Abstract
INTRODUCTION
We asked whether anxiety is associated with prospective risk of dementia, and the extent to which genetic influences mediate this association.
METHODS
Non-demented twins (n=1,082) from the Swedish Adoption Twin Study of Aging (SATSA) completed an assessment of anxiety symptoms in 1984 and were followed for 28 years.
RESULTS
Baseline anxiety score, independent of depressive symptoms, was significantly associated with incident dementia over follow-up (HR = 1.04; 95% CI = 1.01–1.06). There was 48% increased risk of becoming demented for those who had experienced high anxiety at any time compared to those who had not. In co-twin analyses, the association between anxiety symptoms and dementia was greater for dizygotic (HR = 1.11; 95% CI= 1.02–1.20) compared to monozygotic twins (HR = 1.06; 95% CI= 0.95–1.20), indicating genetic mediation.
DISCUSSION
Anxiety symptoms were associated with increased risk of dementia. Genetic factors common to dementia and anxiety partially mediated this association.
Keywords: Anxiety, dementia, genetics, twins, depression
1. Background
The rapid growth of the older adult population continues to make urgent the identification of potentially modifiable risk and protective factors for dementia. Moreover, discovering early and modifiable risk factors is essential to identifying potential targets for treatment and prevention of dementia [1]. Psychiatric symptoms and disorders often co-occur with dementia and may be potentially modifiable. Anxiety disorders and symptoms are the most prevalent psychiatric disorders and symptoms in the elderly [2]. They are associated with negative health outcomes such as increased healthcare costs [3], decreased quality of life and increased disability [4]. Anxiety in later life is associated with worse cognitive functioning and frequently co-occurs with dementia [5]. Some studies have found links between anxiety disorders, posttraumatic stress disorder (one type of anxiety disorder), anxiety symptoms, benzodiazepine use (a routine pharmacological treatment for anxiety), and increased risk of incident dementia [5–12].
Prior research examining anxiety as a risk factor for dementia, however, has produced mixed findings [6–10, 13, 14]. There is a considerable amount of research documenting depression as a risk factor for dementia [15]. It is also unclear whether anxiety, independent of depression, is associated with risk of dementia [10]. Mixed findings have also been observed with respect to the relationship between anxiety and cognitive decline [5, 16, 17].
If anxiety is a risk factor for dementia, little is known about what factors are mediating this association. Past research examining neuroticism (a dimension of personality most associated with anxiety [18]) has posited that genetic factors common to both neuroticism and dementia may be explaining this association [19], but this question has not been investigated with respect anxiety and dementia.
The purpose of this study was to examine whether symptoms of anxiety were associated with an increased risk of developing dementia over a follow-up period of 28 years in a representative sample of Swedish twins. Secondarily, we examined the association between symptoms of anxiety and trajectories of cognitive performance. Lastly, using co-twin analyses, we sought to determine if the association between anxiety and dementia in part reflected genetic factors in common to both phenotypes.
2. Methods
2.1 Data and Study Participants
Data from the Swedish Adoption/Twin Study of Aging (SATSA) were examined for this study. SATSA contains a representative sample of Swedish twins drawn from the population-based Swedish Twin Registry [20]. SATSA consists of two components: questionnaire assessments (Qs) and in-person-testing (IPT). In 1984, participants completed the first questionnaire assessment (Q1), which will be referred to as the baseline for this study. Subsequent questionnaires were completed approximately every three years. Beginning in 1986, a subsample of twins aged 50 and above (n=645) participated in the first IPT assessment including measures of cognition. Participants completed IPTs approximately every three years. Follow-up for dementia continued through 2012. The sampling procedure and methods of the SATSA study have been previously published [20]. SATSA was approved by the Ethics Committee at Karolinska Institutet. All participants provided informed consent for participation.
There were 1,736 participants who completed the baseline questionnaire containing the measurement of anxiety. Of those participants, 1,541 completed the anxiety questionnaire (88.7% response rate). Participants were excluded if they had a dementia diagnosis before the baseline assessment, if they had missing data on other study variables, or if they were younger than 60 years old at their last follow-up assessment, resulting in a final sample of 1,082 participants.
2.2 Assessment of anxiety
State anxiety symptom severity was measured at baseline through Q4 using the state anxiety subscale of the State-Trait Personality Inventory (STPI) [21]. The STPI is a 10-item scale in which participants are asked how they feel “right now.” Items are answered on a 5-point likert type scale (1=Fits me exactly to 5=Does not fit me at all) with higher scores representing greater state anxiety. The STPI contains a subset of items from the state anxiety subscale of the State Trait Anxiety Inventory (STAI). The STAI is reliable and valid with older adults [22].
2.3 Assessment of depression
Depressive symptoms were measured at baseline using the Older American Resources and Services (OARS) depression subscale [23]. This subscale is a 5-item measure in which participants reported dichotomously (No = 0; Yes = 1) if they were experiencing each symptom. The OARS has been found to be a reliable and valid measurement for community dwelling older adults [24]. The OARS was administered at Q1 through Q3. The Center for Epidemiologic Studies Depression scale (CES-D) was available Q2 through Q4 but not at baseline.
