Summary
We recently reported that novel ring-substituted analogs of 3,3′-diindolylmethane (ring-DIMs) have anti-androgenic and growth inhibitory effects in androgen-dependent prostate cancer cells. The objectives of this study were to confirm the ability of 4,4′- and 7,7′-dibromo- and dichloro-substituted ring-DIMs to inhibit androgen-stimulated proliferation of androgen-dependent LNCaP human prostate cancer cells using a non-invasive, real-time monitoring technique. In addition, their ability to induce apoptotic and necrotic cell death in androgen-dependent as well as -independent (PC-3) prostate cancer cells was studied. Prostate cancer cells were treated with increasing concentrations of DIM and ring-DIMs (0.3–30 μM) and effects on cell proliferation were measured in real-time using an xCELLigence cellular analysis system. Chromatin condensation and loss of membrane integrity were determined by Hoechst and propidium iodide staining, respectively. Apoptotic protein markers were measured by immuno-blotting and activation of caspases determined using selective fluorogenic substrates. Intra- and extracellular concentrations of DIM and ring-DIMs were assessed by electrospray ionization tandem mass spectrometry. Ring-DIMs inhibited androgen-stimulated LNCaP cell proliferation and induced apoptosis and necrosis in LNCaP and PC-3 cells with 2–4 fold greater potencies than DIM. DIM and the ring-DIMs increased caspases −3, −8 and −9 activity, elevated expression of Fas, FasL, DR4 and DR5 protein, and induced PARP cleavage in both cell lines. The cytotoxicity of the most potent ring-DIM, 4,4′-dibromoDIM, but not the other compounds was decreased by an inhibitor of caspase −3. The 4,4′-dibromoDIM was primarily found in the extracellular medium, whereas all other compounds were present to a much larger extent in the cell. In conclusion, ring-DIMs inhibited prostate cancer cell growth and induced cell death in LNCaP and PC-3 cells with greater potencies than DIM; they also structure-dependently activated different cell death pathways suggesting that these compounds have clinical potential as chemopreventive and chemotherapeutic agents in prostate cancer, regardless of hormone-dependency.
Keywords: Prostate cancer, Androgen-dependent, Androgen-independent, Diindolylmethane derivatives, LNCaP, PC-3, Apoptosis, Necrosis, Caspase activity, Intracellular, Concentrations
Introduction
Prostate cancer accounts for approximately 30 % of all newly diagnosed cancers in the United States and is the second highest cause of cancer-related deaths in males [1]. Most prostate cancers are initially androgen-dependent (AD) and are treated with a combination of radiotherapy, chemical castration, androgen-receptor (AR) antagonists (hydroxyflutamide, bicalutamide), inhibitors of steroidogenesis (abiraterone) and, in extreme cases, surgery. Unfortunately, a large contingent of AD cancers will progress to become androgen-independent (AI) and treatment-resistant, resulting in the metastases of the tumour and a high incidence of morbidity and mortality. Furthermore, patients treated with either hydroxyflutamide or bicalutamide suffer from severe side-effects as a result of the antihormone therapy [2, 3].
Diet is an important risk factor in the development of prostate cancer and high fruit and vegetable intake is associated with a decreased risk of this disease [4, 5]. DIM (3,3′-diindolylmethane) is produced in the stomach after ingestion of vegetables of the Brassica family, such as broccoli, cauliflower and Brussels’ sprouts, which contain large amounts of indole-3-carbinol (I3C). In the stomach, I3C is converted via acid-catalyzed reactions to various condensation products, including what is considered to be its most biologically active metabolite, DIM [6, 7]. Current evidence indicates that DIM acts as an anticancer agent as it inhibits the growth of a multitude of cancer cell types in vitro and in vivo [8, 9] and has produced positive responses in clinical trials for the treatment of prostate cancer when applied as an absorption-enhanced formulation [10]. DIM is thought to inhibit cancer cell proliferation through several distinct yet overlapping mechanisms. By selectively binding and down-regulating AR activity, reducing AR-specific genes expression [11–13] and by inhibiting signaling through pro-survival regulators such as PI3K, Akt, mTOR and GSK3β, DIM inhibits cancer cell proliferation [14–18]. DIM also induces cancer cell death by increasing the intracellular flux of calcium ions, resulting in the induction of endoplasmic reticulum (ER) stress genes [19–21], and induces apoptosis through up-regulation of Fas and FasL or activation of death receptor 5 (DR5), leading to activation of caspase-dependent extrinsic apoptosis pathways [22–24]. However, despite these observations, several facets of research into this natural compound remain untouched. The diverse molecular targets through which DIM is assumed to exert its anti-proliferative and pro-apoptotic effects have not been identified and DIM-induced necrosis has not been examined. Moreover, the search for compounds exhibiting higher potency and specificity towards prostate tumours is ongoing, and we have studied a number of methyl-substituted derivatives of DIM in other cancerous tissues [19, 25–30]. More recently, we have begun to test halogenated forms of the compound in AD prostate cancer cell models [31].
We have previously shown that a series of 4,4′- and 7,7′-dichlorinated and –dibrominated ring-substituted DIM analogs (ring-DIMs) inhibits the growth of LNCaP AD human prostate cancer cells [31] and that all four ring-DIMs were able to selectively inhibit AR transactivation up to 9 times more effectively than DIM itself. Although the four compounds inhibited androgen-stimulated LNCaP cell proliferation and PSA secretion, only the brominated ring-DIMs decreased expression of AR protein and mRNA as well as nuclear AR accumulation, whereas the chlorinated ring-DIMs did not affect AR expression and nuclear translocation, suggesting these compounds exert their antiandrogenic and growth inhibitory effects via different sets of cellular targets. We hypothesize that in addition to antiandrogenic mechanisms, the ring-DIMs may exert their antiproliferative effect via mechanisms such as stimulation of pro-apoptotic and/or necrotic pathways resulting in cell death. We therefore characterized the cytotoxic effects of four dihalogenated ring-DIMs in AD (LNCaP) and AI (PC-3) human prostate cancer cells.
Materials and methods
Cell lines and reagents
LNCaP cells and PC-3 human prostate cancer cells were purchased from ATCC (Manassas, VA). LNCaP cells were grown in RPMI 1640 supplemented with 10 % fetal bovine serum or 2 % dextran-coated charcoal-stripped FBS, 2 mM L-glutamine, 1 % HEPES, 1 % sodium-pyruvate and 10 ml/L of 100x antibiotic-antimycotic solution while PC-3 cells were grown in a 1:1 mixture of DMEM and Ham’s F12 Nutrient Mixture with either 10 % fetal bovine serum or 2 % dextran-coated charcoal-stripped FBS, 2 mM L-glutamine and 10 ml/L of 100x antibiotic-antimycotic solution (Sigma-Aldrich, St-Louis, MO). Cells were maintained in a humidified atmosphere (5 % CO2) at 37 °C. Ring-substituted 4,4′- and 7,7′-dihaloDIMs were provided by Dr. S. Safe, Texas A&M University, at >95 % purity and were dissolved in DMSO to make 100 mM stock solutions. Dihydrotestosterone (DHT; Steraloids Inc., Newport, RI) was dissolved in DMSO to make 100 mM solutions. The final concentration of DMSO in culture was not greater than 0.2 %. Ac-DEVD-AFC, Ac-IETD-AFC and Ac-LEHD-AFC were purchased from Enzo Life Sciences (Farmingdale, NY) and diluted in DMSO to make 20 mM stock solutions.
