Abstract
This review provides an overview of the current state of mathematical models of cardiac growth and remodeling (G&R). We concisely describe the experimental observations associated with cardiac G&R and discuss existing mathematical models that describe this process. To facilitate the discussion, we have organized the G&R models in terms of (1) the physical focus (biochemical vs. mechanical) and (2) the process that they describe (myocyte hypertrophy vs. extracellular matrix remodeling). The review concludes with a discussion of some possible directions that can advance the existing state of cardiac G&R mathematical modeling.
Keywords: Cardiac growth and remodeling, mathematical modeling, hypertrophy, fibrosis, extracellular remodeling
1. Introduction
The heart is a highly complex living structure consisting of a collection of cells (e.g., myocytes, myofibroblasts, endothelial cells, vascular smooth muscle cells) and extracellular constituents (e.g., collagen fibers and proteoglycans). The primary function of the heart is to cyclically contract in order to generate a pressure gradient to perfuse all body organs including itself. To do so, however, the myocardium must operate as a system where all the individual constituent (or sub-system) operations are tightly orchestrated.
In response to neurohormonal, chemical and mechanical cues, the myocardium can undergo long term adaptive or maladaptive processes that are commonly referred to as “growth and remodeling” (G&R). Growth and remodeling can be a manifestation of many sub-processes that occur at a smaller scale, including cellular hypertrophy, apoptosis, proliferation, and extracellular matrix remodeling1. Collectively, these sub-processes lead to geometrical and functional changes of the heart, which can have significant clinical implications.
Myocardial G&R is widely considered to be an important determinant of the clinical course of heart failure2. As heart failure progresses, the heart size increases and its function deteriorates. Indeed, multiple studies have shown that an increase in heart size is associated with an adverse prognosis in many heart diseases3,4. By the same logic, a reversal of pathological G&R features in the form of a reduction in the heart size is widely considered to be a favorable response to treatments. This process, often referred to as reverse remodeling, and has been observed in numerous treatments that include prolonged support by left ventricular assist device5,6, bio-injection therapies7,8 and cardiac resynchronization therapy9,10.
Despite their clinical importance, our current understanding of the myocardial G&R mechanisms remain rudimentary. For example, the type of mechanical signals that myocytes sense (whether is it stress or strain11 or strain energy) and the way they respond to these signals (how their geometry and function changes) have not been fully elucidated12–14. A clear understanding of the various mechanisms of myocardial G&R and how they interact with one another may provide key insights for developing effective heart failure therapies. Given the complexity of the multitude of G&R pathways and their interactions, mathematical modeling has emerged as a powerful tool to make the problem more tractable. This is especially effective when mathematical models are coupled together with experiments in an iterative fashion to generate and test new hypotheses of G&R.
Here, our goal is to provide an overview of the state of the art in mathematical models of myocardial G&R. We briefly characterize myocardial G&R and then review existing mathematical models. We have categorized the models according to their focus on G&R pathways (biochemical vs. mechanical) and processes (cellular hypertrophy vs. remodeling of the extracellular matrix (ECM)). Finally, we call attention to how these mathematical models can be advanced so that G&R can be understood at a system-level.
2. Myocardial Growth and Remodeling
Myocardial G&R can be broadly defined as changes in the heart geometry and function that occur over a period of time that is significantly longer than a heartbeat. The nature of G&R can be pathological (e.g., in heart diseases) or physiological (e.g., during growth and development, exercise, pregnancy, ageing, etc.). In some cases, the G&R response can be quite similar under both circumstances. For instance, cellular hypertrophy in response to pressure overloading occurs in both hypertensive heart disease and during postnatal heart development. Under pathophysiological conditions, G&R may initially behave as a compensatory mechanism to normalize function in response to pathological stimuli. This is widely believed to be the case when left ventricular (LV) wall thickness increases during pressure overloading, which normalizes the wall stress15. Over time, however, the (usually asymptomatic) compensatory state gives way to a decompensated state that progresses to heart failure. The exact mechanisms responsible for the transition from compensated to decompensated G&R are not fully understood and are under intense investigation16.
Classically, G&R in the myocardium is categorized into two types based on the LV geometry: concentric and eccentric hypertrophy. During concentric hypertrophy, the LV wall thickens and there is little change in the chamber volume whereas during eccentric hypertrophy, the LV wall thins and the chamber volume increases significantly17. The LV also becomes more spherical in the latter. The ultrastructural basis that gives rise to these two types of geometrical features has been associated with the way sarcomeres are added in myocytes. Specifically, thickening of the LV wall and dilation of the LV chamber are due to the parallel and serial addition of sarcomeres in the myocytes, respectively. The fact that the resultant ventricular shape is largely determined by changes in myocyte shape is not surprising for three reasons. First, myocytes account for the bulk (75%) of the myocardial mass18. Second, myocytes are highly organized with their long axis mostly orthogonal to the myocardial transmural direction. Last, myocytes in the adult human heart regenerate at an extremely slow rate in which only about 50% are exchanged during the normal life span19.
In addition to geometrical changes, G&R can also induce functional changes that affect myocardial contraction. These changes can occur in the cardiac tissue’s bulk mechanical and electrical properties, such as contractility, passive stiffness and electrical conduction velocity. During G&R, the myocyte contractile function, which modulates the heart systolic function, may be altered. Myocyte hypertrophy has been associated with both an increase and a decrease in contractile function. This disparity in functional changes during hypertrophy has been attributed to the different phases of G&R; namely, the compensated and the decompensated phases20. In addition to the contractile function, G&R can also perturb the tissue’s passive stiffness. Myocardial stiffness plays a central role in the heart’s diastolic function, whereby an excessive increase in stiffness can impair diastolic filling, a hallmark of diastolic heart failure with preserved ejection fraction. Such an increase in myocardial tissue stiffness has been attributed to a delayed relaxation of the myocyte’s contraction as well as an increase in the intrinsic myocardial stiffness21. The latter may be associated with an alteration of the myocardial collagen fiber network in the ECM21,22.
The highly complex fibrillar collagen network in the myocardial ECM provides structural integrity to the adjoining myocytes. In addition to a network of collagen fiber, the ECM also contains other matrix proteins such as proteoglycans, glycosaminoglycan, and other signaling molecules such as angiotensin II22. Scanning electron microscopy has revealed extensive collagen fibrillar weaves that surround and support individual myocytes23,24. Due to the tight connections between myocytes and the collagen network, the ECM likely serves as a conduit for mechanical (stresses and strains) signal transmission to the cells, in addition to providing structural support for the myocardium. Therefore, any changes in the structure and composition of the ECM would not only contribute to the overall myocardial G&R process (e.g., stiffening of the tissue), but it would also have an impact on the cellular remodeling response. In addition, excessive extracellular collagen fiber accumulation also leads to a decrease in myocardial conduction velocity25, and an increase in arrhythmia26.
The majority of changes during myocardial G&R can be traced to the geometrical and functional changes in the myocytes, as well as changes in the myocardial collagen fiber network. For this reason, mathematical models of myocardial G&R are mainly constructed to describe these events.
3. Biochemical Models of Growth and Remodeling
3.1 Hypertrophy
A variety of hypertrophic stimuli such as mechanical cues (e.g., stresses and strains) and chemical agents (e.g., cytokines, growth factors, catecholamines, vasoactive peptides, hormones and many others) have been identified in myocytes27. Mathematical models have been constructed to describe and elucidate the signaling pathways associated with these hypertrophic stimuli13,28,29. For mechanotransduction pathways, Novak et al.30 proposed a model based on reaction kinetics that predicts the focal adhesion distribution in a cell in response to a force field. In a subsequent work, Grosberg et al.31 extended that model to include the fiber-length force dependency to describe the kinetics of myofibrillogenesis. Other approaches to describe the focal adhesions kinetics include a thermodynamically consistent model proposed by Deshpande et al.32. A number of models have also been constructed to describe the signaling pathways of non-mechanical hypertrophic stimuli. For example, Shin et al.29 constructed a mathematical model to describe the signaling pathway between calcineurin and the nuclear factor of activated T-cells, whereas Cooling et al.28 constructed a model to describe the IP3 signal production by extracellular agonists endothelin-1 and angiotensin-II.