2.4 Diagnosis of dementia
Participants were screened for dementia throughout the study. Participants were identified for dementia work-up if they scored lower than a 24 on the Mini-Mental State Examination (MMSE), declined three or more points from prior MMSE, exhibited poor performance on cognitive testing at an IPT, had a history of dementia documented in medical records, and/or a study nurse or family member offered that the participant had dementia or cognitive problems [25]. Additionally, participants who failed to respond to either a Q or an IPT assessment were contacted and asked to complete a brief telephone cognitive screening interview [26]. When participants were unable to respond, a proxy telephone interview was used.
Participants identified as possible dementia cases through any of these methods were invited to complete a clinical assessment including a medical examination, laboratory tests, and neuropsychological testing. The determination of dementia diagnosis was made by a consensus panel including the assessment team who were blind to twin status [25]. For these participants, diagnostic criteria from the then current Diagnostic and Statistical Manual of Mental Disorders [27] (DSM-III or DSM-IV) were used to diagnose dementia.
Finally, to supplement in-person clinical diagnostic assessments, all twins, including those who refused IPT or telephone screening, were followed until death or December 31, 2012, using the National Patient Registry and National Cause of Death Registry. The National Patient Registry contains information on hospital visits with diagnosis, admission and discharge dates. The Cause of Death Registry has information on the underlying and contributing causes of death. Diagnoses in both registries used the then current International Classification of Diseases (ICD) edition [28–30]. Utilization of NPR/CDR diagnoses has been found to be reliable with acceptable sensitivity/specificity [31]. A total of 43 (25%) of dementia diagnoses came from the NPR/CDR. For these participants, ICD diagnosis of dementia was used.
2.5 Cognitive testing
The SATSA cognitive battery [20] assessed the cognitive domains of perceptual speed (Symbol Digit Test, Figure Identification task), nonverbal memory (Thurstone Picture Memory Test), working memory (Digit Span backwards), attention (Digit Span forwards), and visuospatial abilities (Block Design, Figure Logic, and Card Rotations). Participants completed cognitive testing as many as seven times. Scores were transformed for all tests to percentage of points achieved out of total possible.
2.6 Covariates
Models included covariates known to be associated with risk of dementia including baseline age, sex, education, a physical illness sum score, neuroticism, and benzodiazepine use. The physical illness score consisted of the number of organ systems affected by illness from which the participant was suffering. This score has been used in prior SATSA investigations [32]. Neuroticism was measured using a shortened version of the neuroticism scale of the Eysenck Personality Inventory [33]. The Neuroticism scale contains nine items with higher scores representing more neuroticism. Education was assessed via self-report and dichotomized into elementary education and education beyond elementary school. Self-reported benzodiazepine use (No=0, Yes=1) at baseline was available for a subset of the sample (n=556).
2.7 Statistical Analysis
Descriptive statistics were computed for cases (participants who developed dementia over the follow-up period between 1984 and 2012) and controls (participants who did not develop dementia). The two groups were compared using independent sample t-tests for continuous variables and chi-square tests for categorical variables.
Cox mixed-effects proportional hazards models run with the coxme package [34] in R [35] were used to examine the association between baseline anxiety and incident dementia. A random effect of twin pair was included in the model to adjust for the clustered nature of the data. Age was used as the time scale. First, a univariate Cox model was run to examine the association between STPI score and risk of dementia. To assess the confounding of physical health and demographic variables, a second multivariate Cox model was run including anxiety, sex, education, baseline age, and the physical illness sum score. To examine the association between depressive symptoms, neuroticism, and dementia risk a third model was conducted adjusting for baseline depressive symptoms and neuroticism, in addition to the covariates described in the second model. In order to utilize the multiple assessments of the STPI, additional analyses were run using a dichotomous score (low anxiety =0, high anxiety=1) instead of as a continuous variable. Scoring at or above one standard deviation on the STPI at any of the four assessments was used as the cut point. Here average depressive symptoms from baseline to Q3 were used as a covariate.
Sensitivity analyses were conducted to examine the effects of baseline benzodiazepine use on risk of incident dementia. The proportional hazard models were re-run using the subgroup with benzodiazepine data, adding baseline benzodiazepine use or non-use as a covariate. Lastly, in order to ensure we were examining anxiety and long-term risk of dementia rather than anxiety as a prodromal symptom, the models were re-run to exclude individuals who developed dementia within five years of the baseline.
As secondary analyses of cognitive performance trajectories, mixed-effects age-based quadratic growth models (MIXED procedure in SAS version 9.3 [36]) were fitted to examine the simple relationship between baseline STPI and cognitive performance over age while controlling for baseline age, gender, education, physical health, neuroticism, and depression. Participants were clustered on twin pair to account for non-independence of data. Significant age squared by anxiety interactions were not found so these were removed from the model. Reported models contain main effect of all covariates and the linear age by anxiety interaction. Separate models were run using Q1 STPI followed by the dichotomous STPI score.