Treatment of cells with ring-DIMs, DHT, z-DEVD-fmk, z-IETD-fmk or z-LEHD-fmk
Stock solutions of the ring-DIMs and DHT of 100 mM were made in 100 % dimethyl sulfoxide (DMSO). The ring-DIM stock solutions were diluted 1000-fold to the indicated final concentrations of each compound in the respective culture medium of LNCaP and PC-3 cells supplemented with either 10 % fetal bovine serum or 2 % dextran-coated charcoal-stripped FBS.. In co-exposure experiments with DHT, the hormone was added to LNCaP cells after serial dilution in DMSO to a working stock solution of 100 nM, thus resulting in a final DHT concentration of 0.1 nM in culture medium containing 0.2 % DMSO. Control cells were incubated in 0.1 % or 0.2 % DMSO only, where appropriate. z-DEVD-fmk, z-IETD-fmk or z-LEHD-fmk (BD Biosciences, Franklin Lakes, NJ) were dissolved in 100 % DMSO to produce 10 mM stock solutions and added to cell cultures 4 h prior to treatment with either ring-DIMs or DMSO vehicle, resulting in a final concentration of 5 μM in culture medium containing 0.2 % DMSO.
Growth inhibition of LNCaP cells
LNCaP cells were seeded in 16-well E-plates (Roche Diagnostics, Laval, QC) at a density of 25000 cells per 200 μl medium/well. After 24 h, DHT (0.1 nM) was added along with various concentrations of the ring-DIMs or DMSO vehicle and cells were allowed to grow for an additional 72 h under label-free conditions and measurements were taken in real-time using an xCELLigence cellular analysis system (Roche). The average growth rate of the treated cells was calculated as a percentage of control using measurements from at least 3 replicate experiments.
Apoptosis and necrosis measurements
For cell death measurements (apoptotic and necrotic), LNCaP and PC-3 cells were seeded in 24-well plates in 2 % stripped-FBS. Cells were then treated with (LNCaP) or without (PC-3) 0.1 nM DHT and several concentrations of ring-DIMs or DMSO alone. After 48 h, Hoechst 33342 (Sigma-Aldrich) and propidium iodide (PI; Invitrogen, Carlsbad, CA) were each added at a concentration of 1 μg/ml per well, and the plates were incubated for 15 min at 37 °C. Cells were then observed and counted under a Nikon Eclipse (TE-2000U) inverted fluorescent microscope at 20x magnification. Hoechst-positive and PI-positive cells were visualized using filter cubes with an excitation wavelength of 330–380 nm and 532–587 nm, respectively.
Caspase activity assays
PC-3 and LNCaP cells were seeded in 6-well plates in 2.0 ml culture medium containing 2 % stripped FBS and 24 h later they were treated with various concentrations of ring-DIMs for another 24 h. Proteins were then extracted from harvested cells using 1x RIPA buffer (Millipore, Billerica, MA) containing 1x protease inhibitor cocktail, centrifuged at 13,000 g for 5 min at 4 °C to remove cell debris, and frozen at −80 °C overnight. Protein concentrations were then quantified using a BCA protein detection kit (Thermo Scientific, Waltham, MA). Caspase activities were determined using fluorogenic caspase substrates selective for either caspase-3 (5 μM Ac-DEVD-AFC), caspase-9 (10 μM Ac-LEHD-AFC) or caspase-8 (10 μM Ac-IETD-AFC) in 10 μg of extracted protein suspended in caspase reaction buffer (20 mM PIPES pH 7.2, 30 mM NaCl, 10 mM DTT, 1 mM EDTA, 0.1 % CHAPS, 10 % sucrose). The time-dependent release of 7-amino-4-trifluoromethyl coumarin (AFC) was measured using a SpectroMax M5 microplate reader (Molecular Devices, Sunnydale, CA) at an excitation wavelength of 400 nm and an emission wavelength of 505 nm. Measurements were recorded at 2 min intervals for 90 min. A standard curve of AFC fluorescence was used to calculate the amount of AFC released (in picomoles) during each reaction.
SDS-PAGE and immunoblotting
Crude protein extracts (50 μg) were resolved by electrophoresis using 10 % sodium dodecyl sulfate-polyacrylamide gels and then transferred to a PVDF Immobilon-P membrane (Bio-Rad, Mississauga, ON). Blots were blocked using 5 % milk powder (Brand name, Jean Talon market, Montréal, QC) and incubated with antibodies using a 1:1000 dilution for anti-cleaved PARP (Cell Signaling, Beverly, MA), a 1:1000 dilution for anti-Fas, anti-FasL (Cell Signaling) and anti-DR5 (Cedarlane, Burlington, ON), a 1:500 dilution for anti-DR4 (Millipore), a 1:500 dilution for anti-Bid (Cell Signaling) and a 1:2500 dilution for anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoreactive proteins were exposed to anti-rabbit or anti-mouse horseradish peroxidise-conjugated secondary antibodies (Millipore) that were diluted 1:5000. Antigen-antibody complexes were detected using Immobilon ECL Western Chemiluminescent HRP Substrate (Millipore) and recorded with a Versadoc imaging system (Bio-Rad). Total protein loading was determined using 1x Amido Black staining solution (Sigma-Aldrich).
Mass spectrometric measurement of ring-DIMs and DIM
Mass spectrometry-based analysis of DIM and ring-DIMs was performed using a method previously reported [32]. In brief, the ring-DIMs were extracted by a modified Bligh-Dyer method [32] from cells precipitated by centrifugation for 5 min at 16,000×g at 4 °C and from the supernatant of cultural medium. The extracted lipids were dried under nitrogen and resuspended in chloroform. Immediately prior to injection, the extracted lipids were combined with a 2:1 (v/v) methanol:chloroform mixture supplemented with 0.1 % (v/v) ammonium hydroxide. The samples were injected directly into a Thermo Orbitrap Velos mass spectrometer equipped with a HESI-II ion source (Thermo Scientific, Waltham, MA, USA) at a flow rate of 5 μl/min. Spectra were obtained in positive-ion mode. The source voltage was set to 3.9 kV, a capillary temperature of 275 °C and a sheath gas flow of 5 (arbitrary units). Acquired spectra were analyzed using Xcalibur software (Thermo Scientific).
Statistical analyses
All experiments were performed in triplicate and the data are presented as mean±SEM. Statistically significant differences (P<0.05) between sample groups were calculated using a two-tailed Student’s t-test. IC50 values for growth inhibition and induction of cell death were calculated using a nonlinear curve-fit analysis. All analyses were performed using GraphPad Prism v5.03 (GraphPad Software, San Diego, CA).
Results
Ring-DIMs inhibit DHT-stimulated LNCaP cell proliferation with greater efficiency than DIM
Previously, we showed that ring-DIMs inhibited the growth of DHT-stimulated LNCaP cells [31] with IC50 values between 2- and 3.5-fold lower than DIM. In this earlier study, cell growth was assessed by measuring mitochondrial re-ductase activity after a 96 h exposure to the ring-DIMs. Here, we independently confirmed the inhibitory effects of ring-DIMs on DHT-stimulated LNCaP cell proliferation in real-time using an xCELLigence label-free cell analysis system. Inhibition of androgen-dependent cell proliferation by the ring-DIMs occurred rapidly, before signs of cytotoxicity and was particularly apparent after 48 h of treatment when the stimulatory effects of DHT were increasingly pronounced (Fig. 1a, b) with potencies differing in a structure-dependent manner. Notably, both 7,7′-dibromo-(IC50=0.40±0.23 μM) and 7,7′-dichloroDIM (IC50=0.64±0.18 μM) were most effective at inhibiting DHT-stimulated proliferation of LNCaP cells, with about 2–2.5 times greater potencies than DIM (IC50=0.94±0.26 μM). 4,4′-dibromoDIM (IC50=0.81±0.28 μM), but not 4-4′-dichloroDIM (IC50=1.36±0.42 μM), was still more effective than DIM, but was less potent than the 7,7′-dihaloDIMs.
