Abstract
Background
Viral infection can exacerbate asthma by inducing the accumulation of inflammatory cells in the airway. We have previously reported that double-stranded RNA (dsRNA), a viral product and ligand of the Toll-like receptor-3 (TLR3), activates the transcription factors NF-κB and IRF-3 and upregulates the expression of inflammatory chemokines in airway epithelial cells. Here, we examined the effects of the glucocorticoid fluticasone propionate (FP) on the expression of the inflammatory chemokines CCL5, CXCL8 and CXCL10.
Methods
The airway epithelial cell line BEAS-2B was used for this study. Expression of CCL5, CXCL8 and CXCL10 mRNA and protein was quantified by real-time PCR and ELISA assay, respectively. To examine the association of FP with the physiology of chemokine production, we included several methods. Nuclear translocation of transcription factors was determined by performing Western blot analysis. Histone deacetylase (HDAC) activity in nuclear extracts was measured using a colorimetric assay. Stability of the chemokine mRNAs was examined in cells incubated with actinomycin D. The activities of the CCL5 promoter and the transcription factors NF-κB and IRF-3 were assessed using luciferase reporter assays.
Results
Treatment of BEAS-2B cells with FP significantly and dose-dependently (10−9 to 10−6 M) inhibited dsRNA-induced expression of CCL5, CXCL8 and CXCL10 protein and mRNA, but did not affect mRNA stability. FP also significantly inhibited dsRNA-stimulated CCL5 promoter activity. However, FP had no effect on the activity of HDAC or the nuclear translocation of NF-κB and IRF-3.
Conclusions
FP inhibits the dsRNA-stimulated expression of inflammatory chemokines in airway epithelial cells. FP may act by inhibiting chemokine transcription through an as yet Unidentified mechanism.
Keywords: Airway epithelial cells, Glucocorticoids, Inflammatory chemokines, Virus-induced asthma
Introduction
Respiratory viral infections are the most common cause of acute asthma exacerbations, and one important mechanism by which this is thought to occur is through virus-induced accumulation of inflammatory cells in the airway [1, 2]. Chemokines are a family of small secreted proteins that have potent chemoattractant activity. They are known to be expressed in the airway epithelium and to attract inflammatory cells into the airway during respiratory viral infections. CCL5, also known as RANTES (regulated upon activation normal T cells expressed and secreted), is a chemokine that attracts a number of inflammatory cells including eosinophils into the airways. The increased expression of CCL5 in airway epithelial cells may contribute to the prolonged eosinophilic inflammation and asthma exacerbation observed following infection with viruses such as human rhinovirus (HRV), respiratory syncytial virus (RSV) and influenza virus [3,4].
Toll-like receptors (TLRs) are a family of receptors that recognize a variety of microbial molecules collectively known as pathogen-associated molecular patterns (PAMPS). TLRs play important roles in host defense and contribute to the pathogenesis of certain diseases. TLR3 recognizes double-stranded RNA (dsRNA) synthesized by cells infected with RNA viruses. Airway epithelial cells are known to express TLR3 and bind viral RNA [5–7]. We have previously reported that dsRNA stimulates the expression of inflammatory chemokines, including CCL5/RANTES, CXCL8/IL-8 and CXCL10/IP-10, in airway epithelial cells through binding to TLR3 [8, 9], Stimulation of chemokine secretion occurred, in part, through the activation of the transcription factors nuclear factor-kappa B (NF-κB) and interferon regulatory factor 3 (IRF-3). Although the role of TLR3 in airway inflammation in asthmatic patients is not clear, a recent study using a mouse model suggested that TLR3 may contribute to the exacerbation of asthma caused by viral dsRNA [10].
Glucocorticoids are known to have potent anti-inflammatory actions. Inhalation of corticosteroids is an effective and widely used treatment for asthma [11], but the efficacy of inhaled corticosteroids in the prevention of asthma exacerbation caused by viral infection is not clear. Several reports have shown that glucocorticoids inhibit the expression of inflammatory chemokines induced by viral infection of airway epithelial cells in vitro [12, 13]. In this study, we examined the effects of the glucocorticoid fluticasone propionate (FP) on the expression of inflammatory chemokines in cultured airway epithelial cells, and investigated the molecular mechanism of action of FP in these cells.
