Abstract
Background
Insulin resistance is associated with microalbuminuria among youth with diabetes mellitus. We sought to determine the dose-response effect of insulin sensitivity (IS) on the magnitude of albuminuria and whether there is a threshold below which urine albumin excretion increases.
Methods
These analyses included participants from the SEARCH for Diabetes in Youth Study with incident diabetes who completed a baseline study visit (N = 2988). We estimated IS using a validated equation incorporating waist circumference, HbA1C and fasting serum triglycerides. Multivariate regression analyses were performed to assess the effect of IS on urine albumin creatinine ratio (UACR), stratified by diabetes type. The IS threshold was then determined using segmented regressions within each diabetes type and incorporated into the multivariate model.
Results
There was an association between IS and UACR in type 2 diabetes only (beta = −0.39; p <0.001). There was strong statistical evidence for a threshold effect of IS score on UACR in the group of youth with type 2 (beta = 0.40; p <0.001) but not type 1 diabetes (p = 0.3).
Conclusions
In cross-sectional analyses, there is a negative association between IS and UACR in youth with type 2 but not type 1 diabetes, and this association likely includes a threshold effect of IS on UACR.
Keywords: microalbuminuria, urine:albumin creatinine ratio, insulin resistance, nephropathy, metabolic syndrome
INTRODUCTION
The rising prevalence of diabetes in adolescents and young adults poses a major public health problem, as a greater lifetime diabetes exposure in this group will translate into a greater future burden of microvascular complications [1]. Diabetic kidney disease (DKD) accounts for nearly half of end-stage kidney disease in the USA, and is a major risk factor for cardiovascular events and death [2–4]. Although the precision of microalbuminuria as a predictor for increased risk is less than initially thought, the degree of albuminuria after long diabetes duration is still the strongest available biomarker for the risk of progressive DKD [5]. Albuminuria has also been noted to occur in the presence of obesity, even in the absence of diabetes [6, 7]. Moreover, albuminuria is more prevalent in early type 2 than type 1 diabetes; and differences in body mass index (BMI) and hypertension do not completely explain this difference [8]. It is possible that insulin resistance could explain the higher levels of urinary albumin associated with obesity and type 2 diabetes.
In a previous study from SEARCH, we analyzed the urine albumin:creatinine ratio (UACR) across four etiologic diabetes sub-groups categorized according to diabetes autoantibody (DAA) status (DAA + versus -) and insulin sensitivity (IS) status. We found that the UACR levels among participants in the DAA-/insulin resistant group were significantly higher than those observed in all other groups, even after multivariable adjustment. To explore the underlying factors that accounted for the difference in UACR between the two insulin resistance groups, we performed multivariable regression analyses restricted to these two groups. Only the degree of IS was significantly associated with UACR (β = −0.54; p <0.0001), suggesting that the difference between the insulin resistant DAA+ versus DAA-groups was due to a greater degree of insulin resistance in the DAA-group. Herein, we propose to extend these analyses to determine: 1) whether the association between IS and UACR differs by diabetes type (defined by provider diagnosis), sex or race/ethnicity, and 2) whether there is a threshold of IS below which urine albumin excretion increases.
METHODS
Population
The SEARCH for Diabetes in Youth Study is a multicenter, observational, population-based study of youth with diabetes. A detailed description of study methods has been published previously [9]. SEARCH investigators have conducted population-based ascertainment of incident cases of diabetes in youth aged less than 20 years old from 2002 onwards. Cases were ascertained from geographically defined populations in Ohio, Colorado, South Carolina and Washington, among enrollees in one health plan in California, and Indian Health Service participants from four American Indian populations. Youth identified with incident, non-secondary diabetes were invited for a baseline study visit. Self-reported race and ethnicity were collected using the 2000 U.S. Census questions [10].
Sample selection, design and measurements
This cross-sectional analysis explored the association between diabetes type and the magnitude of albuminuria. Inclusion criteria consisted of having an in-person visit, on average 10 months after the diagnosis of diabetes, where fasting blood was drawn, urine was collected and anthropometric measurements taken (n = 2988). Individuals taking angiotensin converting enzyme inhibitors or angiotensin receptor blockers were excluded from these analyses (n = 38). Diabetes type was defined by the health care provider diagnosis. The study visit included measurement of waist circumference using the National Health and Nutrition Examination Survey protocol [11], systolic and diastolic blood pressures (SBP; DBP), height and weight, as previously described [12]. BMI (kg/m2) was converted to z-scores using the standard Centers for Disease Control and Prevention approach [13].
