Abstract
(−)-Epigallocatechin-3-gallate (EGCg) is a naturally occurring polyphenol found in plant-based foods and beverages such as green tea. Although EGCg can eliminate carbonyl species produced by glucose autoxidation and thus can inhibit protein glycation, it is also reported to be a pro-oxidant that stimulates protein glycation in vitro. To better understand the balance between antioxidant and pro-oxidant features of EGCg, we evaluated EGCg-mediated bioactivities in a human serum albumin (HSA)/glucose model by varying three different parameters (glucose level, EGCg concentration, and time of exposure to EGCg). Measurements of glycation-induced fluorescence, protein carbonyls, and electrophoretic mobility showed that the level of HSA glycation was positively related to the glucose level over the range 10 to 100 mM during a 21-day incubation at 37 °C and pH 7.4. Under mild glycemic pressure (10 mM), long exposure to EGCg enhanced HSA glycation, while brief exposure to low concentrations of EGCg did not. Under high glycemic pressure (100 mM glucose), long exposure to EGCg inhibited glycation. For the first time we showed that brief exposure to EGCg reversed glycation-induced fluorescence, indicating a restorative effect. In conclusion, our research identified glucose level, EGCg concentration, and time of exposure as critical factors dictating EGCg bioactivities in HSA glycation. EGCg did not affect HSA glycation under normal physiological conditions but had a potential therapeutic effect on HSA severely damaged by glycation.
Keywords: Diabetes, polyphenol, tea, fluorescence, carbonyl, AGEs
Introduction
As the incidence of diabetes continues to rise, interest in the potential of food-derived compounds to prevent development of the disease and inhibit its long-term health effects has increased. (−)-Epigallocatechin-3-gallate (EGCg) is a naturally occurring polyphenolic compound that is associated with various chemoprotective effects [1]. Some studies suggest that long term consumption of EGCg-containing beverages, such as green tea, may ameliorate the complications of diabetes by reducing body fat, improving glucose metabolism and maintaining cardiovascular health [2]. EGCg is a potent antioxidant in vitro [3], and dietary antioxidant intake has been associated with reduced risk of diabetes [4]. In addition, EGCg can neutralize carbonyl species [5] and thus protect proteins from glycation [6]. Although EGCg clearly has potential to protect biomolecules from damage, some studies have revealed the pro-oxidant activities of EGCg [7]. Our study was designed to evaluate the potential protective or damaging role of EGCg in protein glycation in vitro in order to provide mechanistic insights into its possible role in diabetes.
Human serum albumin (HSA), as the major plasma protein, is a transporter for various metabolites and is an essential component of the blood antioxidant system [8]. It is subject to modifications by reducing sugars via Maillard reactions during its long in vivo half-life (21 days) [9]. The amount of glycated HSA in individuals with diabetes is typically elevated 2- to 3-fold [10], with over 80 % of the circulating HSA glycated in individuals with severe, uncontrolled diabetes [11]. Glycation alters the tertiary structure of HSA [12], resulting in altered physiological function [13]. Furthermore, glycated HSA can increase cellular oxidative stress and inflammatory activities by activating the receptor for advanced glycation end products (AGEs) [14], consequently contributing to the complications associated with diabetes [15]. Therefore, HSA is a physiologically important target for glycation research.
It is generally accepted that EGCg affects HSA glycation in vitro [5,6,16]. However, three significant knowledge gaps inhibit further understanding of EGCg-mediated biochemical activities under glycemic pressure. There is inadequate information on glycation at physiologically relevant levels of glucose, and a similar lack of information on the role of EGCg concentration. Furthermore, there is little data on how the time of exposure to EGCg affects its activity, a critical consideration for this short-lived molecule. Our work provides unique data on the role of EGCg under physiological conditions.
Glucose level is the first factor that limits our understanding of EGCg-mediated effects on HSA glycation. Most EGCg-related glycation studies have been carried out under high glycemic conditions (sugar > 30 mM), while blood sugar levels in most diabetic patients are in the range 7–10 mM [17]. There is no direct evidence to indicate whether the effects of EGCg on HSA glycation that have been observed under high, experimental glucose levels are meaningful under physiological conditions. We provide essential data to understand the biochemical effects of EGCg on HSA glycation in a system mimicking physiologically feasible conditions.
The second knowledge gap involves EGCg concentration, which might determine the balance between pro- and antioxidant activities of EGCg under glycemic pressure. On the one hand, low concentrations of EGCg (0.25 ~ 10 µM) inhibit glycation-induced fluorescence in an HSA/glucose-fructose system [6]. On the other hand, high concentrations of EGCg could be pro-oxidative. Elbling and colleagues report that 20 µM EGCg increases oxidative stress in cultured mammalian cells, possibly inhibiting cell growth [18]. EGCg (> = 50 µM) can also promote the formation of AGEs, such as N-(carboxymethyl) lysine, in both cell culture and in an HSA model system [16]. No adverse effects were reported for low concentrations of EGCg (<= 10 µM) in the same studies. We speculate that concentration plays a critical role in determining the outcome of EGCg-mediated biochemical activities and provide novel data relevant to the typical low levels of EGCg that can be achieved by oral dosing.
The third knowledge gap relates to the duration and sequence of reactions between EGCg and the glycated protein. EGCg is chemically labile [19], and the parent compound and its degradation products might have different roles in protein glycation reactions. In previous studies, EGCg was incubated with HSA and glucose for at least 6 days [6]. Since EGCg is subject to spontaneous oxidation and decomposition in neutral solution [19], it is reasonable to assume that the degradation products of EGCg, rather than intact EGCg, were responsible for at least some of the biochemical effects in those studies. Glycation is a slow process, taking place over several weeks, but the in vivo half-life of EGCg is only about 4 hours [20]. If intact EGCg plays a role in glycation, it must be during a short time, and must involve glycated protein that has already formed. Previous studies have not examined the effects of intact EGCg on previously glycated protein. To better understand the biochemical activities of EGCg, further work is required to characterize the difference between HSA exposed to EGCg and its degradation products during glycation and HSA briefly exposed to EGCg after glycation. We provide the first direct comparison of these two conditions using physiologically relevant concentrations of the reactants.
In this study, we explored the biochemical activities of EGCg in an HSA/glucose model. Glycation-induced fluorescence, the dinitrophenyl hydrazine (DNPH) assay for carbonyls, and native polyacrylamide gel electrophoresis (PAGE) were used to assess the modification of HSA after 21 days of incubation (37 °C) with various concentrations of glucose (0–100 mM). To evaluate potential time effects on EGCg bioactivities, we incubated EGCg with HSA/glucose samples for 21 days or with glycated HSA samples for 30 min. In both treatments, EGCg concentrations ranged from 0 to 100 µM.