Material and Methods
Cell Culture and Reagents
BEAS-2B is a human airway epithelial cell line transformed with an adenovirus 12-SV40 virus hybrid. We purchased it from the American Type Culture Collection (Manassas, Va., USA). The cells were cultured in DMEM/F12 medium supplemented with 10% FBS, 100 U/ml penicillin and 100 ng/ml streptomycin (Invitrogen, Tokyo, Japan) and maintained at 37°C in a humidified 5% CO2 atmosphere. Cells were treated as described previously [8, 9]. The synthetic dsRNA, poly IC, and the glucocorticoid, FP, were from Sigma-Aldrich (Tokyo, Japan). Recombinant human TNF-α was from R&D Systems (Tokyo, Japan). Firefly luciferase reporter plasmids containing multiple binding sites for NF-κB (pNF-κB-Luc) or IRF-3 (pISRE-Luc) were purchased from BD Biosciences (Tokyo, Japan). The firefly luciferase reporter plasmid containing an 884-bp fragment of the promoter region of the wild-type CCL5/RANTES gene (pRANT-WT) was a kind gift from Dr. Tomas J. Schall. The assay kit for measurement of histone deacetylase (HDAC) activity was purchased from Biomol (Farmingdale, N.Y., USA).
Chemokine ELISA Assays
The concentrations of CXCL8, CXCL10 and CCL5 in cell culture supernatants were measured using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems), as described previously [9]. Standards and samples were added to 96-well microliter plates coated with the appropriate anti-chemokine antibody. After incubation at room temperature for 2 h, each well was washed 5 times with wash buffer. A biotinylated chemokine-specific antibody and horseradish peroxidase-avidin conjugate were added to the wells and incubated at room temperature for 1 or 2 h. The wells were washed 7 times with wash buffer. Substrate solution (stabilized hydrogen peroxide and tetramethylbenzidine) was added to each well, and the plate was incubated at room temperature for 20 or 30 min. Sulfuric acid was added to arrest the reaction, and the absorbance at 450 nm was measured using an ELISA plate reader.
Real-Time PCR
Purification of RNA and synthesis of cDNA were performed as described previously [9]. Predesigned TaqMan probe sets for CCL5, CXCL8 and CXCL10 were purchased from Applied Biosystems (Tokyo, Japan). Each probe was labeled with a fluorescent reporter dye (FAM) at the 5′ end and a downstream quencher dye (TAMRA) at the 3′ end. TaqMan Ribosomal RNA probe, which is labeled with a fluorescent reporter dye (VIC), was used as an internal control. Each reaction consisted of 2X Universal Master Mix (Applied Biosystems), primers, labeled probes and 50 ng cDNA in a total volume of 25 μl. Amplification conditions consisted of an initial incubation at 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Amplification and fluorescence measurements were carried out during the elongation step with an ABI PRISM 7700 Sequence Detection System (Applied Biosystems). Data are shown as fold induction of mRNA compared with non-stimulated control cells.
mRNA Stability Assay
BEAS-2B cells were preincubated with FP or dimethyl acetamide (DMA) vehicle for 30 min and then treated with dsRNA for 8 h. The cells were either harvested (time 0 control condition) or treated with the transcriptional inhibitor actinomycin D (Sigma-Aldrich) for a further 1,4 or 24 h. At the end of these incubations, the expression of CCL5, CXCL8 and CXCL10 mRNAs was determined as described above. Results are expressed as the percent mRNA level compared with the time 0 control cells.
Assay of Histone Deacetylase Activity
Histone deacetylase (HDAC) activity was measured in nuclear extracts using an HDAC Colorimetric Activity Assay/Drug Discovery kit (Biomol). BEAS-2B cells were harvested from culture and nuclear proteins were extracted. The extracts were incubated at 37°C for 30 min with 1 mM of the commercial HDAC substrate in a total volume of 50 μl. After the incubation, 50 μl of the developer solution containing 2 μM of the HDAC inhibitor trichostatin A (TSA) was added, and the samples were incubated at 37°C for 10 min. The deacetylated substrate reacts with the kit developer and produces a yellow color. A standard curve was constructed with dilutions of the deacetylated standard. Absorbance at 405 nM was measured using a microtiter plate reader. HDAC activity was expressed as μM after plotting the absorbance against the concentration of deacetylated standard. HeLa nuclei contained in the kit were used as positive control samples.
