μm, 300 Å, 3.9 × 300 mm2); solvent A: 0.1 M NH4OAc in H2O; solvent B: 0.1 M NH4OAc in CH3CN/H2O (80:20); gradient: 0–32 min, 0–50% B. After the first chromatographic purification and removal of the DMT group, the oligonucleotides were subjected to another HPLC purification step: solvent A: 0.1 M triethylammonium acetate in H2O; solvent B: CH3CN/H2O (80:20); gradient: 0–40 min, 0–20% B. Finally, the purified compounds were desalted by HPLC, analyzed by capillary gel electrophoresis and electrospray ionization mass spectrometry, lyophilized and stored at −20°C.
LC–MS analysis
HPLC–MS analysis was performed on a MSD 1100 system (Agilent) using a Luna C18 column, 3 μm, 120 Å, 2 × 150 mm2 (Waters) at a flow rate of 0.2 ml/min and a 20 mM triethylammonium formate buffer (pH 6). Samples were eluted using a gradient from 40 to 95% acetonitrile within 30 min followed by a column wash at 95% acetonitrile for 10 min. All samples were analyzed in the single quadrupole instrument in both, positive and negative polarity using electrospray ionization. Electrospray conditions were set as follows: capillary exit voltage 80 V, drying gas temperature 325°C, nebulizer pressure 25 psi and needle spray voltage 4 and −4 kV for positive and negative mode, respectively.
Supplementary material
Supplementary information includes the following: 31P NMR spectra of the reaction mixtures obtained from the oxidation of A*pT dimer (5) under various conditions (Figure 1); LC-MS analysis of the oxidation of A*pT dimer (5) with iodine (Figure 2); LC-MS analysis of the oxidation of A*pT dimer (5) with t-butylhydroperoxide (Figure 3); ESI-MS analyses of ODNs ON-1 → ON-11 (Figures 4–15).
RESULTS AND DISCUSSION
The phosphoramidite building blocks of 2′-exo (North) and 3′-exo (South) bicyclo[3.1.0]hexane nucleosides used for solid-phase synthesis were prepared in accordance with the standard protocols for phosphoramidite synthesis (10) starting from the protected bicyclo[3.1.0]hexane nucleosides or abasic pseudosugars (6,8) (Scheme 1). The phosphitylated products proved to be stable under the conditions of work-up and silica gel purification, and could be stored for prolonged time periods without measurable loss of reactivity. Commercially available macroporous polystyrene resin, which has been shown to be well suited for difficult couplings and tolerates a low excess of monomer in the coupling step, was chosen for oligonucleotide synthesis (11,12). Prolonged coupling time periods of 5 min resulted in efficient coupling using 10 and 5 equiv. of the bicyclo[3.1.0]hexane nucleoside monomers for 2 and 20 μmol scale syntheses, respectively. To maximize the amount of monomer available for coupling in the smaller scale synthesis, the coupling of the pseudosugar nucleoside phosphoramidites was performed manually and in batch, and not by automated continuous-flow synthesis.
Use of Beaucage's sulfurizing reagent (13) cleanly converted the phosphite triester linkages to phosphorothioates. For the oxidation of the phosphite to the corresponding phosphate triester, three commonly used reagents were investigated: an iodine-containing oxidizer solution in water/pyridine/THF, (1S)-(+)-CSO in acetonitrile, and t-butylhydroperoxide (TBHP) in acetonitrile/water. Although all reagents worked as expected in oxidizing the internucleotide linkages containing the 3′-exo (South) pseudosugars (4a–c) without significant side reactions, the use of the iodine-containing oxidizer led to a dramatic decrease in yield for the 2′-exo pseudosugars locked in the North conformation (2a–c). In this case, we observed that the color intensity of the cleavage solution containing the DMT-cation decreased dramatically immediately after the introduction of the pseudosugar monomer. A direct comparison between TBHP and CSO for the oxidation of 2b in ON-1 (Table 2) showed very similar MS and HPLC profiles of the oxidized oligo; however, TBHP produced fewer failure sequences at lower retention time periods. Therefore, TBHP was chosen as our standard oxidizing agent.