Blood and single, random urine samples were obtained under conditions of metabolic stability, defined as no episodes of diabetic ketoacidosis in the preceding month and the absence of fever and acute infections. Urine was not collected from girls who were menstruating. Specimens were processed locally and shipped within 24 hours to the central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, Seattle, WA). Measurements of serum cholesterol, triglycerides (TG) and high density lipoprotein (HDL) cholesterol were performed using Roche reagent on a Roche Module P autoanalyzer (Roche Diagnostics, Indianapolis, IN). HbA1c was measured by a dedicated ion exchange high–performance liquid chromatography instrument (TOSOH Bioscience).
A random urine specimen was used for calculation of UACR. Urinary creatinine was measured on the Roche Modular P autoanalyzer by the Roche enzymatic method with calibrator values traceable to the isotope dilution mass spectroscopy reference procedure. Two quality-control samples were analyzed in each run, and the inter-assay coefficient of variation was consistently <2 %. Urine albumin was measured immunochemically using Siemens reagent on a Siemens BNII nephelometer. The inter-assay coefficient of variation was <5 % for the high and <6.5 % for the low-level quality-control sample. We defined albuminuria as a UACR 30 μg/mg as recommended by the American Diabetes Association guidelines [14] and the National Kidney Disease Outcomes Quality Initiative [15].
We estimated IS using a validated equation based on euglycemic hyperinsulinemic clamp data as follows:
This equation was developed and validated using direct measurements of glucose disposal rate (GDR) from euglycemic-hyperinsulinemic clamps conducted among 85 SEARCH participants, and 22 matched non-diabetic control subjects [16]. As previously reported, we defined insulin resistance among SEARCH participants in this study as IS below the 25th percentile for the U.S. general youth population (IS < 8.15) [17].
Blood samples taken at the baseline visit were analyzed for the 65-kD isoform of glutamate decarboxylase and insulinoma-associated-2 antibodies using the NIDDK standardized method [18]. The cutoff values for positivity were 33 DK units/mL for GADA and 5 DK units/mL for IA-2A. The specificity and sensitivity were 97 and 76 %, respectively for GADA, and 99 and 64 %, respectively for IA-2A [18]. Positive titers for either GADA or IA-2A were used to define DAA positivity. Because many participants were treated with insulin, analysis of insulin autoantibodies was not performed.
Statistical analysis
We performed statistical analyses using SAS software, version 9.3 (SAS Institute, Cary, NC) and R (R Core Team, 2015) [19]. The sample sizes for youth from race/ethnic groups other than non-Hispanic white were small. Hence, all racial/ethnic groups other than non-Hispanic white were combined into a single ‘minority’ category. The means and percentages of the demographic and clinical characteristics were compared between diabetes types, based on their health care provider’s diagnosis, using chi square and Wilcoxon two-sample test, which is more robust to deviations from the assumption of normality.
Multivariate regression analyses were performed to assess the relationship between IS and UACR. The logarithm of UACR was used as the outcome. Covariates included age, sex, race/ethnicity, DAA positivity, diabetes duration and SBP z-score. A-priori, the decision was made to leave HbA1c out of the model due to the fact that HbA1c is incorporated into the calculation of IS. Measurements of body size were also left out of the model because waist circumference was also incorporated into the IS calculation, and this measure is collinear with other parameters reflecting BMI.
Interaction analyses
We tested interaction between diabetes type and IS within the combined cohort first, and if the interaction term was significant to p <0.05 all subsequent analyses were stratified by diabetes type. Additional IS*sex and IS*ethnicity interactions were also explored within each diabetes type.
Threshold analyses
We performed threshold estimation using a segmented regression approach. Segmented regressions were fitted using the ‘segmented’ package in R [20]. This package implements an iterative procedure that starts with an initial threshold value, which is repeatedly updated until convergence is reached.
RESULTS
The final sample for these analyses included 2,950 participants. Participants excluded from analysis due to missing urine sample (N = 527 type 1; N = 85 type 2 participants) had a few dissimilarities to those included in the analysis (Supplemental Table 1). The most significant differences were among the participants with type 1 diabetes. Those with a urine sample had lower fasting glucose (165 versus 187 mg/dl, <0.001) and shorter diabetes duration (9.3 versus 10.3 months, p <0.001), than those without a urine sample.