Methods
Materials
O-phenylenediamine was purchased from Acros Organics (Morris Plains, NJ). HSA (≥97%) and methylglyoxal were purchased from MP Biomedicals, LLC (Solon, OH). Amicon 0.5 mL ultrafiltration units (30 kDa molecular weight cut off) and 2,4-dinitrophenylhydrazine (DNPH) were purchased from Fisher Scientific Inc (Pittsburgh, PA). EGCg (>95%) was provided by Lipton Tea Co. (Newark, NJ). 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) and Trolox were obtained from Sigma Chemicals (St. Louis, MO).
HSA and EGCg concentrations were determined spectrophotometrically using their extinction coefficients (ε280 (HSA) = 35750 M−1 cm−1 and ε280(EGCg) = 9700 M−1 cm−1). All solutions were sterile filtered before use, and samples that were contaminated during the 21-day incubation were discarded without analysis.
In vitro glycation of HSA by glucose
HSA was mixed with glucose in 0.2 M sodium phosphate buffer containing 0.1 M sodium chloride (pH 7.4 PBS) to achieve a final concentration of 200 µM HSA and 0, 1, 10 or 100 mM glucose in a 1 mL reaction mixture. The samples were mixed and incubated at 37 °C for 21 days before transferring 450 µL from each sample to an Amicon ultrafiltration unit for 10 min centrifugation at 14000 × g. To further purify each sample, 400 µL of PBS was added to the remaining solution in the filtration device and it was re-filtered as described above. This step was repeated 3 times before collecting the concentrated HSA samples by spinning the filtration devices upside-down in new centrifuge tubes for 2 min at 1000 × g. All samples were adjusted to 200 µM HSA by mixing with 400 µL of PBS, and were kept at 4 °C for further analysis by the DNPH assay, native PAGE and fluorescence as described below.
EGCg treatments
Glucose-HSA solutions were treated with EGCg in two ways. For long term reactions, the mixtures described above were amended to achieve concentrations of 0, 1, 10 or 100 µM EGCg at the start of the 21 day incubation. For short term reactions, EGCg was added to glycated protein that had been purified by ultrafiltration as described above. EGCg was added to these samples to achieve final concentrations of 0, 1, 10 or 100 µM, and the samples were adjusted to 450 µL by PBS before incubation at 37 °C for 30 min. Upon the completion of each EGCg treatment, ultrafiltration was used to remove unreacted EGCg from samples as described above. All samples were adjusted to 200 µM HSA by mixing with 400 µL PBS and were kept at 4 °C for further analysis by the DNPH assay, native PAGE and fluorescence as described below.
Chemical analysis
To determine the glycation-induced fluorescence, each HSA sample was diluted to 30 µM with pH 7.4 PBS. The fluorescence intensity was measured with a LS55 luminescence spectrometer (PerkinElmer Inc., Hebron, KY) with an excitation/emission wavelength pair at 330/410 nm. The fluorescence intensity was corrected by equation 1 to compensate for the inner filter effect due to sample absorbance [21].
| (Equation 1) |
where Fcor and Fobs are the corrected and observed fluorescence intensity, respectively. Abs330 and Abs410 represent the absorbance of samples in a 1 cm path-length cell at the excitation and emission wavelengths. In this study, all fluorescence intensities were corrected prior to data analysis. The anti-glycation effect of EGCg was evaluated by calculating the relative fluorescence intensity (equation 2).
| (Equation 2) |
where Fglycated is the fluorescence intensity of glycated HSA and Fnative is the fluorescence intensity of native HSA.
The carbonyl content of HSA was determined by Levine’s DNPH method [22].
Native PAGE (using the Laemmli system without the SDS) [23] was used to determine changes in the charge density of HSA due to reactions between glucose and amino acid residues such as arginine and lysine [9]. Each purified sample was diluted to 32 µM with PBS before mixing with an equal volume of bromophenol blue/glycerol sample preparation solution, and loading 10 µL (0.16 nmole) per lane of a 12 % or 7.5 % running gel. The gels were stained with Coomassie brilliant blue.
EGCg-mediated methylglyoxal scavenging was determined by post-reaction conversion of remaining methylglyoxal to 1-methylquinoxaline, which was quantitated by high performance liquid chromatography (HPLC) [5]. Methylglyoxal (1 mM) and o-phenylenediamine (1 mM) were prepared fresh in PBS. To prepare the various EGCg samples for analysis, PBS buffer containing 1 mM EGCg was aged at 37 °C for up to 21 days. At intervals throughout the 21-day period, a 100 µL subsample of aged EGCg or PBS (control) was removed and mixed with to 40 µL of 1 mM methyglyoxal and reacted at 37 °C for 60 min. O-phenylenediamine (60 µL, 1 mM) was added to the mixture and after an additional 30 min incubation at 37 °C, the reaction products were quantitated with an Agilent 1100 series HPLC system equipped with a diode array detector (Agilent Technologies, USA). Separation was performed on a Zorbax Eclipse XD8 C8 column (5 µm, 4.6 × 150 mm i.d.) using a gradient of 0.1 % trifluoroacetic acid in water and 0.13 % trifluoroacetic acid in acetonitrile. The product1-methylquinoxaline (tR 28.1 min) was detected at 315 nm and the percentage of methylglyoxal scavenged by EGCg was calculated based on peak area.
Radical scavenging was determined by the ABTS assay [24]. The ABTS radical cation was prepared by oxidation of the neutral species with potassium persulfate, and was stored in the dark until use [25]. EGCg was dissolved in pH 7.4 PBS buffer, and was used immediately or after aging at 37°C for up to 21 days. The colored radical solution was mixed with solutions containing various concentrations of fresh or aged EGCg, and the absorbance at 734 nm was measured after 10 min incubation at room temperature. The data were fit to linear response curves to determine the amount of EGCg required to decolorize 50% of the initial ABTS radical cation (IC50), and compared to the IC50 for Trolox determined under the same conditions.
Statistics
Each glycation reaction and/or EGCg treatment was replicated 5 times. All results were expressed as means +/− SD, and statistical analysis was performed using Sigmaplot 12.5 (Systat Software Inc., San Jose, CA) with P < 0.05 as standard for significance. Two-way ANOVA followed by the Holm-Sidak method[26] was used to analyze interaction between glucose level and EGCg concentration.
Results
In all cases, the glycation reaction was 21 days. Some HSA samples were treated only with glucose (gHSA). The others were reacted with both glucose and EGCg. EGCg was added either at the beginning of the glycation period (Eg-21d), or in a 30 min incubation after the conclusion of the 21 day glycation period (Eg-30m).