To assimilate the various pathways and stimuli of myocyte hypertrophy, Ryall et al.33 described a computational model of the hypertrophic signaling network that integrates many of the established pathways. This extensive network (consisting of 193 reactions and 106 species) was described using logic-based differential equations that represent activation and inhibition of reactions with normalized Hill functions. A sensitivity analysis was performed on the model, which identified species with the greatest effects on myocyte hypertrophy and revealed 12 major functional signaling modules. The model identified Ras as a primary network hub that has the greatest influence on hypertrophy, suggesting that the inhibition of cardiomyocyte hypertrophy could be better achieved by blocking Ras. This finding was validated experimentally in the same study. Contrary to experimental studies focusing on specific isolated hypertrophic signaling pathways34,35, this model demonstrates the ability of mathematical modeling as a tool to analyze a complex system that would otherwise be nearly impossible with experiments that focus on specific hypertrophic signaling pathways. The model could, in principle, also be used to identify drug targets for hypertrophy36. To do so, however, future studies are needed to measure the parameters (which were prescribed with default values for all the reactions in the network) to avoid ad hoc assumptions.
3.2 Extracellular remodeling
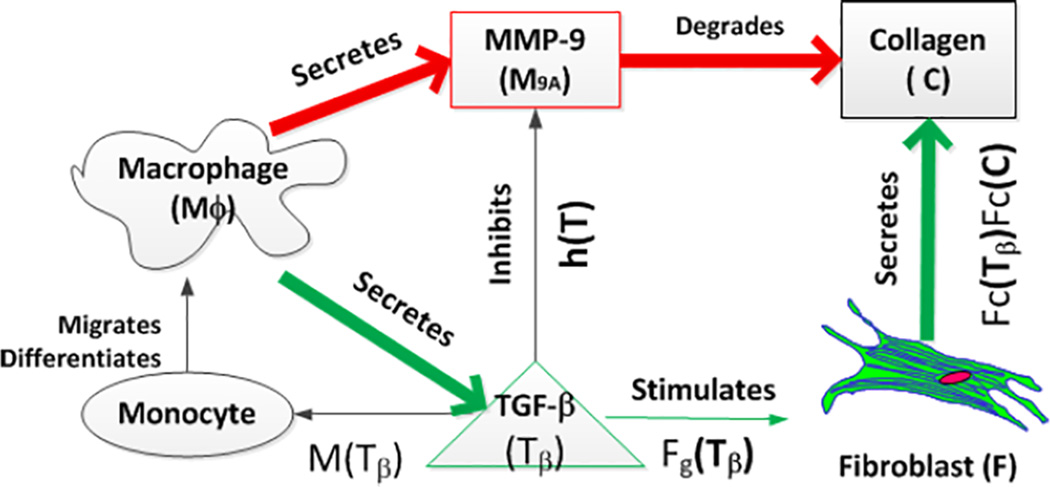
Mathematical models describing the remodeling process in the ECM are scarce. Although some models have been constructed to investigate a specific pathway of ECM remodeling (e.g., degradation of collagen fibers by MMPs37,38), system-level mathematical models that take into account the multiple signaling pathways during ECM remodeling are lacking. Given that this process is most prominent during the development of myocardial infarction, it is not surprising that existing models are mostly developed under this pathological setting. Based on mice with coronary artery ligation, Jin et al.39 developed a mathematical model to describe the post-MI scar formation process. In their ECM model, the time course of key chemical factors post-MI (i.e., macrophage cell density, fibroblast cell density, collagen concentration, TGF-β and activated MMP-9 concentration) were described using a set of coupled ordinary differential equations (ODEs) (Figure 1). The model predictions of macrophage, MMP9 and fibroblast expressions are in good agreement with other published experiments40–42. Using the validated parameter values, the model predicted that an early intervention of elevating the MMP-9 level immediately after myocardial infarction will significantly decrease the resultant collagen density when compared to a later intervention. With further development, the mathematical model may be useful in elucidating the quantitative effects of MMP inhibitors that are currently investigated as a therapy to prevent post-MI LV remodeling43.
Figure 1.
Schematic of the biochemical pathways during scar formation as described by the mathematical model of Jin et al.39
4. Mechanical models of G&R
The models discussed thus far focus primarily on the local cellular-scale biochemical reactions that occur during G&R. These reactions were mostly described using coupled ODEs of first-order reaction kinetics. As such, there is no length scale in the equations for describing the spatial variation of quantities such as the concentration of collagen fibers and size of myocytes. These models also do not directly predict changes in the geometry, structure and mechanical properties of the myocardium during G&R. The geometric, structural and material changes are important as they have direct implications on the cardiac function. Mathematical models of myocardial G&R that can account for these changes are typically constructed at the tissue- and organ-level using the principles of continuum mechanics. These models can be broadly classified based on their origins into: (1) structural adaption theory, (2) volumetric growth theory and (3) constrained mixture theory.
4.1 Structural adaption theory
One of the first cardiac G&R model that considers the (structural) remodeling of myofiber orientation, in addition to geometrical remodeling, was described by Arts et al.44. Using a heart model consisting of adjoining cylindrical shells, the myofiber orientation (in addition to the wall thickness) was adapted in each shell to optimize the local deviation of (1) the fiber shortening during systole and (2) the sarcomere length at the beginning of ejection from their corresponding prescribed set points. Their model yielded a transmural variation of myofiber orientation that is consistent with those found experimentally. A subsequent study by Kroon et al.45 using a finite element model of a truncated ellipsoid also produced the same result, though with a different remodeling law in which the myofibers adapt their direction to minimize the fiber-cross fiber shear strain. We refer readers to the review by Bovendeerd46 for a discussion of this class of models that describe the remodeling of myofiber orientation.
4.2 Volumetric growth theory
First proposed by Rodriguez et al.47, the volumetric growth theory is the most prevalent framework for modeling G&R in myocardial tissue. This theory draws from the concept of decomposing the deformation gradient tensor multiplicatively into an elastic component and a plastic component in finite strain plasticity48. Analogous to this, the deformation gradient tensor F is split into an elastic deformation gradient tensor Fe and a growth deformation gradient tensor Fg in the volumetric growth theory as:
| (1) |
The growth deformation gradient tensor Fg is in general, “incompatible", which means that it is impossible to derive a unique displacement field from the tensor Fg itself. The compatibility condition as required by the deformation gradient tensor F is restored by the elastic component of the deformation gradient tensor Fe. In this theory, the direct consequence of the incompatibility of Fg is that non-homogeneous growth in a body may generate residual stresses and strainsa; i.e., stresses and strains that exist in the body without any external load49. This prediction is consistent with the fact that a residual stress/strain field is always present in the intact ventricular walls49,50. However, the existence of residual stress/strain may not only be due to G&R, as this feature can also be induced by other competing mechanisms51 such as tissue swelling52.
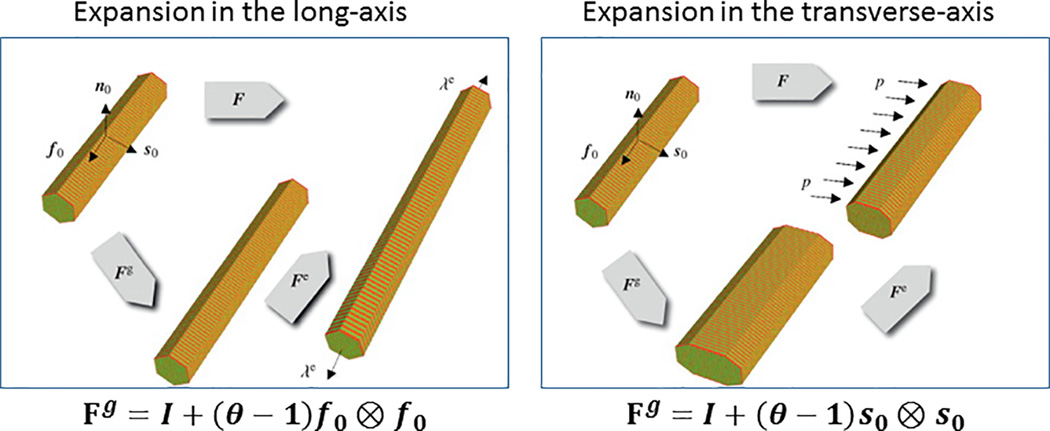
The growth deformation gradient tensor Fg can be interpreted as a change in the tissue stress-free configuration. The geometric changes associated with G&R during cellular hypertrophy and atrophy are most naturally described by this configurational change in which the elastic strains (i.e., strains that generate stresses) are taken with respect to this new configuration. Specifically, the tensorial form of Fg reflects changes in cell geometry due to the parallel and/or serial addition of sarcomeres as they undergo hypertrophy or atrophy (Figure 2). A constitutive relationship is required for the growth deformation gradient tensor Fg to link the geometrical changes (effects) to the stimulus (cause).