Socio-demographic and clinical characteristics were significantly different between type 1 and type 2 participants (Table 1). Participants with type 2 diabetes were older, predominantly female and from racial/ethnic minority groups. They had a lower IS score and HbA1c, but higher BMI, BP and lipid levels. Although microalbuminuria was more common in the type 2 participants, there was no statistically significant difference in the distribution of UACR by diabetes type, despite a numeric trend toward more elevated levels in type 2.
Table 1.
Baseline characteristics of 2,950 participants in the SEARCH for Diabetes in Youth cohort, stratified by diabetes type.
| Baseline characteristics | Type 1 mean (SD) n=2551 |
Type 2 mean (SD) n=399 |
p-value |
|---|---|---|---|
| Age at exam, years | 11 (4) | 15 (3) | <0.001 |
| Male, n (%) | 1352 (53) | 160 (40) | <0.001 |
| Non-Hispanic White, n (%) | 1938 (76) | 96 (24) | <0.001 |
| Time from diabetes diagnosis, months | 10 (6) | 11 (7) | 0.001 |
| Insulin sensitivity score | 10.5 (3.3) | 4.4 (2.2) | <0.001 |
| UACR, μg/mg (median, IQR) | 7.0 (7.9) | 7.4 (14.1) | 0.6 |
| Albuminuria, n (%) | 202 (10) | 53 (17) | <0.001 |
| HbA1c, % | 7.7 (1.6) | 7.2 (2.1) | <0.001 |
| Body mass index z-score | 0.53 (1.02) | 2.13 (0.69) | <0.001 |
| Overweight, n (%) | 816 (32) | 371 (93) | <0.0001 |
| SBP z-score, mmHg | −0.5 (0.9) | 0.39 (1.09) | <0.001 |
| DBP z-score, mmHg | 0.12 (0.81) | 0.52 (0.48) | <0.001 |
| Total cholesterol, mg/dl | 160 (30) | 171 (30) | <0.001 |
| Triglycerides, mg/dl | 67 (55) | 161 (182) | <0.001 |
| HDL, mg/dl | 55 (13) | 41 (10) | <0.001 |
| LDL, mg/dl | 92 (24) | 102 (30) | <0.001 |
UACR = urine albumin:creatinine ratio; Albuminuria = UACR ≥ 30 μg/mg; SBP = systolic blood pressure; DBP = diastolic blood pressure; HDL = high density lipoprotein; LDL = low density lipoprotein
The interaction between IS and diabetes type was statistically significant (p <0.001) and therefore, all other analyses were stratified. IS was significantly associated with UACR in participants with type 2 diabetes (p <0.001), but not type 1 diabetes (p = 0.25). Interactions between IS and sex and IS and ethnicity on UACR were not statistically significant within either diabetes type.
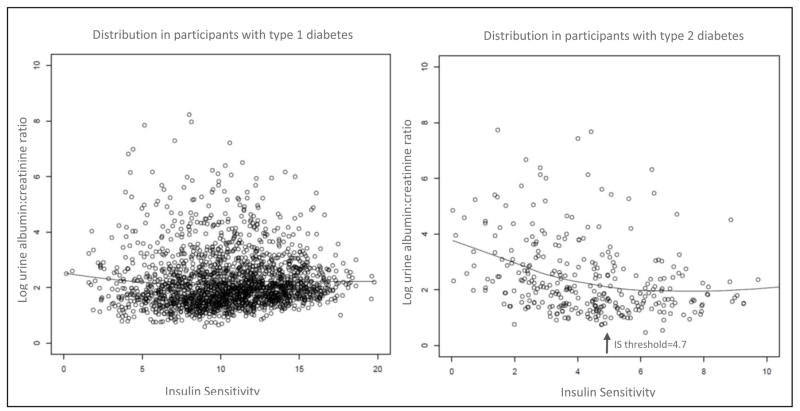
Using a segmented regression approach, a threshold for the relationship between IS and UACR was detected at IS = 6.7 and IS = 4.7 for participants with type 1 and type 2 diabetes, respectively (Figure 1). There was clear statistical evidence for a threshold effect of IS on UACR in participants with type 2 (p <0.001), but not type 1 diabetes (p = 0.09). Output from these multivariate regression analyses are displayed in Table 2. These data indicate that in participants with type 2 diabetes there is an inverse relationship between IS and UACR, and below the IS threshold of 4.7 the relationship becomes even stronger. The performance of the models including versus omitting the threshold value are available in Supplemental Table 2.
