Abstract
INTRODUCTION
The objective of our study was to investigate cross-sectional associations of atrial fibrillation with neuroimaging measures of cerebrovascular disease and Alzheimer’s disease, and their interactions with mild cognitive impairment (MCI).
METHODS
MRI scans of individuals from a population-based study were analyzed for infarctions, total grey matter, hippocampal and white matter hyperintensity (WMH) volumes. A subsample underwent PET imaging.
RESULTS
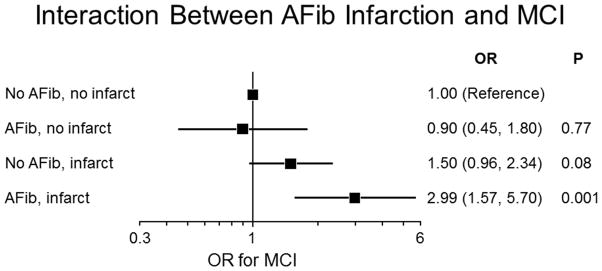
Atrial fibrillation was associated with infarctions and lower total grey matter volume. Compared to subjects with no atrial fibrillation and no infarction, the odds ratio (95% confidence intervals) for MCI was 2.99 (1.57, 5.70; p = 0.001) among participants with atrial fibrillation and infarction, 0.90 (0.45, 1.80; p= 0.77) for atrial fibrillation and no infarction, and 1.50 (0.96, 2.34; p= 0.08) for no atrial fibrillation and any infarction.
DISCUSSION
Participants with both atrial fibrillation and infarction are more likely to have MCI than participants with either infarction or atrial fibrillation alone.
Keywords: Atrial fibrillation, mild cognitive impairment, stroke, Alzheimer’s disease, cerebrovascular disease
1. Introduction
The lifetime prevalence of atrial fibrillation among individuals age 40 and older is approximately 25% [1]. The prevalence of dementia among those over 71 is approximately 13.9% [2]; (10% in Mayo Clinic Study of Aging [MCSA] [3]. Both atrial fibrillation and dementia increase with age. Numerous studies have reported an association between atrial fibrillation and dementia [4, 5] or cognitive impairment[6–8]. The association remains after controlling for both a history of clinical stroke [8–10] and shared risk factors of atrial fibrillation and dementia. A number of mechanisms have been postulated for this independent association including cerebral hypoperfusion [11, 12], silent infarction [5], and even Alzheimer’s disease [13]. Similar to prior studies, the MCSA reported that atrial fibrillation was associated with mild cognitive impairment (MCI), specifically non-amnestic MCI [14]. The objective of this study was to investigate the cross-sectional associations of atrial fibrillation with multimodality neuroimaging measures of cerebrovascular disease and Alzheimer’s disease (AD)–related pathology, and their interactions with regard to MCI.
2. Methods
2.1 Study Participants
Participants were enrolled in the MCSA, a longitudinal population based study designed to investigate predictors of MCI and dementia. The study design of the MCSA has been published previously [15]. Briefly, at the study onset, Olmsted County residents aged 70–89 years were identified using the Rochester Epidemiology Project medical records-linkage system. An age and sex stratified sampling strategy was used to randomly select non-demented subjects for participation in the study. In 2005, participants were invited to undergo MRI imaging. In 2006, participants were invited to undergo PET imaging.
2.2 Cognitive evaluation
Participants were evaluated at baseline and every 15 months by a study coordinator, physician, and neuropsychometrist. The neuropsychometrist administered nine tests covering four domains including memory, executive function, language, and visuospatial skills. The diagnosis of MCI was based on published criteria [16]. The diagnosis of MCI was made by a consensus decision by the behavioral neurologist, neuropsychologists, and study coordinator who saw the participant, after consideration of education level, occupation, and sensory impairment (hearing or vision)[15] [3].
2.3 Clinical data acquisition
Clinical data including history of hypertension, smoking, diabetes, dyslipidemia, history of stroke, coronary artery disease factors were obtained by nurse abstraction of information from the detailed medical records included in the medical records–linkage system [17]. Silent infarction was defined as no history of clinical stroke in the medical record or Hachinski score but the presence of stroke on neuroimaging.
2.4 Criteria for atrial fibrillation
Criteria for atrial fibrillation were based on a physician diagnosis, electrocardiographic evidence of atrial fibrillation and/or treatment for atrial fibrillation using the medical records linkage system from the Rochester Epidemiology Project. Using this method, patients with postoperative atrial fibrillation were included. These data were abstracted by trained research nurses.
