Abstract
Background
Cataracts are a major cause of childhood blindness globally. Although surgically treatable, it is unclear whether children would benefit from such interventions beyond the first few years of life, which are believed to constitute `critical' periods for visual development.
Aims
To study visual acuity outcomes after late treatment of early-onset cataracts and also to determine whether there are longitudinal changes in postoperative acuity.
Methods
We identified 53 children with dense cataracts with an onset within the first half-year after birth through a survey of over 20 000 rural children in India. All had accompanying nystagmus and were older than 8 years of age at the time of treatment. They underwent bilateral cataract surgery and intraocular lens implantation. We then assessed their best-corrected visual acuity 6 weeks and 6 months after surgery.
Results
48 children from the pool of 53 showed improvement in their visual acuity after surgery. Our longitudinal assessments demonstrated further improvements in visual acuity for the majority of these children proceeding from the 6-week to 6-month assessment. Interestingly, older children in our subject pool did not differ significantly from the younger ones in the extent of improvement they exhibit.
Conclusions and relevance
Our results demonstrate that not only can significant vision be acquired until late in childhood, but that neural processes underlying even basic aspects of vision like resolution acuity remain malleable until at least adolescence. These data argue for the provision of cataract treatment to all children, irrespective of their age.
INTRODUCTION
Cataracts are reported to be responsible for approximately 20% of cases of childhood blindness globally.1–6 Unlike in most developed (OECD) nations where cases of congenital cataracts are treated within a few months of birth, children with this condition in the developing world often go untreated for several years. Lack of awareness, access and monetary resources deprive many blind children of corrective surgery. Their blindness profoundly limits their prospects for education, social integration and eventual employment. Even basic survival is threatened by blindness; the WHO estimates that over 50% of the children die within 2 years of going blind.7,8 In the face of this humanitarian crisis, an obvious question is whether these children can be helped, given that they have been blind for several years since birth. Even if we could tackle the logistical challenges involved in providing surgeries to these children, would they actually benefit from the treatment?
The goal of the present study was to address the issue of visual outcomes after extended early-onset blindness with a large and well-characterised subject pool. The specific questions we sought to answer were
What are the visual acuity outcomes following surgery in children with dense bilateral early-onset cataracts when intervened after 8 years of age?
Do acuity outcomes correlate with age at treatment or other patient characteristics such as family history and nature of cataracts?
Are there longitudinal changes in postoperative acuity?
In addition to these primary questions, we also investigated whether low-vision optical aids could provide any benefits postoperatively to these children. This is relevant for developing approaches to integrate the formerly blind into social and educational mainstreams.
METHODS
We undertook a prospective study to analyse visual outcomes in children with dense bilateral cataracts who received late surgical intervention. This study was conducted over a period of 4 years (January 2008 to December 2011) with approval of the institutional review boards at Dr. Shroff's Charity Eye Hospital, New Delhi (SCEH), and the Massachusetts Institute of Technology (MIT).
Study site and study population
Outreach activities, in the form of community and blind school ophthalmic screening camps, were conducted in various states of north India as part of Project Prakash.9,10 All children who were identified as treatably blind were referred to SCEH, a tertiary eye care centre in north India after consultation with their parents.
Ophthalmic examination
At SCEH, a detailed ophthalmic work-up was undertaken as follows:
-
▶
detailed history as to the duration and severity of visual loss;
-
▶
family history of similar disorder;
-
▶
best-corrected visual acuity (BCVA) in finger counting wherever possible; perception and projection of light in other cases;
-
▶
detailed anterior segment evaluation to rule out associated ocular malformations;
-
▶
presence or absence of strabismus and nystagmus;
-
▶
dilated detailed slit lamp biomicroscopy for anterior segment evaluation with special regards to density and morphology of cataract;
-
▶
examination of the posterior segment including dilated indirect ophthalmoscopy wherever possible; ultrasonography B-scan in all cases.
Appropriate treatment was offered to all those who required it; children who fulfilled the following criteria were included in the study.