Transcription Factor Nuclear Translocation Assay
Nuclear proteins were extracted from BEAS-2B cells as previously described [14]. Translocation of NF-κB and TRF-3 was monitored by Western blot analysis of nuclear extracts, as described previously [15]. Proteins in nuclear extracts were resolved by performing 10% Tris-glycine gradient gel electrophoresis (Novex, San Diego, Calif., USA) and transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). The membranes were blocked using 5% nonfat milk powder in TBST (50 mM Tris, 0.15 M NaCl and 0.05% Tween 20), incubated with 1 μg/ml rabbit anti-NF-κB or anti-IRF-3 antibody (Cell Signaling Technology, Tokyo, Japan) in TBST for 3 h, washed with TBST, and then incubated with a secondary horseradish peroxidase-conjugated antirabbit Ig antibody (Amersham Biosciences, Tokyo, Japan) for 1 h. After extensive washing with TBST, the membranes were incubated with a chemiluminescent substrate (ECL Western blot detection system, Amersham Biosciences) and subjected to autoradiography.
Transient Transfections and Luciferase Assays
BEAS-2B cells were transfected with the luciferase reporter plasmids pNF-κB-Luc, pTSRE-Luc and pRANT-WT, as described previously [8,9], and incubated for 24 h. The cells were then incubated with combinations of vehicle, dsRNA, TNF-α, FP and DMA for 24 h. The cells were harvested and stored at −80°C until tested using the Dual-Luciferase Assay system (Promega, Tokyo, Japan). The firefly luciferase activity of each sample was normalized to the renilla luciferase activity, and activity was expressed as fold induction relative to the appropriate control sample.
Statistical Analysis
Data are expressed as the mean ± standard error of the mean (SEM). Statistical differences were determined by analysis of variance with the Fisher PLSD post hoc test. The data were analyzed with StatView IV software (Abacus Concepts, Berkeley, Calif., USA).
Results
Inhibition of Chemokine Protein Production by FP
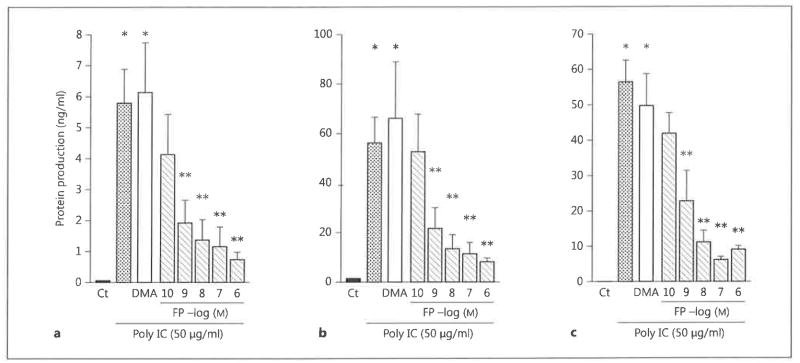
We first examined the effects of FP on chemokine secretion by BEAS-2B cells stimulated with the synthetic dsRNA poly IC. The cells were preincubated with 10−10 to 10−6 M FP for 30 min and then stimulated with 50 μg/ml poly IC for 24 h. FP decreased the production of CCL5, CXCL8 and CXCL10 in a dose-dependent manner (fig. 1). The FP vehicle DMA had no effect on chemokine production. The inhibitory effects of FP were statistically significant at concentrations between 10−9 and 10−6 M (p < 0.05 compared with DMA-treated cells) (fig. 1).
Fig. 1.
The effect of FP on chemokine secretion in BEAS-2B cells. BEAS-2B cells were incubated with the indicated concentrations of FP or with the vehicle DMA for 30 min and then stimulated with the synthetic dsRNA poly IC (50 μg/ml) for 24 h. Concentrations of CCL5/RANTES (a), CXCL8/IL-8 (b) and CXCL10/IP-10 (c) in the culture supernatants at 24 h were quantified by performing ELISA. The data are the mean ± SEM of 3 independent experiments. * p < 0.05 compared with nonstimulated control cells; ** p < 0.05 compared with DMA-treated, dsRNA-stimulated cells. Ct = Control.
Inhibition of Chemokine mRNA Expression by FP
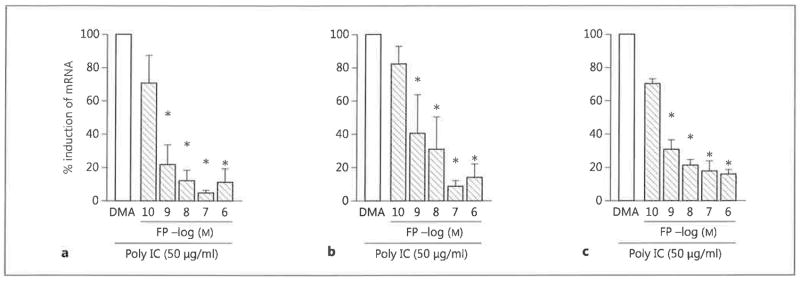
Preincubation of BEAS-2B cells with FP for 30 min reduced the poly IC-stimulated increase in CCL5, CXCL8 and CXCL10 mRNA levels (fig. 2). The inhibitory effects of FP were statistically significant at concentrations between 10−9 and 10−6 M (p < 0.05 compared with DMA-treated cells) (fig. 2). These results are consistent with the observed effects of FP on chemokine protein production, indicating that the block in chemokine expression occurs at the mRNA level.