Figure 2.
Tensorial form of the growth deformation tensor Fg reflecting cellular shape changes. Adapted from Goktepe et al.59
In recent years, a number of constitutive models have been developed under the volumetric growth framework to simulate myocardial G&R under various physiological and pathological conditions. Taber and colleagues have constructed growth constitutive models to describe G&R in the developing heart53,54. In their models, growth was described by an ODE equation that can be generalized as Ḟg = A: (S − S0)55, where the rate of change of the growth deformation gradient tensor Ḟg is driven by a deviation of the growth stimulus S from its homeostatic or target value S0. The growth stimulus was prescribed to be the stress tensor in their model and the fourth-order tensor A contains coefficients that control the growth-rate. This particular form has also been used by Kerckhoffs56 and Kroon et al.57 to simulate cardiac growth in the LV, but with myofiber and cross-myofiber normal strains as the growth stimuli. The unloaded configuration (which typically does not exist in vivo) was used as a reference state for the calculation of the strains as an input in most strain-driven growth constitutive models. This reference state is likely to change during G&R. As discussed by Rodriguez et al.47 and Taber58, however, the issue of which reference state to use would not arise if Cauchy stress, which does not depends on the choice of a reference configuration, is used as a growth stimuli.
The form Ḟg = A: (S − S0) assumes that (1) the growth rate is a linear function of the stimulus and (2) the amount of growth is not constrained by any physiological limit; i.e., growth (or shrinkage) continues as long as S ≠ S0. Models not adhering to these assumptions have also been developed. Based on the conventional hypothesis that myocardial wall stress and strain are, respectively, the stimulus causing concentric and eccentric cardiac hypertrophy during pressure and volume overload, Goktepe et al.59 described separate growth constitutive models to simulate these two conditions. In their model, growth in the myofiber and cross-fiber directions were driven by a deviation of the myofiber stretch and the Mandel stress from their corresponding homeostatic set point. A rate-limiting function was used to restrict growth to within prescribed physiological limits. Goktepe et al.59 showed that key G&R features during pressure overload (wall thickening) and volume overload (chamber dilation) can be predicted using their models. This strain-driven growth model was further extended by Lee et al.60 to simulate a reversal of G&R in response to a reduction in hemodynamic load that can be found, for example, after the implantation of left ventricular assist device. This model has also been integrated with cardiac electromechanics61. Contrary to the prevailing hypothesis that concentric and eccentric hypertrophy are caused separately by an elevated myocardial stress and strain, respectively, Kerckhoffs et al.62 constructed a growth constitutive model in which the different components of myocardial strain tensors are used as growth stimuli. In their model, growth rates in the myofiber and cross myofiber directions were described by nonlinear sigmoidal functions of growth stimuli, which were the deviation of myofiber and cross myofiber strains from their corresponding homeostatic set points. In this model, growth was also restricted to lie within a prescribed physiological limit. Kerckhoffs et al.62 showed that by using these quantities as growth stimuli, the model can reproduce key G&R features associated with both concentric and eccentric hypertrophy. The issue of myocardial stress or strain as a mechanical stimulant for G&R is still under debate11, although a number of studies have leaned towards strain or stretch as the responsible mechanical stimulant for G&R63,64. In one of these studies, Holmes14 found that features of the time-varying signals, which include strain-related LV cavity volume amplitude and its rate of change, were significantly altered during early volume overload in rats. Based on this findings, he concluded that these mechanical signals are candidates for G&R mechanical stimuli. The issue of the true mechanical stimulant for G&R is not easily resolvable. But with the development of more growth constitutive models, various hypothesis can be formulated and tested by corroboration with experiment data.
Although growth constitutive models constructed based on a volumetric growth framework can predict geometrical changes, they cannot directly account for changes in the tissue mechanical properties found in the heart during G&R. These changes can be associated with a change in the ratio of the tissue constituent (e.g., fibrosis1,65) or a change in the intrinsic material property of the constituent (e.g., a change in myocyte stiffness66 and contractile function67). As described by Taber58, existing tissue material constitutive models describing its stress-strain relationship need to be reformulated with an additional input from the growth tensor Fg in order for volumetric G&R–based models to account for any change in the tissue mechanical properties during G&R. To date, none of the volumetric growth-based constitutive models have accounted for this change and this remains a laudable goal of future efforts.
4.3 Constrained mixture theory
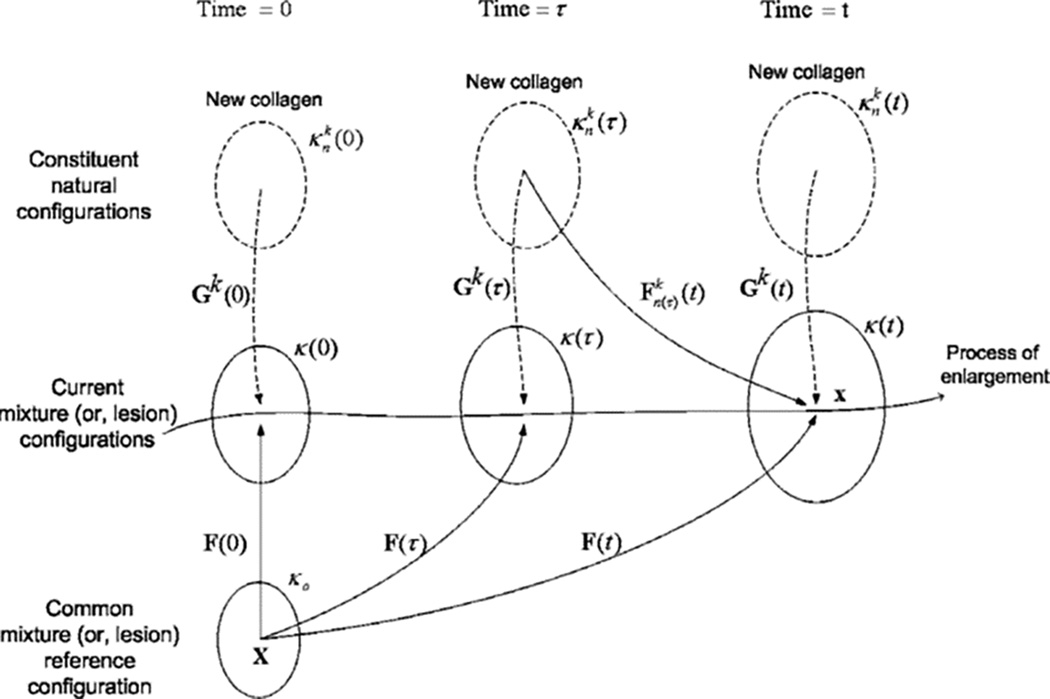
Subsequent to the volumetric growth theory, a separate theoretical framework based on the constrained mixture theory was formulated by Humphrey and Rajagopal68 to model G&R. This theoretical framework was built upon the hypothesis that each tissue constituent, such as muscle cell and collagen fiber, can turnover at different rates and possess different natural/stress-free configurations that evolve during G&R while being constrained to deform as a continuum within the mixture (henceforth the name “constrained mixture”). One of the key hypotheses in this theory is that the deposition of new constituents such as collagen fibers always occurs at the current configuration. Referring to Figure 3, a constituent of type k (e.g., collagen fibers) deposited at time τ with a homeostatic stretch Gk (τ) will experience a deformation described by Fn(τ)(t) = F(t)F−1(τ)Gk(τ) at a later time t in the configuration κ(t). Thus, newly deposited constituents will experience a different stress/strain state than that experienced by previously deposited constituents, even if they are of the same type. By consolidating the effects of all the constituents produced up to the current configuration at time t, the partial stress associated with constituent type k is given as:
| (2) |
Here, m(τ) is the mass production rate, G(t − τ) is the prescribed “survival” function for the constituent produced at time τ and f(·) is the stress response function. The “survival function” describes the constituent’s degradation time-course starting from birth time at τ to current time t. A key feature of this model is that it allows the tissue mechanical property to change during G&R without the need to reformulate the existing material constitutive descriptor (stress-strain relationship) of the constituents.
Figure 3.
Schematic of the constrained mixture-based G&R theory. Adapted from Baek et al.69
Compared to the volumetric growth theory, the constrained mixture model is relatively more complex to work with, in part, because of the need to track the evolving natural configuration of the constituents during G&R. As such, the application of constrained mixture G&R framework has, so far, been mostly confined to modeling arterial G&R using simplified geometry; e.g., approximating the arterial wall as a thin-wall membrane69–71. Although this model has not been applied to the heart, the theoretical framework is highly relevant and should be adapted to model myocardial G&R. This is because collagen deposition and degradation, the major focus of this theory, is an important aspect of myocardial G&R that has been observed in many heart diseases. For a more general review of continuum mixture-based models, including those applied to other biological tissues, we refer the readers to the review by Ateshian and Humphrey72.