For the co-twin analysis, twin pairs discordant for dementia (one twin developed dementia and the other twin did not) were included in the co-twin control analysis (17 monozygotic (MZ) and 46 dizygotic (DZ) twin pairs). Because twin pairs share factors in common, such as early life environment, maternal factors, their segregating genes (50% DZ and 100% MZ), the co-twin design minimizes confounding of these variables. Likewise, because MZ twins share 100% of segregating genes while DZ twins share only half, if a difference is reduced within MZ pairs compared to DZ pairs it is suggestive of significant genetic confounding [37].
Dementia cases and their co-twin controls were compared on study measures using t-tests and chi-square tests. Twins were classified as endorsing more or less anxiety than their co-twin based on any intra-pair difference on the STPI. Secondarily, conditional logistic regression models (PHREG procedure in SAS version 9.3 [36]) including twin pairs as stratum were used to examine the association between anxiety and risk of dementia using three alternative outcomes: STPI score, dichotomous anxiety symptom severity, and endorsing more (or less) anxiety than their co-twin at baseline. Models were run including all discordant twins followed by separate analyses among MZ and DZ twins.
3. Results
3.1. Participant Characteristics
On average, participants were 60.86 (SD = 11.15) years old at the baseline assessment. Descriptive characteristics are presented in table 1. In total, 172 (15.8%) participants developed dementia. Onset of dementia occurred an average of 14.7 (SD = 6.70) years after baseline.
Table 1.
Baseline descriptive statistics and comparisons between cases and controls.
| Dementia N=172 M (SD) or % (N) |
Dementia N=910 M (SD) or % (N) |
t or X2 | p | |
|---|---|---|---|---|
| Baseline Age (Years) | 67.61 (7.63) | 59.58(11.25) | 8.98 | <0.01 |
| Female | 65.1 (112) | 54.9 (500) | 5.68 | 0.02 |
| Education | ||||
| Elementary education | 73.3 (126) | 58.6 (533) | 12.49 | <0.01 |
| Beyond elementary education | 26.7 (46) | 41.4 (377) | ||
| Years to dementia following baseline | 14.65 (6.70) | - | - | - |
| Baseline Physical illness score | 2.05 (1.74) | 1.80 (1.54) | 1.92 | 0.06 |
| Baseline STPI score | 20.06 (8.05) | 18.45 (7.60) | 2.53 | 0.01 |
| STPI Higher than one standard deviation Q1 thru Q4 | 47.1 (81) | 36.5 (322) | 6.46 | 0.02 |
| Baseline neuroticism | 2.76 (2.30) | 2.60 (2.30) | 0.80 | 0.42 |
| Baseline depressive symptoms | 1.10 (1.23) | 1.14 (1.24) | 0.38 | 0.70 |
| Average depressive symptoms Q1 thru Q3 | 1.19 (1.74) | 1.11 (1.54) | 1.92 | 0.06 |
STPI= state anxiety subscale of the State-Trait Personality Inventory
3.2. Incident Dementia
Results from Cox proportional hazard models are presented in table 2. In the univariate tests, each point increase on the STPI was associated with three percent increased likelihood of incident dementia in the univariate model (hazard ratio [HR] = 1.03; 95% confidence interval [CI], 1.01–1.05). After adjusting for baseline age, sex, education, baseline depressive symptoms, baseline neuroticism, and baseline physical illness, the STPI remained a significant predictor of incident dementia (HR = 1.04 95% CI = 1.01–1.05). In univariate analyses, depressive symptoms at baseline (HR = 1.01; 95% CI= 0.87–1.16, not shown in table 2) or averaged across the three assessments (HR= 1.08; 95% CI = 0.92–1.25, not shown in table 2) were not associated with incident dementia. Substituting CES-D averaged Q2-Q4 did not change the conclusion.
Table 2.
Results from Cox mixed effects proportional hazards models.