Fig. 2.
The effect of FP on chemokine mRNA levels in BEAS-2B cells. BEAS-2B cells were incubated with the indicated concentrations of FP or with vehicle DMA for 30 min and then stimulated with poly IC (50 μg/ml) for 24 h. RNA was isolated from the cells and CCL5/RANTES (a), CXCL8/IL-8 (b) and CXCL10/IP-10 (c) mRNA levels were measured by real-time PCR. The data are presented as percent induction compared with control cells and are the mean ± SEM of 3 independent experiments. * p < 0.05 compared with DMA-treated, dsRNA-stimulated control cells.
Effects of FP on the Stability of Chemokine mRNA
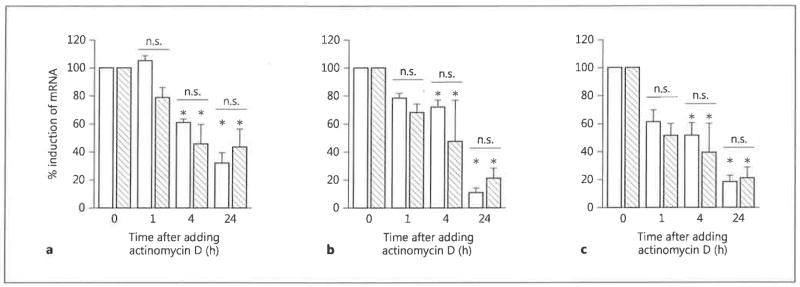
To determine whether FP affects chemokine mRNA stability, BEAS-2B cells were stimulated with poly IC in the presence of FP or DMA for 8 h, and then further transcriptional activity was blocked by the addition of actinomycin D. The decay in chemokine mRNA was then examined over the following 24 h. CCL5, CXCL8 and CXCL10 mRNA levels decreased following the treatment with actinomycin D (fig. 3). However, there was no significant difference in the decay rate in cells treated with dsRNA plus FP or with dsRNA plus DMA. These data indicate that FP had no effect on chemokine mRNA stability, and therefore suggest that FP may block dsRNA-stimulated chemokine production at the transcriptional level.
Fig. 3.
The effect of FP on chemokine mRNA stability in BEAS-2B cells. BEAS-2B cells were treated with poly IC (50 μg/ml) in combination with either FP (10−7 M; hatched bars) or DMA (open bars) for 8 h and then harvested immediately (time 0 control) or further treated with actinomycin D for 1,4 or 24 h. RNA was isolated from the cells and CCL5/RANTES (a), CXCL8/IL-8 (b) and CXCL10/ IP-10 (c) mRNA levels were quantified by real-time PCR. The data are presented as percent induction compared with cells collected at time 0 and are the mean ± SEM of 3 independent experiments. * p < 0.05 compared with cells at time 0. n.s. = Not significant.
Role of HDAC in the Inhibitory Effects of FP
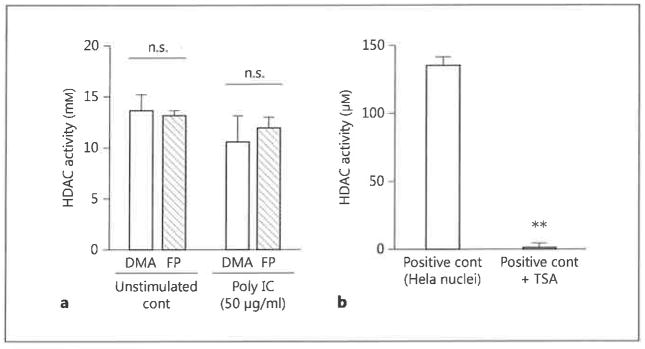
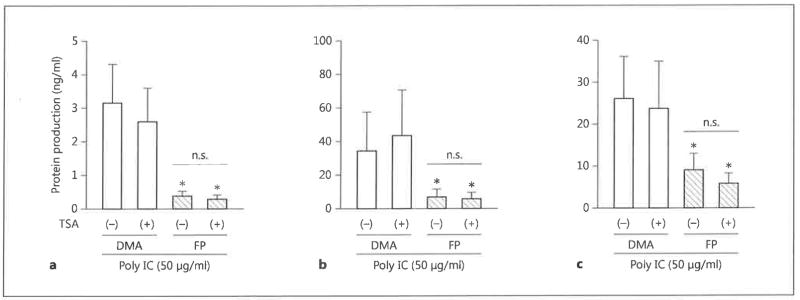
Histone acetylation is an important epigenetic modulator of gene expression. We hypothesized that FP might act on BEAS-2B cells by increasing HDAC activity, thereby reversing histone acetylation and repressing gene transcription. However, incubation of BEAS-2B cells with FP did not affect HDAC activity in either unstimulated or dsRNA-stimulated cells (fig. 4a). To confirm that HDAC is not involved in the effects of FP, we blocked HDAC activity by the treatment of cells with TSA. We first confirmed that the TSA used in our experiments was able to suppress HDAC in HeLa-derived nuclei (fig. 4b). However, treatment of BEAS-2B cells with TSA did not reverse the inhibitory effects of FP on dsRNA-stimulated chemokine secretion (fig. 5). These data indicate that FP inhibition of chemokine transcription is not mediated through an effect on HDAC activity.