Table 2. Sequence and modification of oligonucleotides containing carbocyclic pseudosugars.
| Entry | Sequence (5′ → 3′) | Modification Y | MWcalc | MWfound | Yielda |
|---|---|---|---|---|---|
| ON-1 | CGCGYATTCGCG | North methanocarba dA | 3656.5 | 3656 | 20.4 |
| ON-2 | ACTGYTCTCCCTATAGTGAGTCGTATTA | North methanocarba dA | 8563.7 | 8561.2 | 13.1 |
| ON-3 | CGCGYATTCGCG | South methanocarba dA | 3656.5 | 3658 | 19.3 |
| ON-4 | CGCGYYTTCGCG | South methanocarba dA | 3666.5 | 3668 | 8.5 |
| ON-5 | CGCGAAYTCGCG | North methanocarba T | 3656.5 | 3655.8 | 25.9 |
| ON-6 | CTACGCYYYCCACGCACAG | North methanocarba T | 5738.9 | 5738.2 | 30.7 |
| ON-7 | CGCGAAYTCGCG | South methanocarba T | 3656.5 | 3658 | 26.3 |
| ON-8 | CGCGAAYYCGCG | South methanocarba T | 3666.5 | 3668 | 21.9 |
| ON-9 | CGCGAYTTCGCG | South abasic | 3523.4 | 3522.2 | 15.4 |
| ON-10 | ATTGCGCATTCYGGATCCGCGATC | South abasic | 7204.7 | 7203.1 | 18.9 |
| ON-11 | AATGAGCCmeAAAAAAAAAAYGGCTCAA | South abasic; _ = PSb | 8050.8 | 8048.8 | 10.8 |
aIsolated yield (% theoretical value) after HPLC purification and desalting.
bPhosphorothioate backbone.
Analysis of crude ODNs containing one North methanocarba-dA residue (derived from the coupling of 2b) by mass spectrometry revealed that the major side product was a 5′-phosphorylated fragment, which lacked the 5′ segment of the sequence starting with the pseudosugar-containing nucleoside. These observations imply that the poor yields obtained with the North bicyclo[3.1.0]hexane synthons were neither the result of incomplete coupling of the pseudosugar-containing monomers nor the result of cleavage of the backbone during the final ammonolysis step due to incomplete oxidation. Under these two scenarios, one would not expect to obtain fragments with 5′-phosphates, and the latter would not have caused a decrease in the amount of DMT-cleaved product during deprotection. Therefore, we concluded that the observed cleavage of the backbone occurred during the P(III) → P(V) oxidation with iodine.
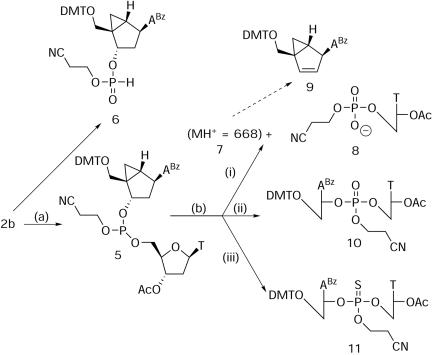
To investigate further the mechanism of strand cleavage, the formation of a model A*pT dimer (A* = North methanocarba-dABz) and the subsequent oxidation of its internucleosidic linkage were monitored by 31P NMR. By adding tetrazole to a solution of 2b and 3′-O-acetylthymidine, the phosphoramidite (δ 149.2) was converted to the expected diastereomers of the A*p(III)T dimer 5 (δ 141.0 and 140.7) and the H-phosphonate diester 6, the product of hydrolysis of the phosphoramidite, represented by a set of two upfield signals (δ 8.7 and 8.6) (Scheme 2).