Inclusion criteria
-
▶
age 8–18 years;
-
▶bilateral symmetrical cataracts on presentation in association with
-
-BCVA less than finger counting at 1.5 m in both eyes;
-
-cataract extent more than 3 mm around visual axis;
-
-sensory nystagmus;
-
-history from parent/caregiver of visual deprivation beginning very early in life (first half-year of life);
-
-absence of any retinal pathology as evidenced by an ultrasonography B-scan preoperatively.
-
-
Exclusion criteria
-
▶
age less than 8 years;
-
▶
BCVA of more than finger counting at 1.5 m in either eye;
-
▶
uniocular, asymmetrical or partial cataract;
-
▶
presence of retinal disease as evidenced by USG B-scan preoperatively and indirect ophthalmoscopy postoperatively in either eye;
-
▶
any history of trauma.
Intervention
Keratometry and biometry of all children was performed under general anaesthesia just before the surgery. A single surgeon (SG) performed all surgeries. A complete circular capsulorhexis was done after instilling methyl cellulose in the anterior chamber and nucleus aspirated with bimanual irrigation and aspiration technique or by phacoaspiration in calcified thick-plate cataracts. All children underwent a primary posterior capsulorhexis through the anterior route with capsulorhexis forceps or vitrector in cases with thick fibrous posterior capsule plaques. A foldable acrylic posterior chamber intraocular lens (PCIOL) was implanted in the bag. Both of the eyes were operated within a period of 4 weeks.
Postoperative Follow-up
At 6 weeks after second eye surgery, distance visual acuity was reassessed using the Lea Symbols logMAR chart at a distance of 6 feet and the best refractive correction was prescribed to all children. The Lea chart comprises four symbols each with an aspect ratio of approximately 1:1. Use of Lea symbols circumvents the lack of English letter literacy of the participants in this study. For all children who completed 6 months follow-up, BCVA at distance and near were again recorded. Near acuity at 30 cm was assessed using a Lea symbols chart.
Prescription of low-vision devices
In children with BCVA less than 20/60 at 6 months, low-vision devices (LVD) were prescribed depending on the needs of the patient. Visual acuity improvement with LVD for distance and near was measured.
RESULTS
A total of 22 398 children were screened as part of community screening camps and screening of 710 blind schools in north India; 1192 children with treatable blindness were identified and referred to base hospital of SCEH. Of these, 427 children were diagnosed as having congenital or developmental cataracts and advised surgery. Families of 237 of these children opted for the surgeries; these children were operated at the hospital. In 184 cases, the cataracts were partial or had late onset, or the children were younger than 8 years of age; the remaining 53 children fulfilled the inclusion criteria and were included in the study. Characteristics of the study cohort are summarised in table 1. The mean age of the patients was 12±2.9 years. In all children, the associated nystagmus was of large amplitude. Additional ocular abnormalities were present in 29 (54.7%). Cataract was divided into total, membranous, polar and zonular (nuclear or lamellar) based on Merin's classification.11,12 Median follow-up period was 7 months (IQR 6–10 months).
Table 1.
Participant characteristics
| Gender | |
| Male | 40 (73.6%) |
| Female | 13 (26.4%) |
| Age (years) | |
| <14 | 33 (62.3%) |
| >14 | 20 (37.7%) |
| Camp type | |
| Community | 30 (56.6%) |
| Blind school | 23 (43.4%) |
| Cataract type | |
| Membranous | 22 (41.5%) |
| Total | 22 (41.5%) |
| Zonular | 20 (37.7%) |
| Polar | 5 (9.4%) |
| Family history | |
| Positive | 27 (50.9%) |
| Negative | 26 (49.1%) |
| Comorbidities | |
| Strabismus | 12 (22.6%) |
| Microcornea | 10 (18.9%) |
| Keratoconus | 4 (7.5%) |
Visual outcomes
Prior to surgery, all patients (n=53) were categorised as the second most severe level of blindness, according to the WHO's taxonomy for visual impairment (ranging from `near normal' to blindness with no light perception) (WHO 2010. International Classification of Diseases, 10th revision). The taxonomy consists of six categories of visual impairment:
Level 0: mild or no visual impairment (acuity better than 6/18 or 20/70)
Level 1: moderate visual impairment (acuity ranging from 6/18 to 6/60 (20/70–20/200))
Level 2: severe visual impairment (acuity ranging from 6/60–3/60 (20/200–20/400)
Level 3: blindness (acuity ranging from 3/60–1/60 (20/400–20/1200) or finger counting at 1 m)
Level 4: blindness (acuity ranging from 1/60 (20/1200) or finger counting at 1 m to light perception)
Level 5: blindness (no light perception).
