Abstract
Environmental temperature greatly affects lifespan in a wide variety of animals, but the exact mechanisms underlying this effect are still largely unknown. A moderate temperature decrease from 22°C to 16°C extends the lifespan of the monogonont rotifer Brachionus manjavacas by up to 163%. Thermodynamic effects on metabolism contribute to this increase in longevity, but are not the only cause. When rotifers are exposed to 16°C for four days and then transfered to 22°C, they survive until day 13 at nearly identical rates as rotifers maintained at 16°C continuously. This persistence of the higher survival for nine days after transfer to 22°C suggests that low temperature exposure alters the expression of genes that affect the rate of aging. The relative persistence of the gene regulation effect suggests that it may play an even larger role in slowing aging than the thermodynamic effects. The life extending effects of these short-term low temperature treatments are largest when the exposure happens early in the life cycle, demonstrating the importance of early development. There is no advantage to lowering the temperature below 16°C to 11° or 5°C. Rotifers exposed to 16°C also displayed increased resistance to heat, starvation, oxidative and osmotic stress. Reproductive rates at 16°C were lower than those at 22°C, but because they reproduce longer, there is no significant change in the lifetime fecundity of females. To investigate which genes contribute to these effects, the expression of specific temperature sensing genes was knocked down using RNAi. Of 12 genes tested, RNAi knockdown of four eliminated the survival enhancing effects of the four-day cold treatment: TRP7, forkhead box C, Y-box factor, and ribosomal protein S6. This demonstrates that active gene regulation is an important factor in temperature mediated life extension, and that these particular genes play an integral role in these pathways. As a thermoresponsive sensor, TRP7 may be responsible for triggering the signaling cascade contributing to temperature mediated life extension. The TRP genes may also provide especially promising candidates for targeted gene manipulations or pharmacological interventions capable of mimicking the effects of low temperature exposure. These results support recent theories of aging that claim rate of aging is determined by an actively regulated genetic mechanism rather than an accumulation of molecular damage.
Keywords: Rotifera, lifespan, aging, temperature, temperature sensing genes, TRP, forkhead box C, Y-box factor, ribosomal protein S6, RNAi, gene expression
Introduction
Many environmental and genetic factors interact to affect aging rate, and a deeper understanding of these mechanisms is integral to discovering new interventions to slow the rate of aging (Malgalhaes et al. 2012). Emerging evidence is shifting the traditional views of aging as an accumulation of molecular damage to an actively regulated signaling network (Gems & Partridge 2013). Even small changes affecting the interconnected pathways can lead to large changes in longevity, especially when applied over the entire lifespan (Conti 2008).
Temperature is a key environmental factor that greatly affects lifespan in all animals studied. There are many cases where modestly lower environmental temperatures greatly increase the lifespan of ectotherms. For example, a 5°C decrease in water temperature extends lifespan of a small, South American fish (genus Cyanolebias) by nearly 100% (Rikke & Johnson 2004). In another fish model, lowering environmental temperature from 25°C to 22°C caused not only a significant increase in mean and maximum lifespan, but also delayed the onset of aging markers such as diminished cognitive ability and lipofuscin accumulation (Valenzo et al. 2006). In lakes with thermal gradients, Daphnia that prefer the colder waters have lower metabolic rates and live several times the usual lifespan (Pietrzak et al. 2013). Because of the internal mechanisms for maintaining a constant temperature, these effects are harder to study in homeotherms (Carrillo & Flouris 2011). However, transgenic mice with a 0.3°C to 0.5°C reduction in core body temperature had 12 to 20% longer lifespans than controls, regardless of diet (Conti et al. 2006).
Although the exact mechanism of temperature mediated life extension remains largely unknown, it is thought to be related to the same mechanisms by which caloric restriction (CR) extends lifespan. In homeotherms, CR is associated with a lower core body temperature because it lowers metabolic rates (Conti 2008). The magnitude of the body temperature response to CR varies with different genetic strains of mice (Rikkel & Johnson 2004). In the fish Cynolebias, CR combined with reduced environmental temperature produced an additive effect on longevity, extending lifespan by nearly 300% (Rikke & Johnson 2004). Several models suggest overlapping mechanisms by which CR and body temperature affect longevity. These include genetic regulation, reductions in inflammatory response, decreased oxidative damage, increased metabolic efficiency, and hormone regulation (Carrillo and Flouris 2011; Conti 2008).
These regulatory pathways are likely highly interconnected and active gene regulation seems to play a major role in temperature mediated extension of longevity (Lee and Kenyon 2009). It is also appreciated that passive thermodynamic effects contribute to the temperature effect, but how much remains to be quantified. In C. elegans, it was found that life extension by lower environmental temperatures is dependent on a thermosensitive genetic pathway, a TRP channel, a calcium sensitive PKC, SGK, and the DAF-16/FOXO transcription factor (Xiao et al. 2013). The DAF-16/FOXO pathway is linked to the nutrient sensing pathway, further connecting the mechanisms of temperature and CR. TRP channels are further known to play a role in external and internal environmental sensing and longevity regulation (Linford et al. 2011). Additionally, the expression of many genes is known to be regulated by changes in temperature. In zebrafish, a shift from 25°C to 12°C increased expression of ependymin, suggesting a role in cold acclimation (Tang et al. 1999). In the rotifer Brachionus manjavacas, a one hour 40°C heat shock increased the expression of four heat shock proteins (HSPs) which play an essential role in thermotolerance and stress protection (Smith et al. 2014). In plants, a 15 minute exposure to 4°C increases the expression of CBF (cold-repeat binding factors), transcriptional factors that trigger a signaling cascade inducing freezing tolerance (Thomashow 2010).
Members of the phylum Rotifera are a useful model for studying aging mechanisms (Snell 2014). Rotifers are members of the understudied supra-phylum Lophotrochozoa. They have a short lifespan of about 12 days at 22°C, which allows full life tables experiments to be performed in a short time (Snell et al. 2014 a). RNAi can be used to effectively knockdown gene expression of selected genes, and biological effects can be observed to better understand the pathway or mechanism by which these genes are acting (Snell et al 2014 b). In this study, we use the rotifer B. manjavacas to explore the effects of different temperature treatments on longevity and identify genes important to temperature-mediated changes in lifespan. We hypothesized that exposure to lower temperatures would greatly extend rotifer lifespan, through both thermodynamic effects and gene regulation. Thermodynamic effects alone cannot account for the observed life extension. We also hypothesized that knockdown in the expression of temperature sensing genes can lead to large changes in longevity and reproduction.
Materials and Methods
Rotifer Culture
All experiments were performed using the rotifer species Brachionus manjavacas. This strain was originally collected from the Azov Sea in Russia, and has been continuously propagated in the laboratory since 1983, with resting eggs being periodically collected, dried, and stored (Rico-Martinez & Snell 1995). Before each experiment, B. majavacas resting eggs were hatched in 25mL, 15ppt artificial sea water (ASW, Instant Ocean) under constant fluorescent illumination (2000 lux) at 25°C. Under these conditions, hatching begins after 18–20 hours, resulting in a uniform cohort of neonates. For particular treatments, resting eggs were hatched at 16°C. At this temperature, hatching was delayed until 48 hours. Hatchlings were fed the green alga Tetraselmis suecica cultured in modified F medium (Guillard 1983) in a 560mL chemostat with 25% daily medium replacement, at 25°C and constant fluorescent illumination of 2000 lux. For experiments investigating the generality of cold response, resting eggs were harvested from three additional rotifer strains; B. manjavacas from Pettaquamscutt, Rhode Island (Suatoni et al 2006), B. plicatilis from China (Snell 2014), and B. rotundiformis from Hawaii (Rico-Martinez & Snell 1995). These strains were cultured under the same conditions as B. manjavacas (Russian).
Experimental Design and Treatments
Full cohort life table experiments were conducted with 120 newly hatched female B. manjavacas per treatment. Animals were kept in 24-well plates with each well containing 5 females in 1mL of medium. Medium contained 6×105 T. suecica cells/mL in 15ppt ASW and 20μM 5-fluoro-2-deoxyuridine (FDU), added to prevent the hatching of asexual eggs (Snell et al 2014b), with the exception of B. rotundiformis medium which contained only 8μM FDU. Plates were checked daily and mortality was recorded until all animals were dead. All animals were transferred to new plates with fresh medium every 8 to 16 days as needed to replenish food supply.
Reproductive life table experiments were performed in a similar fashion, with a few modifications. Single females were kept in each well of 24-well plates in 1mL of medium, for a total of 24 females per treatment. Medium contained 2×105 T. suecica cells/mL in 15ppt ASW, and no FDU was added to allow normal egg hatching. Offspring were produced parthenogenetically and were counted and removed daily. The original maternal females were transferred to new plates every 6 to 12 days, depending on food supply.
All plates were kept in the dark at the appropriate temperature in Percival I-41VL incubators. Standard control temperature was 22°C, with low temperature treatments of 20, 18, 16, 11 and 5°C. 22°C is used as standard incubation temperature because at this temperature, reproductive experiments can be scored accurately at 24 hour intervals. The plates were removed from the incubators once a day and scored at room temperature (22°C). Care was taken to minimize the time the plates were removed from the appropriate temperature treatment.