| HR | SE | 95% CI | ||
|---|---|---|---|---|
| Model 1 | ||||
| Baseline STPI score | 1.03 | 0.01 | 1.01 | 1.05 ** |
|
| ||||
| Model 2 | ||||
| Q1 Age | 0.94 | 0.01 | 0.91 | 0.96 ** |
| Female | 0.92 | 0.19 | 0.64 | 1.35 |
| Beyond elementary education | 0.75 | 0.21 | 0.50 | 1.11 |
| Baseline physical illness | 1.03 | 0.06 | 0.92 | 1.15 |
| Baseline STPI score | 1.03 | 0.01 | 1.01 | 1.05 ** |
|
| ||||
| Model 3 | ||||
| Q1 Age | 0.93 | 0.01 | 0.91 | 0.96 ** |
| Female | 0.96 | 0.20 | 0.65 | 1.42 |
| Beyond elementary education | 0.76 | 0.21 | 0.50 | 1.14 |
| Baseline physical illness | 1.05 | 0.06 | 0.93 | 1.17 |
| Baseline neuroticism | 1.01 | 0.05 | 0.92 | 1.12 |
| Baseline depressive symptoms | 0.89 | 0.09 | 0.74 | 1.07 |
| Baseline STPI score | 1.04 | 0.01 | 1.01 | 1.06 * |
STPI= state anxiety subscale of the State-Trait Personality Inventory
denotes p < 0.01
denotes p<0.05
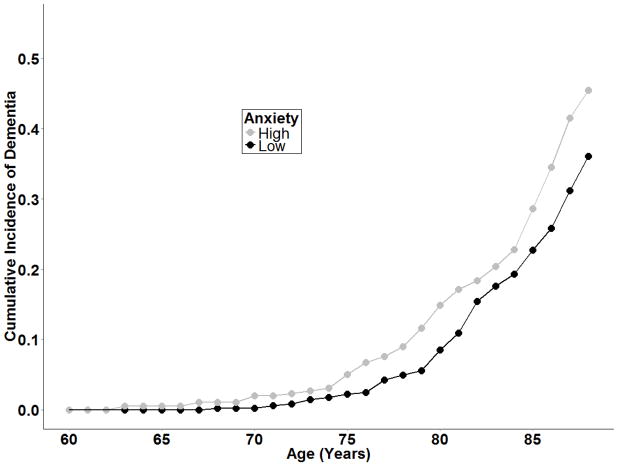
Endorsing at or above one standard deviation on the STPI at any assessment was associated with increased likelihood of dementia in the univariate (HR=1.44; 95% CI=1.01–2.05) and multivariate models (HR = 1.48; 95% CI = 1.01–2.18) including average depression over time as a covariate. Figure 1 displays the cumulative incidence of dementia by age and for high and low anxiety.
Figure 1.
Cumulative incidence of dementia by anxiety group. High anxiety represents participants scoring one or more standard deviations above the mean of STPI at any assessment, low anxiety represents participants scoring below one standard
Sensitivity analyses conducted with the subset of participants who had benzodiazepine data suggested that baseline benzodiazepine use was not associated with incident dementia (HR = 0.95; 95% CI = 0.47–1.92). When adding baseline benzodiazepine use to the model, the size of the HR of the STPI was unchanged. The STPI score remained a significant predictor of dementia when excluding participants who developed dementia within five years of baseline (HR = 1.04; 95% CI = 1.01–1.07).
3.3 Cognitive Performance
Results from the mixed-effects age-based quadratic growth models suggested that baseline STPI was associated with greater declines in Symbol Digit (STPI X Age β = −0.005; 95% CI = −0.01 – −0.001), and Figure Identification (STPI X Age β = −0.01; 95% CI = −0.02 – −0.002) performance over age. A significant main effect of STPI was found on Thurstone Picture Memory (STPI β = −0.15; 95% CI = −0.30 – −0.02), and Block Design (STPI β = −0.17; 95% CI = −0.32 – −0.02, suggesting higher anxiety was associated with worse performance on these tests across all ages. Similarly, on the dichotomous STPI variable, compared to low anxiety, high anxiety at any time point was associated with greater declines in performance over age on Figure Identification (STPI X Age β = −0.18; 95% CI = −0.30 – −0.06), and Thurstone (STPI X Age β = −0.21; 95% CI = −0.38 – −0.04).
3.3. Co-Twin Analysis
When examining discordant pairs only, the twin who developed dementia was more likely to score higher on the baseline STPI, score at or above the one standard deviation at any time on the STPI, and have a higher STPI score relative to their co-twin (table 3). In the regression analyses, STPI score (HR = 1.08; 95% CI = 1.02–1.16), and reporting a higher score on the STPI than the co-twin (HR = 1.96; 95% CI = 1.10–3.50) were associated with higher risk of dementia, but the dichotomous STPI variable was not (HR = 1.91; 95% CI= 0.92–3.96).
Table 3.
Anxiety comparisons for co-twin analyses.