2.5 MRI Acquisition
FLAIR-MRI and MPRAGE images were acquired with 3T MRI scanners and the complete details of the acquisitions can be found elsewhere [18]. THE MPRAGE (structural MRI) images were used to obtain the total grey matter volume estimates and hippocampal volumes using Freesurfer (version 5.3)[19]and the hippocampal volume was adjusted for total intracranial volume as previously described[20]. The FLAIR-MRI was used to ascertain three components of vascular disease – subcortical infarcts, cortical infarcts and white matter hyperintensities. All the brain infarcts were assessed by a trained image analyst and confirmed by a radiologist (K.K.) blinded to all clinical information. Subcortical infarcts included infarcts in white matter (WM), deep grey matter nuclei, cerebellum, and brain stem not involving the hemispheric infarcts. Cortical infarcts were ≥ 1 cm in largest diameter. Intra-rater reliability of this assessment is excellent (proportion in agreement 0.98 for cortical and 0.94 for subcortical infarcts)[21]. White matter hyperintensities (WMH) on FLAIR images were segmented using an automated slice-based seed initialization and region growing methods as previously described[22]. We used WMH divided by the total intracranial volume (TIV) as a measure of white matter disease.
2.6 18F-FDG PET and 11C-PiB PET Acquisition
PET images were acquired with a PET/CT operating in 3-dimensional mode (septa removed). The complete details of PET acquisition were described previously [23, 24]. The global cortical PiB PET retention ratio was calculated by averaging the PiB retention ratio from AD signature regions normalized to cerebellar uptake[25]. Similarly, an AD signature 18F-FDG PET ratio was calculated for brain glucose metabolism from an AD signature meta-ROI with normalization to pons glucose uptake [26] [27] [28]. We performed the analysis with and without partial volume correction.
2.7 Standard protocol approvals, registrations, and patient consents
Study protocols are approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. All participants provided informed written consent prior to participation in the study protocols.
2.8 Statistical analysis
Differences in characteristics between cases and controls were assessed using chi-square test for dichotomous variables and Wilcoxon Rank Sum test for continuous variables. The associations of atrial fibrillation with i) categorical imaging measures (cortical and subcortical infarctions) were assessed using multivariable logistic regression models, and ii) with continuous imaging measures including FDG-PET, PiB-PET, adjusted hippocampal volumes, and log-transformed continuous MRI measurements (total grey matter, and white matter hyperintensity volumes) were assessed using multivariable linear regression models. White matter hyperintensity was scaled by TIV. All models were adjusted for age, sex, APOE ε4 carrier status and education. To investigate possible biases due to non-participation in neuroimaging, we used the propensity score method as was done in our previous studies[3] [29]. Briefly, we used logistic regression models to estimate the effect of age on neuroimaging participation and estimated a predicted probability of participation (propensity score). We then computed the reciprocal of these predicted probabilities as weights (propensity score); participants who were more similar to non-participants received more weight. The regression models were adjusted for the propensity scores. In additional analyses, we used logistic models to investigate the association between atrial fibrillation and MCI, and examined the interaction between atrial fibrillation and MRI measures with regard to MCI. The basis for the interaction analyses is that the association of atrial fibrillation with cognitive impairment may vary by the presence or absence of existing pathology attributable to infarctions rather than to atrial fibrillation alone. Due to the small sample sizes, we did not have power to investigate interactions of atrial fibrillation and PET with MCI.
3. Results
3.1 Demographics
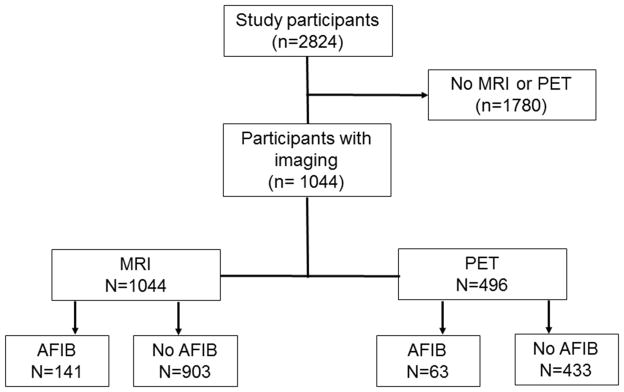
Among 1,044 participants with MRI analyzed, 141 of these had atrial fibrillation, 496 had PET scans analyzed and 63 had atrial fibrillation. Figure 1 represents the flowchart of our study. There were no differences in sex, education, the presence of cognitive impairment or APOE ε4 status among participants with or without MRI scans, with the exception that the group without MRI’s were older (p<.0001).
Figure 1.