Scheme 2. Reagents and conditions: (a) 20 mg (0.023 mmol) 2b in CH3CN, 1.5 equiv. 3′-O-acetylthymidine, 5 equiv. tetrazole (0.5 M in CH3CN); (b-i) 5 equiv. I2 (0.02 M in pyridine/H2O), (b-ii) 0.2 ml of 10% t-BuOOH in CH3CN/H2O (6:1), (b-iii) 5 equiv. Beaucage's Reagent (0.2 M in CH3CN).
Oxidation of the mixture with aqueous iodine (Scheme 2, b-i) yielded a major signal at δ 0.01 and a minor peak slightly upfield at δ −0.13. The reaction was complete within the time required for handling of the sample (∼3 min). The observed pattern suggested the formation of a phosphate diester 8 resulting from cleavage of the internucleotide linkage (major signal) and the oxidation of the H-phosphonate diester 6, the side product formed during the coupling reaction (minor signal). In contrast, the oxidation with t-butylhydroperoxide (Scheme 2, b-ii) was slower (15–20 min) and gave a set of two peaks (δ −1.10 and −1.24) representing the two diastereomers formed by the successful oxidation of the internucleotide linkage leading to dimer 10. As expected, the H-phosphonate 6, a hydrolysis by-product produced during the coupling of the phosphoramidite (δ 8.7 and 8.6), was not converted to the corresponding phosphate diester under the milder oxidation conditions. Alternatively, the use of Beaucage's sulfurizing reagent (Scheme 2, b-iii) cleanly and quantitatively converted the phosphite to the corresponding phosphorothioate 11 (δ 68.50 and 68.05) without any evidence of cleavage of the internucleotidic linkage.
To confirm the 31P NMR results and to characterize further the products obtained under the different oxidation conditions, LC–MS analysis of the reaction mixtures (Scheme 2, oxidations b-i and b-ii) was performed. The results of this study are summarized in Table 1. For the oxidation with iodine (i), the LC-profile showed three major peaks, two at early retention time periods between 2 and 3 min and one at a retention time period of ∼30 min. Mass spectrometric analysis (negative mode) revealed that the fractions with early retention time period contain two detectable products with m/z 283.05 and 416.05 corresponding to the starting nucleoside 3′-O-acetylthymidine (T-OAc) and the phosphorylated cleaved fragment 8, respectively. The signal at ∼30 min was found to contain a species with m/z 668.3 (positive mode detection), a mass expected for either 1b or a different cleavage product 7 (vide infra). A minor signal at 20.84 min contained one or more compounds with m/z 650.3 (positive detection mode) and m/z 694.3 (negative detection mode) which were not fully identified. The m/z of 650.3 suggested olefin 9 possibly derived from 7. So far, however, we have not been able to confirm the presence of 9 by NMR.
Table 1. Oxidation of the A*pT dimer: LC–MS analysis of the reaction mixtures.
| Oxidationa | ES–MS mode | RTb (min) | m/z | Product |
|---|---|---|---|---|
| i | Negative | 2.91–3.45 | 416.05 | 8 |
| 283.05 | T-OAc | |||
| i | Positive | 20.84 | 650.3 | 9 |
| i | Positive | 30.35 | 668.3 | 7 |
| ii | Negative | 2.21 | 283.0 | T-OAc |
| ii | Negative | 3.63 | 763.15 | A*pT–DMT |
| ii | Negative | 23.58 | 783.25 | 6 (diast. 1) |
| 24.15 | 783.25 | 6 (diast. 2) | ||
| ii | Positive | 3.28 | 765.2 | A*pT–DMT |
| ii | Positive | 20.99 | 650.2 | 9 |
| ii | Positive | 22.02 | 1067.3 | 10 (diast. 1) |
| 22.69 | 1067.3 | 10 (diast. 2) | ||
| ii | Positive | 23.59 | 785.2 | 6 (diast. 1) |
| 24.14 | 785.2 | 6 (diast. 2) | ||
| ii | Positive | 30.18 | 668.3 | 8 |
aOxidation with (i) iodine and (ii) tBuOOH.
bLC retention time.