Fig. 1.
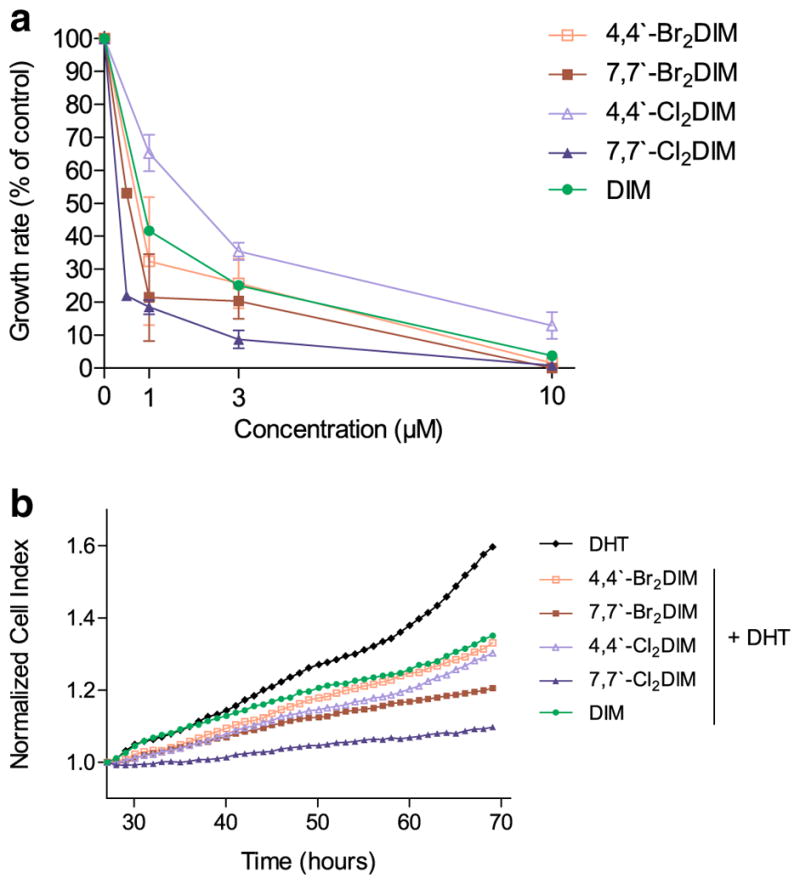
Ring-DIMs and DIM inhibit proliferation of LNCaP human prostate cancer cells. a Relative growth rates of LNCaP cells grown in stripped RPMI 1640 medium without phenol-red and co-treated with 0.1 nM DHT and increasing concentrations (0.5–10 μM) of 4,4′-dibromoDIM (□), 7,7′-dibromoDIM (■), 4,4′-dichloroDIM (△), 7,7′-dichloroDIM (▲) or DIM (●). Growth rates are expressed as percentages of the growth rate obtained with 0.1 nM DHT alone and are presented as mean±SEM of three determinations per treatment. b Effect of 1.0 μM of the ring-DIMs or DIM on 0.1 nM DHT-stimulated LNCaP cell proliferation in real-time using an xCELLigence cellular analysis system
Ring-DIMs induce apoptosis and necrosis in LNCaP and PC-3 prostate cancer cells
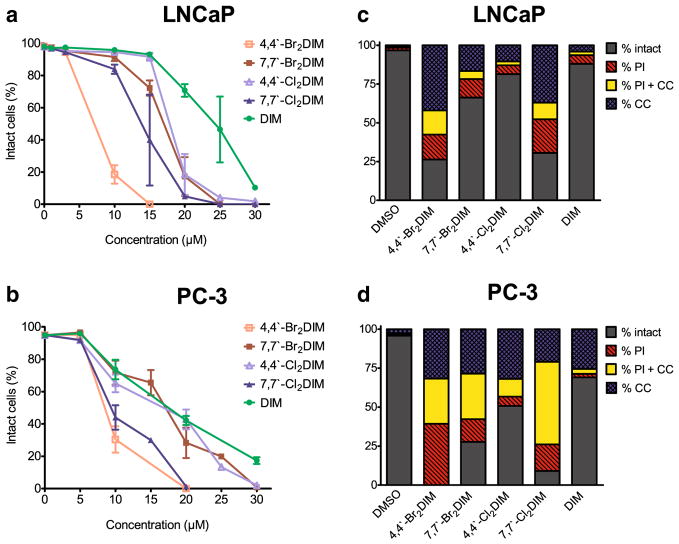
A 48 h exposure to the ring-DIMs resulted in a concentration-dependent decrease in the number of intact LNCaP (Fig. 2a) or PC-3 (Fig. 2b) cells and the ring-DIMs were consistently more effective at killing these cells than DIM (IC50 values were 23.8 μM ±0.4 in LNCaP cells and 18.3 μM±1.0 in PC-3 cells). 4,4′-dibromoDIM was the most potent inducer of LNCaP and PC-3 cell death, with IC50 values of 7.5±0.1 μM and 8.9±0.3 μM, respectively, while IC50 values for 4,4′-dichloro (18.1±0.3 μM and 16.0 ±1.3 μM), 7,7′-dibromo (16.8±0.3 μM and 16.6±0.8 μM), and 7,7′-dichloro (13.9±0.2 μM and 10.5±0.9 μM) were lower than observed for DIM. DIM and ring-DIMs induced nuclear chromatin condensation (CC), a hallmark of apoptosis, and increased the number of cells with permeabilized membranes (PI positive), indicative of necrosis, in both LNCaP (Fig. 2c) and PC-3 (Fig. 2d) cells. Furthermore, a population of cells with combined condensed chromatin and permeabilized membranes (CC+PI) was observed (Fig. 2c and d) and 4,4′-dibromoDIM produced the largest number of these cells in LNCaP and PC-3 cell lines.
Fig. 2.
Ring-DIMs kill AD and AI prostate cancer cells via apoptotic and necrotic forms of cell death. The percentage of intact LNCaP cells (a) and PC-3 cells (b) that do not have fragmented nuclei, condensed chromatin, or propidium iodide staining was calculated 48 h after treatment with increasing concentrations (5–30 μM) of 4-4′-dibromo-DIM (□), 7-7′-dibromo-DIM (■), 4,4′-dichloroDIM (△), 7,7′-dichloroDIM (▲) or DIM (●). Fragmented nuclei and condensed chromatin are indicative of apoptosis, whereas propidium iodide staining is indicative of plasma membrane permeabilization and necrosis. Percentages are represented as mean ± SEM (n=3–5). (c) The percentage of LNCaP cells showing condensed chromatin (CC), propidium iodide staining (PI), or both (CC+PI) after a 48 h treatment with either 10 μM 4,4′-dibromoDIM, 15 μM 7,7′-dibromoDIM, 15 μM 4,4′-dichloroDIM, 15 μM 7,7′-dichloroDIM or 15 μM DIM. (D) The percentage of PC-3 cells showing CC, PI, or CC+PI after a 48 hourtreatment with either 20 μM of the ring-DIMs or DIM
Ring-DIMs increase the levels of caspase-3 activity in LNCaP and PC-3 cells
As indicated above, DIM and ring-DIMs induced both apoptotic- and necrotic forms of cell death. To discern whether the main executioner proteases of apoptosis, caspase-3 was involved in the cytotoxicity of the ring-DIMs, its catalytic activity was measured using the selective fluorogenic substrate, Ac-DEVD-AFC.