Stressor Challenge Experiments
Five stressor challenge experiments were conducted to test the effects of low temperature exposure on resistance to starvation, oxidative stress, UV exposure, osmotic shock, and heat shock. All animals were kept in 6-well plates with each well containing several dozen animals in 5mL medium. Medium consisted of 6×105 T. suecica cells/mL in 15ppt ASW with 20μM FDU. Plates were stored in the dark at 16°C for either 0, 1, 2 or 4 days before being transferred to 22°C. On the fourth day, animals were rinsed with 15ppt ASW to clear away algae, and challenged with one of five stress treatments.
Rotifers in the starvation treatment were transferred to 15ppt ASW and deprived of food for 72 hours. Osmotic shocked animals were transferred from 15ppt to 60ppt ASW in a small petri dish for 1 hour, then returned to 15ppt. Heat shocked animals were transferred to a petri dish where they were incubated at 40°C for 1 hour then returned to 22°C. UV stressed animals were transferred into a small petri dish in 5mL 15ppt ASW and exposed to UV-B radiation for 20 minutes. The dish was 25cm from an 8W light source (UVP, model UVM-28EI) with an intensity peak at 302nm. After undergoing these stress treatments, these animals were transferred to 24-well plates with a total of 120 animals per treatment. Each well consisted of 10 animals and 1mL of 15ppt ASW with 20μM FDU. In the oxidative stress treatment, the medium in the plates also contained 0.1μM juglone. These stress treatments were chosen to produce considerable, but not 100%, mortality in the control. All plates were stored in the dark at 22°C, and scored for survival after 72 hours. Survival is reported as the average percentage surviving in each treatment.
Synthesizing and Purifying dsRNA
Genes putatively related to temperature sensing pathways were derived from the B. manjavacas Transcriptome Shotgun Assembly Project. These sequences are available at DDBJ/EMBL/GenBank under the accession GARS01000000. Twelve genes were selected based on sequence similarity to genes identified by Xiao et al (2013) and other temperature sensing pathways. Primers were designed to amplify a 500bp region from each rotifer gene, and DNA product was sequenced using Eurofins Genomics SimpleSeq to ensure proper amplification of the genes of interest. Primers and accession numbers for all genes can be found in Table 1.
Table 1.
List of putative temperature sensing genes and the primer sequences used in PCR. T7 primers contain an additional T7 adapter at the 5’ end. All genes can be found within the Brachionus manjavacas transcriptome shotgun assembly (GARS01000000) using the accession numbers listed.
| Gene | Abreviati on |
GB Accession | Related Gene/Path way |
Forward | Reverse |
|---|---|---|---|---|---|
| Protein Kinase C 1 |
PKC1 | GARS010028 68.1 |
SGK (Human) |
GGCTGGTTCAAACTGTT GGT |
ATCGCCACCGTTGATGT ATT |
| Protein Kinase C 2 |
PKC2 | GARS010021 61.1 |
PKC (Human) |
TTGAAGAATTGCGTCAC AGG |
CGCACATTGTAGGTCTT GGA |
| Ribosomal Protein S6 Polypepti de 2 |
S6P | GARS010030 02.1 |
SGK (Human) |
GCGATGACGACAATAT CACG |
TCCAACTGCATGAACA GCTC |
| Ribosomal Protein S6 Kinase Alpha-1 |
S6Ka | GARS010025 35.1 |
SGK (Human) |
CAAGCCGACTCCATCTC ATT |
CAGGCTTTAGATCGCG GTAA |
| Transient Receptor Potential Cation Subfamily Member 1 |
TRP1 | GARS010022 38.1 |
TRP (Human) |
GCTGGCCAGACAAATA CTCG |
ATACGCCGAAAGCAAA GAAA |
| Transient Receptor Potential Cation Subfamily Member 7 |
TRP7 | GARS010121 97.1 |
TRP (C elegans) |
CAATGTTTTGGTCCTTA TTTGG |
CCAAATGGAATTGATC CGATA |
| Forkhead Transcript ion Factor n1 4 Transcript Variant 1 |
FhTF | GARS010023 02.1 |
Daf 16-g (C elegans) |
CCCAAGTGGCTATGAG AAGC |
TTAGCTTGCGTTGACA GGTG |
| Forkhead Box C-like Protein |
FhBC | GARS010060 72.1 |
Daf 16-a (C elegans) |
AACGACTGCTTCTGCAA GGT |
GACCGAAAACGACGAG TAGG |
| Fork-head Box j2 3 Transcript ion Factor |
FhBJ | GARS010055 85.1 |
Daf 16-h (C elegans) |
CATCAATGGAATGGTT GTCG |
AACCCTTTCCAGGGTCA TCT |
| Y-box Factor |
YBoxF | GARS010015 40.1 |
Temperatur e Sensitivity |
TGTCGGATACCGCAGT ACAA |
TTTGGTGTCGTCACGG TTTA |
| Y box Binding Protein 1 |
YBoxBP | GARS010028 78.1 |
Temperatur e Sensitivity |
TTGAACCAGTGTCCCAA CAA |
GGCTGTCTTGGTGGTC TGTT |
| Mammali an Ependymi n-related Protein |
MERP | GARS010097 84.1 |
MERP (D rerio) |
ACCATGCACATCTCCTC CTC |
CGATTCCTGGAACAAT GTCG |
To collect DNA for dsRNA synthesis, 0.5μM of forward and reverse primers with a T7-motif (TAATACGACTCACTATAGG) were used with Go-Taq DNA polymerase (Promega), 1mM MgCl2, 100mM dNTP (Promega), 10× buffer, and 40ng of genomic B. manjavacas DNA. The resulting PCR products were purified using a QIAquick PCR purification kit (Qiagen). dsRNA was synthesized from the T7 tagged DNA and purified using a MEGAscript® RNAi kit (Invitrogen). Purified dsRNA was quantified using a NanoDrop 1000 (Thermo Scientific) and visualized on a 1% agarose gel to verify band size.
To serve as a control for mortality or changes in expression due to exposure to transfection reagents, a scrambled sequence of the rotifer TOR gene was synthesized by the company IDT and used in place of genomic DNA in the initial PCR, as described by Snell et al. (2014). The scramble sequence is not found in the known B. manjavacas genome, and was used as a control in all RNAi experiments.
RNAi Knockdown Experiments
Four types of RNAi experiments were performed to screen for biological effects of gene expression knockdown. The biological effects tested were ability of resting eggs to hatch, female survival, reproduction, and cold sensitivity. For all experiments, a transfection solution was prepared with 47uL PBS, 1uL FuGene6 (Promega), and 2uL (200ng) dsRNA and incubated at room temperature for 20 minutes (modified from Snell et al. 2010). For hatching, survival, and cold sensitivity tests, B. manjavacas neonates hatching from resting eggs were collected within 2 hours of their births, and transferred to 500uL 15ppt ASW in a 24-well plate. Roughly 100 animals were transferred to each well. 50uL of transfection solution was added to each well, and the plate was incubated at room temperature for 4 hours before animals were transferred to each experimental treatment.
For the survival experiments, animals were transferred to 24-well plates with 1mL 6×105 T. suecica cells/mL, 20μM FDU, and 5 females in each well. One full plate was used for each treatment, and plates were incubated at 28°C in the dark for eight days. On days 2, 4, and 6, plates were treated with 5uL additional FDU (1mg/mL) to prevent asexual egg hatching, and on day 6 plates were fed 100uL of 6×106 T. suecica cells/mL per well. After 8 days, the number of living animals was counted and survival was recorded as average percent surviving in each treatment.
In the reproductive experiments, animals were transferred from the transfection solution into 24-well plates with 1mL 2×105 T. suecica cells/mL and 1 female in each well. One full plate was used for each treatment, and the plates were incubated in the dark at 25°C for 72 hours. Offspring were counted and removed daily, and population growth rate r was calculated at 72 hours.
To screen for changes in lifespan due to cold sensitivity, animals were transferred into 24-well plates with 1mL 6×105 T. suecica cells/mL, 20μM FDU, and 5 females in each well. For each treatment, 3 full plates were prepared. One of these plates was incubated at 22°C continuously, another was incubated at 16°C continuously, and the third was incubated at 16°C for the first four days and then transferred to 22°C. On days 4, 8, and 12, each well was treated with 5uL additional FDU (1mg/mL), and on day 8 each well was fed 100uL 6×106 T. suecica cells/mL. Survival was recorded daily until day 15, and the data was used to generate right-censored survival curves.
To test the effects of RNAi knockdown on hatching, diapausing B. manjavacas embryos (resting eggs) were decapsulated using the protocol of Snell et al. (2010), and then transferred to 24-well plates with 500uL 15ppt ASW, 25uL transfection solution, and 20 resting eggs in each well. Six replicate wells were used for each treatment. Plates were incubated at 25°C under constant fluorescent illumination (2000 lux). Number of eggs hatched in each well was recorded after 48 hours.
Statistics
Survival curves from full life tables and cold sensitivity screens were evaluated using the JMP Pro 11 (SAS Institute) reliability and survival analysis with a Wilcoxon’s test to compare control and treatments. Stress challenge, hatching, 8 day survival, and reproductive screens were compared by ANOVA with Dunnett’s test to compare treatments to control.