In the case of the t-BuOOH oxidation (ii), two major products with retention time periods of 22.02 and 22.6 min and with the same m/z value 1067.3 were obtained. These correspond to the (M+H)+ ions expected for the diastereomers of the A*pT dimer 10. Another set of two peaks at 23.58 and 24.15 min with m/z o785.2 could be detected, indicating the occurrence of the two diastereomers of the H-phosphonate diester 6. Using negative mode detection, a signal at 2.2 min was identified as the starting nucleoside T-OAc (m/z 283.0). Another small signal at 3.3 min was found to have m/z values of 765.2 and 763.2 in the positive and negative mode, respectively, corresponding to the (M+H)+ and (M−H)− ions of the A*pT dimer without the DMT protecting group. Two small but detectable signals, one at 21.09 min (m/z 650.2) and one at 30.18 min (m/z 668.3), suggests that cleavage of the phosphate backbone also occurred during the oxidation with t-BuOOH, but to a much lesser extent.
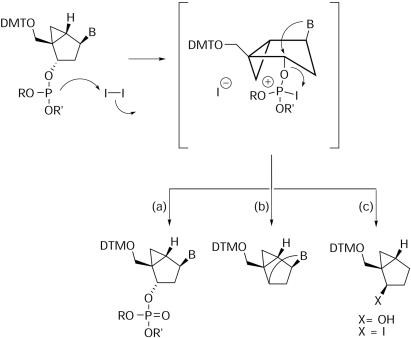
As mentioned already, the cleavage of the internucleotide linkage during the oxidation with iodine does not occur to a significant degree with the corresponding 3′-exo (South) pseudosugars. This difference in stability was consistently observed for all South and North isomers synthesized during this study, including abasic pseudosugars (1a and 3a), methanocarba-dA (1b and 3b) and methanocarba-T (1c and 3c). We propose that the reason for these contrasting behaviors lies in the restricted geometry of the bicyclo[3.1.0]hexane scaffold, which in the case of a 2′-exo (North) pseudonucleoside favors ejection of the excellent leaving group formed when iodine reacts with the phosphite. Under these Mitsunobu-like conditions displacement of the leaving group can occur via intramolecular attack of the base leading to an anhydronucleoside or via simple nucleophilic attack by solvent or other nucleophiles (Scheme 3). We have already reported that in a conventional Mitsunobu reaction, a 2′-exo (North)-methanocarba thymidine analog—similar to 2c—formed exclusively a 2,3′-anhydronucleoside (nucleoside numbering), whereas the same reaction starting with the equivalent 3′-exo (South) analog of 3c failed to give an equivalent anhydronucleoside (14). In the specific case of the iodine-mediated oxidation described here, we envision two likely outcomes from the reactive intermediate in Scheme 3. The first is the normal oxidation of P(III) to P(V) (path a), which appears to have been suppressed. Alternatively, the discharge of the newly formed leaving group can occur via nucleobase participation (B = T or ABz) by an intramolecular process (path b), followed by the formation of further degradation products from this second intermediate, such as olefin formation. In the case when B = H (2a) one can expect release of the leaving group to occur via an SN2 attack by OH− or I− at C-3 (path c). Although 2a could have provided a simpler model to study this reaction, this North abasic sugar was not made in sufficient amounts because it was more difficult to obtain (15) and because in terms of biological importance only the South abasic sugar (4a) proved to be valuable (6). Hence, no additional oligonucleotides were made with this compound (Table 2).
Scheme 3.