Establishing the effects of polyphenols such as EGCg on biological systems can be analytically challenging, because these compounds interfere with many chemical and spectroscopic analytical techniques [27]. In control experiments, we found that the fluorescence of EGCg aged for 30 min or for 21 days in the presence or absence of glucose much lower than that of HSA. The F/Fo calculated for EGCg relative to control HSA was <0.3 for all conditions, so we concluded that EGCg did not interfere with our fluorescence measurements. Control experiments also demonstrated that EGCg did not react with DNPH, used to assess protein carbonyls, or with o-phenylenediamine, used to evaluate methyl glyoxal scavenging.
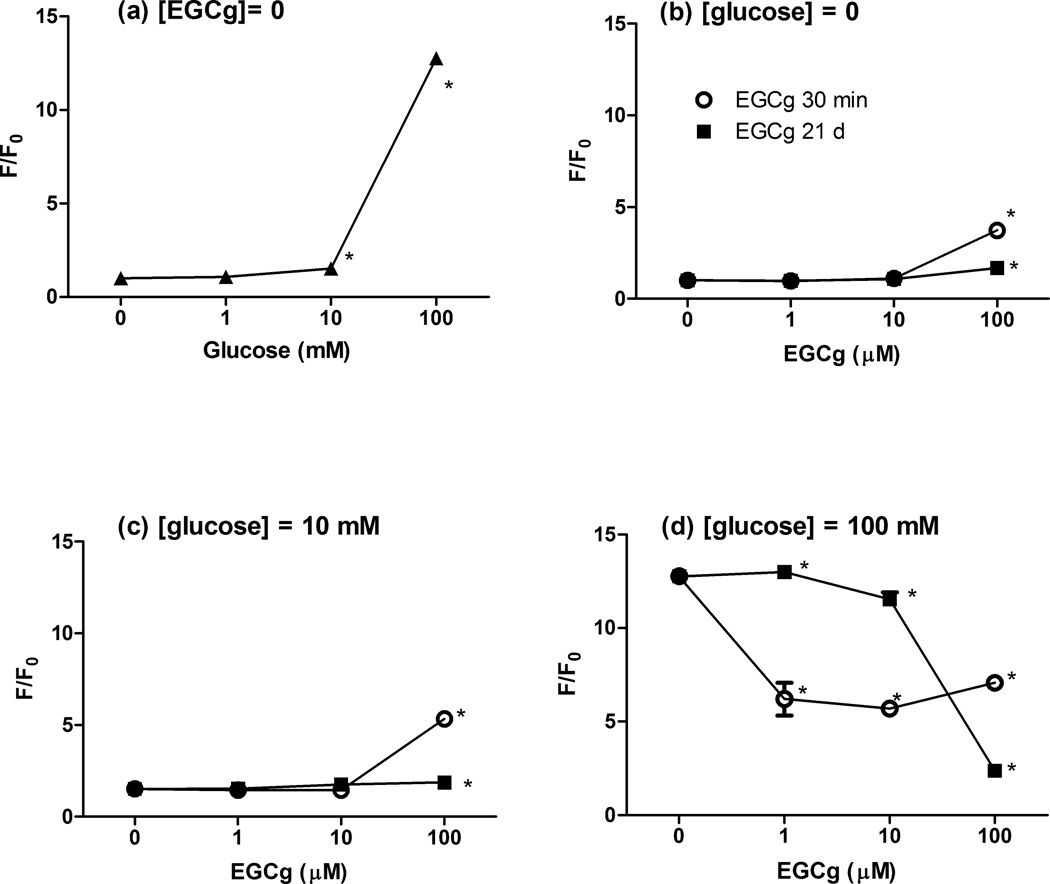
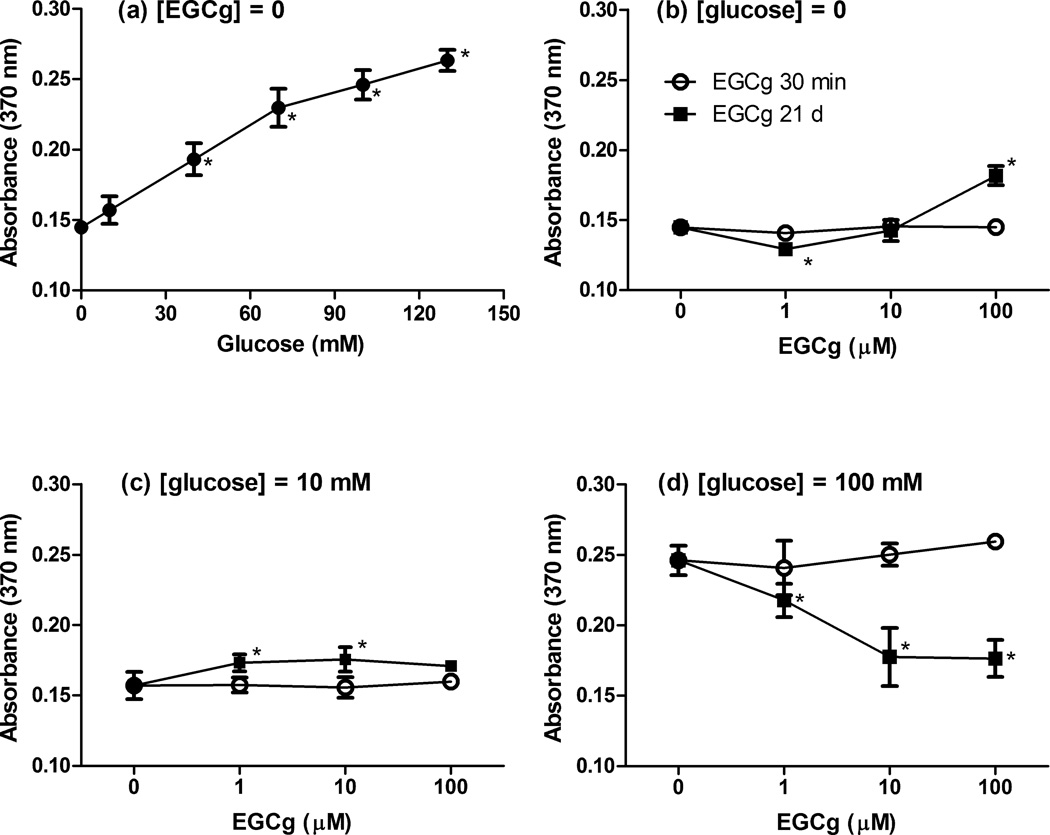
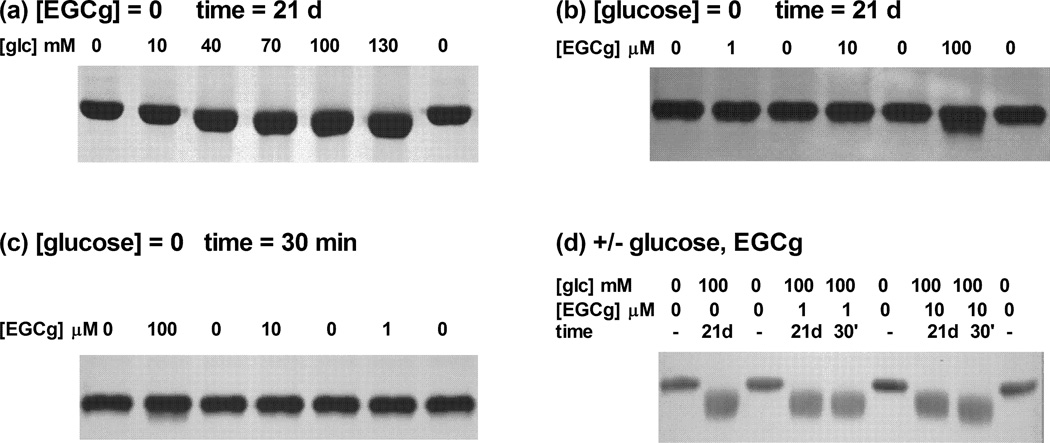
HSA glycation was positively associated with glucose concentrations over the range 10–100 mM. Very low levels of glucose (1 mM) did not modify HSA during the 21 day reaction, but significant glycation-induced fluorescence was noted after treatment with 10 mM glucose, with the reaction product yielding an RFI value ~1.5-fold higher than that of the native protein, while 100 mM glucose yielded a 15-fold increase (Fig. 1a). The DNPH assay and native PAGE were less sensitive than fluorescence, with detectable carbonyls or mobility shifts noted after reaction with at least 40 mM glucose (Fig. 2a, 3a).
Figure 1. Effects of EGCg on the fluorescence of native or glycated HSA.
HSA (200 µM) was incubated for 21 days with different levels of glucose (0–100 mM) in pH 7.4 PBS at 37 °C. Reactions were carried out without added EGCg (a) or with EGCg (b–d) added during the 21 day reaction (■) or after the glycation reaction (○). RFI was calculated as the ratio of the fluorescence of the glycated protein at λex = 330 nm, λem = 410 nm to that of the native HSA, * indicates a statistical difference between the sample protein and its respective control: native HSA (a, b); HSA treated with 10 mM glucose (c) or HSA treated with 100 mM glucose (d).
Figure 2. Effects of EGCg on the carbonyl content of native or glycated HSA.
HSA (200 µM) was incubated for 21 days with different levels of glucose (0–100 mM) in pH 7.4 PBS at 37 °C. Reactions were carried out without added EGCg (a) or with EGCg (b–d) added during the the 21 day reaction (■) or after the glycation reaction (○). Carbonyl content was measured as DNPH reaction products at 370 nm. * indicates a statistical difference between the sample protein and its respective control: native HSA (a, b); HSA treated with 10 mM glucose (c) or HSA treated with 100 mM glucose (d).
Figure 3. Effects of EGCg on the electrophoretic mobility of native or glycated HSA.