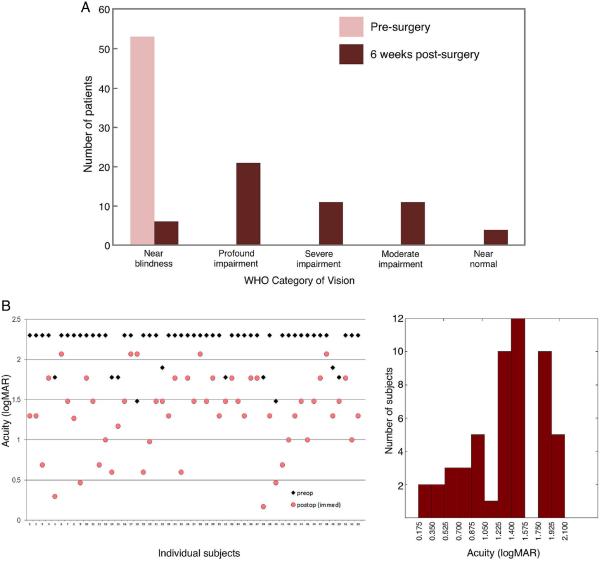
As illustrated in figure 1A, after surgery, 90.6% of the children (48/53) transitioned out of the `near-blindness' category.
Figure 1.
(A) Distribution of patients into WHO categories based on measurements of best-corrected distance vision 6 weeks after surgery. (B) Pre- and 6-week postoperative acuities of 53 congenitally blind children. (C) A frequency histogram of best-corrected visual acuity for the study group 6 weeks after surgery.
Mean preoperative logMAR acuity for distance vision was 2.19, which improved to 1.32±0.46 (95% CI=1.19 to 1.45) after surgery. Mean preop logMAR acuity for near vision was 1.09±0.04, which improved to 0.41±0.3 after surgery. Visual acuity of 20/200 or better was achieved in 28.3% (15/53) children (figure 1A). Figure 1B summarises the quantitative results.
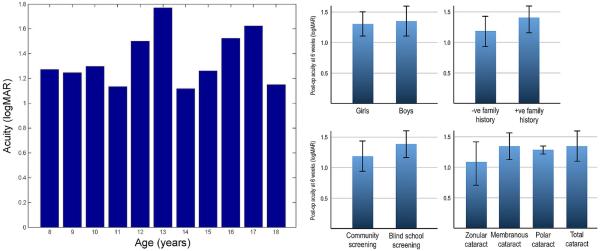
We investigated the relationships between acuity outcomes and various patient variables. Figure 2 summarises the results. Our data showed no significant relationship between 6-week postop BCVA and age, sex, family history, type of cataract and type of screening camp where patient was first identified. It is worth pointing out that zonular cataracts can be developmental and hence might start with lower opacity than the other cataract types. Although not statistically significant, there appears to be a small trend towards marginally better outcomes following treatment of zonular cataracts. Future studies with larger numbers of subjects may be able to examine whether this trend is statistically and clinically significant.
Figure 2.
Influence of five factors on acuity outcomes at 6 weeks postsurgery: age, gender, family history of cataracts, screening camp type and type of cataracts. None of these factors significantly change the outcomes (error bars indicate ±1 SD).
Longitudinal changes in BCVA
BCVA at 6 months was recorded in 43 children, 10 were lost to follow-up. Mean postop logMAR BCVA (n=43) at 6 months was 1.07±0.50 (95% CI=0.91 to 1.22). As shown in figure 3, a majority of the children exhibited improvement in BCVA at 6 months relative to their acuity at the 6-week assessment (paired t test, p<0.01).