Results
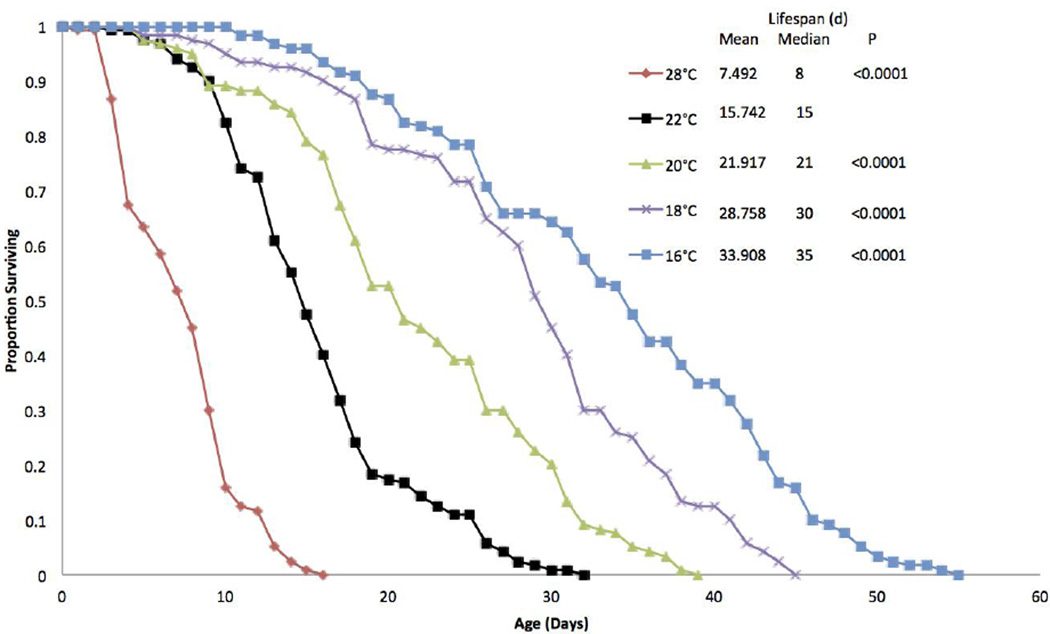
Small changes in environmental temperature lead to large changes in rotifer lifespan (Figure 1). A 2°C decrease from 22°C to 20°C extended lifespan by 39%. A further 2°C decrease to 18°C extended lifespan by 83%, and a 6°C decrease from 22°C to 16°C extended lifespan by 115% compared to the 22°C control. Similarly, a 6°C increase in temperature to 28°C decreased lifespan by 52%. All of these temperature induced changes in survival are significantly different from the 22°C control (P<0.0001) by a Wilcoxon test.
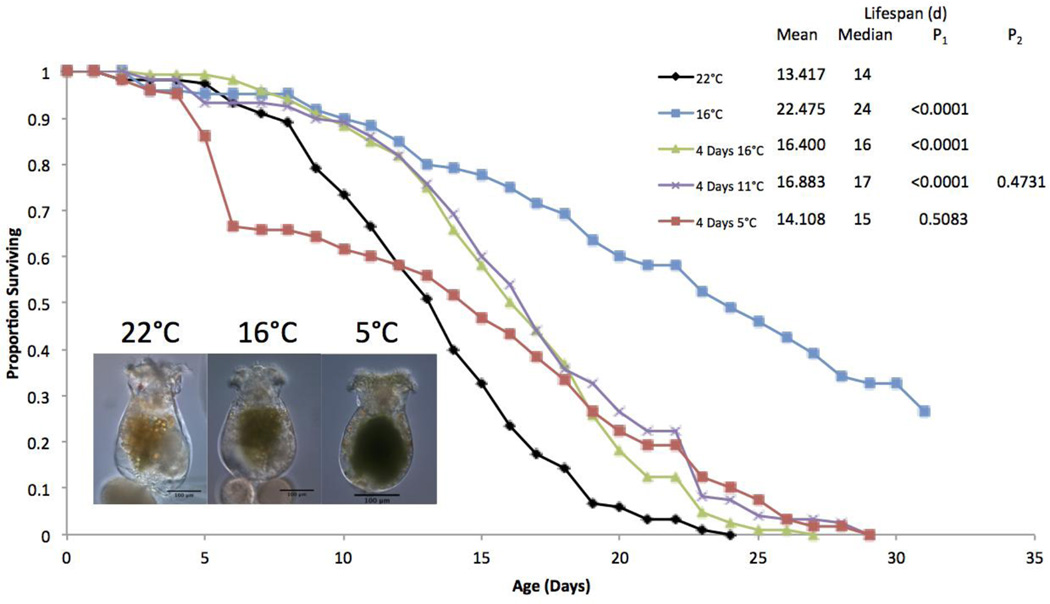
Figure 1.
Survival curves for B. manjavacas incubated continuously at 5 different temperatures. Proportion surviving represents the fraction of an initial cohort of 120 females surviving to each age. Mean and median lifespan are reported in days, and P represents the Wilcoxon test probability that the treatment curves are similar to the 22°C control.
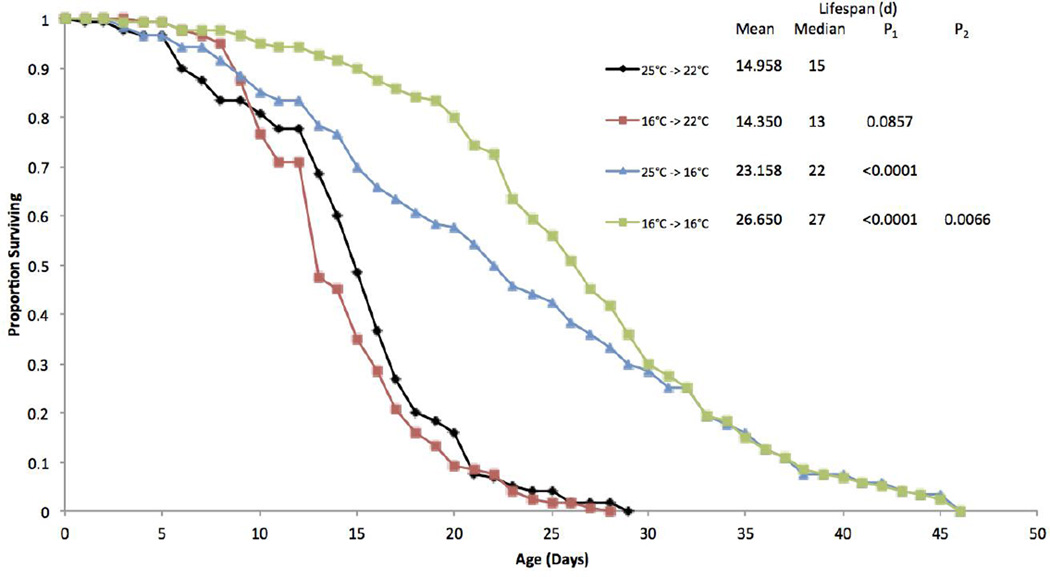
A second experiment examined the effects of hatching the resting eggs at different temperatures on adult survival and lifespan (Figure 2). Hatching resting eggs at 16°C and incubating the newborns at 22°C produced lifespans not significantly different from control animals hatched at 25°C and incubated at 22°C. Animals hatched at 25°C and incubated at 16°C produced significant life extension of 63% as compared to control. However, animals that were hatched at 16°C and maintained at 16°C experienced an 86% longer lifespan, significantly greater than rotifers hatched at 25°C and transferred to 16°C.
Figure 2.
Survival curves for B. manjavacas hatched and incubated at different temperatures. The first number of the treatment refers to the temperature resting eggs were hatched in and the second refers to the incubation temperature after hatching. Proportion surviving represents the fraction of an initial cohort of 120 females surviving to each age. Mean and median lifespan are reported in days. P1 represents the Wilcoxon test probability that the treatment curves are similar to the 25°C–>22°C control, and P2 represents the Wilcoxon test probability that the curve is similar to the 25°C–>16°C treatment.