HSA (200 µM) was incubated with different levels of glucose (0–130 mM) and/or EGCg (0–100 µM) and/or EGCg in pH 7.4 PBS at 37 °C. Reaction times were 21 days for glucose (a) or 21 days (b) or 30 min (c) for EGCg. For samples treated with both glucose and EGCg (d) the glycation time was 21 days, and treatment with EGCg was either during glycation (21 days) or after glycation (30 min). Electrophoretic mobility was evaluated with native PAGE gels (a, 12% gel; b–d, 7.5% gel) followed by Coomassie blue staining.
While low concentrations of EGCg (1 and 10 µM) in the absence of glucose did not affect native HSA, 100 µM EGCg altered the protein in ways that were qualitatively similar to glucose-induced glycation. The RFI and carbonyl content of native HSA did not increase after treatment with 1 or 10 µM EGCg (Fig.1b and 2b), consistent with results from native PAGE (Fig.3b and 3c). Interestingly, HSA incubated 21 days with 1 µM EGCg and no glucose had significantly lower carbonyl content than the native protein, suggesting that very low levels of EGCg might protect proteins against some spontaneous oxidative damage. In samples incubated with 100 µM EGCg, RFI, carbonyl content, and electrophoretic mobility were significantly increased, indicating pro-oxidative reactions between EGCg and HSA (Fig. 1b, 2b, 3b, 3c). One exception to this trend was noted for Eg-30m with 100 µM EGCg, which did not have an elevated carbonyl content.
When the glucose concentration was 10 mM, HSA glycation was somewhat enhanced in Eg-21d samples at the highest concentration of EGCg (Fig. 1c). The carbonyl contents of Eg-21d samples for 1 µM and 10 µM EGCg were only slightly higher than the levels for gHSA (Fig. 2c), but the increases were significant. However, there was no change in carbonyls at the highest level of EGCg (Fig. 2c).
Under the same mild glycemic pressure, Eg-30m did not affect carbonyl formation or glycation induced fluoresence at low concentrations of EGCg (1 and 10 µM). The 30 min treatment with 100 µM EGCg substantially increased glycation-induced fluorescence but not carbonyl levels (Fig.1c and 2c).
When the glucose level was increased to 100 mM, incubation with EGCg for the entire 21 day glycation period exerted protective activity in our HSA/glucose model. The development of glycation-induced fluorescence was inhibited in a dose-dependent manner by EGCg between 10 to 100 µM (Fig. 1d). The RFI value of gHSA samples was 12.8-fold larger than that of the native protein. Addition of 100 µM EGCg reduced the RFI value to 2.4, suggesting suppression of 80% of the glycation-derived fluorophores. The carbonyl contents were significantly lower in Eg-21d samples than in gHSA samples (Fig. 2d). The lowest concentration of EGCg slightly inhibited the formation of protein carbonyls under high glycemic pressure, while more inhibition was achieved at 10 and 100 µM EGCg. However, Eg-21d did not inhibit changes in the electrophoretic mobility of HSA samples (Fig. 3d).