Figure 1.
The relationship between urine albumin:creatinine ratio and insulin sensitivity (IS) in type 1 versus type 2 diabetic participants in the SEARCH cohort. The threshold detected in the type 2 participants is noted by the arrow, and represents the point at which further decreases in insulin sensitivity are associated with even greater degrees of albuminuria.
Table 2.
Output from multivariate linear regression ‘segmented regression threshold model’ analysis of type 2 diabetic participants.
| Covariate | Type 1 Diabetes | Type 2 Diabetes | ||
|---|---|---|---|---|
| Beta Coefficient | p-value | Beta Coefficient | p-value | |
| Intercept | 2.366 | <0.001 | 4.051 | <0.001 |
| Threshold | 0.063 | 0.09 | 0.397 | <0.001 |
| Insulin sensitivity score | −0.038 | 0.25 | −0.385 | <0.001 |
| Age at diagnosis | 0.012 | 0.1 | −0.023 | 0.5 |
| Male gender | −0.341 | <0.001 | 0.010 | 0.9 |
| NonHispanic White Ethnicity | −0.020 | 0.7 | −0.1545 | 0.3 |
| Diabetes autoantibodies | 0.007 | 0.9 | 0.194 | 0.4 |
| Systolic blood pressure Z-score | −0.002 | 0.9 | 0.009 | 0.9 |
| Diabetes Duration | 0.004 | 0.9 | 0.007 | 0.5 |
DISCUSSION
Type 2 diabetes in adolescents and young adults is a growing pandemic, making it imperative to better understand the potential causative factors for DKD in this inadequately studied population. Our findings provide novel evidence that early in the course of type 2 diabetes in youth, the degree of albuminuria is strongly and inversely associated with the IS score, a validated estimate of insulin sensitivity. Moreover, the nature of this relationship in type 2 diabetes likely includes a threshold effect, below which the association becomes even stronger. In youth with type 1 diabetes, the UACR was not significantly associated with the IS score.
The association of IS and albuminuria in diabetes has been noted previously [21–27], however several aspects of our study are novel. This is the first time the association between IS and albuminuria has been directly compared across diabetes types. Moreover, this is the only investigation of IS and albuminuria in an inception cohort of diabetes, giving insight into IS as a potential pathogenic factor early in the natural history of albuminuria.
The association of albuminuria and low IS, or a surrogate abdominal adiposity, has been recognized in type 2 diabetes [24–30], in type 1 diabetes [21, 23, 31–35] and in populations without diabetes [7, 36, 37]. The few negative studies have been from populations with distinct characteristics such as hypertensive individuals or those without significant representation of overweight [25, 29]. The basis for a lack of association between albuminuria and IS in our youth with type 1 diabetes can only be speculated about, but it may be due, in part, to a relative lower prevalence of obesity, insulin resistance and albuminuria at this early stage of type 1 diabetes (Table 1). As shown in Figure 1, the association of IS and albuminuria in type 2 diabetes is most significant in the lowest portion of IS, and very few participants with type 1 diabetes were within this range. Previous SEARCH data have indicated that, although the prevalence of overweight is higher in youth with type 1 diabetes than in non-diabetic youth, the prevalence of obesity is actually somewhat lower [38]. The Epidemiology of Diabetes Interventions and Complications Study documented weight gain and increased abdominal adiposity in type 1 patients over time, as well as its association with increased vascular disease [39]. Studies of adult type 1 diabetic populations have clearly shown low IS to be predictive of subsequent micro- and macroalbuminuria [21, 23, 31–35]. Similarly, higher levels of IS in older type 1 diabetic populations predicted regression of microalbuminuria [40]. These studies were conducted in populations with type 1 diabetes with a duration of 10 – 20 years, mean BMI of 25 – 30 and a prevalence of microalbuminuria of 18 – 44 %; all characteristics very distinct from our population [23, 31, 32].