Flow chart of study participants
MRI-magnetic resonance imaging, PiB-Pittsburgh compound B, FDG-fluorodeoxyglucose, AFIB-atrial fibrillation
Table 1 summarizes the clinical features of participants who underwent MRI scanning by history of atrial fibrillation. Participants with atrial fibrillation were older, had a higher frequency of type 2 diabetes, hypertension, coronary artery disease, stroke, and cognitive impairment.
Table 1.
Characteristics of participants who underwent MRI by atrial fibrillation status
| No atrial fibrillation | Atrial fibrillation | Total | p-value | |
|---|---|---|---|---|
|
| ||||
| Age at Baseline, median (IQR) | 77.2 (73.6, 82.0) | 80.7 (76.2, 83.3) | 77.8 (73.8, 82.2) | <0.0001 |
|
| ||||
| Education years, median (IQR) | 13 (12, 16) | 13 (12, 16) | 13 (12, 16) | 0.85 |
|
| ||||
| Male, n, (%) | 446 (49.4) | 93 (66.0) | 539 (51.6) | 0.0003 |
|
| ||||
| APOE ε24/ε34/ε44, n, (%)* | 234 (26.1) | 36 (25.5) | 270 (26.0) | 0.89 |
|
| ||||
| Diabetes, n, (%) | 151 (16.7) | 38 (27.0) | 189 (18.1) | 0.003 |
|
| ||||
| Hypertension, n, (%) | 639 (70.8) | 124 (87.9) | 763 (73.1) | <0.0001 |
|
| ||||
| Dyslipidemia, n, (%) | 720 (79.7) | 111 (78.7) | 831 (79.6) | 0.78 |
|
| ||||
| Current smoking, n, (%) | 41 (4.5) | 6 (4.3) | 47 (4.5) | 0.88 |
|
| ||||
| Coronary Artery Disease, n, (%) | 303 (33.6) | 93 (66.0) | 396 (37.9) | <0.0001 |
|
| ||||
| Clinical Stroke, n, (%) | 42 (4.7) | 14 (9.9) | 56 (5.4) | 0.010 |
|
| ||||
| Sleep apnea, n, (%) | 269 (11.5%) | 102 (21.1%) | 371 (13.1%) | <0.0001 |
|
| ||||
| CHADS2 score, median (IQR)^ | 2 (1–2) | 2 (2–3) | 2 (1–2) | <.0001 |
|
| ||||
| No medication, n, (%) | 372 (41.2) | 14 (9.9) | 386 (37.0) | <0.0001 |
| Aspirin only, n, (%) | 516 (57.1) | 74 (52.5) | 590 (56.5) | |
| Coumadin only, n, (%) | 13 (1.4) | 36 (25.5) | 49 (4.7) | |
| Both, n, (%) | 2 (0.2) | 17 (12.1) | 19 (1.8) | |
|
| ||||
| Mild Cognitive Impairment, n, (%) | 127 (14.1) | 33 (23.4) | 160 (15.3) | 0.004 |
6 subjects in no atrial fibrillation group had missing data
CHADS2 score estimates the risk of stroke in atrial fibrillation based on the following risk factors (Congestive heart failure, Hypertension, Age >75 years, Diabetes mellitus, Stroke/transient ischemic attack)
The median time from MRI to PET was 39.9 months.
3.2 Association of neuroimaging features with atrial fibrillation
Table 2 summarizes the associations of baseline neuroimaging features with atrial fibrillation. Among participants who underwent MRI (median age, 77.8, 51.6% male), 13.5% had atrial fibrillation. Of those with atrial fibrillation, 30.4% had silent infarcts. Among those who underwent PET imaging (median age, 76.1, 54.6% male), 12.7% had atrial fibrillation. Presence of atrial fibrillation was associated with infarction odds ratio [OR], 1.87; p=0.002), subcortical infarctions (odds ratio [OR], 1.85; p=0.003), and lower total grey matter volume (Beta [β], −0.022, p=0.001) after controlling for age, education, sex, APOE e4 carrier status, coronary artery disease, diabetes, history of clinical stroke, and hypertension. There was a trend for cortical infarctions (OR, 1.68; p=0.11). Since infarctions may influence estimates for the total grey matter volume, we performed a sensitivity analysis that investigated the association of atrial fibrillation with total grey matter volume restricted to subjects with no infarctions and found that atrial fibrillation remained associated lower gray matter volume ([β], −0.03, p=0.001). However, atrial fibrillation was not associated with white matter hyperintensity volume, hippocampal volume, Alzheimer’s pattern of FDG hypometabolism or PiB uptake (β-amyloid accumulation) with or without partial volume correction. With partial volume correction, FDG hypometabolism was not associated with atrial fibrillation for model 1 (Beta [β] = 0.023, p=0.09) but the association was significant for model 2 (Beta [β] = 0.03, p=0.02).