Fig. 4.
HDAC activity in FP-treated BEAS-2B cells, a BEAS-2B cells were stimulated with poly IC (50 μg/ml) in combination with either FP (10−7 M) or DMA for 24 h. Nuclear extracts were then prepared and assayed for HDAC activity, b Nuclei isolated from HeLa cells were either untreated or incubated with 100 nM of the HDAC inhibitor TSA for 30 min. Nuclear extracts were then assayed for HDAC activity. The data are the mean ± SEM of 3 independent experiments. ** p < 0.05 compared with positive control HeLa nuclei without TSA. cont = Control; n.s. = not significant.
Fig. 5.
The effect of HDAC inhibition on chemokine secretion in FP-treated BEAS-2B cells. BEAS-2B cells were incubated with FP (10−7 M) or DMA in the presence or absence of 100 nM TSA for 30 min. Cells were then stimulated with poly IC (50 μg/ml) for 24 h. Concentrations of CCL5/RANTES (a), CXCL8/IL-8 (b) and CXCL10/IP-10 (c) in the culture supernatants at 24 h were quantified by ELISA. The data are the mean ± SEM of 3 independent experiments. * p < 0.05 compared with DMA-treated cells in the presence or absence of TSA. n.s. = Not significant.
Effects of FP on Nuclear Translocation of Transcription Factors
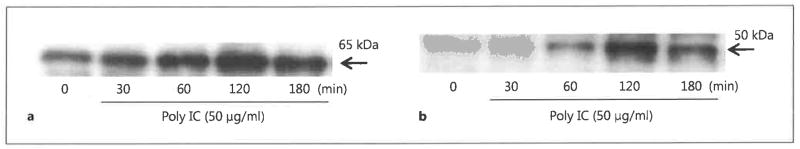
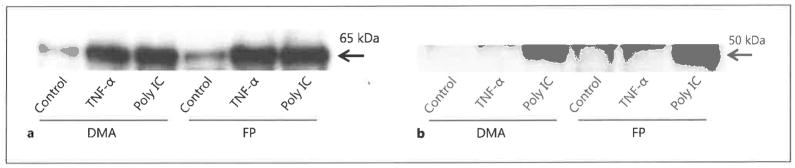
We next examined whether FP interfered in the activation of NF-κB and IRF-3, which have previously been shown to be key transcriptional activators of CCL5, CXCL8 and CXCL10 expression in response to dsRNA stimulation [8, 9]. The translocation of NF-κB and IRF-3 into the nuclei of BEAS-2B cells was monitored by Western blotting of nuclear extracts following cell stimulation with poly IC. Peak translocation was observed after 120 min stimulation with dsRNA (fig. 6). Unexpectedly, we found that FP treatment had no effect on the nuclear translocation of either NF-κB or IRF-3 at 120 min after the addition of dsRNA (fig. 7). TNF-α was used as the positive control for NF-κB activation.
Fig. 6.
Nuclear translocation of transcription factors in dsRNA-stimulated BEAS-2B cells. BEAS-2B cells were stimulated with poly IC (50 μg/ml) for the indicated times before nuclear proteins were extracted and subjected to Western blot analysis of NF-κB p65 (a) or IRF-3 (b).
Fig. 7.
The effect of FP on nuclear translocation of transcription factors in BEAS-2B cells. BEAS-2B cells were incubated with FP (10−7 M) or DMA for 30 min and then stimulated with poly IC (50 μg/ml) or TNF-α (10 ng/ml) for 120 min. Nuclear proteins were extracted and subjected to Western blot analysis of NF-κB p65 (a) or IRF-3 (b).