| Control M (SD) or N (%) |
Case M (SD) or N (%) |
t or χ2 | p | |
|---|---|---|---|---|
| All discordant twin pairs (63 cases 63 controls) | ||||
| Baseline STPI score | 17.11 (5.77) | 21.01 (8.55) | 3.00 | <0.01 |
| Higher than one standard deviation at any time point | 20 (39.5%) | 32 (61.5%) | 4.72 | 0.03 |
| Baseline anxiety greater than co-twin | 18 (31.6.9%) | 39 (68.4%) | 15.47 | <0.01 |
| Discordant MZ twins (17 cases 17 controls) | ||||
| STPI score | 18.88 (6.56) | 21.47 (9.52) | 0.92 | 0.36 |
| Higher than one standard deviation at any time point | 6 (42.9%) | 8 (57.1%) | 0.49 | 0.49 |
| Baseline anxiety greater than co-twin | 6 (42.9%) | 8 (57.1%) | 0.57 | 0.45 |
| Discordant DZ twins (46 cases 46 controls) | ||||
| Baseline STPI score | 16.45 (5.38) | 20.84 (8.27) | 3.01 | <0.01 |
| Higher than one standard deviation at any time point | 14 (36.8%) | 24 (63.2%) | 4.48 | <0.03 |
| Baseline anxiety greater than co-twin | 12 (27.9%) | 31 (72.1%) | 16.79 | <0.01 |
STPI= state anxiety subscale of the State-Trait Personality Inventory
Control = co-twin without dementia
Case = co-twin who developed dementia
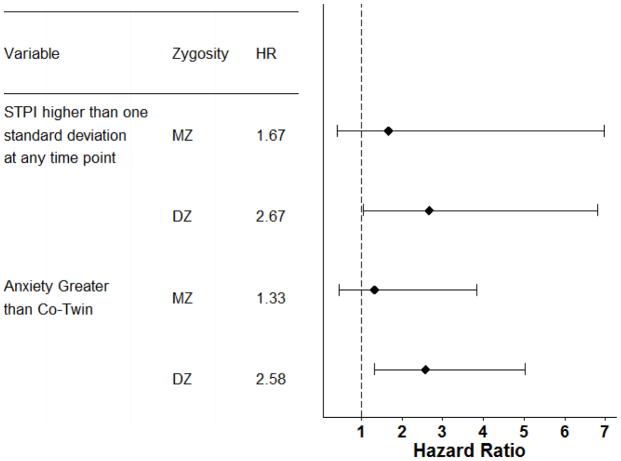
When considered by zygosity, as shown in figure 2, the association between anxiety and risk of dementia was substantially larger for DZ twins when compared with MZ twins. For DZ twins, all three anxiety indicators were associated with significantly increased likelihood of dementia. However, in MZ twins the effect size of anxiety diminished by approximately one half and was no longer statistically significant. This pattern of findings suggests that genetic factors at least partially account for the observed relations between anxiety and dementia risk.
Figure 2.
Hazard ratios with 95% confidence ratios from the co-twin control conditional logistic regression analyses.
4. Discussion
The principal findings of this prospective study are that anxiety symptoms were associated with increased risk of developing dementia. This association was independent of depressive symptoms, neuroticism and other variables associated with dementia. Co-twin control analyses demonstrated an attenuation of the effect of anxiety on dementia in MZ as compared to DZ twins. This finding indicates that genetic and familial factors in common to anxiety and dementia are partially driving the association. No prior studies to our knowledge have examined the role of shared genetic factors. Secondary analyses supported the association between anxiety and decline in cognitive performance, predominately perceptual speed and nonverbal memory.
There are several possible explanations as to why more symptoms of anxiety are associated with greater risk of developing dementia. One possible mechanism is chronic stress that is associated with anxiety, which results in hyperactivation of the hypothalamic-pituitary-adrenal (HPA) axis, which may damage the hippocampus and frontal cortex [38]. Other possible mechanisms include disruptions to the GABAergic system and thyroid abnormalities. GABAergic abnormalities are implicated in both anxiety and in AD [39]. Likewise, thyrotoxicosis is associated with increased anxiety as well as decrements in cognition [40]. Thyrotoxicosis typically occurs in early life and develops into hypothyroidism in later life. These GABAergic and thyroid abnormalities may first manifest as anxiety symptoms followed by severe cognitive deficits later in life.
Further, because the association between anxiety and dementia was partially driven by genetic factors in common to anxiety and dementia, the mechanism may reflect genes that have been implicated in both anxiety and dementia. The brain-derived neurotrophic factor (BDNF) is a protein implicated in neurogenesis and synaptic plasticity. The Val66Met polymorphism of BDNF gene is associated with decreased secretion of BDNF and has been implicated as a possible risk allele for anxiety [41] and worse cognitive functioning [42]. The Apolipoprotein E ε4 variant is the largest known genetic risk for Alzheimer’s disease and also has been implicated with anxiety [43] and stress [44]. Likewise genes associated with serotonin reuptake and the dopaminergic systems have been implicated with anxiety and cognitive performance [45–47]. The complex association between these candidate genes and the phenotypes of interest in this study (e.g. anxiety, dementia, and cognitive functioning) need to be explored further in order to elucidate the biological mechanisms contributing to this association.
Although this study has several strengths (e.g., large sample size of closely followed participants, ability to look at genetic underpinnings, and the long follow-up period) it is important to discuss some limitations. First, it is plausible that the temporal precedence of this association is reversed, with individuals’ awareness of their cognitive decline causing increased anxiety regarding declining abilities. Although this explanation cannot be completely ruled out, anxiety did remain a risk factor when participants who developed dementia within five years of the baseline assessment were excluded. Further, the average onset was 14 years following the baseline, suggesting that it is unlikely that participants would have been experiencing significant cognitive decline at the initial assessment. Second, while the self-reported measure of physical illness has been used in previous research and is commonly utilized in large epidemiological studies, more objective indicators of physical health would have been preferred.