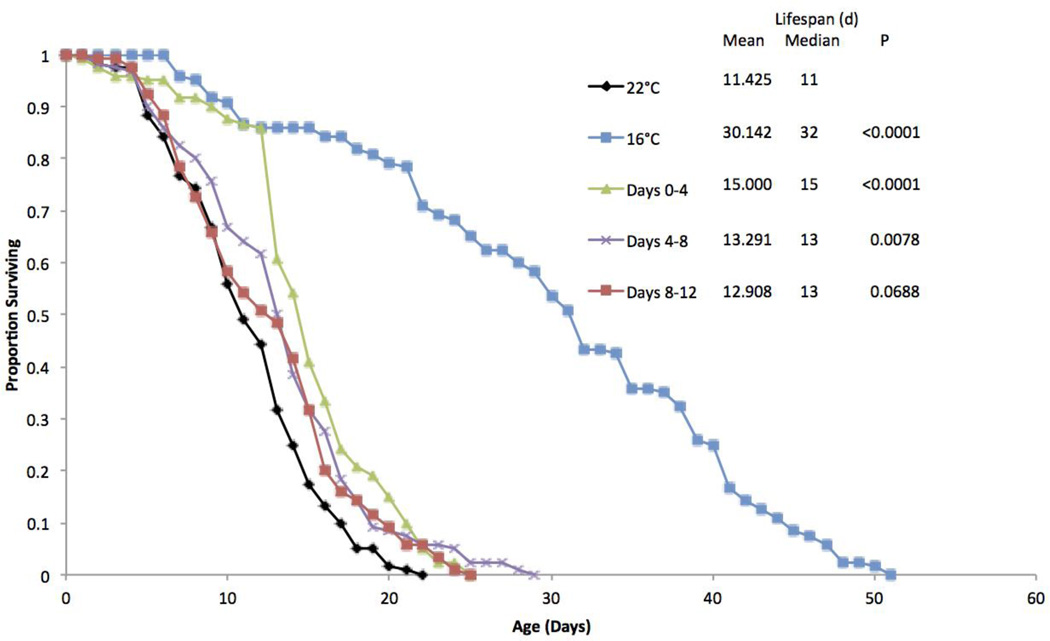
A third life table experiment was performed to explore the effects of 16°C exposure at various life stages (Figure 3). Continuous 16°C exposure extended lifespan by 163%. Exposing rotifers to 16°C for only their first four days significantly extended lifespan by 31%. Even though exposure to 16°C only lasted until day four, the survival curve closely followed that of the 16°C continuous treatment until day 13. Exposing the animals to 16°C from days 4 to 8 also extended lifespan by 16%, but was not nearly as effective at extending lifespan as the same exposure in the early age classes. Exposure to 16°C from days 8 to 12 provided no significant life extension.
Figure 3.
Survival curves for B. manjavacas incubated at different temperatures during different stages of the lifespan. The first two treatments remained at a single continuous temperature. The other three treatments were incubated at 16°C for the days labeled and 22°C for all other days. Proportion surviving represents the fraction of an initial cohort of 120 females surviving to each age. Mean and median lifespan are reported in days, and P represents the Wilcoxon test probability that the treatment curves are similar to the 22°C continuous control.
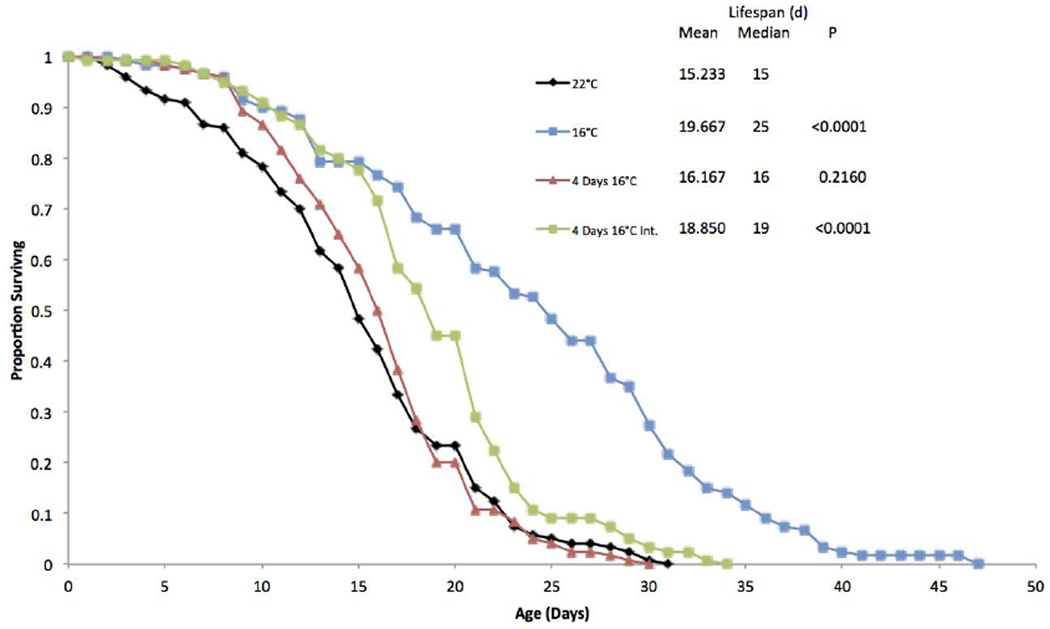
A fourth life table experiment was performed to investigate whether additional exposure to 16°C could extend the protective effect of the initial four day 16°C exposure (Figure 4). In this experiment, 16°C exposure for the first four days did not significantly increase median lifespan, but survival was essentially the same as the 16°C continuous treatment until day 11. However, a 16°C exposure for the first four days, followed by alternating four days at 22°C and then four days at 16°C for the remainder of the lifespan significantly extended lifespan by 24%, and the survival curve followed that of the 16°C continuous treatment until day 16.
Figure 4.
Survival curves for B. manjavacas incubated at different temperature treatments. 4 Days 16°C was exposed to 16°C only for the first 4 days of life, while the Int. treatment was exposed for 4 days intermittently throughout the lifespan. Proportion surviving represents the fraction of an initial cohort of 120 females surviving to each age. Mean and median lifespan are reported in days, and P represents the Wilcoxon test probability that the treatment curves are similar to the 22°C control.
The effects of a range of early life cold treatments were examined in another life table (Figure 5). In this experiment, a four day exposure to 16°C extended lifespan by 22%. A four day exposure to 11°C similarly extended lifespan by 26%, but provided no significant additional benefit beyond exposure to 16°C. A four day exposure to 5°C on the other hand, provided no life extension, and the survival curve displayed an unusually large number of deaths in the early age classes. The rotifers exposed to 5°C were also an average of 42% smaller than those incubated at 22°C, and in many of them, the pseudocoelom was dark green, suggesting that the gut ruptured allowing algae to escape into the pseudocoelom.
Figure 5.
Survival curves for B. manjavacas incubated at different temperatures for the first four days of the lifespan. All four day treatments were transferred to 22°C on day 5. Proportion surviving represents the fraction of an initial cohort of 120 females surviving to each age. Mean and median lifespan are reported in days. P1 represents the Wilcoxon test probability that the treatment curves are similar to the 22°C control, and P2 represents the Wilcoxon test probability that the curve is different from the 4 days at 16°C treatment. Photos show 5 day old animals from different temperatures.
The 22°C continuous control treatments in these five independent life table experiments displayed small amounts of experimental variation (Table 3). The average lifespan of the control animals was 14.2 days with a 12.4% coefficient of variation. The average maximum lifespan of the longest-lived 10% of animals was 23.7 with a 15.1% coefficient of variation.
Table 3.
Data on the sample size, mean lifespan, and lifespan of the longest-lived 10% of individuals from all control treatments. Each experiment represents an independent cohort followed for the entire lifespan. Control treatments were incubated continuously at 22°C with 6×105 cells/mL T. suecica and 20uM FDU.
| Experiment | n | Mean | Max (10%) |
|---|---|---|---|
| 1 | 120 | 15.7 | 27.8 |
| 2 | 120 | 15.0 | 24.3 |
| 3 | 120 | 11.4 | 19.3 |
| 4 | 120 | 15.2 | 26.3 |
| 5 | 120 | 13.4 | 21.0 |
| Mean | 14.2 | 23.7 | |
| Standard Deviation | 1.8 | 3.6 | |
| Coefficient of Variation | 12.4 | 15.1 | |
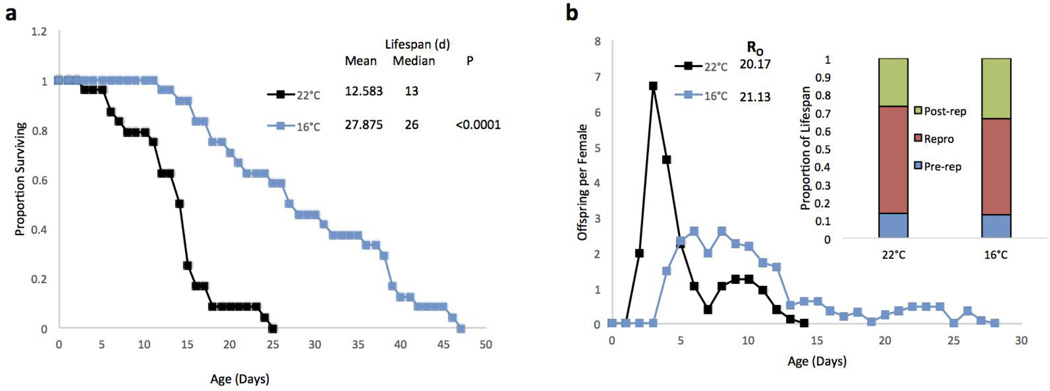
A reproductive life table was performed to observe the effect of a 16°C temperature on lifespan and reproduction. In the absence of FDU, the 16°C treatment extended lifespan by 122% as compared to the 22°C control (Figure 6a). The reproductive curves of the two treatments were very different, with the 22°C treatment peaking at nearly 7 offspring per female per day and earlier (day 4), whereas the 16°C treatment produced fewer offspring (peak: 2.5 offspring per female per day, days 6–8) for a longer period of time (Figure 6b). Nonetheless, the mean total offspring produced by a female over her lifespan (R0) was not significantly different between the two temperatures. The average proportions of the life span spent in pre-reproductive, reproductive, and post-reproductive stages also did not differ significantly between treatments.