Another potential explanation for our findings is the difference in age of onset and consequent pubertal diabetes duration. The vast majority of SEARCH participants with type 2 diabetes were in the peri- or post-pubertal phase at examination (age >12 years), whereas the minority of participants with type 1 diabetes had reached puberty. Data regarding the effect of puberty on diabetic complications has mostly emanated from studies of type 1 diabetes [41]. Several older studies suggested that pre-pubertal diabetes conferred protection against diabetic complications. Moreover, the pubertal phase may exacerbate complications due to changes in the hormonal balance and increasing adiposity and insulin resistance seen in puberty. While we did adjust our analyses for age and diabetes duration, we did not specifically address pubertal phase. We intend to explore this issue further with subsequent longitudinal data, when more participants have passed the pubertal phase.
The association between IS and albuminuria is likely multifactorial. Hypotheses include increased inflammatory mediators produced in visceral adipocytes, increased circulating levels of insulin and impaired insulin signaling within the podocyte [42–45]. Decreasing IS results in aberrations in adipocytokines, including decreased adiponectin and increased leptin and resistin concentrations [42]. Low levels of adiponectin found in obese states have been linked to increased reactive oxygen species, podocyte foot process effacement, endothelial cell dysfunction and albuminuria [43]. Leptin levels rise with insulin resistance, even independent of BMI, and have been found to result in increased TGF-β and collagen deposition [42, 44].
Insulin infusion has been cited to induce transient albuminuria, and its overproduction has been hypothesized as a mechanism by which albuminuria occurs in the insulin resistant state. Insulin stimulates the renin-angiotensin system, growth factors and reactive oxygen species [46]. Alternatively, aberrant insulin signaling has direct implications on the function of the podocyte which, in its normal state, is an insulin responsive cell type [45]. Supportive of this notion that aberrant insulin signaling in the kidney may mediate the association between IS and albuminuria, are numerous small studies in animals and humans showing insulin sensitizers to reduce urinary albumin excretion [47].
Our study has several limitations. Most importantly, only a single, random UACR was available for analysis. Random urine samples carry a higher inter-individual variability than first morning samples, and multiple urine samples are required to maximize precision in quantifying albuminuria [48, 49]. This is not likely to have affected our conclusions, as the variability in UACR is not dependent upon diabetes type or insulin sensitivity. This was also a cross-sectional study design and hence only associations, rather than causality, can be inferred from the results.
Strengths of our study include the large sample size and the ethnic and geographic diversity of the SEARCH study. SEARCH participants were enrolled soon after diagnosis of diabetes, allowing insight into the early natural history of both IS and albuminuria. Moreover, to our knowledge, this is the only study to examine and compare the relationships between IS and albuminuria in type 1 and type 2 diabetes. Our equation estimating IS was developed and validated in a subset of 85 SEARCH participants and 22 nondiabetic controls undergoing euglycemic clamps, and explained 74 % of variance of the glucose disposal rate [16]. This is superior to other currently available glucose disposal rate estimating equations [21, 23]. Although the euglycemic-hyperinsulinemic clamp study is the gold standard for measurement of IS, such an invasive, costly and time-consuming method is impractical for use in large, epidemiologic studies such as the full SEARCH study.
In conclusion, we report that in cross-sectional analyses, insulin sensitivity is significantly associated with albuminuria in children and adolescents with type 2 but not type 1 diabetes of short duration. There is likely a threshold effect of IS on UACR in type 2 diabetic adolescents and young adults, below which the association between IS and urine albumin excretion is stronger. Whether this threshold also portends an increased risk for subsequent micro- and macrovascular complications longitudinally, is a direction for future study.
Supplementary Material
Acknowledgments
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families, and their health care providers, whose participation made this study possible.
Grant Support: SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Site Contract Numbers: Kaiser Permanente Southern California (U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Children’s Hospital Medical Center (Cincinnati) (U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01 DP000254, and U18DP002708), University of Washington School of Medicine (U58/CCU019235-4, U01 DP000244, and U18DP002710-01), Wake Forest University School of Medicine (U48/CCU919219, U01 DP000250, and 200-2010-35171).