Table 2.
Association of neuroimaging features with atrial fibrillation
| Estimates of association | ||||
|---|---|---|---|---|
| Dependent | Beta Estimate (β) | Standard error | 95% CI | P value |
| Total Grey Matter volume (1037) | ||||
| Model 1 | −0.027 | 0.007 | (−0.040, −0.014) | <.0001 |
| Model 2 | −0.022 | 0.007 | (−0.036, −0.009) | 0.001 |
| Hippocampal volume (1038) | ||||
| Model 1 | −0.121 | 0.070 | (−0.258, 0.017) | 0.09 |
| Model 2 | −0.125 | 0.072 | (−0.267, 0.016) | 0.08 |
| WMH/TIV (959) | ||||
| Model 1 | 0.132 | 0.065 | (0.005, 0.259) | 0.04 |
| Model 2 | 0.119 | 0.066 | (−0.011, 0.249) | 0.07 |
| FDG AD ROI (494) | ||||
| Model 1 | −0.0001 | 0.016 | (−0.031, 0.031) | 0.99 |
| Model 2 | 0.006 | 0.016 | (−0.026, 0.038) | 0.70 |
| PIB global Ratio (495) | ||||
| Model 1 | −0.038 | 0.029 | (−0.094, 0.019) | 0.19 |
| Model 2 | −0.043 | 0.030 | (−0.102, 0.015) | 0.15 |
| Cortical Infarction (60/975) | Estimate | Odds ratio | 95% CI | p-value |
|---|---|---|---|---|
| Model 1 | 0.72 | 2.05 | (1.13, 3.73) | 0.02 |
| Model 2 | 0.52 | 1.68 | (0.89, 3.17) | 0.11 |
| Subcortical infarction (206/975) | ||||
| Model 1 | 0.60 | 1.83 | (1.23, 2.72) | 0.003 |
| Model 2 | 0.62 | 1.85 | (1.23, 2.80) | 0.003 |
| Any infarction (233/975) | ||||
| Model 1 | 0.62 | 1.86 | (1.27, 2.74) | 0.002 |
| Model 2 | 0.63 | 1.87 | (1.25, 2.81) | 0.002 |
Model 1: adjusted for age, sex, education, and APOE ε4 status.
Model 2: adjusted for age, sex, education, APOE ε4 status, hypertension, diabetes mellitus, history of clinical stroke, coronary artery disease.
Total number of scans analyzed for the continuous variables in parenthesis
Proportion with infarction for categorical variables in parenthesis
3.3 Relationship between neuroimaging, cognitive impairment and atrial fibrillation
There was an interaction of cortical infarction (p for interaction=0.04) and a trend for interaction of subcortical infarction (p for interaction =0.08) with atrial fibrillation with regards to relative odds of MCI in multivariate models adjusting for age, sex, education, APOE ε4 status, hypertension, diabetes mellitus, history of clinical stroke, coronary artery disease. There was no interaction of total grey matter volume with atrial fibrillation with regards to odds of MCI (p for interaction= 0.49). The OR (95% confidence intervals [CI]) for MCI was 2.99 (1.57, 5.70; p = 0.001) among participants with atrial fibrillation and any infarction, 0.90 (0.45, 1.80; p= 0.77) for atrial fibrillation and no infarction, and 1.50 (0.96, 2.34; p= 0.08) for no atrial fibrillation and any infarction (Figure 2). There was a significant interaction of any infarction with atrial fibrillation with regards to odds of MCI (p for interaction = 0.004). The OR (95% confidence intervals [CI]) for MCI was 6.91 (2.32, 20.61; p = 0.0005) among participants with atrial fibrillation and cortical infarction, 1.15 (0.67, 1.94; p= 0.62) for atrial fibrillation and no cortical infarction, and 1.43 (0.65, 3.12; p= 0.37) for no atrial fibrillation and any cortical infarction. The OR (95% confidence intervals [CI]) for MCI was 3.05 (1.58, 5.90; p = 0.0009) among participants with atrial fibrillation and subcortical infarction, 0.90 (0.46, 1.76; p= 0.76) for atrial fibrillation and no subcortical infarction, and 1.43 (0.89, 2.27; p= 0.14) for no atrial fibrillation and any subcortical infarction. There was no significant association of atrial fibrillation with MCI among the MRI group (OR, 1.38, 95% CI, 0.87, 2.16; p=0.17).