Figure 6.
a) Survival curves for B. manjavacas incubated continuously at 2 different temperatures. b) Reproductive curves for B. manjavacas incubated at 2 different temperatures. Proportion surviving represents the fraction of an initial cohort of 24 females surviving to each age. Mean and median lifespan are reported in days, and P represents the Wilcoxon test probability that the treatment curves are similar to the 22°C control. Offspring per female refers to the average number of offspring produced each day by a cohort of 24 females. R0 is the mean total offspring produced by a female over the lifetime. Pre-rep refers to the average proportion of the lifespan a female spends in the pre-reproductive stage, Repro is the proportion spend reproducing, and Post-rep is the proportion spent in the post-reproductive stage.
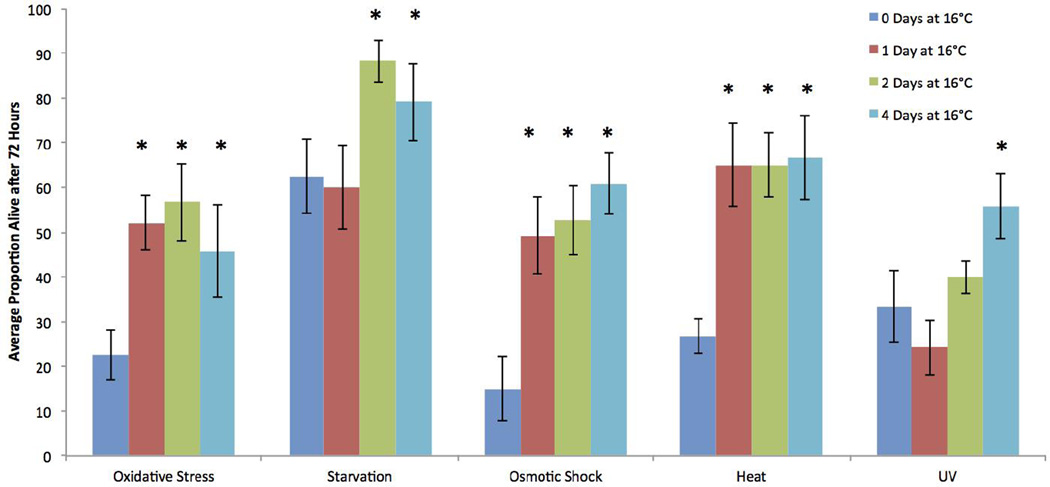
Another effect of exposure to 16°C was to provide protection from different stressors, as demonstrated in five stress challenge experiments (Figure 7). Four days of continuous 16°C exposure provided significant protection from all five stressors as compared to animals incubated at 22°C. One or two days of 16°C exposure followed by 22°C incubation until the stress tests on day 4 also provided significant protection from oxidative, osmotic, and heat stress. Two days of 16°C exposure provided protection from starvation, but only one day exposure did not. Neither one nor two days of 16°C treatment was enough to provide protection from UV light.
Figure 7.
The effects of different stressors on B. manjavacas survival. All treatments were incubated at 22°C when not in the 16°C treatment specified, and all stress challenges were applied on day 4. Oxidative stress exposed animals to 0.1μM juglone for 72 hours. Animals in the starvation test were removed from all food for 72 hours. Osmotic shock exposed animals to 60ppt ASW for 1 hour. Heat shock exposed animals to 40°C for 1 hour. UV treatment animals were exposed to UV light for 20 minutes. Variance is represented by the standard error bars on each column. Asterisks denote a P-value of <0.05 calculated from an ANOVA with Dunnett’s test comparing the treatments to the control for each stressor.
RNAi was used to explore the importance of potential temperature sensitive genes in mediating these life extension effects. Twelve rotifer genes were chosen based on sequence similarity to genes known to be involved in temperature sensing pathways in C. elegans (Table 1). A series of screens were used to assess the different types of biological responses to RNAi knockdown of these genes (Table 2). Only PKC1 knockdown had any significant effect on resting egg hatching, decreasing the mean number of eggs hatched after 48 hours by 73%. RNAi knockdown of PKC1, S6P, TRP7 and YBoxBP genes all significantly decreased average number of offspring produced by asexual mothers after 72 hours. Most interestingly, S6Kα, TRP7, and FhBC knockdown increased mean survival after eight days at 28°C by 36%, 23% and 29%, respectively.
Table 2.
The effects of RNAi mediated knockdown of temperature related genes on B. manjavacas. Hatching refers to the average number of resting eggs hatched after 48 hours. Reproduction refers to average number of offspring per female at 72 hours. Survival refers to the average proportion of animals surviving after 8 days at 28°C. NS denotes no significance difference from the scramble treated control (P>0.05) as calculated by ANOVA with Dunnett’s test. Percentages refer to significant increase or decrease as compared to control.
| Gene | Hatching | Reproduction | Survival |
|---|---|---|---|
| PKC1 | ↓72.5% | ↓15.8% | NS |
| PKC2 | NS | NS | NS |
| S6P | NS | ↓16.1% | NS |
| S6Ka | NS | NS | ↑36.2% |
| TRP1 | NS | NS | NS |
| TRP7 | NS | ↓17.2% | ↑23.2% |
| FhTF | NS | NS | NS |
| FhBC | NS | NS | ↑29.0% |
| FhBJ | NS | NS | NS |
| YBoxF | Ns | NS | NS |
| YBoxBP | NS | ↓21.9% | NS |
| MERP | NS | NS | NS |
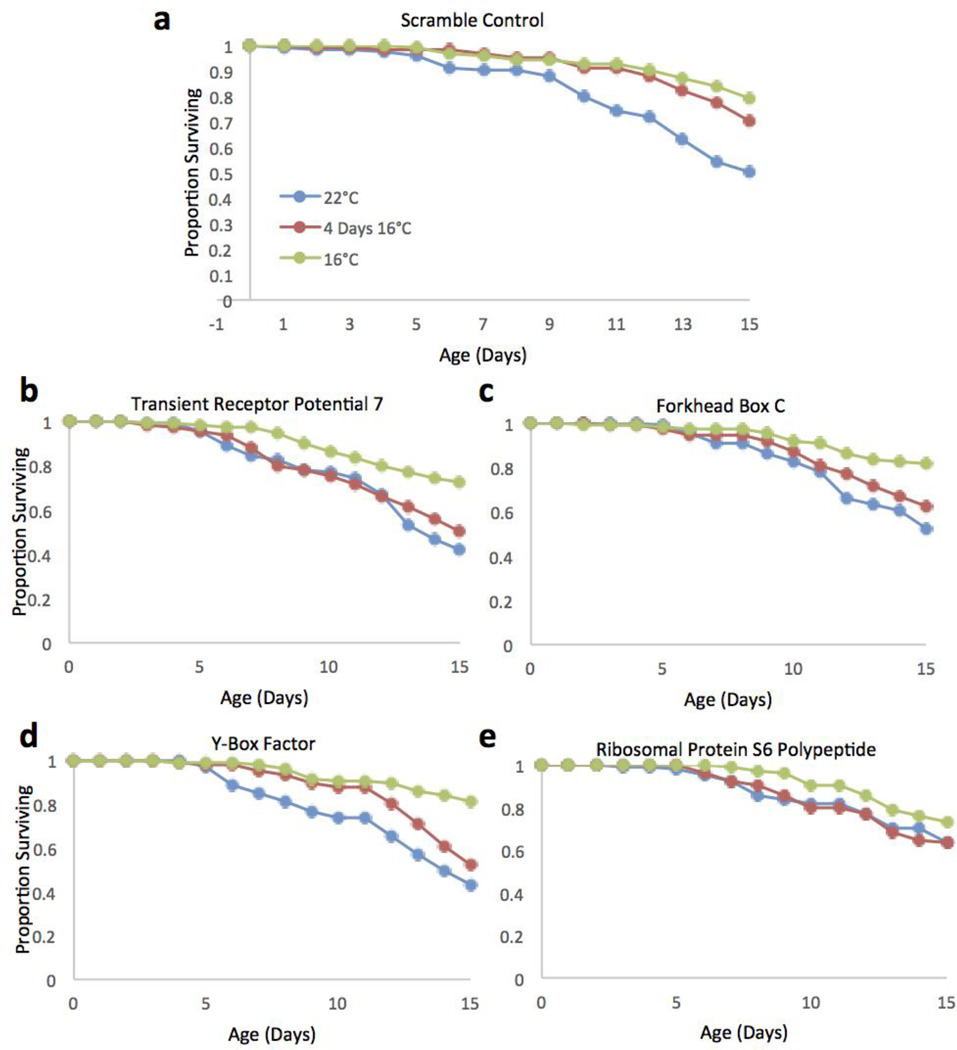
To assess the role of these genes in rotifer temperature sensing more specifically, screens were conducted to compare the survival of RNAi treated animals at 22°C continuous, 16°C continuous, and a four day 16°C exposure before being transferred to 22°C (Figure 8). Control animals were treated with a scrambled dsRNA from the rotifer TOR gene. A Wilcoxon’s test showed that both the four day and continuous 16°C treatments have very similar survival, and that both treatments survived significantly better than the 22°C treatment. However, four of the RNAi knockdown treatments markedly altered the survival curves. When rotifers were exposed to dsRNA of TRP7, FhBC, YBoxF, and S6P genes, the four day 16°C exposure curve showed significantly lower survival than the 16°C continuous, and was much more similar to 22°C continuous exposure. This shift in the effect of a 16°C exposure on rotifer survival demonstrates that these four genes likely have a key role in sensing and responding to low temperatures by rotifers.