The authors wish to acknowledge the involvement of General Clinical Research Centers (GCRC) at the South Carolina Clinical & Translational Research (SCTR) Institute, at the Medical University of South Carolina (NIH/NCRR Grant number UL1RR029882); Seattle Children’s Hospital (NIH CTSA Grant UL1 TR00423 of the University of Washington); University of Colorado Pediatric Clinical and Translational Research Center (CTRC) (Grant Number UL1 TR000154) and the Barbara Davis Center at the University of Colorado at Denver (DERC NIH P30 DK57516); and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8 UL1 TR000077; and the Children with Medical Handicaps program managed by the Ohio Department of Health.
Footnotes
Ethics statement
The study protocol was reviewed and approved by local institutional review boards (IRB) that had jurisdiction over the local study populations. Consent was obtained from parents of all participants under age 18 years and assent from participants according to the specific age requirement deemed by the local IRB.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Disease
Reference list
- 1.Dabelea D, Bell RA, D’agostino RB, Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 2.Ninomiya T, Perkovic V, De Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J ADVANCE Collaborative Group. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, De Boer IH. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, Chen JT, Cope E, Gipson D, He K, Herman W, Heung M, Hirth RA, Jacobsen SS, Kalantar-Zadeh K, Kovesdy CP, Leichtman AB, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, O’hare AM, Pisoni R, Plattner B, Port FK, Rao P, Rhee CM, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, Eggers PW, Agodoa LY, Abbott KC. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2015;66:Svii, S1–305. doi: 10.1053/j.ajkd.2015.05.001. http://www.usrds.org/adr.aspx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caramori ML, Fioretto P, Mauer M. Enhancing the predictive value of urinary albumin for diabetic nephropathy. J Am Soc Nephrol. 2006;17:339–352. doi: 10.1681/ASN.2005101075. [DOI] [PubMed] [Google Scholar]
- 6.Chang A, Van Horn L, Jacobs DR, Jr, Liu K, Muntner P, Newsome B, Shoham DA, Durazo-Arvizu R, Bibbins-Domingo K, Reis J, Kramer H. Lifestyle-related factors, obesity, and incident microalbuminuria: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Kidney Dis. 2013;62:267–275. doi: 10.1053/j.ajkd.2013.02.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanad M, Gharib A. Evaluation of microalbuminuria in obese children and its relation to metabolic syndrome. Pediatr Nephrol. 2011;26:2193–2199. doi: 10.1007/s00467-011-1931-9. [DOI] [PubMed] [Google Scholar]
- 8.Maahs DM, Snively BM, Bell RA, Dolan L, Hirsch I, Imperatore G, Linder B, Marcovina SM, Mayer-Davis EJ, Pettitt DJ, Rodriguez BL, Dabelea D. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care. 2007;30:2593–2598. doi: 10.2337/dc07-0450. [DOI] [PubMed] [Google Scholar]
- 9.SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25:458–471. doi: 10.1016/j.cct.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Ingram DD, Parker JD, Schenker N, Weed JA, Hamilton B, Arias E, Madans JH. United States Census 2000 population with bridged race categories. Vital Health Stat. 2003;2:1–55. [PubMed] [Google Scholar]
- 11.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, Imperatore G, Williams DE, Bell RA, Wadwa RP, Palla SL, Liu LL, Kershnar A, Daniels SR, Linder B. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for diabetes in youth study. Diabetes Care. 2006;29:1891–1896. doi: 10.2337/dc06-0310. [DOI] [PubMed] [Google Scholar]