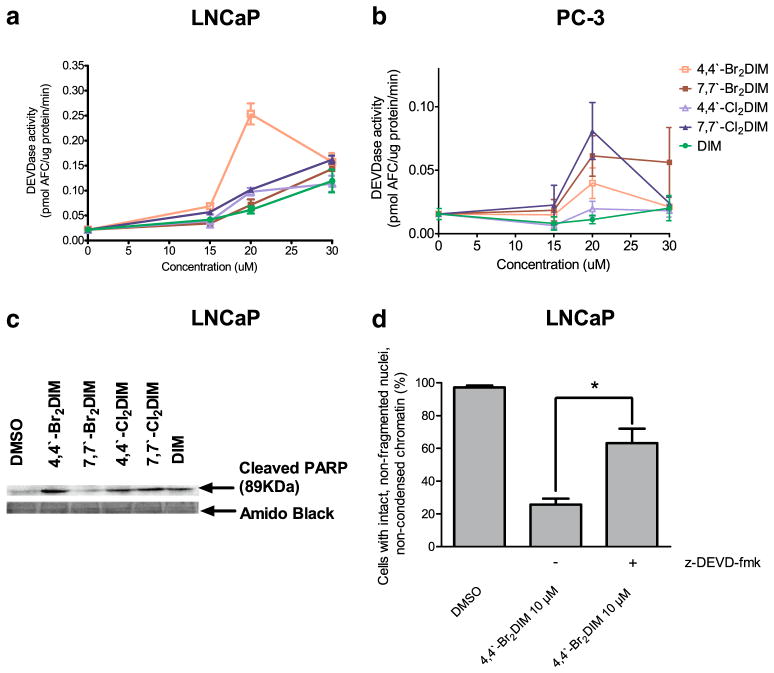
We observed increases of DEVDase activity in both LNCaP and PC-3 cells after 24 h of exposure to various concentrations of DIM and the ring-DIMs, with catalytic activity significantly increased by the most toxic ring-DIM, 4,4′-dibromoDIM at 20 μM (Fig. 3a). The other ring-DIMs and DIM were less effective, indicating a correlation between caspase-3 activation and the degree of cell death induced by these compounds. In PC-3 cells only modest increases in DEVDase activity were measured after exposure to the ring-DIMs. In these cells, 7,7′-dichloro- and 7,7′-dibromoDIM increased DEVDase activity most effectively at 20 μM, and not 4,4′-dibromoDIM, although the ring-DIMs were still more effective than DIM. The increased efficacy of 7,7′-dichloroDIM to induce caspase-3 activity in PC-3 cells relative to the other ring-DIMs (Fig. 3b) is consistent with its relatively greater cytotoxic potency in this cell line (Fig. 2b) compared to LNCaP cells (Fig. 2a). PC-3 cells treated with 20 μM of the least toxic compounds, DIM and 4,4′-dichloroDIM, had the lowest levels of DEVDase activity, Thus, with the exception of 4,4′-dibromoDIM, there was a correlation between the degree of cytotoxicity of the ring-DIMs and their ability to activate effector caspase-3. We also found increases in PARP cleavage in LNCaP cells treated with 20 μM of each ring-DIMs as well as DIM (Fig. 2c). Moreover, by pre-incubating cells with the selective caspase-3 inhibitor z-DEVD-fmk, we found that the number of apoptotic LNCaP cells was decreased after exposure to cytotoxic concentrations of 4,4′-dibromoDIM (Fig. 3d), but not 7,7′-dibromoDIM, 4,4′-dichloroDIM, 7,7′-dichloroDIM or DIM (not shown).
Fig. 3.
Ring-DIMs increase the activity of executioner caspase-3 in AD and AI prostate cancer cells. Activity of caspase-3 was measured by cleavage of the fluorogenic substrate Ac-DEVD-AFC in response to a 24 h treatment of LNCaP cells (a) and PC-3 cells (b) with increasing concentrations (15–30 μM) of 4,4′-dibromoDIM (□), 7,7′-dibromoDIM (■), 4,4′-dichloroDIM (△), 7,7′-dichloroDIM (▲) or DIM (●). (c) Cleavage of poly(ADP-ribose) polymerase after a 24 h exposure of LNCaP cells to 20 μM of ring-DIMs or DIM. (d) Inhibition of LNCaP cell death after a 24 h exposure to 10 μM 4,4′-dibromoDIM in the presence of 10 μM of the membrane permeable caspase-3 inhibitor z-DEVD-fmk. In A, B and D, data are represented as mean±SEM (n=3–5); (*) indicates a statistically significant difference between the two treatment groups (Student t-test; p<0.05)
Ring-DIMs trigger extrinsic and intrinsic pathways of cell death
Caspase-3 can be activated in a variety of ways, most notably through activation of the intrinsic and extrinsic apoptotic pathways. The extrinsic pathway involves activation of cell surface death receptors, with recruitment of the initiator caspase-8 to the plasma membrane where it is cleaved, dimerized and activated [33–35]. The extrinsic pathway can be linked to the intrinsic pathway, where cytochrome c released from the mitochondria results in activation of caspase-9 [36–39]. In both pathways, the activation of the initiator caspases-8 and -9 results in the downstream cleavage and activation of caspase-3 [40, 41].
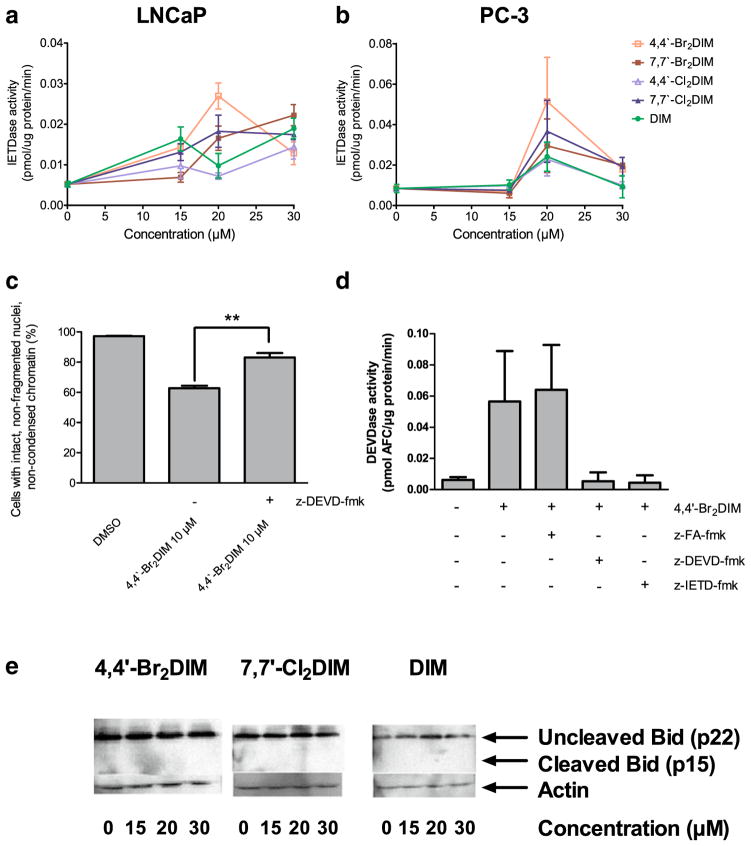
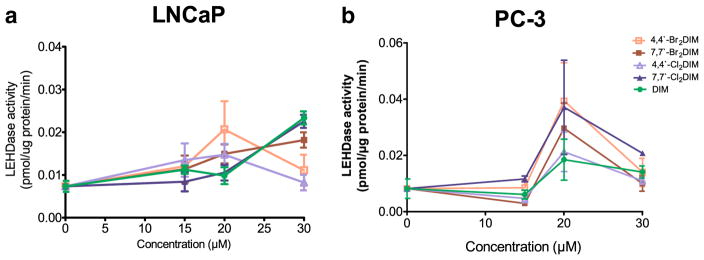
We found concentration-dependent increases in caspase-8 and -9 activity in LNCaP and PC-3 cells treated with 20 μM DIM or the ring-DIMs, with highest levels of activity coinciding with cytotoxic concentrations of the compounds (Figs. 4 and 5). As observed for caspase-3, 4,4′-dibromoDIM-treated cells had the greatest caspase-8 and -9 activity at a concentration of 20 μM, compared to treatment with the other compounds. The post-maximal decrease of caspases activity observed in 4,4′-dibromoDIM-treated LNCaP and PC-3 cells coincided with loss of cells due to its potent cytotoxicity; this was also observed with the second most cytotoxic ring-DIM, 7,7′-dichloroDIM, in LNCaP cells. We found that cell death induced by 4,4′-dibromoDIM was, at least partially, due to activation of caspase-8, as treatment of LNCaPs with both 4,4′-dibromoDIM and z-IETD-fmk resulted in a decrease in the number of apoptotic cells (Fig. 4c). Moreover, treatment of LNCaPs with 4,4′-dibromoDIM and z-IETD-fmk resulted in a marked reduction in the activity of caspase-3, while treatment with z-FA-fmk did not (4D). However, treatment with either brominated or chlorinated ring-DIMs, or DIM itself, did not increase the levels of the 15KDa fragment of Bid (Fig. 4e).
