Abstract
Objective:
To assess volumes and microstructural integrity of deep gray matter structures in a homogeneous cohort of patients with neuromyelitis optica spectrum disorder (NMOSD).
Methods:
This was a cross-sectional study including 36 aquaporin-4 antibody-positive (AQP4 Ab-positive) Caucasian patients with NMOSD and healthy controls matched for age, sex, and education. Volumetry of deep gray matter structures (DGM; thalamus, caudate, putamen, globus pallidus, hippocampus, amygdala, nucleus accumbens) was performed using 2 independent automated methods. Microstructural integrity was assessed based on diffusion tensor imaging.
Results:
Both volumetric analysis methods consistently revealed similar volumes of DGM structures in patients and controls without significant group differences. Moreover, no differences in DGM microstructural integrity were observed between groups.
Conclusions:
Deep gray matter structures are not affected in AQP4 Ab-positive Caucasian patients with NMOSD. NMOSD imaging studies should be interpreted with respect to Ab status, educational background, and ethnicity of included patients.
Neuromyelitis optica spectrum disorders (NMOSD) are autoimmune CNS conditions that are associated with antibodies (Abs) targeting astrocytic aquaporin-4 (AQP4) water channels in the majority of patients.1 Hallmark clinical symptoms are optic neuritis (ON) and longitudinally extensive transverse myelitis (LETM), but other clinical symptoms, mostly brainstem-associated, might also be present.2 Accordingly, clinical routine MRI can show edema of the optic nerve and longitudinally extensive spinal cord lesions, but also—mostly nonspecific—cerebral white matter lesions.3 Imaging studies using diffusion tensor imaging (DTI) or ultra-high-field MRI have revealed white matter damage predominantly affecting the visual pathway and the corticospinal tract. Results regarding abnormalities of deep gray matter (DGM) structures are inconsistent, with some studies reporting normal volumes while other studies observed hippocampal and thalamic atrophy. These conflicting results might be driven by differences between studies or heterogeneity within patient cohorts regarding sample size, ethnicity, educational background, Ab status, and applied imaging analysis methods. We therefore studied DGM structures in a large, well-characterized cohort of AQP4 Ab–positive Caucasian patients with NMOSD fulfilling the latest diagnostic criteria.2 First, we used the 2 most established tools (FSL FIRST and Freesurfer) to perform automated volumetric analyses of DGM structures. Second, we analyzed DGM microstructural integrity using DTI.
METHODS
Patients and controls.
For this cross-sectional observational study, 36 patients fulfilling the 2015 international consensus diagnostic criteria for NMOSD2 were recruited from the outpatient clinics of the NeuroCure Clinical Research Center and the Department of Neurology of Charité-Universitätsmedizin Berlin (table). All patients were tested for the presence of AQP4 and myelin oligodendrocyte glycoprotein (MOG) Abs by an accredited clinical laboratory (Euroimmun, Lübeck, Germany). Inclusion criteria were Caucasian ethnicity and AQP4 Ab seropositivity. Patients with MOG Abs were excluded. The majority of patients (17 of 36; 47%) were characterized by the classic neuromyelitis optica phenotype (ON + acute myelitis/LETM). Two patients exhibited a history of area postrema syndrome in addition to ON/myelitis. Another 2 patients had a history of acute brainstem syndrome concomitant with ON and LETM. Other patients were characterized by isolated or recurrent ON without other symptoms (5), LETM alone (8), and combinations of LETM and acute brainstem syndrome (1) and LETM with area postrema syndrome and acute brainstem syndrome (1). All but one of the patients with NMOSD were treated with immunosuppressive therapy at the time of investigation (rituximab, 17; azathioprine, 12; mycophenolate mofetil, 2; methotrexate, 1; glatiramer acetate 1; stand-alone prednisolone treatment, 1; plasmapheresis, 1). Seven patients had add-on oral prednisolone therapy. All patients were relapse-free and without high-dose corticosteroid treatment at least 2 weeks prior to the MRI investigations.
Table.