Figure 2.
Interaction between Atrial fibrillation, infarction and MCI
The introduction of sleep apnea into the multivariate model did not significantly change the odds of MCI and sleep apnea was not significantly associated with MCI (p=0.89) (even though it is significantly associated with atrial fibrillation).
3.3. Association of atrial fibrillation and infarction by PET status
There was an association of atrial fibrillation with infarction among those that were PiB positive (p=0.004), adjusting for age, sex, education, APOE ε4 status, hypertension, diabetes mellitus, history of clinical stroke, and coronary artery disease. There was no association of atrial fibrillation with infarction among those that were PiB negative (p= 0.59).
There was a trend for an association of atrial fibrillation with infarction among those with Alzheimer’s pattern of FDG hypometabolism adjusting for age, sex, education, APOE ε4 status, hypertension, diabetes mellitus, history of clinical stroke, and coronary artery disease (p=0056) and those without and Alzheimer’s pattern of FDG PET hypometabolism (p=0.07).
4. Discussion
In our cohort, atrial fibrillation was associated with lower total grey matter volume and the presence of infarctions. This association remained significant after controlling for age, APOE and vascular risk factors including history of clinical stroke. In contrast, atrial fibrillation was not associated with hippocampal volume, white matter hyperintensity, PiB uptake (amyloid burden), or an AD pattern of FDG hypometabolism. Thus, the main finding of our study was that atrial fibrillation with infarction (cortical and subcortical) was associated with increased odds of cognitive impairment compared to atrial fibrillation without infarction and infarction without atrial fibrillation.
Epidemiologic studies have attempted to control for strokes by excluding participants with prevalent and/or incident stroke, but the association between cognitive impairment and atrial fibrillation persisted[8, 10]. However, these studies only excluded clinical strokes. Our results show that the association of atrial fibrillation with MCI varies by the presence of silent (subclinical) infarction since we controlled for a history of clinical stroke. The association between silent infarction and MCI is not surprising since silent infarctions are common in atrial fibrillation [30–32] and numerous studies have demonstrated silent infarctions are a risk factor for dementia[33]. Since the risk of infarction in atrial fibrillation can be reduced by the use of anticoagulation, this association is clinically relevant.
The interaction between atrial fibrillation, infarction, and MCI may help us understand the mechanisms that underlie the association of atrial fibrillation with cognitive impairment. The synergistic relationship between infarction and atrial fibrillation may result from microemboli that occur with atrial fibrillation [12, 34]. The presence of cortical and subcortical infarction is associated with concomitant microinfarctions on pathological studies [35, 36]. Thus, microemboli and microinfarctions may explain why patients with atrial fibrillation and visible infarctions on MRI have a greater risk of cognitive impairment than patients with visible infarctions on MRI but no atrial fibrillation.
We did not find an association between hippocampal volumes and atrial fibrillation. Because of the number of participants with atrial fibrillation and MRI, we may have been underpowered to detect a significant association with hippocampal volume. However, a prior study of subjects with atrial fibrillation without evidence of infarctions on MRI reported that atrial fibrillation was associated with lower hippocampal volumes [37]. In that study, the average age was more than 10 years younger than our study cohort and the control group differed significantly in age, sex, and education, which were included as covariates. These study design differences may explain the differences between the two results. In the present study, we found no interaction between hippocampal volumes and atrial fibrillation in regards to odds of cognitive impairment.
In our cohort there was no association between atrial fibrillation and white matter hyperintensity volume. This is in agreement with prior studies [11]. The absence of a relationship between white matter hyperintensities and atrial fibrillation suggests that the embolic mechanism causing infarction must be different from the pathophysiological mechanisms underlying white matter hyperintensities and lacunar stroke[38], which may be more closely linked to lipohyalinosis.
Atrial fibrillation was not associated with amyloid deposition or AD pattern of FDG PET hypometabolism. This finding, in combination with the absence of an association with hippocampal volumes, would suggest that atrial fibrillation promotes cognitive decline independently of Alzheimer’s pathophysiology. Atrial fibrillation likely decreases the threshold for dementia expression in the presence of Alzheimer’s pathophysiology through the additive effect of vascular pathology[39].
The association between grey matter volume and atrial fibrillation has been previously reported [11]. Prior studies in younger participants (mean age 62)[40] did not detect differences in grey matter volume in participants with vs. without atrial fibrillation[37], suggesting that longer duration or earlier onset of atrial fibrillation may be an important determinant of its effects on grey matter volume. A longer exposure period to the effects of atrial fibrillation would logically produce greater neuronal injury, neuronal loss, and reduced grey matter volumes [34, 41]. The relationship was not dependent on the presence of macro-infarcts because atrial fibrillation was still associated with decreased grey matter volume even when subjects with infarction were excluded from the analysis.