Fig. 4.
Ring-DIMs increase the activity of the initiator caspase-8 in AD and AI prostate cancer cells. Activity of caspase-8 was measured by cleavage of the fluorogenic substrate Ac-IETD-AFC in response to a 24 h treatment of LNCaP cells (a) and PC-3 cells (b) with increasing concentrations (15–30 μM) of 4,4′-dibromoDIM (□), 7,7′-dibromoDIM (■), 4,4′-dichloroDIM (△), 7,7′-dichloroDIM (▲) or DIM (●). Inhibition of LNCaP cell death (c) and caspase-3 activity (d) after a 24 h exposure to 10 μM 4,4′-dibromoDIM in combination with 10 μM of the membrane permeable caspase-8 inhibitor z-IETD-fmk. (e) Cleavage of Bid after a 24 h exposure of LNCaP cells to 10 μM 4,4′-dibromoDIM or 20 μM 7,7′-dichloroDIM or DIM. In A, B, C and D, data are represented as mean ± SEM (n=3–5); *p<0.05
Fig. 5.
Ring-DIMs increase the activity of the initiator caspase-9 in AD and AI prostate cancer cells. Activity of caspase-9 was measured by cleavage of the fluorogenic substrate Ac-LEHD-AFC in response to a 24 h treatment of LNCaP cells (a) and PC-3 cells (b) with increasing concentrations (15–30 μM) of 4,4′-dibromoDIM (□), 7,7′-dibromoDIM (■), 4,4′-dichloroDIM (△), 7,7′-dichloroDIM (▲) or DIM (●)
Intra/extracellular distribution of ring-DIMs
We also investigated whether differences in cytotoxic potency and possibly mechanism of action of DIM and ring-DIMs were due to differences in intracellular concentrations attained in cell culture. We found a significant percentage of DIM and each ring-DIM inside both LNCaP and PC-3 cells after 24 h of exposure (Table 1). About 20–30 % of DIM was found in the cellular fraction of both cell lines. Whereas exposure to 7,7′-dibromo-, 4,4′-dichloro- or 7,7′-dichloroDIM resulted in intracellular levels of ≥38 % (LNCaP) and ≥29 % (PC-3) of the amount of compound added to the cell culture media, cells treated with 4,4′-dibromoDIM contained 2–5 times lower intracellular levels of this most cytotoxic derivative compared to the other ring-DIMs (Table 1). Moreover, in LNCaP cells, intracellular levels of 7,7′-dihaloDIMs were twice as high as that of DIM.
Table 1.
Extra/intracellular distribution of ring-DIMs and DIM in LNCaP and PC-3 human prostate cancer cells in culture. Cells were separated from cultural media and the compounds were extracted and their concentrations measured by mass spectrometry as described in Materials and Methods. Percentages are presented as means±SD of three independent experiments
| Cell Line | Compound | % of Compound Recovered
|
|
|---|---|---|---|
| Medium | Cells | ||
| LNCaP | 4-4′-Br2DIM | 85.18±5.83 | 14.82±5.83 |
| 7-7′-Br2DIM | 32.05±0.47 | 67.94±0.47 | |
| 4-4′-Cl2DIM | 61.80±2.06 | 38.19±2.06 | |
| 7-7′-Cl2DIM | 40.29±4.99 | 59.71±4.99 | |
| DIM | 63.92±3.26 | 36.08±3.26 | |
| PC-3 | 4-4′-Br2DIM | 93.73±2.60 | 6.27±2.60 |
| 7-7′-Br2DIM | 76.17±9.40 | 23.83±9.40 | |
| 4-4′-Cl2DIM | 67.21±5.99 | 32.78±5.99 | |
| 7-7′-Cl2DIM | 70.51±11.35 | 29.49±11.35 | |
| DIM | 77.2±3.74 | 22.80±3.74 | |
Ring-DIMs up-regulate expression of cell surface death receptors
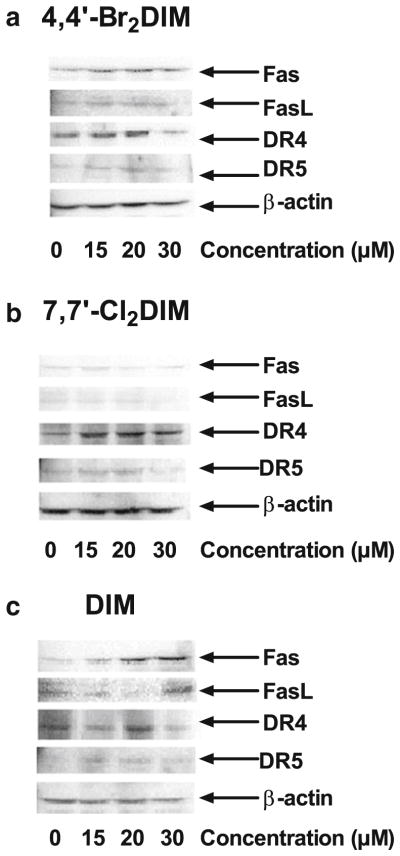
We found that exposure of LNCaP cells to DIM and ring-DIMs resulted in a marked increase in the protein levels of the cell surface death receptors DR4/TRAIL-R1 and DR5/TRAIL-R2, and Fas (Fig. 6). Exposure of LNCaP cells to 4,4′-dibromoDIM (Fig. 6a) and DIM (Fig. 6c) resulted in a concentration-dependent increase in protein levels of Fas and FasL, with 4,4′-dibromoDIM being effective at a lower concentration than DIM. Interestingly, no increase in expression of Fas or FasL was found in cells treated with 7,7′-dichloroDIM (Fig. 6b). However, LNCaP cells treated with either 4,4′-dibromoDIM, 7,7′-dichloroDIM or DIM all had moderately increased expression levels of DR4 and DR5.
Fig. 6.