Under the same high glycemic conditions, treatment with EGCg for 30 min after the 21 day glycation reaction exerted a restorative effect in our HSA/glucose model. Eg-30m at all tested EGCg concentrations quenched ~50 % of the glycation-induced fluorescence of gHSA. The RFI values for Eg-30m samples with 1 and 10 µM EGCg were much smaller than those of Eg-21d samples with the same levels of EGCg (Fig. 1d). There was no evidence for a dose-dependent response to EGCg in the 30 min incubation. Furthermore, Eg-30m could not eliminate protein carbonyls derived from glycation (Fig. 2d) or restore electrophoretic mobility to glycated HSA (Fig. 3d).
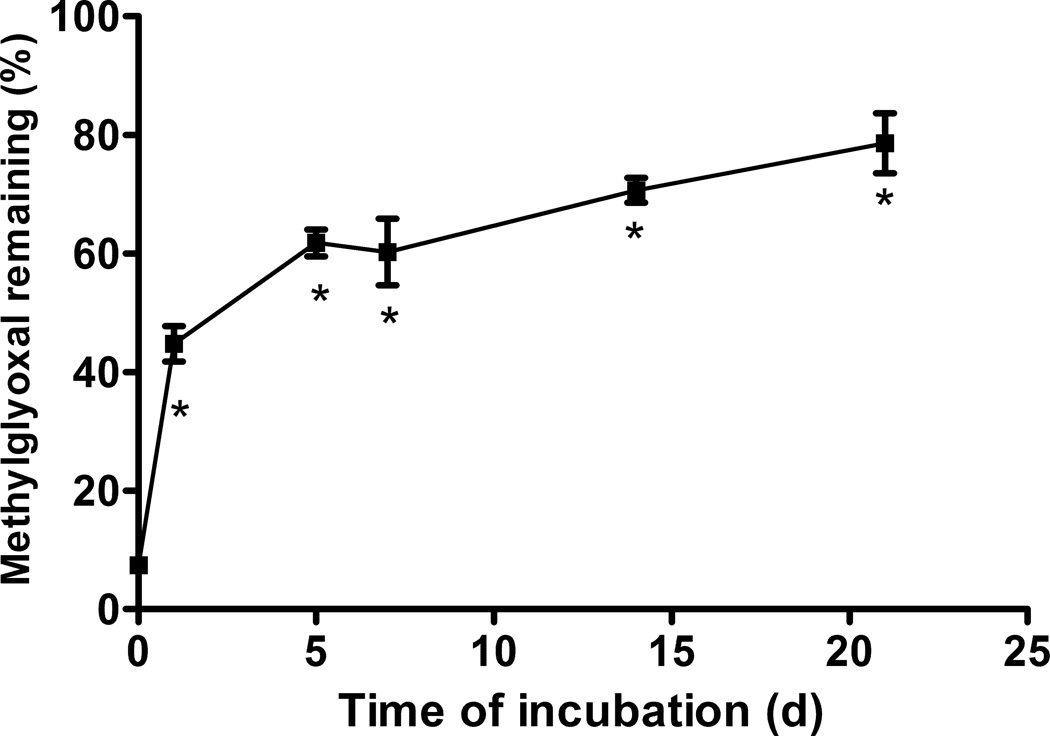
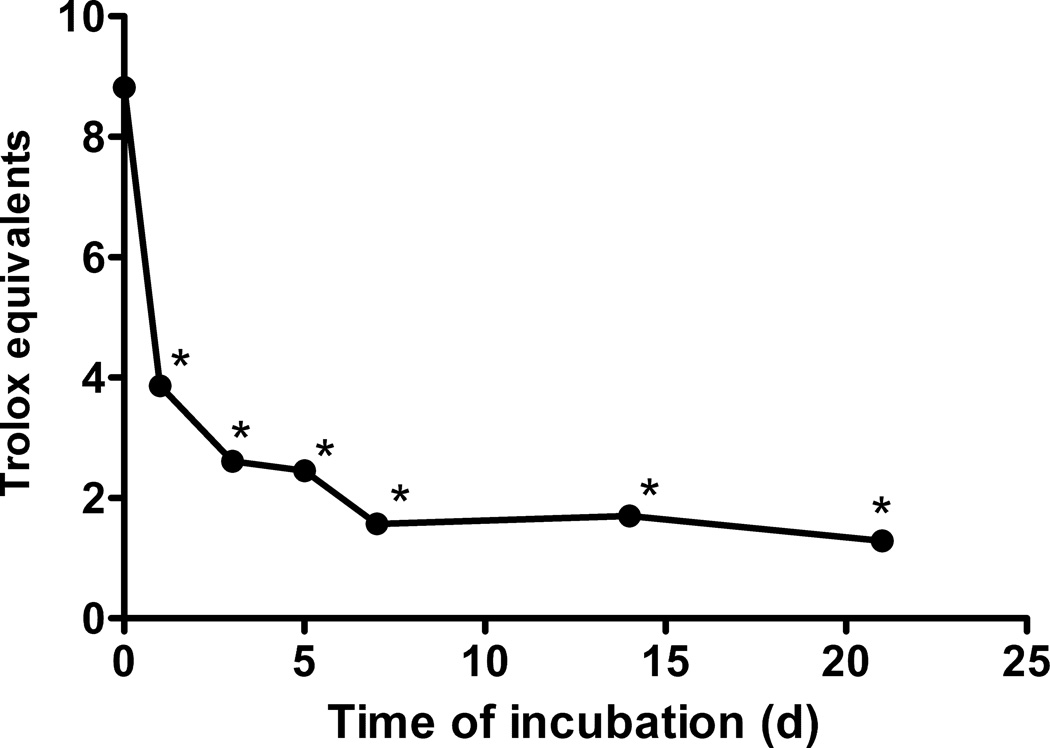
The ability of aged EGCg to scavenge methylglyoxal was diminished in a time-dependent fashion (Fig. 4). Fresh EGCg destroyed 93% of the added methylgyoxal, but after 7 days of aging, EGCg could only eliminate 40% of the methylglyoxal. Scavenging ability continued to decline over the remainder of the incubation, with only 21% of the methyl glyoxal scavenged by 21 day old EGCg (Fig. 4). Antioxidant activity also diminished with age of the EGCg solution (Fig. 5).
Figure 4. Effect of incubation time on the methylglyoxal scavenging ability of EGCg.
EGCg (1 mM) was incubated for up to 21 days in pH 7.4 PBS at 37 °C before determining methyl glyoxal scavenging activity.. * indicates a statistical difference between the sample and fresh EGCg.
Figure 5. Effect of incubation time on the antioxidant activity of EGCg.