Third, in our analyses, depressive symptoms were not significantly associated with dementia risk. This finding is inconsistent with the majority of research that concludes that depression is a risk factor for dementia [48, 49]. It is possible the OARS, which is both brief and non-comprehensive, may not have been sensitive enough to detect a significant effect of depression on risk of dementia. However, results were unchanged using CES-D. At the same time, one limitation of prior research is that anxiety symptoms were rarely assessed and/or controlled for. Therefore, in the studies that do not control for anxiety, the finding that depression is a risk factor for lower cognitive performance may really be picking up on anxiety [7]. Given the high comorbidity and similarity in presentation between anxiety and depression [50], disentangling the differences between the two can be challenging. An issue here is that the overlapping symptoms of depression and anxiety are potentially confounding when including them in the same statistical model. However, the OARS and the STPI have no overlapping content suggesting that confounding by including these in the same model was minimal.
Fourth, although the co-twin control analyses were consistent with genetic confounding, the sample size for the MZ discordant cases was about one-third that of the DZ twin pairs. Fifth, although we were able to rule out baseline benzodiazepines as an explanation for the findings, we do not know which participants may have received benzodiazepines after baseline or other treatments for anxiety that may have influenced cognitive performance. Finally, a complete psychiatric history for the participant and participant’s family was not available; therefore, we were unable to examine the association between history of anxiety disorders and risk of dementia.
The finding that anxiety is associated with increased risk of dementia has important clinical implications. When conducting cognitive assessments with older adults reporting subjective and objective cognitive complaints, it is important to assess for anxiety. Healthcare providers often misdiagnose late life anxiety; this study demonstrates that these symptoms may be a particularly important risk factor of future dementia. Moreover, our results suggest that even subclinical anxiety is a risk factor for dementia, suggesting that clinicians should pay attention to any anxiety (not just diagnosable anxiety disorders) in older adults. Anxiety is a potentially modifiable risk factor for dementia. Research needs to examine the effect of successfully preventing or treating anxiety and if such interventions decrease the risk of developing dementia. Lastly, our findings that genetic factors in common to both anxiety and dementia partially explain this association may be relevant to designing interventions to reduce anxiety and to understanding the pathophysiology of dementia.
Acknowledgments
SATSA was supported by grants R01 AG04563, R01 AG10175, the MacArthur Foundation Research Network on Successful Aging, the Swedish Council For Working Life and Social Research (FAS) (97:0147:1B, 2009-0795) and Swedish Research Council (825-2007-7460, 825-2009-6141).
Dementia assessments were supported by R01 AG08724.
Dr. Petkus was partially supported by a Ruth L. Kirschstein National Research Service Award (NRSA) fellowship awarded by the National Institute on Aging (1F31AG042218-01).
Abbreviations
- SATSA
Swedish Adoption Twin Study of Aging
- Q
questionnaire assessments
- IPT
in-person-testing assessments
- STPI
State Trait Personality Inventory
- OARS
Older American Resources Services
- MZ
monozygotic
- DZ
Dizygotic
- NPR/NCR
National Patient Registry and National Cause of Death Registry
- HR
hazard ratio
- 95% CI
95% Confidence Interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew J. Petkus, Email: petkus@usc.edu.
Chandra A. Reynolds, Email: chandra.reynolds@ucr.edu.
Julie Loebach Wetherell, Email: jwetherell@ucsd.edu.
William S. Kremen, Email: wkremen@ucsd.edu.
Nancy L. Pedersen, Email: nancy.pedersen@ki.se.
Margaret Gatz, Email: gatz@usc.edu.