Ring-DIMs up-regulate expression of cell surface death receptors. LNCaP cells were treated with increasing concentrations (15–30 μM) of either 4,4′-dibromoDIM (a), 7,7′-dichloroDIM (b) or DIM (c) for 24 h and protein levels of Fas, FasL, DR4, and DR5 were assayed by western blot
Discussion
In this study we report that dihalo-ring-substituted derivatives of DIM inhibit androgen-dependent prostate cancer cell growth with significantly greater potency than DIM. We previously showed that ring-DIMs are potent inhibitors of androgen-dependent LNCaP prostate cancer cell growth and that these effects could, in part, be explained by antagonistic actions at the level of androgen receptor nuclear translocation, transactivation and gene transcription [31]. Our present study expands these findings by demonstrating that the ring-DIMs, at slightly greater concentrations than those that inhibit androgen-dependent cell proliferation, induce cell death in androgen-independent PC-3 cells as well as LNCaP cells and that this occurs via apoptotic and necrotic mechanisms in both cell types.
Ring-DIMs structure-dependently inhibit androgen-dependent prostate cancer cell growth
This study shows that brominated and chlorinated synthetic analogs of DIM inhibit androgen-dependent prostate cancer cell proliferation with a greater potency than DIM (Fig. 1), as previously reported [31]. The brominated and chlorinated derivatives of DIM inhibited AR-dependent cellular signalling through different mechanisms, and results of this study show that their effects on cellular proliferation also differ. Whereas the mechanism of inhibition of AR signalling appeared to be dependent on the type of halogen atoms at the 4,4′ or 7,7′ positions of the DIM base structure with the brominated DIMs, but not the chlorinated DIMs, blocking AR nuclear translocation, their effects on proliferation appeared to depend primarily on the location of the halogen atoms. This finding implies that certain ring-DIMs, such as 7,7′-dibromo-DIM, a moderate inhibitor of AR trans-activation but potent antiproliferative compound, might antagonize other growth and survival pathways not necessarily dependent on the AR. These antiproliferative pathways, which appear to be independent of the cell death induction discussed below, are currently under further investigation.
Ring-DIMs induce death of androgen-dependent and –independent prostate cancer cells
Results of this study show that brominated and chlorinated DIMs were more effective than DIM at inducing apoptosis and necrosis in LNCaP and PC-3 cells. Previous studies have found greater pro-apoptotic responses to other synthetic DIM derivatives. For example, a set of 1,1-bis(3′-indolyl)-1-(p-substituted-phenyl)methanes (C-DIMs), carrying para-trifluoromethyl-, t-butyl-, phenyl-, cyano-, or methoxy-groups have previously been shown to activate anti-proliferative and pro-apoptotic pathways, and this was due in part to their activity as ligands for peroxisome proliferator-activated receptor γ (PPARγ) and NR4A1 (TR3/Nur77); these same compounds also induced ER stress in pancreatic, ovarian and colon cancer cells [19, 25, 27–29]. Other chemically modified dietary compounds such as brominated vanillin (a flavour compound) and chlorinated flavonoids have been shown to kill Jurkat leukemia cells and hepatocarcinoma cells, respectively, at concentrations lower than the parent molecule by activating down-stream effectors of apoptosis such as caspase-3, resulting in nuclear DNA fragmentation and chromatin condensation [42, 43]. We observed similar effects with brominated and chlorinated ring-DIMs, since all four compounds not only induced chromatin condensation and increased plasma membrane permeability more effectively than DIM (Fig. 2), but they also induced caspase-3 activity to higher levels (Fig. 3a, b). Moreover, there appears to be an AR-independent component to ring-DIM induced cell death since both AR positive (LNCaP) and AR negative (PC-3) cells were sensitive to similar concentrations of the ring-DIMs. The 4,4′-dibromoDIM was the most potent cytotoxic compound and strongest caspase activator in each cell line, and inhibition of LNCaP cell death using the cell permeable caspase-3 inhibitor z-DEVD-fmk was only observed when using this synthetic DIM derivative. This suggests that addition of bromine atoms to the 4- and 4′-carbon of DIM confers a greater affinity to this compound for molecular targets involved in initiating the observed caspase-dependent cell death. In contrast, the other derivatives and DIM itself did not display this cytotoxic characteristic, favouring caspase-independent apoptosis instead. Indeed, caspase-9 was activated in both cell lines treated with any of the five compounds (Fig. 5a, b), an observation consistent with mitochondrial outer membrane permeabilization (MOMP) and fragmentation. Such fragmentation would lead to the release from the mitochondrial inner membrane of not just cytochrome c, which is required for formation of the apoptosome and activation of caspase-9, but other pro-apoptotic proteins, such as apoptosis inducing factor (AIF) and endonuclease G, that do not require caspase activation in order to initiate apoptosis [44–47]. Also, although 4,4′-dibromoDIM was the strongest activator of caspase-9 in LNCaP and PC-3 cells, inhibition of cell death with z-DEVD-fmk was incomplete. Therefore, although 4,4′-dibromoDIM induced higher levels of caspase-dependent cell death than other ring-DIMs, induction of caspase-independent pathways is also important for the activity of this DIM analog.
The fact that the ring-DIMs increase caspase-8 activity in LNCaP and PC-3 cells (Fig. 4c, d) indicates that, in addition to activating the intrinsic mitochondria-controlled pathway of apoptosis, they also induce cell death via cell surface death receptors. Several death receptors have been implicated in DIM-induced apoptosis in studies of cancer cells other than those of prostate, most notably Fas and death receptor 5 (DR5 or TRAIL-R2). We found that DIM at 30 μM increased levels of Fas and FasL in LNCaP cells, possibly through activation of ATF3 as was observed with a 1-(p-substituted-phenyl)methane derivative of DIM in pancreatic cells [30], and treatment with z-IETD-fmk, a cell permeable caspase-8 inhibitor, abrogated DIM-induced apoptosis in LNCaP cells [22]. Although inhibition of caspase-3 did not reduce cell death by DIM and three of the four ring-DIMs, it is possible that the penultimate step in cell death occurs via caspase-8 activation as cell death and caspase-3 activation induced by 4,4′-dibromoDIM were abrogated in the presence of the caspase-8 inhibitor, z-IETD-fmk. However, activation of caspase-8 and caspase-9 likely occurs via separate pathways as Bid was not found to be cleaved by any of the ring-DIMs or DIM. Moreover, our results are consistent with these prior studies as treatment of LNCaP cells with the most potent ring-DIM, 4,4′-dibromoDIM or DIM, but not 7,7′-dichloroDIM, resulted in increased levels of Fas and FasL. Treatment with any of these three compounds elevated the levels of DR4, an observation that has not been reported previously in any cell type exposed to DIM, and of DR5, which has not been reported in prostate cancer cells exposed to DIM or any of its various derivatives. In pancreatic and colon cancer cells, DIM and 1,1-bis(3′-indolyl)-1-(p-tert-butylphenyl)methane were shown to induce a thapsigargin(Tg)-like unfolded protein response (UPR), as they significantly increased markers of ER stress, such as calcium release from the ER into the cytoplasm, cleavage of ATF6 and levels of GRP78 and CHOP, leading to a CHOP-dependent increase in DR5 expression and concomitant activation of caspase-8 [19, 27]. Moreover, in the prostate cancer cell line DU145, addition of DIM at a concentration of 25 μM was shown to mobilise stores of calcium from the ER, and cell death induced by DIM was dependent on cytoplasmic increases in calcium [20]. It is possible then that the ring-DIMs, particularly 4,4′-dibromoDIM, may have greater potencies than DIM itself due to a combination of factors, including: 1) stimulation of a greater UPR, 2) elevation of expression levels of pro-apoptotic death receptors proteins, such as DR4, DR5, Fas and its ligand FasL, 3) increased ability to directly activate cell surface receptors DR4, DR5 and/or Fas (which is suggested by the greater preference of 4,4′-dibromoDIM for the exterior cell environment), and 4) specific targeting of mitochondrial membrane disruption through Bcl-2 downregulation or Bax upregulation, thereby initiating the intrinsic pathway of apoptosis independent of DR4, DR5 and Fas (a possible preferred mechanism of 7,7-dihaloDIMs, which attain high intracellular levels).