Inhibition of CCL5 Promoter Activity by FP
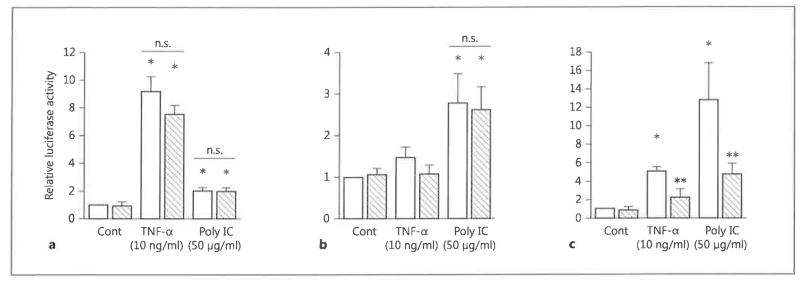
Finally, we examined the effect of FP on the transcriptional regulation of chemokine expression using luciferase reporter plasmids. Consistent with the lack of effect on nuclear translocation of NF-κB and IRF3, we found that FP did not inhibit dsRNA-stimulated activity of the NF-κB and IRF-3 luciferase reporter constructs (fig. 8a, b). However, FP had a moderate but statistically significant inhibitory effect on dsRNA- and TNF-α-stimulated CCL5 promoter activity (fig. 8c). These data suggest that FP inhibits chemokine production via a transcriptional mechanism.
Fig. 8.
The effect of FP on reporters for NF-κB and IRF-3, and CCL-5 promoter activity in BEAS-2B cells. BEAS-2B cells were transfected with luciferase reporter plasmids and the control vector pRL-TK and 48 h later were incubated with FP (10−7 M; hatched bars) or DMA (open bars) for 30 min and then stimulated in the absence (Cont) or presence of poly IC (50 μg/ml) or TNF-α (10 ng/ ml) for 24 h. Cells were then harvested and subjected to dual firefly and renilla luciferase assays. The relative luciferase activity in cells expressing pNF-κB-Luc (reporter for NF-κB) (a), pISRE-Luc (reporter for IRF-3) (b) and pRANT-WT (CCL5/RANTES promoter) (c) was calculated as fold induction compared with the appropriate control value. The data are the mean ± SEM of 3 independent experiments. * p < 0.05 compared with nonstimulated control cells. ** p < 0.05 compared with DMA-treated cells stimulated with poly IC or TNF-α. Cont = Control; n.s. = not significant.
Discussion
We showed here that the glucocorticoid FP significantly suppressed dsRNA induction of inflammatory chemokines in airway epithelial cells and that the effect of FP was mediated, at least in part, by inhibiting chemokine mRNA expression. However, we could not detect an effect of FP on several well-known mechanisms of glucocorticoid action, including destabilization of mRNA, activation of HDAC activity and direct inhibition of nuclear translocation of the transcription factors NF-κB and IRF-3 [11,16]. On the other hand, we found that FP suppressed the dsRNA-stimulated increase in CCL5 promoter activity, indicating that it may inhibit the transcription of chemokines induced with dsRNA using other, less well-characterized mechanisms.
Glucocorticoids suppress the expression of multiple inflammatory genes via several different molecular mechanisms. For example, they recruit HDAC to acetylated gene complexes and thereby prevent transcription. They also inhibit nuclear translocation of transcription factors that activate target gene transcription. Several reports have shown that glucocorticoids inhibit TLR3 signaling through the inhibition of phosphorylation of transcription factors [17, 18]. It has also been reported that they decrease the stability of mRNA. However, our data are inconsistent with these reported mechanisms of action. There are several possible reasons for this discrepancy, including differences in cell type, genes of interest and experimental conditions. Others have reported that glucocorticoids inhibit mitogen-activated protein (MAP) kinase activity and subsequent target gene expression by inducing MAP kinase phosphatase 1 [11, 16]. However, activation of MAP kinase does not appear to be involved in dsRNA induction of inflammatory chemokines in the airway epithelial cells used in our experiments [9].