EGCg (1 mM) was incubated for up to 21 days in pH 7.4 PBS at 37 °C before determining radical scavenging activity with the ABTS assay. The IC50 values were expressed as Trolox equivalents. * indicates a statistical difference between the sample and fresh EGCg.
Discussion
Our study reveals important effects of EGCg on protein glycation under physiological conditions. We found for the first time that EGCg not only inhibits protein glycation, but also reverses glycation when the time and concentrations of the reactions are controlled in a fashion that mimics the physiological condition. We also found that in the absence of glucose, high concentrations of EGCg damage protein in ways that resemble glycation reactions. These data support the hypothesis that EGCg is a bifunctional molecule that can protect or damage other biomolecules, perhaps because it has both pro- and antioxidant properties with potential to directly protect or damage biomolecules, and to indirectly control stress responses [7].
We devised a new model for testing EGCg that is physiologically more relevant than previous models. EGCg is a molecule with very short half life [19], so that very little EGCg is found in the blood stream 24 h after ingestion. Glycation is a very slow reaction [28], requiring weeks for the protein to accumulate detectable damage. Our system tests EGCg added for 30 min after the glycation reaction, to focus on the physiologically realistic effect of ingested EGCg on pre-existing glycated serum proteins.
We demonstrated that we could detect protein glycation when glucose levels were held within the physiologically normal range for healthy and diabetic individuals (1–30 mM) [17]. Although we could detect glycation damage when glucose levels were as low as 10 mM, the protein was more heavily damaged when glucose levels were artificially high (100 mM), similar to other studies of glycation at various concentrations of sugar [29]. Our methods for determining protein carbonyls or for electrophoretically evaluating changes in protein charge density are less sensitive than fluorescence, but show the same trend with glucose concentration.
We note that our results with EGCg as a modifier of glycation are dependent on the glycation pressure and the EGCg concentration and are a consequence of the interaction between the polyphenol and the protein. When a small amount of EGCg was added for a short period to protein that was slightly glycated (10 mM glucose) there was little effect, but if a larger amount of EGCg was added, glycation damage was increased. This finding is consistent with earlier reports that 100 µM EGCg stimulates protein carbonyl formation in HSA [30]. We propose that the protein binding activity of EGCg [31] provides a mechanistic basis for the concentration-dependent effects of EGCg on lightly glycated protein. We suggest that when the concentration of EGCg is low and the protein is relatively undamaged, the polyphenol binds specifically to the hydrophobic pocket of the protein [32], and does not damage the protein. However, if excess EGCg is added, nonspecific binding to the protein is initiated, leading to damage similar to that induced by glycation. The mechanism for damage includes the well-known formation of covalent bonds between the electron-dense B ring of the flavonoid and electrophilic groups on the protein such as amines or thiols [33,34], analogous to glycation reactions at these residues [9]. Increased carbonyl content is a consequence of the irreversible oxidative deamination of the polyphenol-protein adduct [35], while increases in fluorescence are attributed to the association of the protein with the highly aromatic polyphenol [33,34].
We provide the first evidence that if protein is heavily glycated (100 mM glucose) before treatment with EGCg, then short treatment with EGCg can reverse the damage. A 30 min treatment with 1 µM EGCg quenched 55% of the glycation-induced fluorescence of 200 µM gHSA, which suggests a catalytic mechanism for the polyphenol. The nucleophilic A and/or B rings of the flavonoid might selectively target the positively charged AGEs, such as glyoxal- and methylglyoxal-induced lysine dimers, and might then carry out catalytic desctruction of AGES by mechanisms similar to those reported for some thiazolium and pyridium AGES breakers [36]. An alternative to the catalytic mechanism is transglycation reaction between the nucleophilic polyphenol and the glycosylamine precursor to AGES [37]. Given the potential of EGCg to reverse glycative damage, further experiments to identify potential reaction products that would elucidate mechanism of action are warranted.
However, the EGCg-mediated restorative effect was not reflected by all measures of glycation. Some fluorescent AGEs were resistant to EGCg-mediated restoration even at high levels of EGCg, and the carbonyl content and electrophoretic mobility of glycated HSA were not changed by the short treatment with EGCg. The remaining fluorescent AGEs may include vesperlysines and pentosidine, which are chemically more stable than other AGEs [38]. Carbonylation irreversibly alters the affected protein [39], so persistence of this damage marker despite the EGCg treatment is not surprising. We conclude that EGCg-mediated restoration only applies to certain glycative modifications and is limited by EGCg and AGEs structure and reactivity. More work is needed to establish pathways for destruction of AGEs by brief treatment with polyphenols such as EGCg, and to establish whether destruction of the AGEs has positive effects on protein activity and physiological parameters.
We compared our model to more typical models in which the EGCg is added at the beginning of the several week-long glycation reaction. Effects of EGCg in this reaction system are not due to the parent compound, but to the complex mixture of oxidative and degradative products of EGCg reaction [40]. When EGCg was added to the reaction at the beginning of glycation, the level of glycation-induced damage increased slightly if the glucose concentration was at physiological level. The mechanisms for increased glycation damage may include reactions between EGCg and the protein similar to those described above. A more likely mechanism for increased damage when EGCg is present during the prolonged glycation reaction is reaction of the protein with EGCg degradation products. For example, polyphenols like EGCg participate in the formation of hydrogen peroxide in the presence of trace amounts of reactive metal ions via Fenton reactions [41,42], and hydrogen peroxide facilitates albumin glycation [43]. We propose that long exposure to EGCg enhances HSA glycation by producing hydrogen peroxide. This result contrasts with the typical view of polyphenols as beneficial antioxidants [2,6], but is consistent with previous reports that EGCg damages proteins [18,44]. Because our work shows that long incubation of EGCg converts it to species with pro-oxidant activity, future work should employ methods that mimic the in vivo situation of limited exposure of plasma proteins to the short-lived EGCg molecule.
When the glucose concentration was very high (100 mM), then EGCg added to the glycation reaction protected the protein from damage, similar to previous research [6]. EGCg is an effective scavenger of reactive aldehydes such as methyl glyoxal and is a radical scavenging agent [36]. We demonstrated novel reactivity for the EGCg decomposition products by showing that they are able to scavenge carbonyls and radicals, providing a mechanism for long-term protection of protein by a short-lived parent compound. Given the likely importance of EGCg decomposition products in vivo, further characterization of their reactivities with important biological species is justified.