References
- 1.Langbaum JB, Fleisher AS, Chen K, Ayutyanont N, Lopera F, Quiroz YT, et al. Ushering in the study and treatment of preclinical Alzheimer disease. Nat Rev Neurol. 2013;9:371–381. doi: 10.1038/nrneurol.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byers AL, Yaffe K, Covinsky KE, Friedman MB, Bruce ML. High occurrence of mood and anxiety disorders among older adults: The National Comorbidity Survey Replication. Arch Gen Psychiatry. 2010;67:489–496. doi: 10.1001/archgenpsychiatry.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasiliadis HM, Dionne PA, Preville M, Gentil L, Berbiche D, Latimer E. The excess healthcare costs associated with depression and anxiety in elderly living in the community. Am J Geriatr Psychiatry. 2013;21:536–548. doi: 10.1016/j.jagp.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Porensky EK, Dew MA, Karp JF, Skidmore E, Rollman BL, Shear MK, et al. The burden of late-life generalized anxiety disorder: effects on disability, health-related quality of life, and healthcare utilization. Am J Geriatr Psychiatry. 2009;17:473–482. doi: 10.1097/jgp.0b013e31819b87b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaudreau SA, O’Hara R. Late-life anxiety and cognitive impairment: a review. Am J Geriatr Psychiatry. 2008;16:790–803. doi: 10.1097/JGP.0b013e31817945c3. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi SU, Kimbrell T, Pyne JM, Magruder KM, Hudson TJ, Petersen NJ, et al. Greater prevalence and incidence of dementia in older veterans with posttraumatic stress disorder. J Am Geriatr Soc. 2010;58:1627–1633. doi: 10.1111/j.1532-5415.2010.02977.x. [DOI] [PubMed] [Google Scholar]
- 7.Burton C, Campbell P, Jordan K, Strauss V, Mallen C. The association of anxiety and depression with future dementia diagnosis: a case-control study in primary care. Fam Pract. 2013;30:25–30. doi: 10.1093/fampra/cms044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zilkens RR, Bruce DG, Duke J, Spilsbury K, Semmens JB. Severe psychiatric disorders in mid-life and risk of dementia in late- life (age 65–84 years): a population based case-control study. Curr Alzheimer Res. 2014;11:681–693. doi: 10.2174/1567205011666140812115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67:608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallacher J, Bayer A, Fish M, Pickering J, Pedro S, Dunstan F, et al. Does anxiety affect risk of dementia? Findings from the Caerphilly Prospective Study. Psychosom Med. 2009;71:659–666. doi: 10.1097/PSY.0b013e3181a6177c. [DOI] [PubMed] [Google Scholar]
- 11.Billioti de Gage S, Moride Y, Ducruet T, Kurth T, Verdoux H, Tournier M, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietrzak RH, Maruff P, Woodward M, Fredrickson J, Fredrickson A, Krystal JH, et al. Mild worry symptoms predict decline in learning and memory in healthy older adults: a 2-year prospective cohort study. Am J Geriatr Psychiatry. 2012;20:266–275. doi: 10.1097/JGP.0b013e3182107e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Bruijn RF, Direk N, Mirza SS, Hofman A, Koudstaal PJ, Tiemeier H, et al. Anxiety is not associated with the risk of dementia or cognitive decline: The Rotterdam study. Am J Geriatr Psychiatry. 2014 doi: 10.1016/j.jagp.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Pink A, Stokin GB, Bartley MM, Roberts RO, Sochor O, Machulda MM, et al. Neuropsychiatric symptoms, APOE epsilon4, and the risk of incident dementia: a population-based study. Neurology. 2015;84:935–943. doi: 10.1212/WNL.0000000000001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF., 3rd Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yochim BP, Mueller AE, Segal DL. Late life anxiety is associated with decreased memory and executive functioning in community dwelling older adults. J Anxiety Disord. 2013;27:567–575. doi: 10.1016/j.janxdis.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Bunce D, Batterham PJ, Mackinnon AJ, Christensen H. Depression, anxiety and cognition in community-dwelling adults aged 70 years and over. J Psychiatr Res. 2012;46:1662–1666. doi: 10.1016/j.jpsychires.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 18.Ormel J, Jeronimus BF, Kotov R, Riese H, Bos EH, Hankin B, et al. Neuroticism and common mental disorders: Meaning and utility of a complex relationship. Clinical Psychology Reviews. 2013;33:686–697. doi: 10.1016/j.cpr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terracciano A, Sutin AR, An Y, O’Brien RJ, Ferrucci L, Zonderman AB, et al. Personality and risk of Alzheimer’s disease: new data and meta-analysis. Alzheimers Dement. 2014;10:179–186. doi: 10.1016/j.jalz.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finkel D, Pedersen N. Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging. Aging, Neuropsychology, and Cognition. 2004;11:325–345. [Google Scholar]
- 21.Spielberger CD. Preliminary manual for the State-Trait Personality Inventory (STPI) Tampa, FL: University of South Florida; 1979. [Google Scholar]
- 22.Potvin O, Bergua V, Meillon C, Le Goff M, Bouisson J, Dartigues JF, et al. Norms and associated factors of the STAI-Y State anxiety inventory in older adults: Results from the PAQUID study. Int Psychogeriatr. 2011:1–11. doi: 10.1017/S1041610210002358. [DOI] [PubMed] [Google Scholar]
- 23.Duke University Center for the Aging and Human Development. Multidemensional functional assessment: The OARS methodology. Durham, NC: Duke University Medical Center; 1978. [Google Scholar]