Demographic information of patients and controls and volumes, mean diffusivity, and fractional anisotropy of deep gray matter structures
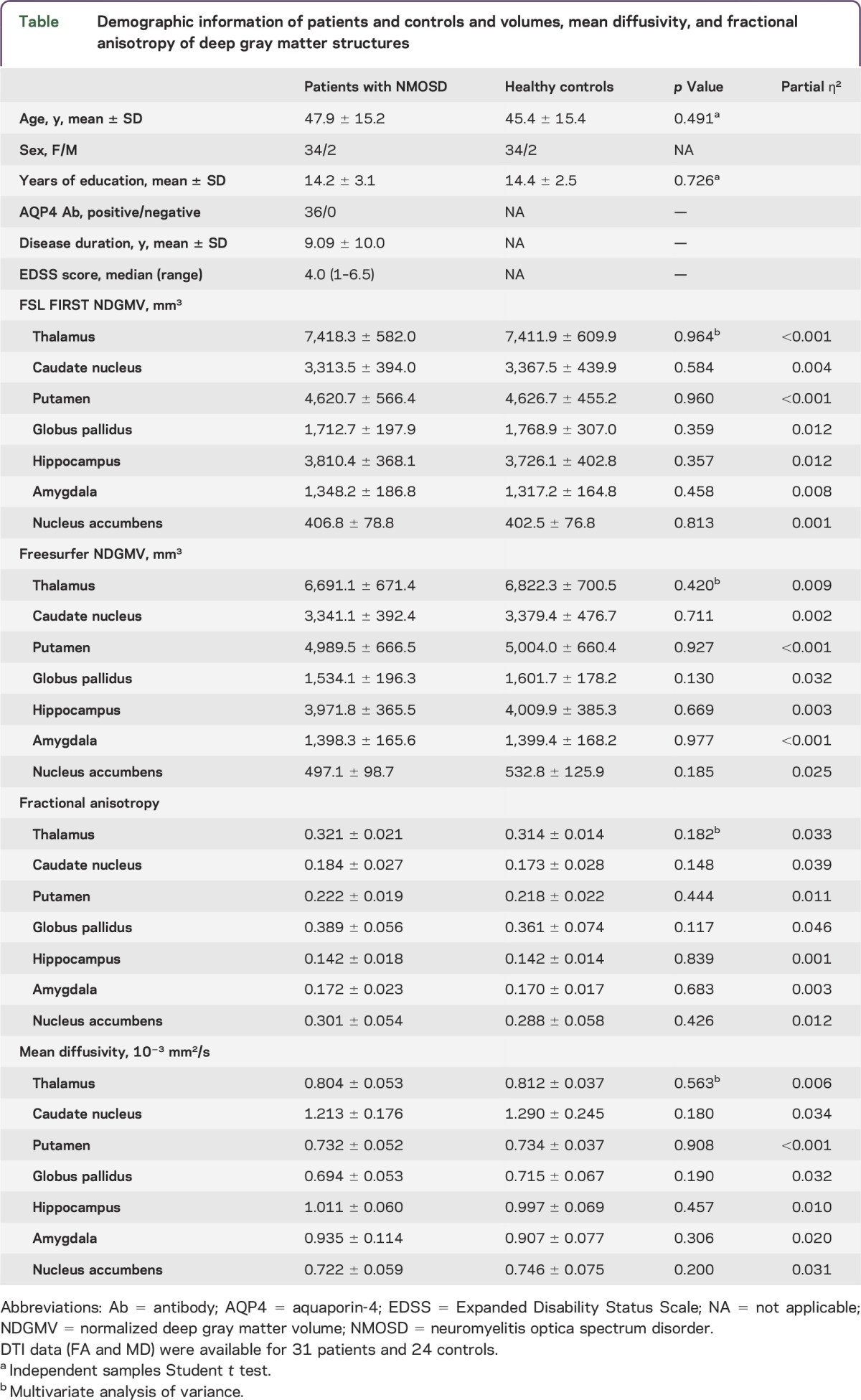
The control group comprised 36 healthy participants from the NeuroCure Clinical Research Center research database who had no history of neurologic or psychiatric diseases and who were matched for age, sex, and years of education (table).
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional review board of the Charité-Universitätsmedizin Berlin. All study participants gave written informed consent.
MRI data acquisition.
MRI data were acquired on a Siemens Magnetom Tim Trio 3T scanner (Siemens, Erlangen, Germany) using previously described sequences4: (1) a high-resolution 3D magnetization-prepared rapid gradient-echo sequence (voxel size 1 × 1 × 1 mm3) and (2) a single-shot echoplanar imaging sequence for the acquisition of DTI data (available for 31 patients and 24 controls).
Analysis of subcortical volumes and microstructural integrity.