5. System Growth and Remodeling
Although mathematical models of myocardial G&R have advanced over the years, much work is needed to refine, improve and integrate these models. The following is a discussion of directions that can be undertaken to advance mathematical models of myocardial G&R.
5.1 Model Testing, Calibration and Validation
Most myocardial G&R models are not calibrated using experimental studies specifically designed for such a purpose. Instead, model parameters are largely estimated using previous experimental studies typically designed for a different purpose. As such, the experiments may not fully explore the variable space that is necessary to calibrate and test the models. For example, ventricular pressure is typically not varied over a large range in chronic experiments beyond hypertensive and baseline levels. This limitation may pose a problem where the number of experimental data points is insufficient to calibrate and test the growth constitutive models, particularly, in models where G&R is stress-mediated.
The number of parameters in the G&R models discussed in this review is listed in Table 1. In general, these parameters can be categorized based on their association with biochemical reaction rates, growth/mass production rates and limits/threshold/homeostatic states associated with G&R. The chemical rates (unit: s−1) can be discerned from the growth/mass production rates (unit: days−1) in terms of their timescales. As shown in the Table, the number of parameters can range between 8 to more than 100 in these models. This large number of parameters can, in effect, render the whole process of model testing, calibration and validation a complex task.
Table 1.
Parameters in the discussed myocardial G&R models
| Models | No. of parameters |
BR | GM | LM | Remarks | ||
|---|---|---|---|---|---|---|---|
| Cellular-based | Hypertrophy | Rydall et al.33 | >= 100 | All | X | X | Note: Although all parameters listed here are biochemical reaction rates, it is unclear as to where the transition from biochemical to growth rates are in the model. |
| ECM remodeling | Jin et al.39 | 15 | 3 | 9 | 3 | ||
| Continuum-based | Hypertrophy | Lin and Taber53 | 9 | X | 9 | X | Focused on developmental G&R |
| Goktepe et al.73 | 8 | X | 2 | 6 | Assumed a different set of parameters for stress and strain-mediated G&R constitutive laws | ||
| Lee et al.60 | 8 | X | 2 | 6 | Assumed a different set of parameters for atrophy and hypertrophy | ||
| Kerckhoff et al.62 | 15 | X | 6 | 4 | |||
| ECM remodeling | Wilson et al.91 | 11 | X | 2 | 9 | Parameters are associated with constituents in the arteries (collagen, elastin and smooth muscle). Only parameters associated with G&R are considered –parameters associated with the mechanical properties are listed here. |
Abbreviations: BR, biochemical reaction rates; GM, growth/mass production rates; LM, limits/threshold/homeostatic states associated with G&R: ECM, extracellular matrix.
Perhaps a way to manage the complexity of this process in some of these models is to reduce them to the simplest form while retaining their primary hypothesis. To illustrate this point, we consider the simplification of a volumetric growth-based stress-mediated G&R constitutive model53,73. The hypothesis in the model is that growth (in the form of a change in wall thickness) occurs for the purpose of normalizing ventricular wall stress. Using standard Laplace’s law to calculate the wall stress, the time-rate of a change in the wall thickness Δh̅ can be reduced to the following ODE:
| (3) |
where K is the growth rate constant, Po is the pre-growth pressure, h̅ is the pre-growth wall thickness and P̅ is the change in pressure (see Appendix for details). Dimensions and pressure in the equation have been normalized to the internal LV chamber radius and Po, respectively. The only model parameter in this model is the growth rate constant K.
5.1.1 Testing the Model Hypothesis
In the model, steady state occurs when the wall stress is restored. Under this condition, we find that the change in wall thickness Δh̅ is a function of the pressure change P̅ and pre-growth wall thickness h̅ by
| (4) |
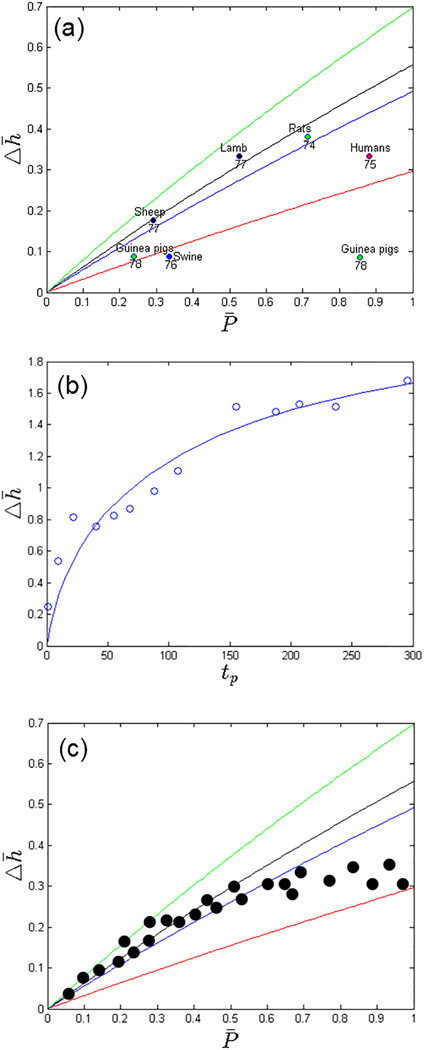
To test whether the model hypothesis agrees with the general observations of G&R, we plot this non-dimensionalized functional relationship (for the experimental range of 0.2 ≤ h̅ ≤ 0.7) with data points collected from different existing experimental and clinical studies of different species during LV pressure-overload (Figure 4a)74–78. These data are given in the Appendix. As shown in the figure, the data gathered from experiments conducted on different species are, in general, compatible with this simplified model and fall within the range of the experimental values of h̅. On the other hand, the disparity between the model prediction and experiment is greater in some cases if the comparison was made based on the specific normalized wall thickness h̅ of each experiment. More data points are needed to ascertain this compatibility. In the plot, we have implicitly assumed that a steady state condition is reached in the experiments, but as a result of LV pressure-overloading. This assumption may not be valid when G&R is decompensatory; i.e., when hypertrophy is insufficient to mitigate the increase in pressure. Nevertheless, the general quantitative agreement between the data points and the prediction of the simplified model provides a certain level of confidence of the model hypothesis and its functional form.
Figure 4.
(a): Normalized pressure change vs. thickness change as predicted by a simplified version of the G&R constitutive model at steady state (lines) and obtained from different experiments (dots). Reference numbers and species are given on the bottom and top of each experimental data point, respectively. (b): Wall thickness change vs. physiological time tp in the fitted model (line) with experimental measurements in the neonatal rat heart (dots). Note: time-variable and wall thickness in the experimental data has been scaled to a physiological time tp based on the body weight of 0.065kg and the internal LV radius of 1.5mm, respectively. (c): Hypothetical example of experimental data (dots) showing the existence of a threshold for hypertrophy.
5.1.2 Model Calibration and Validation
To calibrate the model parameter K (growth rate), longitudinal data of the LV geometry of more than 2 time points during G&R is required. In order to accommodate for interspecies differences in experiments, it may also be helpful to fit the G&R constitutive model in Eq. (1) using physiological time tp rather than the chronological time tc, based on the allometric scaling law tp = tc W−1/4 with W denoting the body weight79. As an example, we fit the model to the experimental longitudinal data of neonatal rats acquired up to 150 days after birth. To do so, we assumed that the fetal pressure is 1/10 of that in the adult rat that has an LV pressure of 120 mm Hg (P̅ = 9)80, and a thickness-to-radius ratio of h̅ = 0.43 (pre-growth wall LV thickness and internal radius of 0.65mm and 1.5mm, respectively). We find that a growth rate K = 6 × 10−4/ day is able to fit the experimental longitudinal data of neonatal rats relatively well80. As shown in Figure 4b, the parabolic growth curve predicted by the model is consistent with the trend found in the experimental data. In the final step to ascertain the predictive capability of the parameterized model, the calibrated growth rate of K = 6 × 10−4/ day needs to be validated against an independent set of measurements that are not used in the calibration process. Sampling techniques such as random subsampling, K-fold cross-validation and leave-one-out-cross-validation can be used to overcome the limitation of a small set of experimental data.81,82
Using a simplified model, we have demonstrated the two important aspects of G&R mathematical model development; i.e., model hypothesis testing and model validation/calibration. In the former, we seek to test whether the model hypothesis and formulation are consistent with existing experimental data, while in the latter, we seek to determine and test parameter values that best-fit the data. The illustrated example also demonstrates the potentiality of approaching the issue of model calibration and validation in a systematic way: (1) reducing the model to its simplest form suitable for validation and calibration, (2) calibrating the simplified model and (3) using the calibrated parameter values as initial guesses for the calibration of a more sophisticated model with realistic ventricular geometry. In this process, one may also find that the model may be inadequate to explain certain features of the experiments and if so, appropriate refinement or reformulation of the model then becomes necessary. For example, a hypothetical scenario where the model prediction of the change in thickness Δh̅ for a given change in pressure P̅ is substantially and consistently higher than those found in the experiment (i.e., when all the experimental data points lie below the curves in Figure 4a) would imply that a change in thickness during G&R is insufficient to normalize the wall stress. In that case, the model would need to be reformulated (e.g., if G&R shows better correlation with another stimulant such as strain or strain energy) or refined (e.g., if experiments imply that there exists a threshold for cellular hypertrophy – See Figure 4c for a hypothetical example). In the latter case, new parameters (e.g., a threshold for hypertrophy) would need to be introduced into the model.