We found that EGCg in the absence of glycating agent altered the protein in ways similar to glycation, especially in the short incubation experiments. As described above, covalent modification of protein by EGCg can yield protein carbonyls and fluorescent species. Although the data demonstrate that EGCg can potentially damage proteins, ingestion of dietary EGCg is normally safe. The amount of EGCg found in a typical diet is low (<50 mg/day) [45], and only a small fraction of the ingested EGCg is absorbed from the digestive tract [20]. Most of the absorbed EGCg is modified by glucuronidation, methylation and sulfation during absorption, which attenuates its reactivity [46]. Furthermore, EGCg has a short half-life in vivo [20], preventing its accumulation. However, our study indicates that using EGCg as a supplement beyond the levels found naturally in foods and beverages could lead to unfavorably high levels in the blood stream with possible negative effects on serum proteins such as HSA.
In conclusion, EGCg is a redox active compound exhibiting both pro-oxidant and antioxidant activities. Our study clearly showed that bioactivities of EGCg in an HSA/glucose model were determined by glucose level, EGCg concentration and time of exposure. In a physiologically relevant environment (mild glycemic pressure, low EGCg concentration and brief exposure of the glycated protein to EGCg), EGCg did not affect carbonyl content of HSA. However, fluorescence of glycated HSA was partially reversed by EGCg, suggesting a potential restorative effect. Although in vivo experimentation will be required to test the therapeutic implications of our observations, our in vitro study provides preliminary evidence for an important positive effect of food-derived polyphenols within reaction constraints imposed by instability of the polyphenols. However, our work also suggests that dietary supplements of reactive polyphenols such as EGCg should be used with caution to avoid pro-oxidant, damaging activities in individuals with normal or mildly elevated glycemic pressure.
Acknowledgments
The work was partially supported by NIDDK R15DK069285.
Footnotes
Declaration of interest. The authors report no declarations of interest.
References
- 1.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochemical Pharmacology. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thielecke F, Boschmann M. The potential role of green tea catechins in the prevention of the metabolic syndrome - A review. Phytochemistry. 2009;70:11–24. doi: 10.1016/j.phytochem.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Hagerman AE, Riedl KM, Rice RE. Tannins as biological antioxidants. In: Gross GG, Hemingway RW, Yoshida T, editors. Plant Polyphenols 2: Chemistry, Biology, Pharmacology, Ecology. New York City: Kluwer Academic/Plenum; 1999. pp. 495–505. [Google Scholar]
- 4.Montonen J, Knekt P, Jarvinen R, Reunanen A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care. 2004;27:362–366. doi: 10.2337/diacare.27.2.362. [DOI] [PubMed] [Google Scholar]
- 5.Sang S, Shao X, Bai N, Lo CY, Yang CS, Ho CT. Tea polyphenol (−)-epigallocatechin-3-gallate: A new trapping agent of reactive dicarbonyl species. Chemical Research in Toxicology. 2007;20:1862–1870. doi: 10.1021/tx700190s. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa T, Yokozawa T, Terasawa K, Shu S, Juneja LR. Protective activity of green tea against free radical- and glucose-mediated protein damage. Journal of Agricultural and Food Chemistry. 2002;50:2418–2422. doi: 10.1021/jf011339n. [DOI] [PubMed] [Google Scholar]
- 7.Yiannakopoulou EC. Targeting oxidative stress response by green tea polyphenols: clinical implications. Free Radical Research. 2013;47:667–671. doi: 10.3109/10715762.2013.819975. [DOI] [PubMed] [Google Scholar]
- 8.Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: From bench to bedside. Molecular Aspects of Medicine. 2012;33:209–290. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Rondeau P, Bourdon E. The glycation of albumin: Structural and functional impacts. Biochimie. 2011;93:645–658. doi: 10.1016/j.biochi.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Yoshiuchi K, Matsuhisa M, Katakami N, Nakatani Y, Sakamoto K, Matsuoka T, Umayahara Y, Kosugi K, Kaneto H, Yamasaki Y, et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocrine Journal. 2008;55:503–507. doi: 10.1507/endocrj.k07e-089. [DOI] [PubMed] [Google Scholar]
- 11.Kisugi R, Kouzuma T, Yamamoto T, Akizuki S, Miyamoto H, Someya Y, Yokoyama J, Abe I, Hirai N, Ohnishi A. Structural and glycation site changes of albumin in diabetic patient with very high glycated albumin. Clinica Chimica Acta. 2007;382:59–64. doi: 10.1016/j.cca.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Saito K, Hamano K, Nakagawa M, Yugawa K, Muraoka J, Kuba H, Furukawa K, Azuma T. Conformational analysis of human serum albumin and its non-enzymatic glycation products using monoclonal antibodies. Journal of Biochemistry. 2011;149:569–580. doi: 10.1093/jb/mvr007. [DOI] [PubMed] [Google Scholar]
- 13.Shaklai N, Garlick RL, Bunn HF. Nonenzymatic glycosylation of human-serum albumin alters its conformation and function. Journal of Biological Chemistry. 1984;259:3812–3817. [PubMed] [Google Scholar]
- 14.Cohen MP, Shea E, Chen S, Shearman CW. Glycated albumin increases oxidative stress, activates NF-kappa B and extracellular signal-regulated kinase (ERK), and stimulates ERK-dependent transforming growth factor-beta(1) production in macrophage RAW cells. Journal of Laboratory and Clinical Medicine. 2003;141:242–249. doi: 10.1067/mlc.2003.27. [DOI] [PubMed] [Google Scholar]
- 15.Nin JW, Jorsal A, Ferreira I, Schalkwijk CG, Prins MH, Parving H-H, Tarnow L, Rossing P, Stehouwer CD. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes a 12-year follow-up study. Diabetes Care. 2011;34:442–447. doi: 10.2337/dc10-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujiwara Y, Kiyota N, Tsurushima K, Yoshitomi M, Mera K, Sakashita N, Takeya M, Ikeda T, Araki T, Nohara T, et al. Natural compounds containing a catechol group enhance the formation of N-(carboxymethyl)lysine of the Maillard reaction. Free Radical Biology and Medicine. 2011;50:883–891. doi: 10.1016/j.freeradbiomed.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 17.McFarland KF, Catalano EW, Day JF, Thorpe SR, Baynes JW. Non-enzymatic glucosylation of serum-proteins in diabetes-mellitus. Diabetes. 1979;28:1011–1014. doi: 10.2337/diab.28.11.1011. [DOI] [PubMed] [Google Scholar]