Figure 3.
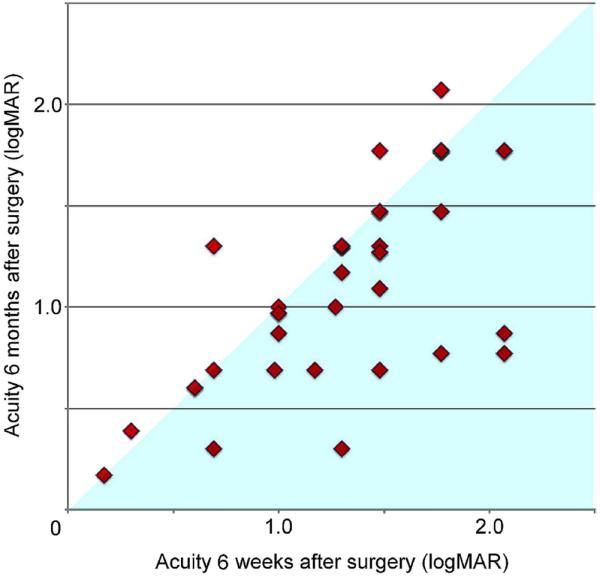
Longitudinal changes in acuity for 43 children whom we followed over several months. As a group, the children show a significant improvement in acuity over the course of 6 months (as indicated in the figure above by the presence of most data points below the diagonal). All measurements are with best refractive correction, but without low-vision aids.
Impact of low-vision aids
All children were given a trial of various low-vision devices for distance and near-vision and low-vision aids were prescribed as needed. Most commonly dispensed LVD for distance was 4× telescope (58.3%) and for near was dome magnifier (73%). Low-vision trial was done for 40 children (77%) with postoperative best-corrected visual acuity worse than 20/60.
Distance vision
Effective visual acuity improved in all but one patient (98.3%) and remained unchanged in 1(1.7%). Mean logMAR vision improved to 1.0±0.56 for distance. In terms of the WHO taxonomy of visual impairment, with the low-vision devices, 25% patients (n=10) further improved to the no impairment category (≥20/60).
Near vision
Near vision was N36 or worse in 100% patients before surgery. After surgery, only 24% of these had near vision worse than N36, while the remaining 76% had near vision better than N36. LVDs for near-vision tasks were prescribed for 15 patients whose near vision was worse than N8. It was observed that after using the near-vision devices, a significant number of patients (9, 53.4%) achieved better than N8 vision.
DISCUSSION
Results of several animal studies involving rearing under visual deprivation regimens13 have provided evidence for the notion of `critical periods' in visual development. Normal visual input during a short time window early in the lifespan appears to be necessary for normal visual development. The critical period in animals like mice and kittens is believed to last for a few weeks or months.13–15 By extrapolation, this period for humans is assumed to transpire within the first 6–8 years of life,13,16 although direct data for this extrapolation are weak.
Implications of the critical period idea are far-reaching. If visual outcomes in early blind children are likely to be poor beyond the first 8 years of life, then it may be counterproductive to subject children older than 8 years to risks associated with surgery. As a matter of health and social policy, for individuals past this assumed critical window, resources should only be directed towards non-surgical assistance (eg, blindness-aids, advocacy). But if there were evidence for positive visual outcomes even after late surgical intervention, the policy with which such cases are approached would need to be reappraised. However, empirical data addressing this issue have hitherto been very limited, and those that do exist do not yield a consistent picture. Some studies suggest poor visual outcomes in children with bilateral congenital cataracts even with a delay of just a little over 1 year.17 On the other hand, studies from western India,18 Nepal19 and Tanzania20 report visual improvement in cases of delayed cataract treatment. Some of the apparent contradictions across these studies stem from their small sample sizes, variable definitions of `delay' and the inclusion of both early and late-onset cataracts.