5.2 Model integrations across scales
As discussed, a clear distinction can be found in existing mathematical models of G&R that have focused on either the associated biochemical reactions or mechanics. There is still a disconnection between these two types of models, especially in terms of the spatial and temporal scales. Biochemical models of G&R were largely constructed based on experiments conducted using in vitro cell culture studies usually lasting a few days, whereas the mechanics models of G&R were largely constructed based on in vivo animal studies usually lasting for a few weeks.
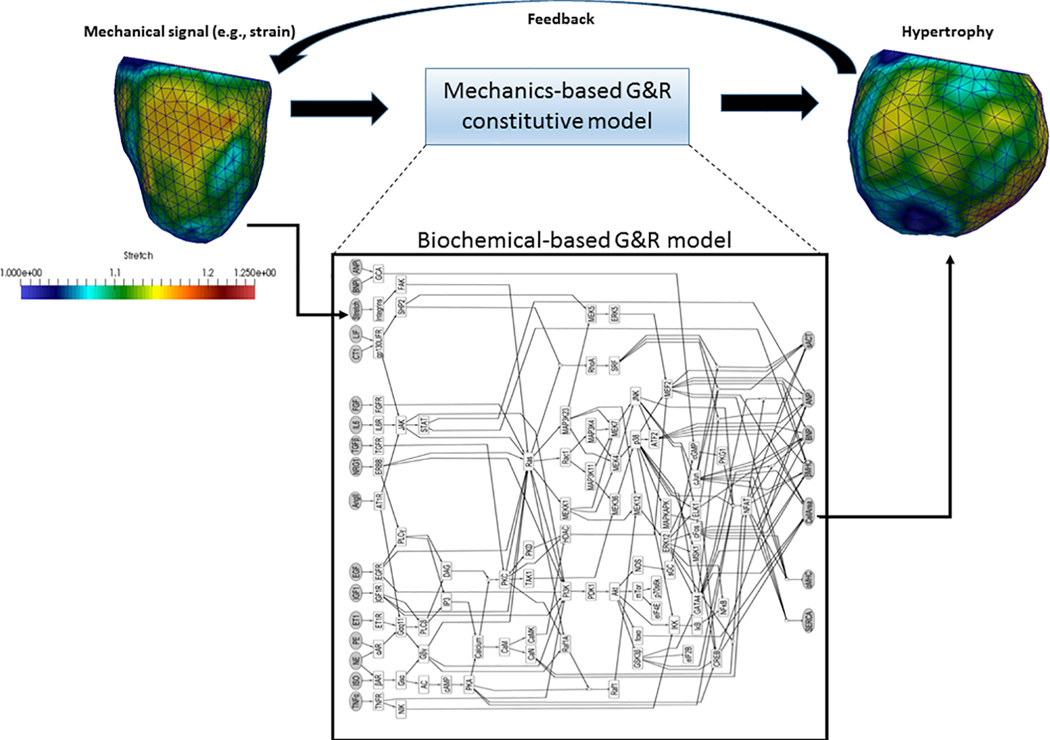
These different mathematical models need to be integrated to achieve a system understanding of G&R under both physiological and pathological conditions. This is especially so because mechanics-based models can provide physiologically realistic input such as animal or patient-specific myocardial strain/stress to the biochemical models, whereas biochemical models can provide a more physical G&R signaling pathways for the mechanics-based models. The integration can be achieved via a simple relation between the parameters of the two different models (e.g., biochemical reaction rates -> growth rate) or a complete integration of the biochemical model to the mechanics-based model as illustrated in Figure 5.
Figure 5.
An example of integrating a biochemical-based G&R model to a mechanics-based G&R model. In the example, the biochemical hypertrophic signaling network model is taken from Ryall et al.33, with mechanical stretch as one of the inputs and the cell area as one of the outputs. These quantities can be associated with those from existing mechanics-based strain-mediated G&R constitutive models60,62,73 in which mechanical strain and the growth deformation gradient tensor are the input and output, respectively. As such, the biochemical-based model can be integrated easily into mechanics-based G&R models.
5.3 A need for multiscale G&R modeling
It is clear that a multiscale G&R model can be a potentially useful research and clinical tool. Specifically, such a model can be useful in (1) developing a more in-depth understanding of the various G&R mechanisms, and in future, (2) a tool personalized treatment. The connection of G&R models of different timescale is essential in predicting how short term (acute) events can influence the long-term (chronic) course of a disease and/or treatment. On the other hand, connecting G&R models at different spatial scales will enable one to predict the large-scale effects arising from small-scale molecular events. A multiscale G&R model has the potential to unravel how minute changes at the small-scale level can lead to significant long-term large-scale changes, which is analogous to the so-called “butterfly effects”b in Chaos theory83. Such a model would be especially useful in understanding and predicting the progression of a number of heart diseases and potential treatments. We discuss two areas where such a multiscale G&R could potentially be useful.
Cardiac resynchronization therapy
Reverse remodeling in the form of a reduction in LV end-systolic volume (by ≥ 15%) is a clinically-relevant indicator of a positive response to cardiac resynchronization therapy (CRT)84. Approximately, 30% of patients, however, do not improve after therapy and the identification of potential responders to CRT remains a challenge. One of the hypothesis is that CRT can help to attenuate the early systolic stretch, a potential G&R stimulant, found in the late activated region as a result of left branch bundle block85. This effect can become confounded, however, by other factors such as a depressed contractility in ischemic patients. Computational modeling, with its inherent ability to integrate all the confounding factors, have already been used to understand the short-term effects of this treatment86. A multiscale G&R model could potentially be used to understand and predict the long-term effects of CRT.
Familial Hypertrophic Cardiomyopathy
Familial hypertrophic cardiomyopathy is a disease associated with the genetic mutation of sarcomeric proteins87. Some of the features associated with this disease include myocyte hypertrophy and disarray, fibrosis, diastolic dysfunction, and thickening of the ventricular wall. These genetic mutations can alter sarcomere function, which support some of the prevailing hypotheses that the resultant large-scale G&R features are compensatory responses to this alteration.87 In this aspect, a multiscale G&R model that is able to connect small-scale molecular-level changes caused by mutation to large-scale ventricular G&R features could be extremely useful in testing these hypotheses. Given that pharmacological drugs operate at the molecular level, such a multiscale model could, in principle, be also used as an in silico clinical trial to test the long-term efficacy of new drugs.
6. Conclusion
Since the first observation by Sandler and Dodge88, who speculated that the “heart may hypertrophy as a result of increased tension to maintain wall stress within certain limits as yet undefined”, the past 50 years has seen significant advances in our knowledge about G&R in the myocardium. Indeed, it is becoming clearer that myocardial G&R is a very complex process – its mechanism is certainly not as simple as the one originally suggested by Sandler and Dodge88. There is little doubt that mathematical modeling can play an important role in our quest to elucidate the complex mechanisms of G&R. There are many challenges in improving the current state of mathematical cardiac G&R models and synergistically integrating them with experiments and clinical data. Overcoming these challenges will require significant effort. But with heart failure still affecting a large population, the benefits are likely to be enormous.