To gain further insight into the proposed mechanism, a simpler model reaction was investigated (Scheme 4). Protected North methanocarba-dA (1b) was first reacted with dibenzyl diisopropylphosphoramidite and tetrazole. In situ oxidation with t-BuOOH gave 14, which can be regarded as a simple version of the A*pT dimer 10 (Scheme 2). When the same reaction was performed in the presence of aqueous iodine as oxidant, an unidentified compound was isolated as the major component (23%) from a complex mixture of products. Based on mass spectrometry, we originally assumed that the structure of this product was the starting nucleoside 1b (MH+ = 668); however, the NMR spectrum was vastly different. Since this was the only isolable product from the reaction mixture, we thought it would be useful to perform additional experiments to identify it.
Scheme 4. Reagents and conditions: (a-i) (CH3)3SiCl, pyridine; (a-ii) PhCOCl, pyridine; (a-iii) conc. NH4OH; (b) DMTCl, pyridine, DMAP; (c-i) dibenzyl diisopropylphosphoramidite, tetrazole, THF; (c-ii) t-BuOOH (Luperox® 98%); (d-i) dibenzyl diisopropylphosphoramidite, tetrazole, CH3CN; and (d-ii) 0.02 M of I2 in pyridine/H2O (75:25).
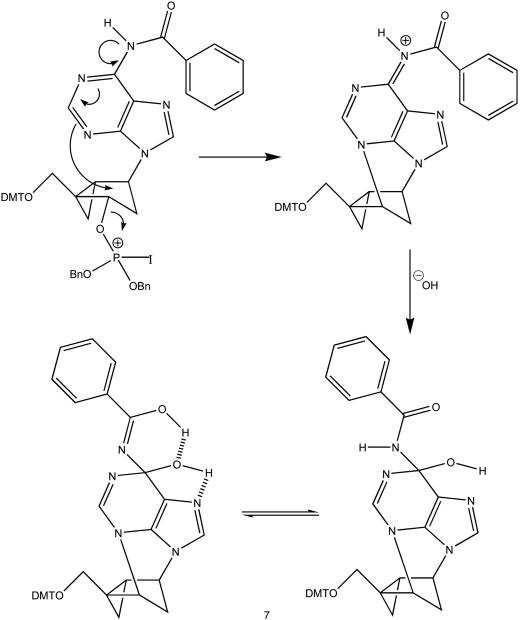
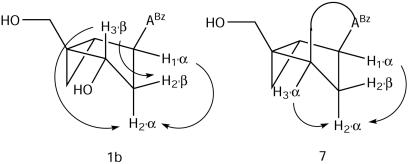
First, all attempts to acetylate this product under standard conditions consistently failed, suggesting that no free hydroxyl group was present in the molecule. A comparison of the COSY spectra of 1b and this product proved that it was epimeric to 1b at C-3′ (nucleoside numbering). An inverted configuration at C-3′ would have a vanishingly small coupling constant between H3′α and H2′β due to the specific geometry of the fixed torsion angles in the bicyclo[3.1.0]hexane system (torsion angle = 90°); only the coupling between H3′α and H2′α was observed for this compound (Figure 2). For the same reason (torsion angle = 90°), the coupling constant between H1′α and H2′β was zero, and the only coupling detected from that system was between H1′α and H2′α. Therefore, H3′α and H1′α are coupled exclusively to the low-field doublet corresponding to the H2′α proton, which is located under the same concave face of the bicyclo[3.1.0]hexane ring. This was demonstrated by the appearance of cross peaks between these signals and the H2′α doublet in the COSY spectrum. By way of comparison, the situation with the starting material (1b) was totally different: H3′β was coupled to both H2′α and H2′β multiplets, while H1′α appeared coupled only to the now high-field H2′α multiplet—cis to the 3′-OH group (Figure 2). In addition, the COSY data for the isolated compound revealed a very unusual coupling between two exchangeable protons at δ 8.7 and δ 10.4. This could not be rationalized by either 1b, or its simple C-3′ epimer, since the exchangeable protons (NH and OH) are too far removed for a scalar coupling interaction. Hence, it would appear that these two protons are connected by less than five bonds. Additional evidence in support of a novel structure was obtained from the 1H and 13C chemical shifts. The chemical shift of the putative H-8 proton appeared shifted to a much lower field than in a standard purine, indicating a change in the electronic character of the base ring-system, and the peak at 85.7 ppm in the 