We had the unique opportunity to address these issues through a study of visual outcomes in well-characterised children treated late in life for bilateral early-onset cataracts. Parental reports and the presence of nystagmus were consistent with the existence of profound visual impairment coincident with or soon after birth. The results of our study show that significant visual improvement was achieved for the majority of our patients, notwithstanding the delayed treatment. There was significant improvement in both distance and near vision after surgery and low-vision devices. Following cataract extraction, 61% of the children in our study cohort were able to read N12 with glasses alone and 24% improved further with LVDs. These surgeries have had a qualitative impact on the educational opportunities available to several of the children we worked with. In total, 9 out of 23 children (39%) screened from blind schools were shifted to normal schools with special educator facilities. Also, 4 out of the remaining 14 (17.3%) who remained in blind schools started learning print along with studying Braille.
Most (81%) of the children in our study group completed the 6 months postoperative follow-up. This is notable in the context of the high drop-out rates previous studies18–20 have reported when following patients treated for long-standing cataracts. There was significant improvement in BCVA at 6 months follow-up for a majority of the children. This indicates not only that significant vision can be acquired until late in childhood, but that neural processes underlying even basic aspects of vision like resolution acuity remain malleable until at least adolescence.
This study has focused on an important but very specific aspect of visual performance—resolution acuity. A comprehensive picture of the impact of late cataract treatments should include other aspects of vision as well, such as performance on recognition, navigation and visuomotor coordination tasks. Furthermore, quality-of-life changes following these surgeries need to be assessed. Besides highlighting the need for these additional studies, our results also point to several intriguing open questions. A significant one relates to outcome variabilities. Why is it that some children are able to progress from near blindness to near normalcy, while others show more limited improvements in acuity? What factors govern the visual outcomes? Similarly, although the majority of the children showed longitudinal gains in acuity, a few exhibited small, but measurable decrements. Causes of the decrements are not clear. Some of this variability of outcomes may be attributable to small differences in the opacity of cataracts that children started out with. Even as multiple considerations mentioned earlier strongly point towards congenitality of the cataracts, the precise cataract densities at birth are unknown. The lack of such records is an inevitable limitation of a study like this one where the participants have been entirely deprived of modern medical care for the first several years of life. Nevertheless, it is encouraging to note the positive visual outcomes across the majority of these cases.
Another question concerns the observed lack of correlation between age at treatment and acuity outcome. We had expected acuity outcomes to be systematically better the younger the child at the time of treatment. However, we find no evidence of such a correlation. Is it the case that the age window over which such a correlation might exist occurs well before the age of 8 years (which was the lower-bound for our cohort)? This possibility is consistent with the idea that a `critical period' in visual development may extend only to the first few years of development; treatment at different ages within this period may well elicit different acuity outcomes. But once this period has elapsed, then the relationship between outcome and age may flatten out, leading to a weakening or even elimination of correlation between the two variables. Conducting a study similar to the one reported here with children younger than 8 years of age would help resolve this issue.
To summarise, we have found that children blind due to early-onset cataracts show significant gains in visual acuity after cataract removal surgery. These results are encouraging from the social healthcare perspective and argue for the provision of cataract treatment to all children, irrespective of their age. Although treatment ought to be provided at the earliest possible opportunity, a significant measure of visual recovery can be obtained even in late childhood. Furthermore, given the notable improvements with low-vision aids, the ideal intervention for children with long-term congenital cataracts should include surgery as well as appropriate visual rehabilitation. This will help ensure that treatably blind children are given the best chance to be integrated into the world of sight.
Acknowledgements
We are thankful to the children who participated in these studies and Prakash outreach team members Naval Chauhan, Harvinder Dhillon, Zishan Muhammad and Shakeela Bi, and optometrists Tapojwal Bannerjee, Sonia Sharma and Sonia Srivastav. Funding for Project Prakash was provided by the National Eye Institute (NEI) of the National Institutes of Health (NIH) via grant R01EY020517, and the James McDonnell Foundation. This support covers all aspects of the work described here including study design, surgeries, data collection and analysis, and manuscript preparation.
Footnotes
Contributors All authors designed the study; SG performed the surgeries; PA assessed acuity outcomes and compiled all data; PA, SS, TG, AK, GC and PS analysed data; all authors contributed to writing the manuscript.
Competing interests None.