Acknowledgments
The authors thank Pamela Derish in the Department of Surgery, UCSF, for proofreading the manuscript. This work was supported by NIH Grants R01-HL-077921 (J.M. Guccione) and R01-HL-118627 and U01-HL-119578 (J.M. Guccione and G.S. Kassab).
Appendix
Derivation of simplified G&R constitutive model for pressure overload
The simplified constitutive law was derived from previous 3D constitutive models of stress-mediated G&R53,73. Those models were constructed based on the hypothesis that G&R occurs for the purpose of restoring ventricular wall stress. A simplified version of these models can be written as follows:
| (A1) |
Here, Δh/ri is the change in thickness during G&R normalized with respect to the pre-growth LV inner radius. This quantity has the same physical meaning as the growth deformation gradient tensor in the volumetric G&R framework. In Equation (A1), K is the growth rate constant, σ is the wall stress as a result of a new pressure Pnew, and σ* is the wall stress in the pre-growth configuration under the previous pressure Pold, which can be assumed to be a homeostatic set point. Using Laplace’s law and assuming that (1) the LV can be described by an ellipsoid, (2) the cavity volume does not change (i.e., ri is a constant) and (3) a thick ventricular wall with homogeneous stress distribution across the thickness, the pre- and post-growth meridional wall stresses can be approximated as89,90
| (A2,3) |
Although one can also compute for the circumferential wall stress here, the calculation requires information about the LV major- and minor-axis dimensions. These information are not given in most of the experimental studies.
Letting h̅ = h/ri, Δh̅ = Δh/ri, P̅ = (Pnew − Pold)/Pold, and substituting (A2) and (A3) into (A1) yields
| (A4) |
Equation (A4) can be solved with an initial condition of Δh̅ = 0 to obtain a time-evolution of the wall thickness. At steady state, and the change in wall thickness Δh̅ can be computed from Equation (A4) as a function of P̅ and h̅ by:
| (A5) |
We note that two solutions exist for Δh̅ in Eq. (A4) under a steady state condition. However, only one solution yields a physically meaningful (Δh̅ > 0) solution.
Table A1.
Experimental measurements of the long-term LV geometrical changes due to pressure overload of various species from the literature.
| Species | Pold (mmHg) | Pnew (mmHg) | P̅ | ri (mm) | h (mm) | Δh (mm) |
h̅ | Δh̅ |
|---|---|---|---|---|---|---|---|---|
| Rats | 115 | 197 | 0.71 | 2.45 | 2.8 | 0.8 | 1.14 | 0.33 |
| Humans | 117 | 220 | 0.88 | 24.00 | 9.0 | 8.0 | 0.38 | 0.33 |
| Swine | 134 | 179 | 0.34 | 12.50 | 8.9 | 1.1 | 0.71 | 0.09 |
| Lamb | 95 | 145 | 0.53 | 12.00 | 10.0 | 4.0 | 0.83 | 0.33 |
| Sheep | 110 | 142 | 0.29 | 17.00 | 14.0 | 3.0 | 0.82 | 0.18 |
| Guinea pigs | 71 | 88 | 0.24 | 2.26 | 2.6 | 0.2 | 1.15 | 0.09 |
| Guinea pigs | 62 | 115 | 0.85 | 2.33 | 2.6 | 0.2 | 1.12 | 0.09 |
Note: ri in the results from the guinea pigs are calculated from LV cavity area measurements.
Footnotes
There are special cases in which non-homogeneous growth does not generate residual stresses.
The “butterfly effect” is a term coined by the mathematician Edward Lorenz in describing how a slight variation in the initial conditions of a complex nonlinear system can have a tremendous impact on the future outcomes. To describe this phenomenon, he gave an example of how the flapping of a butterfly’s wing in Brazil can set off a tornado in Texas.
References
- 1.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83(6):1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 2.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J. Am. Coll. Cardiol. 2000;35(3):569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 3.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76(1):44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 4.Hamer AW, Takayama M, Abraham KA, Roche AH, Kerr AR, Williams BF, Ramage MC, White HD. End-systolic volume and long-term survival after coronary artery bypass graft surgery in patients with impaired left ventricular function. Circulation. 1994;90(6):2899–2904. doi: 10.1161/01.cir.90.6.2899. [DOI] [PubMed] [Google Scholar]
- 5.Levin H, Oz M, Chen J, Packer M, Rose E, Burkhoff D. Reversal of chronic ventricular dilation in patients with end-stage cardiomyopathy by prolonged mechanical unloading. Circulation. 1995;91(11):2717–2720. doi: 10.1161/01.cir.91.11.2717. [DOI] [PubMed] [Google Scholar]
- 6.Burkhoff D, Klotz S, Mancini DM. LVAD-induced reverse remodeling: basic and clinical implications for myocardial recovery. J. Card. Fail. 2006;12(3):227–239. doi: 10.1016/j.cardfail.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ. Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee LC, Wall ST, Klepach D, Ge L, Zhang Z, Lee RJ, Hinson A, Gorman JH, Gorman RC, Guccione JM. Algisyl-LVR™ with coronary artery bypass grafting reduces left ventricular wall stress and improves function in the failing human heart. Int. J. Cardiol. 2013;168:2022–2028. doi: 10.1016/j.ijcard.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu CM, Bleeker GB, Fung JWH, Schalij MJ, Zhang Q, van der Wall EE, Chan YS, Kong SL, Bax JJ. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation. 2005;112:1580–1586. doi: 10.1161/CIRCULATIONAHA.105.538272. [DOI] [PubMed] [Google Scholar]
- 10.Yu CM, Fung W-H, Lin H, Zhang Q, Sanderson JE, Lau CP. Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol. 2003;91:684–688. doi: 10.1016/s0002-9149(02)03404-5. [DOI] [PubMed] [Google Scholar]
- 11.Omens JH. Stress and strain as regulators of myocardial growth. Prog. Biophys. Mol. Biol. 1998;69(2–3):559–572. doi: 10.1016/s0079-6107(98)00025-x. [DOI] [PubMed] [Google Scholar]
- 12.Omens JH, McCulloch AD, Lorenzen-schmidt I. Mechanotransduction in Cardiac Remodeling and Heart Failure. 2007 [Google Scholar]
- 13.Sheehy SP, Grosberg A, Parker KK. The contribution of cellular mechanotransduction to cardiomyocyte form and function. Biomech. Model. Mechanobiol. 2012;11(8):1227–1239. doi: 10.1007/s10237-012-0419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes JW. Candidate mechanical stimuli for hypertrophy during volume overload. J. Appl. Physiol. 2004 May;97:1453–1460. doi: 10.1152/japplphysiol.00834.2003. 2004. [DOI] [PubMed] [Google Scholar]
- 15.Grossman W. Cardiac Hypertrophy : Pathologic Process? Useful Adaptation or. Am. J. Med. 1980 Oct;69:576–584. doi: 10.1016/0002-9343(80)90471-4. [DOI] [PubMed] [Google Scholar]
- 16.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122(25):2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 18.Vliegen HW, van der Laarse A, Cornelisse CJ, Eulderink F. Myocardial changes in pressure overload-induced left ventricular hypertrophy. A study on tissue composition, polyploidization and multinucleation. Eur. Heart J. 1991;12(4):488–494. doi: 10.1093/oxfordjournals.eurheartj.a059928. [DOI] [PubMed] [Google Scholar]
- 19.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaub MC, Hefti MA, Zuellig RA, Morano I. Modulation of contractility in human cardiac hypertrophy by myosin essential light chain isoforms. Cardiovasc. Res. 1998;37(2):381–404. doi: 10.1016/s0008-6363(97)00258-7. doi:S0008-6363(97)00258-7 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Kass DA, Bronzwaer JGF, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ. Res. 2004;94(12):1533–1542. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 22.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol. Rev. 2007;87(4):1285–12342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 23.Weber KT. Cardiac Interstitium in Health and Disease : The Fibrillar Collagen Network. J. Am. Coll. Cardiol. 1989;13(7):1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 24.Robinson TF, Cohen-Gould L, Factor SM, Eghbali M, Blumenfeld OO. Structure and function of connective tissue in cardiac muscle: collagen types I and III in endomysial struts and pericellular fibers. Scanning Microsc. 1988;2:1005–1015. [PubMed] [Google Scholar]