Interestingly, subcortical infarctions were associated with atrial fibrillation and the presence of subcortical and cortical strokes with atrial fibrillation increased the odds of cognitive impairment. Typically, subcortical strokes are secondary to small vessel disease (mainly lipohyalinosis), rather than embolism. Even after controlling for typical risk factors of small vessel disease such as diabetes and hypertension, the association persisted, suggesting a possible embolic source for some subcortical infarcts. This finding is congruent with a recent MRI study that demonstrated “silent cerebral ischemia (SCI)”, defined as sharply outlined areas of hyperintensity on FLAIR imaging, to be associated with atrial fibrillation and cognitive impairment even after controlling for vascular risk factors[42].
Current guidelines for initiating anticoagulation use the CHADS2 [43] or the CHA2DS2-VASc[44] scoring systems which incorporate points for clinical stroke history and have been validated to predict future clinical stroke risk. However, these scoring systems, and the clinical decisions based on them, fail to take into account silent brain infarctions. Silent infarction is present in 15% of atrial fibrillation subjects using non-contrast head CT[30], a less sensitive imaging modality. More recent estimates suggest that silent infarction may occur in up to 28.3% of atrial fibrillation subjects using MRI, similar to the numbers in our study, and silent infarction predicts future stroke risk[45]. Furthermore, 23.1% of these atrial fibrillation subjects with silent stroke have a CHADS2 score of 0 or 1 when the silent infarction is not considered for the grading. Thus, a substantial proportion of patients at risk for cognitive decline are likely being undertreated. In our sample, approximately 53% of those with atrial fibrillation were on anticoagulation which is similar to other large atrial fibrillation samples [46].
Recent evidence suggests that increased time outside the therapeutic window (INR: 2–3) for anticoagulation increases the risk for dementia[47]. In this cross-sectional study, we were unable to assess the quality of anticoagulation with regards to cognitive impairment because INR values were not available but it would be of interest to determine whether the association is independent of infarction.
The strengths of the current study include the comprehensive in-person cognitive evaluations and use of a multi-modality imaging approach to identify neuroimaging abnormalities in a population-based sample. Our study however has some limitations. The cross-sectional nature of the study limits our ability to investigate the temporal relationships among atrial fibrillation, infarction and cognitive status. In addition, we could not examine which imaging features predict future cognitive decline or the trajectory of neuroimaging changes after a diagnosis of atrial fibrillation. Longitudinal studies will be needed to determine whether anticoagulation or dual therapy with antiplatelet agents is associated with the risk of cognitive impairment. Similar to other studies, our method of ascertaining atrial fibrillation likely underestimates cases of paroxysmal atrial fibrillation which are often asymptomatic. Additional individuals may have developed atrial fibrillation after inclusion in the study or may harbor undetected paroxysmal atrial fibrillation only recognizable by prolonged ambulatory monitoring [48]. We did not distinguish which patients had persistent versus paroxysmal atrial fibrillation. However, stroke risk is similar in persistent and paroxysmal atrial fibrillation [49] so this likely did not affect our findings. It would have been of interest to investigate the effect of duration of atrial fibrillation as well as echocardiogram features on cognitive, but our ascertainment method could not accurately record duration and echocardiograms were not performed as part of this study. In this study, we did not find an association of atrial fibrillation with cognitive impairment among patients with MRI, despite the association of atrial fibrillation with nonamnestic mild cognitive impairment in patients with and without MRI in a previous publication [14]. This may be due to limited power or alternatively, to non-participation bias due to a higher prevalence of silent infarctions among participants with atrial fibrillation and without MRI than in patients with atrial fibrillation and MRI. A larger number of participants with naMCI would have allowed us to examine the association with non-amnestic MCI.
5. Conclusion
In summary, these data highlight that presence of both atrial fibrillation and infarctions is associated with a greater odds of MCI compared to atrial fibrillation alone. Therefore, our study suggests that subclinical infarctions (both cortical and subcortical) may mediate cognitive impairment in patients with atrial fibrillation. While the influence of other mechanisms (such as inflammation, endothelial dysfunction, hypoperfusion) still need to be explored since atrial fibrillation is associated with lower grey matter volume even in the absence of visible infarctions on MRI, our cross-sectional findings do not support the notion that atrial fibrillation increases the likelihood of MCI in the absence of brain infarctions. However, our study does suggest that Alzheimer pathology is not associated with atrial fibrillation, but certainly the additive effects of multiple pathologies will logically decrease the threshold of dementia. While differences in the distribution, size, and number of infarctions may explain the increased risk of MCI in patients with infarctions and atrial fibrillation compared to infarctions and no atrial fibrillation, the most important practical implication of our study is that anticoagulation may reduce the risk of infarctions and thereby, confer substantial protection against cognitive decline related to AF, though the extent of this protection needs to be investigated formally through longitudinal studies.