Our data indicate that FP inhibits CCL5 transcription in airway epithelial cells stimulated with dsRNA; however, FP did not inhibit the nuclear translocation of NF-κB or IRF-3. These results suggest that FP may interfere with the interactions between transcription factors and cofactors specific for the chemokine promoter. Glucocorticoids are known to interact with transcriptional coregulators, including glucocorticoid receptor-interacting protein 1 (GRIP1) [19]. GRIP1 binds to transcription factors, including NF-κB and IRF-3, and coactivates target gene transcription. Interactions between GRIP1 and p-TEFb (positive-transcription elongation factor b) enhance gene transcription after transcription factors have bound to the promoter region [20, 21]. Further studies are needed to clarify the molecular mechanisms underlying the effects of FP observed in this report, although it is possible that FP may interfere with the function of transcriptional coregulators. In support of this, we have observed that p-TEFb is involved in the induction of inflammatory chemokines in dsRNA-stimulated airway epithelial cells (data not shown).
Numerous clinical studies have shown that treatment with inhaled corticosteroids reduces exacerbations in patients with asthma. However, inhaled corticosteroids do not significantly improve the course of illness in virus-infected asthma patients [1, 22]. This observation contrasts with those of other studies, including ours, which show that glucocorticoids have potent anti-inflammatory effects in systems using virus or viral RNA in vitro [12, 13]. One reason for this discrepancy may be that glucocorticoids suppress the host defense, including production of the anti-viral cytokine interferon. An ideal therapy for virus-induced asthma would thus balance the anti-inflammatory effects of glucocorticoids with the need to maintain the host defense [2,23]. Understanding the molecular mechanisms underlying the anti-inflammatory effects of glucocorticoids may thus contribute to improved therapies for asthma.
Acknowledgments
The authors would like to thank Dr. Tomas J. Schall for providing the CCL5/RANTES promoter plasmid. This work was supported by the Ministry of Health, Labour and Welfare, Environmental Restoration and Conservation Agency, and GlaxoSmithKline.
Footnotes
Disclosure Statement
The authors declare that no financial or other conflict of interest exists in relation to the contents of this article.
References
- 1.Kim WK, Gern JE. Updates in the relationship between human rhinovirus and asthma. Allergy Asthma Immunol Res. 2012;4:116–121. doi: 10.4168/aair.2012.4.3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singanayagam A, Joshi PV, Mallia P, Johnston SL. Viruses exacerbating chronic pulmonary disease: the role of immune modulation. BMC Med. 2012;10:27. doi: 10.1186/1741-7015-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsukura S, Kokubu F, Kubo H, Tomita T, Tokunaga H, Kadokura M, Yamamoto T, Kuroiwa Y, Ohno T, Suzaki H, Adachi M. Expression of RANTES by normal airway epithelial cells after influenza virus A infection. Am J Respir Cell Mol Biol. 1998;18:255–264. doi: 10.1165/ajrcmb.18.2.2822. [DOI] [PubMed] [Google Scholar]
- 4.Matsukura S, Kokubu F, Noda H, Tokunaga H, Adachi M. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J Allergy Clin Immunol. 1996;98:1080–1087. doi: 10.1016/s0091-6749(96)80195-3. [DOI] [PubMed] [Google Scholar]
- 5.Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280:5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 6.Slater L, Bartlett NW, Haas JJ, Zhu J, Message SD, Walton RP, Sykes A, Dahdaleh S, Clarke DL, Belvisi MG, Kon OM, Fujita T, Jeffery PK, Johnston SL, Edwards MR. Co-ordinated role of TLR3, RIG-I and MDA5 in the innate response to rhinovirus in bronchial epithelium. PLoS Pathog. 2010;6:el001178. doi: 10.1371/journal.ppat.1001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol. 2005;79:3350–3357. doi: 10.1128/JVI.79.6.3350-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ieki K, Matsukura S, Kokubu F, Kimura T, Kuga H, Kawaguchi M, Odaka M, Suzuki S, Watanabe S, Takeuchi H, Schleimer RP, Adachi M. Double-stranded RNA activates RANTES gene transcription through co-operation of nuclear factor-kappaB and interferon regulatory factors in human airway epithelial cells. Clin Exp Allergy. 2004;34:745–752. doi: 10.1111/j.1365-2222.2004.1941.x. [DOI] [PubMed] [Google Scholar]