- 13.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 14.Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49:S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Dabelea D, D’agostino RB, Jr, Mason CC, West N, Hamman RF, Mayer-Davis EJ, Maahs D, Klingensmith G, Knowler WC, Nadeau K. Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia. 2011;54:78–86. doi: 10.1007/s00125-010-1911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabelea D, Pihoker C, Talton JW, D’agostino RB, Jr, Fujimoto W, Klingensmith GJ, Lawrence JM, Linder B, Marcovina SM, Mayer-Davis EJ, Imperatore G, Dolan LM. Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2011;34:1628–1633. doi: 10.2337/dc10-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonifacio E, Yu L, Williams AK, Eisenbarth GS, Bingley PJ, Marcovina SM, Adler K, Ziegler AG, Mueller PW, Schatz DA, Krischer JP, Steffes MW, Akolkar B. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab. 2010;95:3360–3367. doi: 10.1210/jc.2010-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Accessed on 2015]. http://www.R-project.org/ [Google Scholar]
- 20.Muggeo VM. Estimating regression models with unknown break-points. Stat Med. 2003;22:3055–3071. doi: 10.1002/sim.1545. [DOI] [PubMed] [Google Scholar]
- 21.Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE. Nephropathy in type 1 diabetes: a manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int. 2002;62:963–970. doi: 10.1046/j.1523-1755.2002.00507.x. [DOI] [PubMed] [Google Scholar]
- 22.Bjornstad P, Maahs DM, Cherney DZ, Cree-Green M, West A, Pyle L, Nadeau KJ. Insulin sensitivity is an important determinant of renal health in adolescents with type 2 diabetes. Diabetes Care. 2014;37:3033–3039. doi: 10.2337/dc14-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjornstad P, Snell-Bergeon JK, Rewers M, Jalal D, Chonchol MB, Johnson RJ, Maahs DM. Early diabetic nephropathy: a complication of reduced insulin sensitivity in type 1 diabetes. Diabetes Care. 2013;36:3678–3683. doi: 10.2337/dc13-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parvanova AI, Trevisan R, Iliev IP, Dimitrov BD, Vedovato M, Tiengo A, Remuzzi G, Ruggenenti P. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55:1456–1462. doi: 10.2337/db05-1484. [DOI] [PubMed] [Google Scholar]
- 25.Groop L, Ekstrand A, Forsblom C, Widen E, Groop PH, Teppo AM, Eriksson J. Insulin resistance, hypertension and microalbuminuria in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1993;36:642–647. doi: 10.1007/BF00404074. [DOI] [PubMed] [Google Scholar]
- 26.De Cosmo S, Minenna A, Ludovico O, Mastroianno S, Di Giorgio A, Pirro L, Trischitta V. Increased urinary albumin excretion, insulin resistance, and related cardiovascular risk factors in patients with type 2 diabetes: evidence of a sex-specific association. Diabetes Care. 2005;28:910–915. doi: 10.2337/diacare.28.4.910. [DOI] [PubMed] [Google Scholar]
- 27.Chan JC, Tomlinson B, Nicholls MG, Swaminathan R, Cheung CK, Woo J, Cockram CS. Albuminuria, insulin resistance and dyslipidaemia in Chinese patients with non-insulin-dependent diabetes (NIDDM) Diabet Med. 1996;13:150–155. doi: 10.1002/(SICI)1096-9136(199602)13:2<150::AID-DIA33>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 28.Hsu CC, Chang HY, Huang MC, Hwang SJ, Yang YC, Tai TY, Yang HJ, Chang CT, Chang CJ, Li YS, Shin SJ, Kuo KN. Association between insulin resistance and development of microalbuminuria in type 2 diabetes: a prospective cohort study. Diabetes Care. 2011;34:982–987. doi: 10.2337/dc10-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emoto M, Nishizawa Y, Maekawa K, Kawagishi T, Kogawa K, Hiura Y, Mori K, Tanaka S, Ishimura E, Inaba M, Okuno Y, Morii H. Insulin resistance in non-obese, non-insulin-dependent diabetic patients with diabetic nephropathy. Metabolism. 1997;46:1013–1018. doi: 10.1016/s0026-0495(97)90271-7. [DOI] [PubMed] [Google Scholar]
- 30.Pinkney JH, Denver AE, Mohamed-Ali V, Foster C, Yudkin JS. Insulin resistance in non-insulin-dependent diabetes mellitus is associated with microalbuminuria independently of ambulatory blood pressure. J Diabetes Complications. 1995;9:230–233. doi: 10.1016/1056-8727(95)80009-4. [DOI] [PubMed] [Google Scholar]