13C spectrum suggested the presence of an sp3 hybridized carbon atom bound to more than one heteroatom. These data, along with preliminary long-range 1H−13C correlations, led us to postulate structure 7 (Scheme 5). Compound 7 could be derived from a mechanism similar to that outlined in Scheme 3 (path b). Consistent with the mechanism of anhydronucleoside formation reported for the case of the Mistunobu reaction with the thymine analog of 1c (14), activation of P(III) by iodine would be followed by an intramolecular nucleophilic attack at C-3′ by the purine N-3 atom. This reaction would be facilitated by the nitrogen atom of the N-benzoyl group at C-6, which extends the conjugation of the amide system into the pyrimidine aromatic ring. Hydration of C-6 with hydroxide ion under the reaction conditions would yield compound 7 as a mixture of tautomers. Although most of the evidence points to this structure, the formation of compound 7 during this reaction is still tentative and additional data will be collected to unequivocally prove this postulate. A similar iodine-mediated oxidation of the thymine analog (2c) led to the formation of the already known anhydronucleoside (14), thus providing additional support for the proposed mechanism responsible for the cleavage of the internucleotide linkage during the oxidative step of DNA synthesis.
Figure 2.
Schematic representation of coupled protons (arrows) in 1b and in 7 (inverted stereochemistry).
Scheme 5.
Using the optimized synthesis conditions for the incorporation of the bicyclo[3.1.0] pseudonucleoside synthons, several oligonucleotides were successfully synthesized and characterized by HPLC and mass spectrometry (Table 2). The isolated yields after purification and desalting were in the range of those generally obtained for unmodified oligonucleotides. The short self-complementary oligomers ON-1, 3–5 and 7–9 were prepared for structural elucidation involving NMR studies and crystallography. ON-2, ON-6, ON-10 and ON-11 were made to study the effect of sugar conformation on the binding and activity of certain nucleic acid-binding enzymes in order to achieve a greater understanding of the preference that biologically important enzymes have for specific nucleoside sugar puckers. The results from these studies will be reported in due course.
CONCLUSION
In this paper, we describe the synthesis and characterization of oligonucleotides containing conformationally constrained sites based on bicyclo[3.1.0]hexane pseudosugar synthons. The conditions of oligonucleotide synthesis via phosphoramidite chemistry had to be adjusted to take into account the altered structure and reactivity of the novel monomers compared to standard phosphoramidites. In particular, iodine, a commonly used reagent for the oxidation of internucleotide triester linkages had to be replaced by a milder reagent, t-butylhydroperoxide, in order to avoid strand cleavage and the generation of 5′-phosphate-containing oligonucleotide fragments. Our studies revealed that this cleavage reaction, which does not occur to a measurable extent with the South pseudosugars, might be due to an intramolecular Mitsunobu-type reaction with participation of the nucleobase.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at NAR Online.