- 9.Matsukura S, Kokubu F, Kurokawa M, Kawaguchi M, Ieki K, Kuga H, Odaka M, Suzuki S, Watanabe S, Takeuchi H, Kasama T, Adachi M. Synthetic double-stranded RNA induces multiple genes related to inflammation through Toll-like receptor 3 depending on NF-kappaB and/or IRF-3 in airway epithelial cells. Clin Exp Allergy. 2006;36:1049–1062. doi: 10.1111/j.1365-2222.2006.02530.x. [DOI] [PubMed] [Google Scholar]
- 10.Torres D, Dieudonne A, Ryffel B, Vilain E, SiTahar M, Pichavant M, Lassalle P, Trottein F, Gosset P. Double-stranded RNA exacerbates pulmonary allergic reaction through TLR3: implication of airway epithelium and dendritic cells. J Immunol. 2010;185:451–459. doi: 10.4049/jimmunol.0902833. [DOI] [PubMed] [Google Scholar]
- 11.Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29–43. doi: 10.1111/j.1476-5381.2010.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skevaki CL, Christodoulou I, Spyridaki IS, Tiniakou I, Georgiou V, Xepapadaki P, Kafetzis DA, Papadopoulos NG. Budesonide and formoterol inhibit inflammatory mediator production by bronchial epithelial cells infected with rhinovirus. Clin Exp Allergy. 2009;39:1700–1710. doi: 10.1111/j.1365-2222.2009.03307.x. [DOI] [PubMed] [Google Scholar]
- 13.Edwards MR, Johnson MW, Johnston SL. Combination therapy: synergistic suppression of virus-induced chemokines in airway epithelial cells. Am J Respir Cell Mol Biol. 2006;34:616–624. doi: 10.1165/rcmb.2005-0385OC. [DOI] [PubMed] [Google Scholar]
- 14.Matsukura S, Stellato C, Plitt JR, Bickel C, Miura K, Georas SN, Casolaro V, Schleimer RP. Activation of eotaxin gene transcription by NF-kappa B and STAT6 in human airway epithelial cells. J Immunol. 1999;163:6876–6883. [PubMed] [Google Scholar]
- 15.Homma T, Matsukura S, Hirose T, Ohnishi T, Kimura T, Kurokawa M, Ieki K, Odaka M, Suzuki S, Watanabe S, Sato M, Kawaguchi M, Schleimer RP, Adachi M. Cooperative activation of CCL5 expression by TLR3 and tumor necrosis factor-alpha or interferon-gamma through nuclear factor-kappaB or STAT-1 in airway epithelial cells. Int Arch Allergy Immunol. 2010;152(suppl 1):9–17. doi: 10.1159/000312120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stellato C. Glucocorticoid actions on airway epithelial responses in immunity: functional outcomes and molecular targets. J Allergy Clin Immunol. 2007;120:1247–1263. doi: 10.1016/j.jaci.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 17.McCoy CE, Carpenter S, Palsson-McDermott EM, Gearing LJ, O’Neill LA. Glucocorticoids inhibit IRF3 phosphorylation in response to Toll-like receptor-3 and -4 by targeting TBK1 activation. J Biol Chem. 2008;283:14277–14285. doi: 10.1074/jbc.M709731200. [DOI] [PubMed] [Google Scholar]
- 18.Hara Y, Shiraishi A, Kobayashi T, Kadota Y, Shirakata Y, Hashimoto K, Ohashi Y. Alteration of TLR3 pathways by glucocorticoids may be responsible for immunosusceptibility of human corneal epithelial cells to viral infections. Mol Vis. 2009;15:937–948. [PMC free article] [PubMed] [Google Scholar]
- 19.O’Neill LA. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Kino T, Slobodskaya O, Pavlakis GN, Chrousos GP. Nuclear receptor coactivator pl60 proteins enhance the HIV-1 long terminal repeat promoter by bridging promoter-bound factors and the Tat-P-TEFb complex. J Biol Chem. 2002;277:2396–2405. doi: 10.1074/jbc.M106312200. [DOI] [PubMed] [Google Scholar]
- 21.Dobrovolna J, Chinenov Y, Kennedy MA, Liu B, Rogatsky I. Glucocorticoid-dependent phosphorylation of the transcriptional coregulatorGRIPl. Mol Cell Biol. 2012;32:730–739. doi: 10.1128/MCB.06473-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunberg K, Sharon RF, Sont JK, In’t Veen JC, Van Schadewijk WA, De Klerk EP, Dick CR, Van Krieken JH, Sterk PJ. Rhinovirus-induced airway inflammation in asthma: effect of treatment with inhaled corticosteroids before and during experimental infection. Am J Respir Crit Care Med. 2001;164:1816–1822. doi: 10.1164/ajrccm.164.10.2102118. [DOI] [PubMed] [Google Scholar]
- 23.Davies JM, Carroll ML, Li H, Poh AM, Kirkegard D, Towers M, Upham JW. Budesonide and formoterol reduce early innate anti-viral immune responses in vitro. PLoS One. 2011;6:e27898. doi: 10.1371/journal.pone.0027898. [DOI] [PMC free article] [PubMed] [Google Scholar]