- 25.King JH, Huang CLH, Fraser JA. Determinants of myocardial conduction velocity: Implications for arrhythmogenesis. Front. Physiol. 2013 Jun 4;:1–14. doi: 10.3389/fphys.2013.00154. June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen TP, Qu Z, Weiss JN. Cardiac fibrosis and arrhythmogenesis: The road to repair is paved with perils. J. Mol. Cell. Cardiol. 2014;70:83–91. doi: 10.1016/j.yjmcc.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hefti MA, Harder BA, Eppenberger HM, Schaub MC. Signaling pathways in cardiac myocyte hypertrophy. J. Mol. Cell. Cardiol. 1997;29(11):2873–2892. doi: 10.1006/jmcc.1997.0523. [DOI] [PubMed] [Google Scholar]
- 28.Cooling M, Hunter P, Crampin EJ. Modeling hypertrophic IP3 transients in the cardiac myocyte. Biophys. J. 2007;93(10):3421–3433. doi: 10.1529/biophysj.107.110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin SY, Choo SM, Kim D, Baek SJ, Wolkenhauer O, Cho KH. Switching feedback mechanisms realize the dual role of MCIP in the regulation of calcineurin activity. FEBS Lett. 2006;580(25):5965–5973. doi: 10.1016/j.febslet.2006.09.064. [DOI] [PubMed] [Google Scholar]
- 30.Novak IL, Slepchenko BM, Mogilner A, Loew LM. Cooperativity between cell contractility and adhesion. Phys. Rev. Lett. 2004;93(26 I):2–5. doi: 10.1103/PhysRevLett.93.268109. [DOI] [PubMed] [Google Scholar]
- 31.Grosberg A, Kuo P-L, Guo C-L, Geisse Na, Bray M-A, Adams WJ, Sheehy SP, Parker KK. Self-organization of muscle cell structure and function. PLoS Comput. Biol. 2011;7(2):e1001088. doi: 10.1371/journal.pcbi.1001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deshpande VS, Mrksich M, McMeeking RM, Evans AG. A bio-mechanical model for coupling cell contractility with focal adhesion formation. J. Mech. Phys. Solids. 2008;56(4):1484–1510. [Google Scholar]
- 33.Ryall KA, Holland DO, Delaney Ka, Kraeutler MJ, Parker AJ, Saucerman JJ. Network reconstruction and systems analysis of cardiac myocyte hypertrophy signaling. J. Biol. Chem. 2012;287(50):42259–42268. doi: 10.1074/jbc.M112.382937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19(23):6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molkentin JD, Dorn GW. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu. Rev. Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 36.Majkut S, Dingal PCDP, Discher DE. Stress sensitivity and mechanotransduction during heart development. Curr. Biol. 2014;24(10):R495–R501. doi: 10.1016/j.cub.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karagiannis ED, Popel AS. A theoretical model of type I collagen proteolysis by matrix metalloproteinase (MMP) 2 and membrane type 1 MMP in the presence of tissue inhibitor of metalloproteinase 2. J. Biol. Chem. 2004;279(37):39105–39114. doi: 10.1074/jbc.M403627200. [DOI] [PubMed] [Google Scholar]
- 38.Vempati P, Karagiannis ED, Popel AS. A biochemical model of matrix metalloproteinase 9 activation and inhibition. J. Biol. Chem. 2007;282(52):37585–37596. doi: 10.1074/jbc.M611500200. [DOI] [PubMed] [Google Scholar]
- 39.Jin YF, Han HC, Berger J, Dai Q, Lindsey ML. Combining experimental and mathematical modeling to reveal mechanisms of macrophage-dependent left ventricular remodeling. BMC Syst. Biol. 2011;5(1):60. doi: 10.1186/1752-0509-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2006;290(1):H232–H239. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- 41.Yang F, Liu Y, Yang X, Xu J, Kapke A, Carretero OA. Myocardial infarction and cardiac remodelling in mice. Exp. Physiol. Transl. Integr. 2002;87(5):547–555. doi: 10.1113/eph8702385. [DOI] [PubMed] [Google Scholar]
- 42.Fang L, Gao XM, Moore XL, Kiriazis H, Su Y, Ming Z, Lim YL, Dart AM, Du XJ. Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. J. Mol. Cell. Cardiol. 2007;43(5):535–544. doi: 10.1016/j.yjmcc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Gajarsa JJ, Kloner RA. Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail. Rev. 2011;16(1):13–21. doi: 10.1007/s10741-010-9181-7. [DOI] [PubMed] [Google Scholar]
- 44.Arts T, Prinzen FW, Snoeckx LHEH, Rijcken JM, Reneman RS. Adaption of cardiac structure by mechanical feedback in the environment of the cell: A model study. Biophys. J. 1994;66(4):953–961. doi: 10.1016/S0006-3495(94)80876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kroon W, Delhaas T, Bovendeerd PHM, Arts T. Computational analysis of the myocardial structure: Adaptation of cardiac myofiber orientations through deformation. Med. Image Anal. 2009;13(2):346–353. doi: 10.1016/j.media.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Bovendeerd PHM. Modeling of cardiac growth and remodeling of myofiber orientation. J. Biomech. 2012;45(5):872–881. doi: 10.1016/j.jbiomech.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez E, Hoger A, Mcculloch AD. Stress-dependent finite growth in soft elastic tissues. J. Biomech. 1994;27(4):455–467. doi: 10.1016/0021-9290(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 48.Lee EH. Elastic-Plastic Deformation at Finite Strains. J. Appl. Mech. 1969;36(1):1–6. [Google Scholar]
- 49.Genet M, Rausch MK, Lee LC, Choy JS, Zhao X, Kassab GS, Kozerke S, Guccione JM, Kuhl E. Heterogeneous growth-induced prestrain in the heart. J. Biomech. 2015 doi: 10.1016/j.jbiomech.2015.03.012. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omens JH, Fung YC. Residual strain in rat left ventricle. Circ. Res. 1990;66:37–45. doi: 10.1161/01.res.66.1.37. [DOI] [PubMed] [Google Scholar]
- 51.Lanir Y. Mechanisms of residual stress in soft tissues. J. Biomech. Eng. 2009;131(4):044506. doi: 10.1115/1.3049863. [DOI] [PubMed] [Google Scholar]
- 52.Lanir Y, Hayam G, Abovsky M, Zlotnick AY, Uretzky G, Nevo E, Ben-Haim SA. Effect of myocardial swelling on residual strain in the left ventricle of the rat. Am. J. Physiol. 1996;270(5 Pt 2):H1736–H1743. doi: 10.1152/ajpheart.1996.270.5.H1736. [DOI] [PubMed] [Google Scholar]
- 53.Lin IE, Taber LA. A model for stress-induced growth in the developing heart. J. Biomech. Eng. 1995;117(3):343–349. doi: 10.1115/1.2794190. [DOI] [PubMed] [Google Scholar]
- 54.Taber LA, Chabert S. Theoretical and experimental study of growth and remodeling in the developing heart. Biomech. Model. Mechanobiol. 2002;1(1):29–43. doi: 10.1007/s10237-002-0002-3. [DOI] [PubMed] [Google Scholar]
- 55.Taber LA. Towards a unified theory for morphomechanics. Philos. Trans. A. Math. Phys. Eng. Sci. 2009;367(1902):3555–3583. doi: 10.1098/rsta.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kerckhoffs RCP. Computational modeling of cardiac growth in the post-natal rat with a strain-based growth law. J. Biomech. 2012;45(5):865–871. doi: 10.1016/j.jbiomech.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kroon W, Delhaas T, Arts T, Bovendeerd P. Computational modeling of volumetric soft tissue growth: application to the cardiac left ventricle. Biomech. Model. Mechanobiol. 2009;8(4):301–309. doi: 10.1007/s10237-008-0136-z. [DOI] [PubMed] [Google Scholar]
- 58.Taber LA. Biomechanics of Growth, Remodeling, and Morphogenesis. Appl. Mech. Rev. 1995;48(8):487–545. [Google Scholar]