Figure 8.
Survival curves for RNAi treated B. manjavacas incubated at 3 different temperatures. a) Typical curves for the scramble treated control animals at each temperature. b–e) Survival curves for expression knockdown treatments where the persistent survival effect of a 4 day 16°C exposure is eliminated. Proportion surviving represents the fraction of an initial cohort of 120 females surviving to each age.
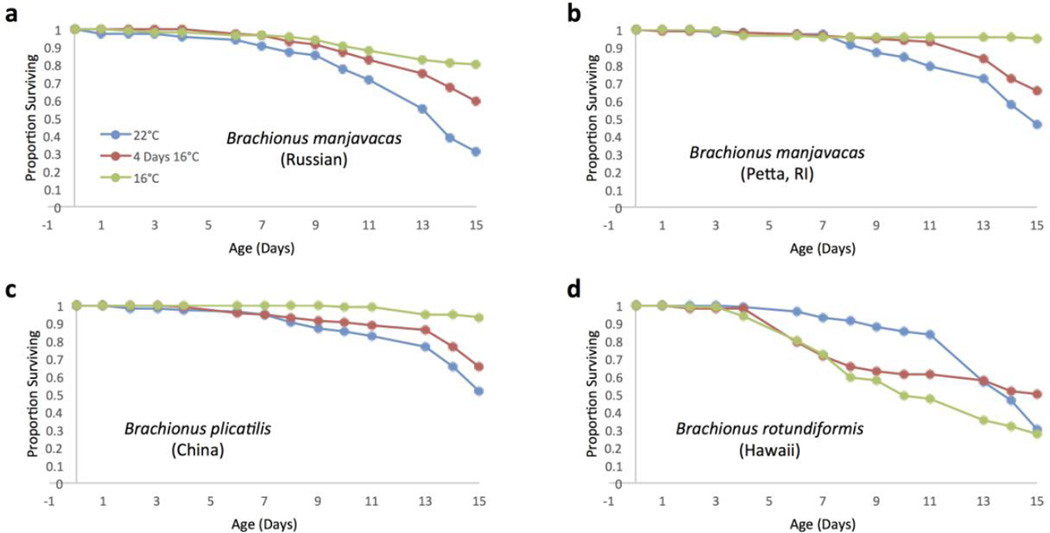
To investigate the generality of this life extension response by animals treated at 16°C for four days, similar screens were performed on four different Brachionus strains (Figure 9). In B. manjavacas (Russian) and B. manjvacas (Petta), both the four day treatment and continuous 16°C treatment survived significantly better than the 22°C control. B. plicatilis (China), followed a similar pattern, but the persistent survival effect of the four day treatment was not as strong. In B. rotundiformis (Hawaii), the response to cold treatment was much different. The animals at 22°C continuously actually survived significantly better than those cultured at 16°C continuously. Those treated at 16°C for four days survived significantly better than those at 16°C continuously, and did not differ significantly from the 22°C treatment.
Figure 9.
Survival curves for Brachionus rotifers incubated at 3 different temperature treatments. a) Typical curves for B. manjavacas (Russian) at each temperature. b–d) Survival curves for other Brachionus strains and species. Proportion surviving represents the fraction of an initial cohort of 120 females surviving to each age.
Discussion
The initial life table experiment demonstrated that even modest changes in temperature (9–27%) could greatly extend rotifer lifespan (Figure 1). Each 2°C reduction in temperature resulted in roughly 40% lifespan extension. A 6°C temperature difference from 22°C to 16°C provided the greatest lifespan increase up to 163%. Although a 6°C difference in core body temperature would be sizeable in a homeotherm, it lies well within the thermal tolerance range for a Brachionus rotifer (Walker 1981).
It is well documented that across a variety of species including microbes, ectotherms, and endotherms, metabolic rate decreases with decreasing temperature by reducing the rates of biochemical reactions (Gillooly et al 2001). In tropical plants, temperatures below 15°C disrupt vital physiological processes including photosynthesis and respiration, slowing and in some cases completely inactivating metabolism (Lutkatkin et al 2012). Insects are able to rapidly acclimate to cold temperatures through a variety of physiological mechanisms (Teets and Denlinger 2013). These responses include accumulation of cryoprotectants such as glycerol, a metabolic shift towards glycolysis, increases in membrane phospholipid unsaturation, and changes in calcium levels (Lee and Denlinger 1991; Teets and Denlinger 2013; Murray et al 2007). However, these responses alone are not likely sufficient to explain the life extension effects found in our experiments.
A key finding from this study is the persistence of higher survival after removal from 16°C. Survival rates for the four day 16°C treatment matched that of continuous 16°C exposure until day 12. This demonstrates that the mechanism of low temperature life extension cannot solely be a passive thermodynamic effect. It is quite likely that low temperature exposure modulated the expression of specific genes, thereby slowing aging processes. Clearly, identifying these genes and how they impact aging processes is a high priority in future work. Based on the duration of this persistent survival effect, and our findings that knockdown of specific genes can completely eliminate this effect, it is possible that genetic regulation elicited by changes in temperature plays an even larger role in temperature mediated life extension than passive thermodynamic effects. However, quantifying the relative magnitude of each mechanism requires additional research.
Although complete enumeration of the genetic pathways responsible requires further investigation, the life extending responses to lowered temperature are likely caused by a complex signaling cascade regulated by a sensory receptor. The mechanism of temperature sensation in plants remains incompletely understood, but within only 15 minutes of a 4°C exposure, CBF transcription factors are induced. These transcription factors signal a large cascade of other genes involved in cold acclimation and stress resistance (Thomashow 2010; Miura and Furumoto 2013). In C. elegans, temperature mediated life extension was found to be dependent on the expression of the TRP channel TRPA-1 (Xiao et al 2013). The TRP superfamily is comprised of a large number of cation channels with a variety of selective activation mechanisms. Many of the TRPs are thermosensory receptors that respond to a wide range of temperatures to trigger signaling cascades that regulate body temperature and avoid tissue damage (Flockerzi and Nilius 2007). TRP thermoreceptors likely control this temperature mediated signaling cascade in rotifers as well. Because of the linkage between temperature, aging, and metabolism, it is likely that part of this cascade involves nutrient sensing pathways like TOR as well.
A series of RNAi experiments was used to further explore which genes may play an integral role in the thermosensory life extension of rotifers. The genes investigated in these experiments were all derived from the B. manjavacas transcriptome based on sequence similarity to genes found in temperature sensing pathways from other animals (Table 1). The high similarity between these genes suggests that they have been highly conserved evolutionarily and likely play an important role in metabolism. Of the 12 genes knocked down, only three (TRP7, FhBC, and S6Ka) caused a significant change in survival, and these effects were not nearly as large as the effects produced by a continuous 16°C exposure (Table 2). Changing the expression of only one gene at a time was not sufficient to mimic the effects of a continuous low temperature treatment, further supporting the idea that multiple pathways are involved in temperature mediated life extension.
The persistent effect of a four day 16°C treatment on survival was effectively eliminated in four of the gene knockdown treatments; TRP7, FhBC, YBoxF, and S6P. This emphasizes the likely role of these genes in temperature mediated life extension. FhBC and S6P are related to the DAF-16 and SGK genes, respectively. DAF-16 is a key regulator of both lifespan and stress resistance, and SGK is an upstream regulator of DAF-16 (Riedel et al 2013; Xiao et al 2013). TRP7 is a member of the TRP channel family, which are ancient, multifunctional thermosensory channels that can respond to both extracellular or intercellular activators (Linford et al 2011). TRP7 specifically is related to the C. elegans TRPA-1 which detects environmental temperature drops to regulate a signaling cascade (Lee & Kinyon 2009; Xiao et al 2013). Finding ways to artificially manipulate sensory signaling through pharmacological or gene treatments could be a key to capturing the benefits of low temperature treatments on longevity without the need for prolonged low temperature exposure (Linford et al 2011). The TRP channels provide excellent pharmacological targets for further research because there are several drugs that are already known to bind to mammalian thermosensory TRPs (Flockerzi and Nilius 2007). Further experimentation is necessary to understand these types of gene-environment interactions and to discover new aging interventions (Magalhães et al 2012).
The stress challenge experiments demonstrated that a 16°C temperature treatment provides protection to a variety of stressor types (Figure 7). This further supports the idea that temperature mediated longevity is controlled by a variety of interconnected pathways. Many life-extending interventions are associated with increased stress resistance, so it is not surprising that continuous low temperature exposure provides protection (Johnson et al 1996). However, even a one-day exposure to 16°C provides significant protection against oxidative, osmotic, and heat stress four days later. This provides additional evidence that temperature effects may be caused by the induction of specific genes. There is already considerable support for the idea that stress resistance has a genetic basis and that resistance can arise as an adaptation to abiotic environmental factors (Dragosits et al 2013).