Analyses were performed as described previously.4 We used the 2 most established and most commonly applied tools to analyze subcortical volumes to account for possible methodologic differences between previous studies, i.e., FSL FIRST (v5.0.0) and Freesurfer (v5.1). FSL FIRST performs subcortical registration and segmentation using Bayesian shape and appearance models constructed from manually segmented images.5 Volumetric segmentation with Freesurfer includes removal of nonbrain tissue using a hybrid watershed/surface deformation procedure and automated Talairach transformation followed by segmentation of the deep gray matter volumetric structures.6 In both analyses, left and right volumes of DGM structures were averaged. Volumes of all subcortical structures in both analysis pipelines were adjusted for intracranial volume (ICV) by using the following formula:
 |
Microstructural integrity of DGM structures was assessed by DTI using FSL 4.1. After preprocessing, individual fractional anisotropy (FA) and mean diffusivity (MD) maps were calculated by fitting a tensor model to the diffusion data. FA and MD maps were registered to the brain-extracted T1-weighted images. Individual mean MD and FA values of DGM structures were calculated based on DGM masks obtained with FSL FIRST.
Statistical analysis.
Statistical analyses were performed using SPSS 22 (IBM, Armonk, NY). Demographic variables were compared between groups using independent samples t tests. To compare subcortical volumes between patients and controls, multivariate analyses of variance (MANOVA) with group as factor and the 7 DGM structure volumes as dependent variables were performed. Additionally, MANOVAs were repeated with age, sex, and years of education as covariates.
RESULTS
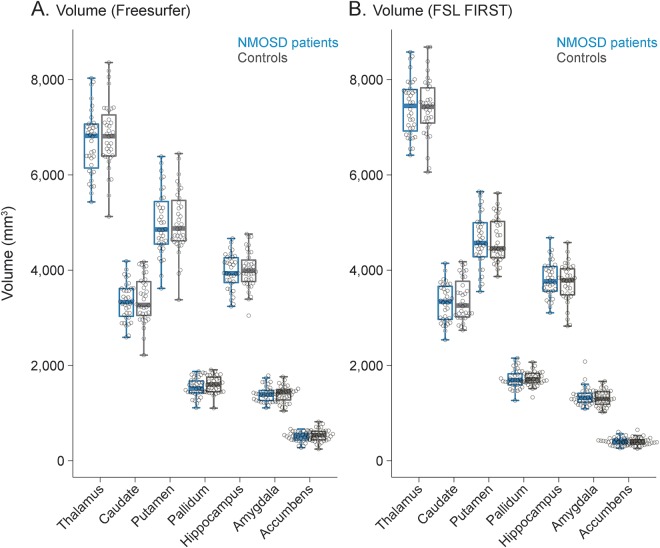
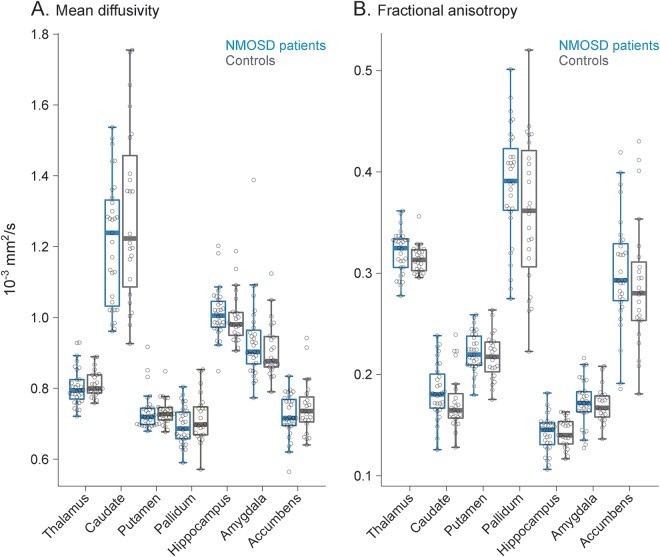
Both volumetric analysis pipelines revealed normal deep gray matter volumes in AQP4 Ab-positive patients with NMOSD in comparison to healthy controls matched for age, sex, and years of education (table, figure 1). For both analyses, observed effect sizes (partial ƞ2) were very low, ranging from <0.001 to 0.032. No significant differences in microstructural integrity were observed between patients and controls for any of the investigated DGM structures (table, figure 2). Similarly, effect sizes were very low (partial ƞ2 <0.001–0.046). Repeated analyses with age, sex, and years of education showed similar results without significant differences between patients and controls and low effect sizes throughout for all volumetric and microstructural integrity measures.
Figure 1. Deep gray matter volumes.
Analysis of deep gray matter volumes with Freesurfer (A) and FSL FIRST (B) in patients with neuromyelitis optica spectrum disorder (NMOSD) and controls matched for age, sex, and years of education revealed no significant group differences.