- 31.Epstein EJ, Osman JL, Cohen HW, Rajpathak SN, Lewis O, Crandall JP. Use of the estimated glucose disposal rate as a measure of insulin resistance in an urban multiethnic population with type 1 diabetes. Diabetes Care. 2013;36:2280–2285. doi: 10.2337/dc12-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Boer IH, Sibley SD, Kestenbaum B, Sampson JN, Young B, Cleary PA, Steffes MW, Weiss NS, Brunzell JD. Central obesity, incident microalbuminuria, and change in creatinine clearance in the epidemiology of diabetes interventions and complications study. J Am Soc Nephrol. 2007;18:235–243. doi: 10.1681/ASN.2006040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaturvedi N, Bandinelli S, Mangili R, Penno G, Rottiers RE, Fuller JH. Microalbuminuria in type 1 diabetes: rates, risk factors and glycemic threshold. Kidney Int. 2001;60:219–227. doi: 10.1046/j.1523-1755.2001.00789.x. [DOI] [PubMed] [Google Scholar]
- 34.Ekstrand AV, Groop PH, Gronhagen-Riska C. Insulin resistance precedes microalbuminuria in patients with insulin-dependent diabetes mellitus. Nephrol Dial Transplant. 1998;13:3079–3083. doi: 10.1093/ndt/13.12.3079. [DOI] [PubMed] [Google Scholar]
- 35.Yip J, Mattock MB, Morocutti A, Sethi M, Trevisan R, Viberti G. Insulin resistance in insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993;342:883–887. doi: 10.1016/0140-6736(93)91943-g. [DOI] [PubMed] [Google Scholar]
- 36.Jang CM, Hyun YY, Lee KB, Kim H. Insulin resistance is associated with the development of albuminuria in Korean subjects without diabetes. Endocrine. 2015;48:203–210. doi: 10.1007/s12020-014-0242-x. [DOI] [PubMed] [Google Scholar]
- 37.Mykkanen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes. 1998;47:793–800. doi: 10.2337/diabetes.47.5.793. [DOI] [PubMed] [Google Scholar]
- 38.Liu LL, Lawrence JM, Davis C, Liese AD, Pettitt DJ, Pihoker C, Dabelea D, Hamman R, Waitzfelder B, Kahn HS. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2010;11:4–11. doi: 10.1111/j.1399-5448.2009.00519.x. [DOI] [PubMed] [Google Scholar]
- 39.Purnell JQ, Zinman B, Brunzell JD. The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation. 2013;127:180–187. doi: 10.1161/CIRCULATIONAHA.111.077487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bjornstad P, Maahs DM, Johnson RJ, Rewers M, Snell-Bergeon JK. Estimated insulin sensitivity predicts regression of albuminuria in Type 1 diabetes. Diabet Med. 2015;32:257–261. doi: 10.1111/dme.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho YH, Craig ME, Donaghue KC. Puberty as an accelerator for diabetes complications. Pediatr Diabetes. 2014;15:18–26. doi: 10.1111/pedi.12112. [DOI] [PubMed] [Google Scholar]
- 42.Briffa JF, Mcainch AJ, Poronnik P, Hryciw DH. Adipokines as a link between obesity and chronic kidney disease. Am J Physiol Renal Physiol. 2013;305:F1629–1636. doi: 10.1152/ajprenal.00263.2013. [DOI] [PubMed] [Google Scholar]
- 43.Christou GA, Kiortsis DN. The role of adiponectin in renal physiology and development of albuminuria. J Endocrinol. 2014;221:R49–61. doi: 10.1530/JOE-13-0578. [DOI] [PubMed] [Google Scholar]
- 44.Alix PM, Guebre-Egziabher F, Soulage CO. Leptin as an uremic toxin: Deleterious role of leptin in chronic kidney disease. Biochimie. 2014;105:12–21. doi: 10.1016/j.biochi.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 45.Jauregui A, Mintz DH, Mundel P, Fornoni A. Role of altered insulin signaling pathways in the pathogenesis of podocyte malfunction and microalbuminuria. Curr Opin Nephrol Hypertens. 2009;18:539–545. doi: 10.1097/MNH.0b013e32832f7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol. 2006;26:232–244. doi: 10.1159/000093632. [DOI] [PubMed] [Google Scholar]
- 47.Sarafidis PA, Bakris GL. Protection of the kidney by thiazolidinediones: an assessment from bench to bedside. Kidney Int. 2006;70:1223–1233. doi: 10.1038/sj.ki.5001620. [DOI] [PubMed] [Google Scholar]
- 48.Mchardy KC, Gann ME, Ross IS, Pearson DW. A simple approach to screening for microalbuminuria in a type 1 (insulin-dependent) diabetic population. Ann Clin Biochem. 1991;28:450–455. doi: 10.1177/000456329102800505. [DOI] [PubMed] [Google Scholar]
- 49.Deeb A, Zaoui P, Le Penven S, Tartry D, Lantelme P, Ducher M, Fauvel JP. Are triplicate urine samples necessary to assess albuminuria? Nephron Clin Pract. 2012;122:80–85. doi: 10.1159/000350429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.