Acknowledgments
Funding:
This work was supported in part by grants U01 AG06786 (Mayo Clinic Study of Aging) and P50 AG016574 (Mayo Alzheimer Center) and by funds from the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program.
Footnotes
Disclosures:
Jonathan Graff-Radford -None
Rosebud Roberts-None
Malini Madhavan-None
Prashanthi Vemuri-receives research support from the NIH (R00 AG037573, P50 AG016574)
Alejandro A. Rabinstein- None
Ruth Cha-None
Michelle Mielke- MMM has provided consulting services for Abbvie and Lilly and receives research support from the NIH (U01 AG06786, U01 37526) and the Michael J. Fox Foundation
Kejal Kantarci- KK serves on the data safety monitoring boards for Pfizer Inc. and Jannsen Alzheimer’s Immunothrapy, Takeda Global Research & Development Center, Inc.; and receives research support from the NIH (R01-AG40042, P50-AG016574, P50-AG44170, U19-AG10483, U01-AG04279) and the MN Partnership for Biotechnology and Medical Genomics
Val Lowe- VJL is a consultant for Bayer Schering Pharma and Piramal Imaging and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, the NIH, the Elsie and Marvin Dekelboum Family Foundation, the MN Partnership for Biotechnology and Medical Genomics, and the Leukemia and Lymphoma Society.
Matthew L. Senjem-None
Jeffrey Gunter-None
David S. Knopman- DSK serves as Deputy Editor for Neurology®; serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the Dominantly Inherited Alzheimer’s Disease Treatment Unit. He is an investigator in clinical trials sponsored by TauRX; and receives research support from the NIH (R01-AG11378, P50 AG16574, U01 AG06786, AG 29550, AG32306, and U01 96917).
Ronald C. Petersen- RCP serves on scientific advisory boards for Elan Pharmaceuticals, Wyeth Pharmaceuticals, and GE Healthcare, and receives research support from the NIH (P50-AG16574, U01- AG06786, R01-AG11378, and U01–24904).
Clifford R. Jack Jr- CRJ has provided consulting services for Janssen Research and Development and Eli Lily. He receives research funding from the US National Institutes of Health (NIH; R01-AG011378, U01- HL096917, U01-AG024904, RO1 AG041851, R01 AG37551, R01AG043392, and U01-AG06786) and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–32. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75:889–97. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer’s dementia. Heart Rhythm. 2010;7:433–7. doi: 10.1016/j.hrthm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. Stroke. 1997;28:316–21. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- 6.O’Connell JE, Gray CS, French JM, Robertson IH. Atrial fibrillation and cognitive function: case-control study. J Neurol Neurosurg Psychiatry. 1998;65:386–9. doi: 10.1136/jnnp.65.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilander L, Andren B, Nyman H, Lind L, Boberg M, Lithell H. Atrial fibrillation is an independent determinant of low cognitive function: a cross-sectional study in elderly men. Stroke. 1998;29:1816–20. doi: 10.1161/01.str.29.9.1816. [DOI] [PubMed] [Google Scholar]
- 8.Thacker EL, McKnight B, Psaty BM, Longstreth WT, Jr, Sitlani CM, Dublin S, et al. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology. 2013;81:119–25. doi: 10.1212/WNL.0b013e31829a33d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marzona I, O’Donnell M, Teo K, Gao P, Anderson C, Bosch J, et al. Increased risk of cognitive and functional decline in patients with atrial fibrillation: results of the ONTARGET and TRANSCEND studies. Cmaj. 2012;184:E329–36. doi: 10.1503/cmaj.111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dublin S, Anderson ML, Haneuse SJ, Heckbert SR, Crane PK, Breitner JC, et al. Atrial fibrillation and risk of dementia: a prospective cohort study. J Am Geriatr Soc. 2011;59:1369–75. doi: 10.1111/j.1532-5415.2011.03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefansdottir H, Arnar DO, Aspelund T, Sigurdsson S, Jonsdottir MK, Hjaltason H, et al. Atrial fibrillation is associated with reduced brain volume and cognitive function independent of cerebral infarcts. Stroke. 2013;44:1020–5. doi: 10.1161/STROKEAHA.12.679381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabatini T, Frisoni GB, Barbisoni P, Bellelli G, Rozzini R, Trabucchi M. Atrial fibrillation and cognitive disorders in older people. J Am Geriatr Soc. 2000;48:387–90. doi: 10.1111/j.1532-5415.2000.tb04695.x. [DOI] [PubMed] [Google Scholar]