REFERENCES
- 1.Neidle S. (2000) Nucleic Acid Structure and Recognition. Oxford University Press, Oxford, New York, Chapter 5. [Google Scholar]
- 2.Dickerson R.E. and Chiu,T.K. (1998) Helix bending as a factor in protein/DNA recognition. Biopolymers, 44, 361–403. [DOI] [PubMed] [Google Scholar]
- 3.Kamath S., Sarma,M.H., Zhurkin,V.B., Turner,C.J. and Sarma,R.H. (2000) In Proceedings of the Eleventh Conversation, University of Albany, SUNY, June 15–29, 1999. J. Biomol. Struct. Dyn., 11, 317–325. [Google Scholar]
- 4.Sinden R.R. (1994) DNA Structure and Function. Academic Press, San Diego, New York, Boston, Chapter 2. [Google Scholar]
- 5.Saenger W. (1984) Principles of Nucleic Acid Structure. Springer-Verlag, New York, Berlin, Heilderberg, Tokyo, Chapter 9. [Google Scholar]
- 6.Wang P., Nicklaus,M.C., Marquez,V.E., Brank,A.S., Christman,J.K., Banavali,N.K. and MacKerell,A.D.,Jr (2000) Use of oligodeoxyribonucleotides with conformationally constrained abasic sugar targets to probe the mechanism of base flipping by HhaI DNA (cytosine C5)-methyltransferase. J. Am. Chem. Soc., 122, 12422–12434. [Google Scholar]
- 7.Altmann K.H., Kesselring,R., Francotte,E. and Rihs,G. (1994) 4′,6′-Methano carbocyclic thymidine: a conformationally constrained building block for oligonucleotides. Tetrahedron Lett., 35, 2331–2334. [Google Scholar]
- 8.Marquez V.E., Siddiqui,M.A., Ezzitouni,A., Russ,P., Wang,J.Y., Wagner,R.W. and Matteucci,M.D. (1996) Nucleosides with a twist. Can fixed forms of sugar ring pucker influence biological activity in nucleosides and oligonucleotides? J. Med. Chem., 39, 3739–3747. [DOI] [PubMed] [Google Scholar]
- 9.Altmann K.H., Imwinkelried,R., Kesselring,R. and Rihs,G. (1994) 1′,6′-Methano carbocyclic thymidine: synthesis, X-ray crystal structure, and effect on nucleic acid duplex stability. Tetrahedron Lett., 35, 7625–7628. [Google Scholar]
- 10.Nielsen J., Marugg,J.E., Taagaard,M., Van Boom,J.H. and Dahl,O. (1986) Polymer-supported synthesis of deoxyoligonucleotides using in situ prepared deoxynucleoside 2-cyanoethyl phosphoramidites. Rec. Trav. Chim. Pays-Bass., 105, 33–34. [Google Scholar]
- 11.McCollum C. and Andrus,A. (1991) An optimized polystyrene support for rapid, efficient oligonucleotide synthesis. Tetrahedron Lett., 32, 4069–4072. [Google Scholar]
- 12.Maier M.A., Yannopoulos,C.G., Mohamed,N., Roland,A., Fritz,H., Mohan,V., Just,G. and Manoharan,M. (2003) Synthesis of antisense oligonucleotides conjugated to a multivalent carbohydrate cluster for cellular targeting. Bioconjug. Chem., 14, 18–29. [DOI] [PubMed] [Google Scholar]
- 13.Iyer R.P., Phillips,L.R., Egan,W., Regan,J.B. and Beaucage,S.L. (1990) The automated synthesis of sulfur-containing oligodeoxyribonucleotides using 3H-1,2-benzodithiol-3-one 1,1-dioxide as a sulfur transfer reagent. J. Org. Chem., 55, 4693–4699. [Google Scholar]
- 14.Marquez V.E., Ezzitouni,A., Russ,P., Siddiqui,M.A., Ford,H.,Jr, Feldman,R.J., Mitsuya,H., George,C. and Barchi,J.J.,Jr (1998) HIV-1 reverse transcriptase can discriminate between two conformationally locked carbocyclic AZT triphosphate analogues. J. Am. Chem. Soc., 120, 2780–2789. [Google Scholar]
- 15.Marquez V.E., Wang,P., Nicklaus,M.C., Maier,M., Manoharan,M., Christman,J.K., Banavali,N.K. and MacKerell,A.D. (2001) Inhibition of (cytosine C5) methyltransferase by oligonucleotides containing flexible (cyclopentane) and conformationally constrained (bicyclo[3.1.0]hexane) abasic sites. Nucleos. Nucleot. Nucleic Acids, 20, 451–459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.