- 59.Göktepe S, Abilez OJ, Parker KK, Kuhl E. A multiscale model for eccentric and concentric cardiac growth through sarcomerogenesis. J. Theor. Biol. 2010;265(3):433–442. doi: 10.1016/j.jtbi.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 60.Lee LC, Genet M, Acevedo-Bolton G, Ordovas K, Guccione JM, Kuhl E. A computational model that predicts reverse growth in response to mechanical unloading. Biomech. Model. Mechanobiol. 2015;14:217–229. doi: 10.1007/s10237-014-0598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee LC, Sundnes J, Genet M, Wenk JF, ST W. An Integrated Electromechanical-Reversible Growth Heart Model for Simulating Cardiac Therapies. Biomech. Model. Mechanobiol. 2015 doi: 10.1007/s10237-015-0723-8. (In press) [DOI] [PubMed] [Google Scholar]
- 62.Kerckhoffs RCP, Omens J, McCulloch AD. A single strain-based growth law predicts concentric and eccentric cardiac growth during pressure and volume overload. Mech. Res. Commun. 2012;42:40–50. doi: 10.1016/j.mechrescom.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Emery JL, Omens JH. Mechanical regulation of myocardial growth during volume-overload hypertrophy in the rat. Am. J. Physiol. 1997;273(3 Pt 2):H1198–H1204. doi: 10.1152/ajpheart.1997.273.3.H1198. [DOI] [PubMed] [Google Scholar]
- 64.Guterl KA, Haggart CR, Janssen PM, Holmes JW. Isometric contraction induces rapid myocyte remodeling in cultured rat right ventricular papillary muscles. Am. J. Physiol. Heart Circ. Physiol. 2007;293(6):H3707–H3712. doi: 10.1152/ajpheart.00296.2007. [DOI] [PubMed] [Google Scholar]
- 65.Weber KT, Anversa P, Armstrong PW, Brilla CG, Burnett JC, Cruickshank JM, Devereux RB, Giles TD, Korsgaard N, Leier CV, Mendelsohn FAO, Motz WH, Mulvany MJ, Strauer BE. Remodeling and Raparation of the Cardiovascular System. J. Am. Coll. Cardiol. 1992;20(1):3–16. doi: 10.1016/0735-1097(92)90130-f. [DOI] [PubMed] [Google Scholar]
- 66.Rain S, Handoko L, Trip P, Gan T-J, Westerhof N, Stienen G, Paulus W, Ottenheijm C, Marcus T, Dorfmüller P, Guignabert C, Humbert M, MacDonald P, dos Remedios C, Postmus P, Saripalli C, Hidalgo C, Granzier H, Vonk-Noordegraaf A, van der Velden J, de Man F. Right Ventricular Diastolic Impairment in Patients With Pulmonary Arterial Hypertension. Circulation. 2013;128:2016–2025. doi: 10.1161/CIRCULATIONAHA.113.001873. doi: [DOI] [PubMed] [Google Scholar]
- 67.Shimkunas R, Makwana O, Spaulding K, Bazargan M, Khazalpour M, Takaba K, Soleimani M, Myagmar B-E, Lovett DH, Simpson PC, Ratcliffe MB, Baker AJ. Myofilament dysfunction contributes to impaired myocardial contraction in the infarct border zone. AJP Hear. Circ. Physiol. 2014;307:H1150–H1158. doi: 10.1152/ajpheart.00463.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Humphrey JD, Rajagopal KR. A Constrained Mixture Model for Growth and Remodeling of Soft Tissues. Math. Model. Methods Appl. Sci. 2002;12:407–430. [Google Scholar]
- 69.Baek S, Rajagopal KR, Humphrey JD. A Theoretical Model of Enlarging Intracranial Fusiform Aneurysms. J. Biomech. Eng. 2006;128(1):142. doi: 10.1115/1.2132374. [DOI] [PubMed] [Google Scholar]
- 70.Valentín A, Holzapfel GA. Constrained mixture models as tools for testing competing hypotheses in arterial biomechanics: A brief survey. Mech. Res. Commun. 2012;42:126–133. doi: 10.1016/j.mechrescom.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gleason RL, Humphrey JD. Effects of a sustained extension on arterial growth and remodeling: A theoretical study. J. Biomech. 2005;38:1255–1261. doi: 10.1016/j.jbiomech.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 72.Ateshian GA, Humphrey JD. Continuum mixture models of biological growth and remodeling: past successes and future opportunities. Annu. Rev. Biomed. Eng. 2012;14:97–111. doi: 10.1146/annurev-bioeng-071910-124726. [DOI] [PubMed] [Google Scholar]
- 73.Göktepe S, Abilez OJ, Parker KK, Kuhl E. A multiscale model for eccentric and concentric cardiac growth through sarcomerogenesis. J. Theor. Biol. 2010;265(3):433–442. doi: 10.1016/j.jtbi.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 74.Litwin SE, Katz SE, Weinberg EO, Lorell BH, Aurigemma GP, Douglas PS. Serial echocardiographic-Doppler assessment of left ventricular geometry and function in rats with pressure-overload hypertrophy. Chronic angiotensin-converting enzyme inhibition attenuates the transition to heart failure. Circulation. 1995;91(10):2642–2654. doi: 10.1161/01.cir.91.10.2642. [DOI] [PubMed] [Google Scholar]
- 75.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Invest. 1975;56(1):56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nediani C, Formigli L, Perna AM, Ibba-Manneschi L, Zecchi-Orlandini S, Fiorillo C, Ponziani V, Cecchi C, Liguori P, Fratini G, Nassi P. Early changes induced in the left ventricle by pressure overload. An experimental study on swine heart. J. Mol. Cell. Cardiol. 2000;32(1):131–142. doi: 10.1006/jmcc.1999.1060. [DOI] [PubMed] [Google Scholar]
- 77.Aoyagi T, Mirsky I, Flanagan MF, Currier JJ, Colan SD, Fujii AM. Myocardial function in immature and mature sheep with pressure-overload hypertrophy. Am. J. Physiol. 1992;262(4 Pt 2):H1036–H1048. doi: 10.1152/ajpheart.1992.262.4.H1036. [DOI] [PubMed] [Google Scholar]
- 78.Laviolle B, Pape D, Verdier M-C, Lavenu A, Bellissant E. Hemodynamic and histomorphometric characteristics of heart failure induced by aortic stenosis in the guinea pig: comparison of two constriction sizes. Can. J. Physiol. Pharmacol. 2009;87(11):908–914. doi: 10.1139/y09-083. [DOI] [PubMed] [Google Scholar]
- 79.Schmid-Nielson K. Scaling: Why Is Animal Size so Important? 1st. Cambridge University Press; 1984. [Google Scholar]
- 80.Anversa P, Olivetti G. Cellular basis of physiological and pathological myocardial growth. Compr. Physiol. 2011:75–144. [Google Scholar]
- 81.Stone M. Cross-Validatory Choice and Assessment of Statistical Predictions. J. R. Stat. Soc. Ser. B. 1974;36:111–147. [Google Scholar]
- 82.Klipp E, Herwig R, Kowald A, Wierling C, Lehrach H. Systems Biology in Practice: Concepts, Implementation and Application. 2005 [Google Scholar]
- 83.Lorenz EN. The Essence of Chaos. Am. J. Phys. 1995;63(9):862. [Google Scholar]
- 84.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J, St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117(20):2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 85.Prinzen FW, Cheriex EC, Delhaas T, Van Oosterhout MFM, Arts T, Wellens HJJ, Reneman RS. Asymmetric thickness of the left ventricular wall resulting from asynchronous electric activation: A study in dogs with ventricular pacing and in patients with left bundle branch block. Am. Heart J. 1995;130:1045–1053. doi: 10.1016/0002-8703(95)90207-4. [DOI] [PubMed] [Google Scholar]
- 86.Kerckhoffs RCP, Lumens J, Vernooy K, Omens JH, Mulligan LJ, Delhaas T, Arts T, McCulloch AD, Prinzen FW. Cardiac resynchronization: Insight from experimental and computational models. Prog. Biophys. Mol. Biol. 2008;97:543–561. doi: 10.1016/j.pbiomolbio.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cirino AL. Familial Hypertrophic Cardiomyopathy Overview. GeneReviews. 2008 [Google Scholar]
- 88.Sandler H, Dodge HT. Left Ventricular Tension and Stress in Man. Circ. Res. 1971;XIII(2):437–445. doi: 10.1161/01.res.13.2.91. [DOI] [PubMed] [Google Scholar]
- 89.Falsetti HL, Mates RE, Grant C, Greene DG, Bunnell IL. Left Ventricular Wall Stress Calculated from One-Plane Cineangiography: an approach to force-velocity in man. Circ. Res. 1970;26(1):71–83. doi: 10.1161/01.res.26.1.71. [DOI] [PubMed] [Google Scholar]
- 90.Yin FC. Ventricular wall stress. Circ. Res. 1981;49(4):829–842. doi: 10.1161/01.res.49.4.829. [DOI] [PubMed] [Google Scholar]
- 91.Wilson JS, Baek S, Humphrey JD. Parametric study of effects of collagen turnover on the natural history of abdominal aortic aneurysms. Proc. Math. Phys. Eng. Sci. 2013;469:20120556. doi: 10.1098/rspa.2012.0556. [DOI] [PMC free article] [PubMed] [Google Scholar]