- 24.Gatz M, Pedersen NL, Harris J. Measurement characteristics of the mental health scale from the OARS. Older Americans Resources and Services. J Gerontol. 1987;42:332–335. doi: 10.1093/geronj/42.3.332. [DOI] [PubMed] [Google Scholar]
- 25.Gatz M, Pedersen NL, Berg S, Johansson B, Johansson K, Mortimer JA, et al. Heritability for Alzheimer’s disease: the study of dementia in Swedish twins. J Gerontol A Biol Sci Med Sci. 1997;52:M117–125. doi: 10.1093/gerona/52a.2.m117. [DOI] [PubMed] [Google Scholar]
- 26.Gatz M, Reynolds C, Nikolic J, Lowe B, Karel M, Pedersen N. An empirical test of telephone screening to identify potential dementia cases. Int Psychogeriatr. 1995;7:429–438. doi: 10.1017/s1041610295002171. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 28.Organization WH. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. World Health Organization; 1993. [Google Scholar]
- 29.Organization WH. International Classification of Diseases-9; Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death. Geneva: World Health Organization; 1975. [Google Scholar]
- 30.Organization WH. International Classification of Diseases-8; Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death. Geneva: World Health Organization; 1965. [Google Scholar]
- 31.Jin YP, Gatz M, Johansson B, Pedersen NL. Sensitivity and specificity of dementia coding in two Swedish disease registries. Neurology. 2004;63:739–741. doi: 10.1212/01.wnl.0000134604.48018.97. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds CA, Zavala C, Gatz M, Vie L, Johansson B, Malmberg B, et al. Sortilin receptor 1 predicts longitudinal cognitive change. Neurobiology and Aging. 2013;34:1710, e1711–1718. doi: 10.1016/j.neurobiolaging.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eysenck HJ, Eysenck SBG. Manual for the Eysenck Personality Inventory. San Diego, CA: Educational and Industrial Testing Service; 1968. [Google Scholar]
- 34.Therneau T. Coxmh: Mixed effects cox models. 2012. [Google Scholar]
- 35.R_Development_Core_Team; Computing RFfS, editor. R: A language and environment for statistical computing. Vienna, Austria: 2012. [Google Scholar]
- 36.SAS Statistical Software. 9.3 ed. Gary, Indiana: SAS Institute; 2012. [Google Scholar]
- 37.McGue M, Osler M, Christensen K. Causal inference and observational research: The utility of twins. Perspect Psychol Sci. 2010;5:546–556. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigues R, Petersen RB, Perry G. Parallels between major depressive disorder and Alzheimer’s disease: role of oxidative stress and genetic vulnerability. Cell Mol Neurobiol. 2014;34:925–949. doi: 10.1007/s10571-014-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Z, Guo Z, Gearing M, Chen G. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzhiemer’s disease model. Nat Commun. 2014;5:4159. doi: 10.1038/ncomms5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samuels MH. Psychiatric and cognitive manifestations of hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2014;21:377–383. doi: 10.1097/MED.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suliman S, Hemmings SM, Seedat S. Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: systematic review and meta-regression analysis. Front Integr Neurosci. 2013;7:55. doi: 10.3389/fnint.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward DD, Summers MJ, Saunders NL, Janssen P, Stuart KE, Vickers JC. APOE and BDNF Val66Met polymorphisms combine to influence episodic memory function in older adults. Behav Brain Res. 2014;271:309–315. doi: 10.1016/j.bbr.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Michels A, Multhammer M, Zintl M, Mendoza MC, Klunemann HH. Association of apolipoprotein E epsilon4 (ApoE epsilon4) homozygosity with psychiatric behavioral symptoms. J Alzheimers Dis. 2012;28:25–32. doi: 10.3233/JAD-2011-110554. [DOI] [PubMed] [Google Scholar]
- 44.Petkus AJ, Wetherell JL, Stein MB, Liu L, Barrett-Connor E. History of sexual assault is associated with greater declines in executive functioning in older adults with APOE epsilon4. J Gerontol B Psychol Sci Soc Sci. 2012;67:653–659. doi: 10.1093/geronb/gbr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynolds CA, Jansson M, Gatz M, Pedersen NL. Longitudinal change in memory performance associated with HTR2A polymorphism. Neurobiol Aging. 2006;27:150–154. doi: 10.1016/j.neurobiolaging.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 46.Lenze EJ, Dixon D, Nowotny P, Lotrich FE, Dore PM, Pollock BG, et al. Escitalopram reduces attentional performance in anxious older adults with high-expression genetic variants at serotonin 2A and 1B receptors. Int J Neuropsychopharmacol. 2013;16:279–288. doi: 10.1017/S1461145712000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proitsi P, Lupton MK, Reeves SJ, Hamilton G, Archer N, Martin BM, et al. Association of serotonin and dopamine gene pathways with behavioral subphenotypes in dementia. Neurobiol Aging. 2012;33:791–803. doi: 10.1016/j.neurobiolaging.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry. 2012;69:493–498. doi: 10.1001/archgenpsychiatry.2011.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Byers AL, Covinsky KE, Barnes DE, Yaffe K. Dysthymia and depression increase risk of dementia and mortality among older veterans. Am J Geriatr Psychiatry. 2012;20:664–672. doi: 10.1097/JGP.0b013e31822001c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petkus A, Lenze E, Wetherell JL. Anxious depression: Application of the transdiagnostic model to older adults. In: Sajatovic M, Lavretsky H, Reynolds CA, editors. Late Life Mood Disorders. New York: Oxford University Press; 2013. [Google Scholar]