One factor that has been mostly overlooked with regards to DIM-induced cell death is the appearance of necrotic cells. Here we show that DIM and ring-DIMs increase the percentage of necrotic cells after a 48 h period of exposure (Fig. 2c, d) and that ability of DIM and ring-DIMs to enter cells is to a certain extent dependent on their molecular structure. In addition to the pro-apoptotic targets that ring-DIMs preferentially act upon, it may be the case that these compounds also have non-specific interactions with prostate cancer cell membranes, thereby inducing a necrotic form of cell death.
Structure-activity relationships of ring-DIMs differ between anti-proliferative and pro-apoptotic effects
Our studies of the inhibition of cell proliferation by ring-DIMs show that the derivatives most potent at arresting androgen-dependent growth of LNCaP cells were 7,7′-dichloro- and 7,7′-dibromoDIM. These data are consistent with the effects previously seen on LNCaP cell proliferation and AR transactivation, as the most effective ring-DIMs were found to be the 7,7′-dihalo-substituted ring-DIMs [31]. Our current study found intracellular levels of these ring-DIMs to be similar in LNCaP cells, suggesting that their anti-proliferative targets are inside the cell. Interestingly, we found the cytotoxic potencies of the ring-DIMs to differ from their ability to inhibit cell proliferation. The 4,4′-dichloroDIM was the least potent ring-DIM with respect to induction of cell death, yet substitution of chlorine by bromine atoms, although decreasing its ability to enter LNCaP and PC-3 cells, increased the potency of the compound to induce cell death compared to the other three analogs. Additionally, 4,4′-dibromoDIM was the most potent inducer of caspase-3 activity and in LNCaP cells it induced Fas and FasL at lower concentrations than the other analogs or DIM. These observations imply that, in contrast to the other compounds, the caspase-dependent pro-apoptotic effects of 4,4′-dibromoDIM are due to its increased ability to interact with and activate death-receptors or other plasma membrane receptors, thereby preferentially activating caspase-8, and decreasing non-specific induction of necrosis. Taken together, these results provide evidence for a dynamic interplay between molecular structural and the ability of a particular ring-DIM to either kill prostate cancer cells or arrest cell growth, where the position of the halogen atom appears to determine the compound’s potency with respect to inhibition of cell growth and –survival pathways. Moreover, the identity of the halogen atom as well as its position on the indole ring are crucial factors in determining the ability of the ring-DIMs to target apoptotic and necrotic pathways, independent of cell cycle machinery.
Conclusions
Our results confirm that ring-DIMs exhibit potent anti-proliferative effects on LNCaP prostate cancer cells. We show that ring-DIMs have pro-apoptotic and pro-necrotic effects on both AD and AI prostate cancer cells and are able to kill these cells with a greater potency and efficacy than DIM. Our studies indicate that 4,4′-dibromoDIM is the most effective at killing prostate cancer cells, and addition of bromine to the 4 and 4′ positions in the indole ring enhances the selectivity of the analog for caspase-dependent targets. Future studies will concentrate on discerning these upstream pro-apoptotic and anti-proliferative targets, and determine the effectiveness and mechanisms of action of the ring-DIMs using in vivo models of prostate cancer.
Acknowledgments
This work was made possible by an operating grant from the Canadian Institutes of Health Research (CIHR grant no. MOP-115019).
Footnotes
The authors declare to have no conflict of interest.
Contributor Information
A. A. Goldberg, INRS – Institut Armand-Frappier, Université du Québec, 531, boulevard des Prairies, Laval, QC, Canada
V. I. Titorenko, Department of Biology, Concordia University, Montreal, QC, Canada
A. Beach, Department of Biology, Concordia University, Montreal, QC, Canada
K. Abdelbaqi, INRS – Institut Armand-Frappier, Université du Québec, 531, boulevard des Prairies, Laval, QC, Canada
S. Safe, Veterinary Physiology and Pharmacology, A&M University, College Station, TX, USA, Institute for Bioscience and Technology, Houston, TX, USA
J. T. Sanderson, INRS – Institut Armand-Frappier, Université du Québec, 531, boulevard des Prairies, Laval, QC, Canada
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Wysowski DK, Freiman JP, Tourtelot JB, Horton ML., 3rd Fatal and nonfatal hepatotoxicity associated with flutamide. Ann Intern Med. 1993;118:860–864. doi: 10.7326/0003-4819-118-11-199306010-00006. [DOI] [PubMed] [Google Scholar]
- 3.McLeod DG. Tolerability of Nonsteroidal Antiandrogens in the Treatment of Advanced Prostate Cancer. Oncologist. 1997;2:18–27. [PubMed] [Google Scholar]
- 4.Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. Journal of the National Cancer Institute. 2000;92:61–68. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2002;42:1–9. doi: 10.1207/S15327914NC421_1. [DOI] [PubMed] [Google Scholar]
- 6.Bjeldanes LF, Kim J-Y, Grose KR, Bartholomew JC, Bradfield CA. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci USA. 1991;88:9543–9547. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Kruif CA, Marsman JW, Venekamp JC, Falke HE, Noordhoek J, Blaauboer BJ, Wortelboer HM. Structure elucidation of acid reaction products of indole-3-carbinol: detection in vivo and enzyme induction in vitro. Chem-Biol Interact. 1991;80:303–315. doi: 10.1016/0009-2797(91)90090-t. [DOI] [PubMed] [Google Scholar]
- 8.Ge X, Yannai S, Rennert G, Gruener N, Fares FA. 3,3′-Diindolylmethane induces apoptosis in human cancer cells. Biochem Biophys Res Commun. 1996;228:153–158. doi: 10.1006/bbrc.1996.1631. [DOI] [PubMed] [Google Scholar]
- 9.Nachshon-Kedmi M, Fares FA, Yannai S. Therapeutic activity of 3,3′-diindolylmethane on prostate cancer in an in vivo model. Prostate. 2004;61:153–160. doi: 10.1002/pros.20092. [DOI] [PubMed] [Google Scholar]
- 10.Heath EI, Heilbrun LK, Li J, Vaishampayan U, Harper F, Pemberton P, Sarkar FH. A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3′-Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. American journal of translational research. 2010;2:402–411. [PMC free article] [PubMed] [Google Scholar]
- 11.Le HT, Schaldach CM, Firestone GL, Bjeldanes LF. Plant-derived 3,3′-diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J Biol Chem. 2003;278:21136–21145. doi: 10.1074/jbc.M300588200. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Kong D, Wang Z, Ahmad A, Bao B, Padhye S, Sarkar FH. Inactivation of AR/TMPRSS2-ERG/Wnt signaling networks attenuates the aggressive behavior of prostate cancer cells. Cancer prevention research. 2011;4:1495–1506. doi: 10.1158/1940-6207.CAPR-11-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang TT, Schoene NW, Milner JA, Kim YS. Broccoli-derived phytochemicals indole-3-carbinol and 3,3′-diindolylmethane exerts concentration-dependent pleiotropic effects on prostate cancer cells: comparison with other cancer preventive phytochemicals. Mol Carcinog. 2012;51:244–256. doi: 10.1002/mc.20774. [DOI] [PubMed] [Google Scholar]