- 18.Elbling L, Weiss R-M, Teufelhofer O, Uhl M, Knasmuller S, Schulte-Hermann R, Berger W, Michksche M. Green tea extract and (−)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. FASEB Journal. 2005;19:807. doi: 10.1096/fj.04-2915fje. [DOI] [PubMed] [Google Scholar]
- 19.Zimeri J, Tong C. Degradation kinetics of (−)-epigallocatechin gallate as a function of pH and dissolved oxygen in a liquid model system. Journal of Food Science. 1999;64:753–758. [Google Scholar]
- 20.Van Amelsvoort JMM, Hof KHV, Mathot J, Mulder TPJ, Wiersma A, Tijburg LBM. Plasma concentrations of individual tea catechins after a single oral dose in humans. Xenobiotica. 2001;31:891–901. doi: 10.1080/00498250110079149. [DOI] [PubMed] [Google Scholar]
- 21.Lakowicz JR. Principles of fluorescence spectroscopy. New York: Springer; 2006. p. 954. [Google Scholar]
- 22.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz Anke G, Ahn B-W, Shaltiel S, Stadtman Earl R. Determination of carbonyl content in oxidatively modified proteins. Methods in Enzymology. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli UK. Cleavage of structural proteins during the assembly of the hear of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 25.Riedl KM, Hagerman AE. Tannin-protein complexes as radical scavengers and radical sinks. Journal of Agricultural and Food Chemistry. 2001;49:4917–4923. doi: 10.1021/jf010683h. [DOI] [PubMed] [Google Scholar]
- 26.Glantz SA. Primer of biostatistics. New York: McGraw Hill; 2002. p. 312. [Google Scholar]
- 27.Wisman KN, Perkins AA, Jeffers MD, Hagerman AE. Accurate assessment of the bioactivities of redox-active polyphenolics in cell culture. Journal of Agricultural and Food Chemistry. 2008;56:7831–7837. doi: 10.1021/jf8011954. [DOI] [PubMed] [Google Scholar]
- 28.van Boekel M. Kinetic aspects of the Maillard reaction: a critical review. Nahrung-Food. 2001;45:150–159. doi: 10.1002/1521-3803(20010601)45:3<150::AID-FOOD150>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Voziyan PA, Khalifah RG, Thibaudeau C, Yildiz A, Jacob J, Serianni AS, Hudson BG. Modification of proteins in vitro by physiological levels of glucose - Pyridoxamine inhibits conversion of Amadori intermediate to advanced glycation end-products through binding of redox metal ions. Journal of Biological Chemistry. 2003;278:46616–46624. doi: 10.1074/jbc.M307155200. [DOI] [PubMed] [Google Scholar]
- 30.Ishii T, Mori T, Ichikawa T, Kaku M, Kusaka K, Uekusa Y, Akagawa M, Aihara Y, Furuta T, Wakimoto T, et al. Structural characteristics of green tea catechins for formation of protein carbonyl in human serum albumin. Bioorganic & Medicinal Chemistry. 2010;18:4892–4896. doi: 10.1016/j.bmc.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Hagerman AE. Fifty years of polyphenol-protein complexes. Recent Advances in Polyphenol Research. 2012;3:71–97. [Google Scholar]
- 32.Li M, Hagerman AE. Role of the flavan-3-ol and galloyl moieties in the interaction of (−)-epigallocatechin gallate with serum albumin. Journal of Agricultural and Food Chemistry. 2014;62:3768–3775. doi: 10.1021/jf500246m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao D, Zhang YJ, Zhang HH, Zhong LW, Qian XH. Systematic characterization of the covalent interactions between (−)-epigallocatechin gallate and peptides under physiological conditions by mass spectrometry. Rapid Communications in Mass Spectrometry. 2009;23:1147–1157. doi: 10.1002/rcm.3985. [DOI] [PubMed] [Google Scholar]
- 34.Trombley JD, Loegel TN, Danielson ND, Hagerman AE. Capillary electrophoresis methods for the determination of covalent polyphenol-protein complexes. Analytical and Bioanalytical Chemistry. 2011;401:1523–1529. doi: 10.1007/s00216-011-4846-1. [DOI] [PubMed] [Google Scholar]
- 35.Ishii T, Ichikawa T, Minoda K, Kusaka K, Ito S, Suzuki Y, Akagawa M, Mochizuki K, Goda T, Nakayama T. Human serum albumin as an antioxidant in the oxidation of (−)-epigallocatechin gallate: Participation of reversible covalent binding for interaction and stabilization. Bioscience Biotechnology and Biochemistry. 2011;75:100–106. doi: 10.1271/bbb.100600. [DOI] [PubMed] [Google Scholar]
- 36.Aldini G, Vistoli G, Stefek M, Chondrogianni N, Grune T, Sereikaite J, Sadowska-Bartosz I, Bartosz G. Molecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end products. Free Radical Research. 2013;47:93–137. doi: 10.3109/10715762.2013.792926. [DOI] [PubMed] [Google Scholar]
- 37.Szwergold BS, Howell SK, Beisswenger PJ. Transglycation - A potential new mechanism for deglycation of Schiff's bases. Annals of the New York Academy of Sciences. 2005;1043:845–864. doi: 10.1196/annals.1333.097. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura K, Nakazawa Y, Ienaga K. Acid-stable fluorescent advanced glycation end products: Vesperlysines A, B, and C are formed as crosslinked products in the maillard reaction between lysine or proteins with glucose. Biochemical and Biophysical Research Communications. 1997;232:227–230. doi: 10.1006/bbrc.1997.6262. [DOI] [PubMed] [Google Scholar]
- 39.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clinical Chemistry. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka T, Watarumi S, Matsuo Y, Kamei M, Kouno I. Production of theasinensins A and D epigallocatechin gallate dimers of black tea, by oxidation-reduction dismutation of dehydrotheasinensin A. Tetrahedron. 2003;59:7939–7947. [Google Scholar]
- 41.Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, Riechel TL. High molecular weight plant polyphenolics (tannins) as biological antioxidants. Journal of Agricultural and Food Chemistry. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- 42.Lambert JD, Kwon SJ, Hong J, Yang CS. Salivary hydrogen peroxide produced by holding or chewing green tea in the oral cavity. Free Radical Research. 2007;41:850–853. doi: 10.1080/10715760601091659. [DOI] [PubMed] [Google Scholar]
- 43.Vlassopoulos A, Lean MEJ, Combet E. Role of oxidative stress in physiological albumin glycation: A neglected interaction. Free Radical Biology and Medicine. 2013;60:318–324. doi: 10.1016/j.freeradbiomed.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Hagerman AE, Dean RT, Davies MJ. Radical chemistry of epigallocatechin gallate and its relevance to protein damage. Archives of Biochemistry and Biophysics. 2003;414:115–120. doi: 10.1016/s0003-9861(03)00158-9. [DOI] [PubMed] [Google Scholar]
- 45.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. American Journal of Clinical Nutrition. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 46.Sang SM, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacological Research. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]