Patient consent Obtained.
Ethics approval MIT Committee on the Use of Humans as Experimental Subjects.
Provenance and peer review Not commissioned; externally peer reviewed.
Copyright of British Journal of Ophthalmology is the property of BMJ Publishing Group and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use.
REFERENCES
- 1.Dandona L, Williams JD, Williams BC, et al. Population-based assessment of childhood blindness in southern India. Arch. Ophthalmol. 1998;116:545–6. [PubMed] [Google Scholar]
- 2.Hornby S, Adolph S, Gothwal VK, et al. Evaluation of children in six blind schools of Andhra Pradesh. Indian J Ophthalmol. 2000;48:195–200. [PubMed] [Google Scholar]
- 3.Gilbert C, Foster A. Blindness in children: control priorities and research opportunities. Br J Ophthalmol. 2001;85:1025–7. doi: 10.1136/bjo.85.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson ME, Trivedi RH. Childhood blindness and pediatric cataract. Cataract and refractive surgery today. 2005 Oct;:52–4. [Google Scholar]
- 5.Ericksen JR, Bronsard A, Mosha M, et al. Predictors of poor follow-up in children that had cataract surgery. Ophthalmic Epidemiol. 2006;13:237–43. doi: 10.1080/09286580600672213. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharjee H, Das K, Borah RR, et al. Causes of childhood blindness in north eastern states of india. Indian J Ophthalmol. 2008;56:495–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Waddell KM. Report of WHO/IAPB scientific meeting. World Health Organization; Geneva: 2000. Preventing blindness in children. [Google Scholar]
- 8.Action plan. 2006–2011. Vision 2020: The Right to Sight. Global Initiative for the elimination of avoidable blindness. [PubMed] [Google Scholar]
- 9.Mandavilli A. Look and Learn. Nature. 2006;441:271–2. doi: 10.1038/441271a. [DOI] [PubMed] [Google Scholar]
- 10.Sinha P. Once blind and now they see. Scientific American. 2013;309:48–55. doi: 10.1038/scientificamerican0713-48. [DOI] [PubMed] [Google Scholar]
- 11.Merin S, Crawford JS. The etiology of congenital cataracts: a survey of 386 cases. Can J Ophthalmol. 1971;6:178–6182. [PubMed] [Google Scholar]
- 12.Merin S. Congenital cataracts. In: Goldberg MF, editor. Genetic and Metabolic Eye Disease. Little, Brown & Co.; Boston: 1974. pp. 337–55. [Google Scholar]
- 13.Daw N. Visual development. 2nd edn Springer; New York: 2006. [Google Scholar]
- 14.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. Journal of Physiology. 1970;206:419–36. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Developmental Brain Research. 1991;58:151–8. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- 16.Olitsky SE, Nelson BA, Brooks S. The sensitive period of visual development in humans. J Pediatr Ophthalmol Strabismus. 2002;39:69–72. doi: 10.3928/0191-3913-20020301-04. [DOI] [PubMed] [Google Scholar]
- 17.Garza-Reyes M, Rodríguez-Almaraz M, Ramírez-Ortíz MA. Long-term visual results in congenital cataract surgery associated with preoperative nystagmus. Arch Med Res. 2000;31:500–4. doi: 10.1016/s0188-4409(00)00101-6. [DOI] [PubMed] [Google Scholar]
- 18.Gogate P, Khandekar R, Shrishrimal M, et al. Delayed presentation of cataracts in children: are they worth operating upon? Ophthalmic Epidemiol. 2010;17:25–33. doi: 10.3109/09286580903450338. [DOI] [PubMed] [Google Scholar]
- 19.Thakur J, Reddy H, Wilson ME, et al. Pediatric cataract surgery in Nepal. J Cataract Refract Surg. 2004;30:1629–35. doi: 10.1016/j.jcrs.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 20.Bowman RJC, Kabiru J, Negretti G. Outcomes of bilateral cataract surgery in Tanzanian children. Ophthalmology. 2007;114:2287–92. doi: 10.1016/j.ophtha.2007.01.030. [DOI] [PubMed] [Google Scholar]