Figure 2. Microstructural integrity of deep gray matter structures.
Diffusion tensor imaging–derived measures, mean diffusivity (A) and fractional anisotropy (B), of deep gray matter microstructural integrity were not significantly different between patients with neuromyelitis optica spectrum disorder (NMOSD) and matched controls.
DISCUSSION
We found normal volumes and microstructural integrity of DGM structures in AQP4 Ab-positive Caucasian patients with NMOSD. Given the homogeneity of the investigated patient cohort and the application of 2 independent automatic volumetric methodologic approaches as well as additional DTI analyses, these results might help to interpret inconsistent findings in the neuromyelitis optica (NMO)/NMOSD imaging literature.
As a key point of this study, several measures were taken to establish a homogeneous study cohort and to assess the effect of different analysis methods.
First, the current study was restricted to Caucasian patients with NMOSD given that ethnicity likely interacts with NMOSD pathogenicity, as indicated by a disproportionate affection of Asian and African American populations. Second, patients and controls were carefully matched with respect to age, sex, and educational background since all 3 variables have been shown to interact with DGM volume, and analyses were additionally performed using the parameters as covariates. Third, only AQP4 Ab-positive patients were recruited. AQP4 Ab-positive and -negative patients may exhibit distinct disease patterns and a subset of seronegative patients harbor MOG Abs that may be associated with a distinct clinical entity.1 Fourth, volumetric analyses were performed using the 2 most established software packages that have previously been used in NMO research, i.e., FSL FIRST and Freesurfer, to account for possible methodologic differences. In addition, DTI was used for a sensitive assessment of DGM structural integrity.
Previous studies that vary along the above discussed disease dimensions observed conflicting results. Investigating 18 AQP4-positive patients with NMO recruited in the United Kingdom, a recent study found no atrophy of the thalamus using FSL FIRST.7 In contrast, a study of 54 patients with NMO (70% AQP4-positive) recruited in China observed reduced volumes of thalamus, hippocampus, and nucleus accumbens in comparison to healthy controls.8 While educational background was not reported in the former study, in the latter study healthy controls had >1 year more formal education than patients, although the difference was not significant for all patient subgroups. Importantly, DGM atrophy was only observed in cognitively impaired patients but not in cognitively intact patients and in a regression analysis cognitive impairment was largely driven by the level of education, which by itself has been associated with lower DGM volumes.9 A study of 30 patients with NMO (21 Italian, 9 Korean; 83% AQP4-positive) that used Freesurfer to assess cortical and deep gray matter found no cortical lesions and only mild thinning of sensorimotor and visual cortex as well as mild thalamic atrophy.10 These changes were interpreted to be caused by retrograde axonal degeneration due to spinal cord damage.
DTI-derived measures FA and MD of gray matter structures are sensitive markers of microstructural integrity and can indicate neuronal damage caused by microscopic barrier disruption and extracellular fluid accumulation. Furthermore, these measures have been shown to differentiate patients from healthy controls and to correlate with clinical impairment.4 Here, we observed normal DGM microstructural integrity in patients with NMOSD. Together with the observation of normal volumes, these results clearly indicate that DGM structures are not affected in our patients with NMOSD. Of note, this finding contrasts with prominent atrophy of deep gray matter structures in multiple sclerosis and suggests a more focal brain pathology in NMOSD.11
While the results of this study cannot be generalized to all NMOSD cohorts, they provide strong evidence that DGM structures are intact in AQP4 Ab-positive Caucasian patients with NMOSD. Thus, future studies should address imaging differences between patients with NMOSD along the proposed dimensions of Ab status, ethnicity, and educational background and correlate these findings with cognitive performance.12
GLOSSARY
- Ab
antibody
- AQP4
aquaporin-4
- DGM
deep gray matter
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- ICV
intracranial volume
- LETM
longitudinally extensive transverse myelitis
- MANOVA
multivariate analysis of variance
- MD
mean diffusivity
- MOG
myelin oligodendrocyte glycoprotein
- NMO
neuromyelitis optica
- NMOSD
neuromyelitis optica spectrum disorder
- ON
optic neuritis
AUTHOR CONTRIBUTIONS
Carsten Finke: study concept and design, data acquisition, analysis and interpretation, writing of the manuscript. Josephine Heine: data analysis, critical revision of the manuscript for intellectual content. Florence Pache: data acquisition and interpretation, critical revision of the manuscript for intellectual content. Anna Lacheta: data acquisition and interpretation, critical revision of the manuscript for intellectual content. Nadja Borisow: data acquisition and interpretation, critical revision of the manuscript for intellectual content. Joseph Kuchling: data acquisition and interpretation, critical revision of the manuscript for intellectual content. Judith Bellmann-Strobl: data acquisition and interpretation, critical revision of the manuscript for intellectual content. Klemens Ruprecht: data interpretation, critical revision of the manuscript for intellectual content. Alexander U. Brandt: study concept and design, data acquisition and interpretation, critical revision of the manuscript for intellectual content. Friedemann Paul: study supervision, critical revision of the manuscript for intellectual content.