Lower temperatures may also confer resistance to stressors through the production of molecular chaperones. Heat shock proteins (HSPs) are well characterized, highly conserved molecular chaperones up-regulated after exposure to high temperatures (Fabbri et al 2008). They stabilize proteins and increase thermotolerance in many animals (Smith et al 2012). There is evidence that under cold stress, plants exhibit both post-transcriptional and post-translational regulation. RNA binding proteins prevent RNA from becoming over-stabilized in misfolded conformations at low temperatures, and cold stress affects an ubiquitin E3 ligase pathway to regulate the proteasome (Miura and Furumoto 2013). In addition, cold stress has been shown to up-regulate HSP70 in Drosophila (Sinclair et al 2007). Furthermore, the activation of thermosensitive TRPs in mammalian skin cells has been found to induce the expression of HSPs (Hsu and Yoshioka 2015).
In the reproductive assay, it was found that while an incubation temperature of 16°C increased lifespan by 122% as compared to a 22°C control group, the lifetime fecundity of an individual female was unchanged (Figure 6). In addition, the proportion of the lifespan spent in the reproductive phase was unchanged by temperature. This demonstrates that lower temperatures increase health-span as well as lifespan. Females at 16°C produced fewer offspring per day, but remained reproductive longer. This is consistent with previous research that found that in B. plicatilis, increasing temperature increased intrinsic rate of increase, but the effect of temperature on fecundity was negligible as long as the temperature was within the normal range for natural habitats (Miracle & Serra 1989). However, it was also discovered that the slope of the relationship between temperature and reproduction was dependent on the genotype of the strain, and there were distinct differences between warm- and cold-adapted genotypes (Miracle & Serra 1989).
This same phenomenon has been observed between rotifer sibling species in both natural populations and laboratory settings. In Spain, it was found that populations of B. plicatilis and two of B. rotundiformis were adapted to different temperature and salinity preferences. As such, the three genotypes demonstrated different responses in sexual reproduction to different temperature treatments (Gómez et al 1997; Serra et al 1998). We have observed that the lifespans of three closely related rotifer species (B. manjavacas, B. plicatilis, and B. calyciflorus) each respond differently to 16°C. While the lifespan of B. manjavacas increases up to 163% as compared to animals at 22°C, B. plicatilis lifespan increased by 84% and B. calyciflorus lifespan was increased by only 32% (unpublished observations).
Furthermore, different strains and species respond differently to the four day 16°C treatment. Both B. manjavacas Russian and Petta show significant persistent enhancement of survival until day 13 (Figure 9). B. plicatilis (China) also demonstrates better survival after a four day 16°C treatment, but the magnitude of the effect is much smaller. The response of B. rotundiformis (Hawaii) is much different, with the 22°C incubated animals surviving significantly better than those at 16°C. This difference is not surprising, as B. rotundiformis is a species adapted to warmer temperatures and higher salinities than B. plicatilis (Serra et al 1998). These differences in ecological specialization and temperature adaptation between closely related species provide insights into the differential response of rotifer strains to low temperature treatments.
Further exploration into the effects of a four day cold treatment on enhanced survival indicated that there was no benefit of reducing temperatures lower than 16°C (Figure 5). A four day exposure to 5°C actually resulted in much poorer survival in early age classes. The rotifers exposed to 5°C were also much smaller and many had algae present in their pseudocoeloms. This suggests disruption of the membrane integrity of the gut, allowing algae to leak into the pseudocoelom. This is consistent with the finding that heat and cold stress caused a decrease in membrane stability in mussels (Yao & Somero 2012).
Enhanced survival from a four day 16°C treatment can be further extended by subsequent intermittent exposure to 16°C (Figure 4). Lifespan was extended in treatments where every four days, the rotifers were transferred from 16°C to 22°C, or vice versa. However, there was no effect of the same transfers being performed every other day or every two days. This suggests that the regulation of the genes responsible for temperature mediated longevity effects require a specific amount of time to be activated.
Temperature during hatching of B. manjavacas is important because this is when embryological development occurs, and the rate of aging likely is determined in early life stages (Carrillo & Flouris 2011). Rotifer resting eggs are arrested in the gastrulation stage, so the majority of development occurs during hatching (Boschetti et al 2011). Upon hatching, rotifers are mostly eutelic and there is no further cell replication until new eggs are produced (Wallace 2002). Exposure to 16°C only during these early developmental stages was not enough to significantly extend lifespan, but 16°C exposure during hatching as well as during the full life span increased longevity more than any other treatment (Figure 2). The importance of early development is further highlighted by the fact that a four day exposure to 16°C is enough to extend lifespan only if it occurs in first four days of life (Figure 3). Exposure to 16°C after 8 days of age provided no life extension. These results support the idea that the rate of aging is determined early in life.
There are several avenues of investigation into the mechanisms and effects of temperature dependent longevity that would benefit from further exploration. Heat-shock proteins (HSPs) are another family of genes that should be a high priority for study. HSPs are upregulated in B. manjavacas at high temperatures (40°C), and in caloric restriction (Smith et al 2012; Yang et al 2014). Another area for further research is intraspecific differences in temperature response. Different genetic isolates of B. plicatilis respond differently to intermittent fasting (Gribble et al 2014). Understanding the basis for the differential response of these naturally occurring strains can provide additional insight into the genetic basis of temperature sensitivity. Also, the RNAi approach taken in this study focused on targeting specific conserved genes. A broader RNAseq approach would provide a more comprehensive look at the genes that are differentially expressed at different temperatures, and could help identify novel genes involved in temperature sensitive pathways.
Recent theories challenge the traditional view of aging as an accumulation of molecular damage, and instead argue that aging is a result of actively regulated genetic pathways (Gems and Partridge 2013; Blagosklonny 2012). Our results further emphasize the importance of such genetic regulation. By activating the low temperature-sensing pathway in B. manjavacas, we observed enhanced survival even after thermodynamic reductions in metabolism were withdrawn. Exposure to 16°C only enhanced longevity when exposures were during early age classes, supporting theories that the rate of aging is likely determined by environmental factors during early development (Carillo and Flouris 2011). Furthermore, our findings support the idea that many species harbor a latent potential for increased longevity, and that potential can be realized by environmental manipulations (Johnson et al 2006). The many interconnected mechanisms that are responsible for temperature mediated life extension offer numerous targets for new aging interventions.
Highlights.
Decreasing temperature from 22°C to 16°C increases lifespan of B. manjavacas by 163%
A four day 16°C exposure enhances survival for nine days after transfer to 22°C
Thermodynamic effects alone cannot account for the persistent survival effect
Four genes, including a TRP, were found to play an important role in this effect
Acknowledgments
We are grateful for the support of the National Institute of Aging, grant R01 AG037960-02. We also express our appreciation for the technical support provided by Julie Wilson and Sharadha Krishnappan, and to Kristin Gribble for her comments that improved the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boschetti C, Leasi F, Ricci C. Developmental stages in diapausing eggs: an investigation across monogonont rotifer species. Hydrobiologia. 2011;662:149–155. [Google Scholar]
- Carrillo AE, Flouris AD. Caloric restriction and longevity: Effects of reduced body temperature. Aging Research Reviews. 2011;10:153–162. doi: 10.1016/j.arr.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Cockett TK, Beehler CC. Protective Effects of Hypothermia in Exploration of Space. Journal of the American Medical Association. 1962;182.10:977–979. doi: 10.1001/jama.1962.03050490001001. [DOI] [PubMed] [Google Scholar]
- Conti B. Considerations on temperature, longevity and aging. Cellular and Molecular Life Sciences. 2008;65:1626–1630. doi: 10.1007/s00018-008-7536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B, et al. Transgenic mice with a reduced core body temperature have an increased lifespan. Science. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- Dragosits M, Mozhayskiy V, Quinones-Soto S, Park J, Tagkopoulos I. Evolutionary potential, cross-stress behavior and the genetic basis of acquired stress resistance in Escherichia coli. Molecular Systems Biology. 2013;9.643:1–13. doi: 10.1038/msb.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri E, Valbonesi P, Franzellitti S. HSP expression in bivalves. Information Systems Journal. 2008;5:135–161. [Google Scholar]
- Flockerzi V, Nilius B. Transient Receptor Potential (TRP) Channels. Handbook of Experimental Physiology. 2007;179:1–615. [PubMed] [Google Scholar]