- 14.Bhuiyan MM, Li Y, Banerjee S, Ahmed F, Wang Z, Ali S, Sarkar FH. Down-regulation of androgen receptor by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B prostate cancer cells. Cancer Res. 2006;66:10064–10072. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- 15.Chinnakannu K, Chen D, Li Y, Wang Z, Dou QP, Reddy GP, Sarkar FH. Cell cycle-dependent effects of 3,3′-diindolylmethane on proliferation and apoptosis of prostate cancer cells. J Cell Physiol. 2009;219:94–99. doi: 10.1002/jcp.21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garikapaty VP, Ashok BT, Tadi K, Mittelman A, Tiwari RK. 3,3′-Diindolylmethane downregulates pro-survival pathway in hormone independent prostate cancer. Biochem Biophys Res Commun. 2006;340:718–725. doi: 10.1016/j.bbrc.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 17.Kong D, Banerjee S, Huang W, Li Y, Wang Z, Kim HR, Sarkar FH. Mammalian target of rapamycin repression by 3,3′-diindolylmethane inhibits invasion and angiogenesis in platelet-derived growth factor-D-overexpressing PC3 cells. Cancer Res. 2008;68:1927–1934. doi: 10.1158/0008-5472.CAN-07-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, Reddy GP, Sarkar FH. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282:21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 19.Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S. 3,3′-diindolylmethane (DIM) and its derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis. 2006;27:717–728. doi: 10.1093/carcin/bgi270. [DOI] [PubMed] [Google Scholar]
- 20.Savino JA, 3rd, Evans JF, Rabinowitz D, Auborn KJ, Carter TH. Multiple, disparate roles for calcium signaling in apoptosis of human prostate and cervical cancer cells exposed to diindolylmethane. Mol Cancer Ther. 2006;5:556–563. doi: 10.1158/1535-7163.MCT-05-0355. [DOI] [PubMed] [Google Scholar]
- 21.Sun S, Han J, Ralph WM, Jr, Chandrasekaran A, Liu K, Auborn KJ, Carter TH. Endoplasmic reticulum stress as a correlate of cytotoxicity in human tumor cells exposed to diindolylmethane in vitro. Cell stress & chaperones. 2004;9:76–87. doi: 10.1379/CSC-2R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho HJ, Park SY, Kim EJ, Kim JK, Park JH. 3,3′-Diindolylmethane inhibits prostate cancer development in the transgenic adenocarcinoma mouse prostate model. Mol Carcinog. 2011;50:100–112. doi: 10.1002/mc.20698. [DOI] [PubMed] [Google Scholar]
- 23.Nachshon-Kedmi M, Yannai S, Haj A, Fares FA. Indole-3-carbinol and 3,3′-diindolylmethane induce apoptosis in human prostate cancer cells. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2003;41:745–752. doi: 10.1016/s0278-6915(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 24.Nachshon-Kedmi M, Yannai S, Fares FA. Induction of apoptosis in human prostate cancer cell line, PC3, by 3,3′-diindolylmethane through the mitochondrial pathway. Br J Cancer. 2004;91:1358–1363. doi: 10.1038/sj.bjc.6602145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chintharlapalli S, Papineni S, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through PPARgamma-dependent and PPARgamma-independent pathways. Mol Cancer Ther. 2006;5:1362–1370. doi: 10.1158/1535-7163.MCT-06-0002. [DOI] [PubMed] [Google Scholar]
- 26.Cho SD, Yoon K, Chintharlapalli S, Abdelrahim M, Lei P, Hamilton S, Khan S, Ramaiah SK, Safe S. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and nuclear receptor-independent pathways. Cancer Res. 2007;67:674–683. doi: 10.1158/0008-5472.CAN-06-2907. [DOI] [PubMed] [Google Scholar]
- 27.Lei P, Abdelrahim M, Cho SD, Liu S, Chintharlapalli S, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through activation of c-jun N-terminal kinase. Carcinogenesis. 2008;29:1139–1147. doi: 10.1093/carcin/bgn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei P, Abdelrahim M, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit ovarian cancer cell growth through peroxisome proliferator-activated receptor-dependent and independent pathways. Mol Cancer Ther. 2006;5:2324–2336. doi: 10.1158/1535-7163.MCT-06-0184. [DOI] [PubMed] [Google Scholar]
- 29.Qin C, Morrow D, Stewart J, Spencer K, Porter W, Smith R, 3rd, Phillips T, Abdelrahim M, Samudio I, Safe S. A new class of peroxisome proliferator-activated receptor gamma (PPARgamma) agonists that inhibit growth of breast cancer cells: 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes. Mol Cancer Ther. 2004;3:247–260. [PubMed] [Google Scholar]
- 30.Yoon K, Lee SO, Cho SD, Kim K, Khan S, Safe S. Activation of nuclear TR3 (NR4A1) by a diindolylmethane analog induces apoptosis and proapoptotic genes in pancreatic cancer cells and tumors. Carcinogenesis. 2011;32:836–842. doi: 10.1093/carcin/bgr040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdelbaqi K, Lack N, Guns ET, Kotha L, Safe S, Sanderson JT. Antiandrogenic and growth inhibitory effects of ring-substituted analogs of 3,3′-diindolylmethane (Ring-DIMs) in hormone-responsive LNCaP human prostate cancer cells. Prostate. 2011;71:1401–1412. doi: 10.1002/pros.21356. [DOI] [PubMed] [Google Scholar]
- 32.Bourque SD, Titorenko VI. A quantitative assessment of the yeast lipidome using electrospray ionization mass spectrometry. Journal of visualized experiments, JoVE. 2009 doi: 10.3791/1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bratton SB, MacFarlane M, Cain K, Cohen GM. Protein complexes activate distinct caspase cascades in death receptor and stress-induced apoptosis. Exp Cell Res. 2000;256:27–33. doi: 10.1006/excr.2000.4835. [DOI] [PubMed] [Google Scholar]
- 34.Lavrik IN, Krammer PH. Regulation of CD95/Fas signaling at the DISC. Cell death and differentiation. 2012;19:36–41. doi: 10.1038/cdd.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. J Clin Immunol. 1999;19:350–364. doi: 10.1023/a:1020546615229. [DOI] [PubMed] [Google Scholar]
- 36.Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- 37.Jourdain A, Martinou JC. Mitochondrial outer-membrane permeabilization and remodelling in apoptosis. Int J Biochem Cell Biol. 2009;41:1884–1889. doi: 10.1016/j.biocel.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Ow YP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nature reviews. Molecular cell biology. 2008;9:532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- 39.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nature reviews. Molecular cell biology. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 40.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H, Dong A, Gao C, Tan C, Xie Z, Zu X, Qu L, Jiang Y. New synthetic flavone derivatives induce apoptosis of hepatocarcinoma cells. Bioorg Med Chem. 2010;18:6322–6328. doi: 10.1016/j.bmc.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 43.Yan YQ, Xu QZ, Wang L, Sui JL, Bai B, Zhou PK. Vanillin derivative 6-bromine-5-hydroxy-4-methoxybenzaldehyde-elicited apoptosis and G2/M arrest of Jurkat cells proceeds concurrently with DNA-PKcs cleavage and Akt inactivation. Int J Oncol. 2006;29:1167–1172. [PubMed] [Google Scholar]
- 44.Cande C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N, Kroemer G. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 2002;84:215–222. doi: 10.1016/s0300-9084(02)01374-3. [DOI] [PubMed] [Google Scholar]
- 45.Kim R, Emi M, Tanabe K. Caspase-dependent and -independent cell death pathways after DNA damage (Review) Oncology reports. 2005;14:595–599. [PubMed] [Google Scholar]
- 46.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 47.Lorenzo HK, Susin SA. Mitochondrial effectors in caspase-independent cell death. FEBS Lett. 2004;557:14–20. doi: 10.1016/s0014-5793(03)01464-9. [DOI] [PubMed] [Google Scholar]