STUDY FUNDING
This study is not industry-sponsored. The study was supported by Deutsche Forschungsgemeinschaft (Exc 257 to F. Paul) and Bundesministerium für Bildung und Forschung (Competence Network Multiple Sclerosis KKNMS to F. Paul, F. Pache, and K.R.).
DISCLOSURE
C. Finke received research support from Berlin School of Mind and Brain. J. Heine reports no disclosures. F. Pache received travel funding from Genzyme, Bayer, Biogen Idec, and ECTRIMS, and received research support from Charite-Universitatsmedizin Berlin, Berlin Institute of Health, Ministry in Germany, and Novartis. A. Lacheta reports no disclosures. N. Borisow received travel funding from Teva. J. Kuchling reports no disclosures. J. Bellmann-Strobl received travel funding and speaker honoraria from Bayer, Sanofi-Aventis/Genzyme, and Teva. K. Ruprecht served on the scientific advisory board for Sanofi-Aventis/Genzyme, Novartis, and Roche; received travel funding and speaker honoraria from Bayer Healthcare, Biogen Idec, Merck Serono, Sanofi-Aventis/Genzyme, Teva, Novartis, and Guthy Jackson; is an academic editor for PLOS One; receives publishing royalties from Elsevier; and received research support from Novartis and German Ministry of Education and Research. A. Brandt served on the scientific advisory board for Biogen VISION study; received travel funding and/or speaker honoraria from Novartis, Bayer, Biogen, and Teva; has a patent pending for Method and system for optic nerve head shape quantification, Perceptive visual computing based postural control analysis, and Multiple sclerosis biomarker; has consulted for Nexus and Motognosis; and received research support from Novartis Pharma, Biogen Idec, BMWI, and MBMF. F. Paul served on the steering committee for Novartis OCTIMS study and MedImmune; received speaker honoraria and travel funding from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai, and MedImmune; is an academic editor for PLoS One; is an associate editor for Neurology®: Neuroimmunology & Neuroinflammation; has consulted for SanofiGenzyme, Biogen Idec, and MedImmune; and received research support from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Alexion, Merck Serono, German Research Council, Werth Stiftung of the City of Cologne, German Ministry of Education and Research (BMBF Competence Network Multiple Sclerosis), and Arthur Arnstein Stiftung Berlin. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation 2012;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HJ, Paul F, Lana-Peixoto MA, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology 2015;84:1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finke C, Kopp UA, Pajkert A, et al. Structural hippocampal damage following anti-N-methyl-D-aspartate receptor encephalitis. Biol Psychiatry Epub 2015 Feb 26. [DOI] [PubMed]
- 5.Patenaude B, Smith SM, Kennedy DN, Jenkinson MA. Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011;56:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 7.Matthews L, Kolind S, Brazier A, et al. Imaging surrogates of disease activity in neuromyelitis optica allow distinction from multiple sclerosis. PLoS One 2015;10:e0137715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Fu Y, Schoonheim MM, et al. Structural MRI substrates of cognitive impairment in neuromyelitis optica. Neurology 2015;85:1491–1499. [DOI] [PubMed] [Google Scholar]
- 9.Noble KG, Grieve SM, Korgaonkar MS, et al. Hippocampal volume varies with educational attainment across the life-span. Front Hum Neurosci 2012;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabrese M, Oh MS, Favaretto A, et al. No MRI evidence of cortical lesions in neuromyelitis optica. Neurology 2012;79:1671–1676. [DOI] [PubMed] [Google Scholar]
- 11.Schoonheim MM, Popescu V, Lopes FCR, et al. Subcortical atrophy and cognition: sex effects in multiple sclerosis. Neurology 2012;79:1754–1761. [DOI] [PubMed] [Google Scholar]
- 12.Zhang N, Li YJ, Fu Y, et al. Cognitive impairment in Chinese neuromyelitis optica. Mult Scler 2015;21:1839–1846. [DOI] [PubMed] [Google Scholar]