- Garrity PA, Goodman MB, Samuel AD, Sengupta P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes and Development. 2010;24:2365–2382. doi: 10.1101/gad.1953710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Partridge L. Genetics of Longevity in Model Organisms: Debates and Paradigm Shifts. Annual Review of Physiology. 2013;75:621–644. doi: 10.1146/annurev-physiol-030212-183712. [DOI] [PubMed] [Google Scholar]
- Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- Gómez A, Carmona MJ, Serra M. Ecological factors affecting gene flow in the Brachionus plicatilis complex (Rotifera) Oecologia. 1997;111:350–356. doi: 10.1007/s004420050245. [DOI] [PubMed] [Google Scholar]
- Gribble KE, Kaido O, Jarvis G, Welch DBM. Patterns of intraspecific variability in the response to caloric restriction. Experimental Gerontology. 2014;51:28–37. doi: 10.1016/j.exger.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillard RRL. Culture of phytoplankton for feeding marine invertebrates. In: Berg CJ Jr, editor. Culture of Marine Invertebrates. Stroudsburg, PA: Hutchinson Ross; 1983. [Google Scholar]
- Hsu WL, Yoshioka T. Role of TRP channels in the induction of heat shock proteins (Hsps) by heating skin. Biophysics. 2015;11:25–32. doi: 10.2142/biophysics.11.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE, Lithgow GJ, Murakami S. Hypothesis: Interventions That Increase the Response to Stress Offer the Potential for Effective Life Prolongation and Increased Health. The Journals of Gerontology. 1996;51A.6:B392–B395. doi: 10.1093/gerona/51a.6.b392. [DOI] [PubMed] [Google Scholar]
- Jones BL, Schneider DM, Snell TW. Thermostable proteins in the diapausing eggs of Brachionus manjavacas (Rotifera) Comparative Biochemistry and Physiology, Part A. 2012;162.3:193–199. doi: 10.1016/j.cbpa.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Lee RE, Jr, Denlinger DL. Insects at Low Temperature. New York: Chapman and Hall; 1991. [Google Scholar]
- Lee SJ, Kenyon C. Regulation of the Longevity Response to Temperature by Thermosensory Neurons in Caenorhabditis elegans. Current Biology. 2009;19:715–722. doi: 10.1016/j.cub.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Begun A, Kaeberlein M. HIF-1 modulates longevity and healthspan in a temperature dependent manner. Aging Cell. 2011;10:318–326. doi: 10.1111/j.1474-9726.2011.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linford NJ, Kuo TH, Chan TP, Pletcher SD. Sensory Perception and Aging in Model Systems: From the Outside In. Annual Review of Cell and Developmental Biology. 2011;27:759–785. doi: 10.1146/annurev-cellbio-092910-154240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkatkin AS, Brazaityte A, Bobinas C, Duchovskis P. Chilling injury in chilling-sensitive plants: a review. Agriculture. 99.2:111–124. [Google Scholar]
- Kristensen TN, Hoffmann AA, Overgaard J, Sørensen JG, Hallas R, Loeschcke V. Costs and benefits of cold acclimation in field-released Drosophila. PNAS. 2008;105.1:216–221. doi: 10.1073/pnas.0708074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães JP, Wuttke D, Wood SH, Plank M, Vora C. Genome-Environment Interactions That Modulate Aging: Powerful Targets for Drug Discovery. Pharmacological Reviews. 2012;64.1:88–101. doi: 10.1124/pr.110.004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta M, Biggiogera M, Zancanaro C. Hypometabolic induced state: a potential tool in biomedicine and space exploration. Environmental Science and Biotechnology. 2007;6:47–60. [Google Scholar]
- Miracle MR, Serra M. Salinity and temperature influence in rotifer life history characteristics. Hydrobiologia. 1989;186:81–102. [Google Scholar]
- Miura K, Furumoto T. Cold signaling and cold response in plants. International Journal of Molecular Sciences. 2013;14:5312–5337. doi: 10.3390/ijms14035312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Johnson TE. A Genetic Pathway Conferring Life Extension and Resistance to UV Stress in Caenorhabditis elegans. Genetics. 1996;143.3:1207–1218. doi: 10.1093/genetics/143.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P, Hayward SAL, Govan GG, Gracey AY, Crossins AR. An explicit test of the phospholipid saturation hypothesis of acquired cold tolerance in Caenorhabditis elegans. PNAS. 2007;104.13:54489–5494. doi: 10.1073/pnas.0609590104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo M, Schaller MD, Feihl F, Ribordy V, Liaudet L. From evidence to clinical practice: Effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest. Critical Care Medicine. 2006;34.7:1865–1873. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PubMed] [Google Scholar]
- Pietrzak B, Bednarska A, Markowska M, Rojek M, Szymanska E, Slusarczyk M. Behavioural and physiological mechanisms behind extreme longevity in Daphnia. Hydrobiologia. 2013;715:125–134. [Google Scholar]
- Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- Rako L, Hoffmann AA. Complexity of the cold acclimation response in Drosophila melanogaster. Journal of Insect Physiology. 2006;52:94–104. doi: 10.1016/j.jinsphys.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Rico-Martinez R, Snell TW. Male discrimination of female Brachionus plicatilis Muller and Brachionus rotundiformis Tschugunoff (Rotifera) J Exp Mar Biol Ecol. 1995;190:39–49. [Google Scholar]
- Riedel CG, et al. DAF-16 employs the chromatin remodeller SWI/SNF to promote stress resistance and longevity. Nature Cell Biology. 2013;15.5:491–501. doi: 10.1038/ncb2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikke BA, Johnson TE. Lower body temperature as a potential mechanism of life extension in homeotherms. Experimental Gerontology. 2004;39:927–930. doi: 10.1016/j.exger.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Serra M, Gómez A, Carmona MJ. Ecological genetics of Brachionus sympatric sibling species. Hydrobiologia. 1998;387:373–384. [Google Scholar]
- Sinclair BJ, Gibbs AG, Roberts SP. Gene transcription during exposure to, and recovery from, cold and desiccation stress in Drosophila melanogaster. Insect Molecular Biology. 2007;16.4:435–443. doi: 10.1111/j.1365-2583.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- Smith HA, Burns AR, Shearer TL, Snell TW. Three heat shock proteins are essential for rotifer thermotolerance. Journal of Experimental Marine Biology and Ecology. 2012;413:1–6. [Google Scholar]
- Snell TW. Rotifers as models for the biology of aging. International Review of Hydrobiology. 2014;99:84–95. doi: 10.1002/iroh.201301707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell TW, Johnston RK. Glycerol extends the lifespan of Brachionus manjavacas (Rotifera) and protects against stressors. Experimental Gerontology. 2014;57:47–56. doi: 10.1016/j.exger.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell TW, Johnston RK, Gribble KE, Welch DBM. Rotifers as experimental tools for investigating aging. Invertebrate Reproduction & Development. 2014a doi: 10.1080/07924259.2014.925516. http://dx.doi.org/10.1080/07924259.2014.925516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell TW, Johnston RK, Rabeneck B, Zipperer C, Teat S. Joint Inhibition of TOR and JNK pathways interacts to extend the lifespan of Brachionus manjavacas (Rotifera) Experimental Gerontology. 2014b;52:55–69. doi: 10.1016/j.exger.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell TW, Shearer TL, Smith HA. Exposure to dsRNA elicits RNA interference in Brachionus manjavacas (Rotifera) Marine Biotechnology. 2010 doi: 10.1007/s10126-010-9295-x. [DOI] [PubMed] [Google Scholar]
- Suatoni E, Vicario S, Rice S, Snell T, Caccone A. An analysis of species boundaries and biogeographic patterns in a cryptic species complex: The rotifer – Brachionus plicatilis. Molecular Phylogenetics and Evolution. 2006;41:86–98. doi: 10.1016/j.ympev.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Sun KH, Sun GH, Lin G, Lin WW, Chuang MJ. Cold-induced ependymin expression in zebrafish and carp brain: implications for cold acclimation. FEBS Letters. 1999;459.1:95–99. doi: 10.1016/s0014-5793(99)01229-6. [DOI] [PubMed] [Google Scholar]
- Teets NM, Denlinger DL. Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiological Entomology. 2013:9–12. [Google Scholar]
- Terblanche JS, Deere JA, Clusella-Trullas S, Janion C, Chown SL. Critical thermal limits depend on methodological context. Proceedings of the Royal Society B. 2007;274:2935–2942. doi: 10.1098/rspb.2007.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MF. Molecular basis of plant cold acclimation: Insights gained from studying the CBF cold response pathway. Plant Physiology. 2010;154:571–577. doi: 10.1104/pp.110.161794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Cattaneo A, Domenici L, Cellerino A. Temperature affects longevity and age-related locomotor and cognitive decay in the short-lived fish Nothobranchius furzeri. Aging Cell. 2006;5:275–278. doi: 10.1111/j.1474-9726.2006.00212.x. [DOI] [PubMed] [Google Scholar]
- Walker KF. A synopsis of ecological information on the saline lake rotifer Brachionus plicatilis Müller 1786. Hydrobiologia. 1981;81:159–167. [Google Scholar]
- Wallace RL. Rotifers: Exquisite Metazoans. Integrated and Computational Biology. 2002;42:660–667. doi: 10.1093/icb/42.3.660. [DOI] [PubMed] [Google Scholar]
- Xiao R, Zhang B, Dong Y, Gong J, Xu T, Liu J, Xu XZS. A Genetic Program Promotes C. elegans Longevity at Cold Temperatures via a Thermosensitive TRP Channel. Cell. 2013;152:806–817. doi: 10.1016/j.cell.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mu Y, Dong S, Jiang Q, Yang J. Changes in the expression of four heat shock proteins during the aging process in Brachionus calyciflorus (Rotifera) Cell Stress and Chaperones. 2014;19.1:33–52. doi: 10.1007/s12192-013-0432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CL, Somero GN. The impact of acute temperature stress on hemocytes of invasive and native mussels (Mytilus galloprovincialis and Mytilus californianus): DNA damage, membrane integrity, apoptosis, and signaling pathways. Journal of Experimental Biology. 2012;215.24:4267–4277. doi: 10.1242/jeb.073577. [DOI] [PubMed] [Google Scholar]