- 13.Goette A, Braun-Dullaeus RC. Atrial fibrillation is associated with impaired cognitive function and hippocampal atrophy: silent cerebral ischaemia vs. Alzheimer’s disease? Eur Heart J. 2008;29:2067–9. doi: 10.1093/eurheartj/ehn343. [DOI] [PubMed] [Google Scholar]
- 14.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. Cardiac disease associated with increased risk of nonamnestic cognitive impairment: stronger effect on women. JAMA Neurol. 2013;70:374–82. doi: 10.1001/jamaneurol.2013.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 17.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–68. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 20.Jack CR, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, et al. Age-specific population frequencies of cerebral ?-amyloidosis and neurodegeneration among people with normal cognitive function aged 50?89 years: a cross-sectional study. The Lancet Neurology. 2014;13:997–1005. doi: 10.1016/S1474-4422(14)70194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantarci K, Petersen RC, Przybelski SA, Weigand SD, Shiung MM, Whitwell JL, et al. Hippocampal volumes, proton magnetic resonance spectroscopy metabolites, and cerebrovascular disease in mild cognitive impairment subtypes. Arch Neurol. 2008;65:1621–8. doi: 10.1001/archneur.65.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raz L, Jayachandran M, Tosakulwong N, Lesnick TG, Wille SM, Murphy MC, et al. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology. 2013;80:911–8. doi: 10.1212/WNL.0b013e3182840c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knopman DS, Jack CR, Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe V, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78:1576–82. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowe VJ, Kemp BJ, Jack CR, Jr, Senjem M, Weigand S, Shiung M, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med. 2009;50:878–86. doi: 10.2967/jnumed.108.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–18. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–9. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–8. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kessler RC, Little RJ, Groves RM. Advances in strategies for minimizing and adjusting for survey nonresponse. Epidemiol Rev. 1995;17:192–204. doi: 10.1093/oxfordjournals.epirev.a036176. [DOI] [PubMed] [Google Scholar]
- 30.Ezekowitz MD, James KE, Nazarian SM, Davenport J, Broderick JP, Gupta SR, et al. Silent cerebral infarction in patients with nonrheumatic atrial fibrillation. The Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. Circulation. 1995;92:2178–82. doi: 10.1161/01.cir.92.8.2178. [DOI] [PubMed] [Google Scholar]
- 31.Chen LY, Lopez FL, Gottesman RF, Huxley RR, Agarwal SK, Loehr L, et al. Atrial Fibrillation and Cognitive Decline-The Role of Subclinical Cerebral Infarcts: The Atherosclerosis Risk in Communities Study. Stroke. 2014 doi: 10.1161/STROKEAHA.114.005243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalantarian S, Ay H, Gollub RL, Lee H, Retzepi K, Mansour M, et al. Association Between Atrial Fibrillation and Silent Cerebral Infarctions: A Systematic Review and Meta-analysis. Ann Intern Med. 2014;161:650–8. doi: 10.7326/M14-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–22. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 34.Georgiadis D, Lindner A, Manz M, Sonntag M, Zunker P, Zerkowski HR, et al. Intracranial microembolic signals in 500 patients with potential cardiac or carotid embolic source and in normal controls. Stroke. 1997;28:1203–7. doi: 10.1161/01.str.28.6.1203. [DOI] [PubMed] [Google Scholar]
- 35.Raman MR, Preboske GM, Przybelski SA, Gunter JL, Senjem ML, Vemuri P, et al. Antemortem MRI findings associated with microinfarcts at autopsy. Neurology. 2014;82:1951–8. doi: 10.1212/WNL.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 37.Knecht S, Oelschlager C, Duning T, Lohmann H, Albers J, Stehling C, et al. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J. 2008;29:2125–32. doi: 10.1093/eurheartj/ehn341. [DOI] [PubMed] [Google Scholar]
- 38.Rost NS, Rahman RM, Biffi A, Smith EE, Kanakis A, Fitzpatrick K, et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology. 2010;75:1670–7. doi: 10.1212/WNL.0b013e3181fc279a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol. 2008;64:168–76. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004;63:1591–9. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 41.Kumral E, Balkir K, Uzuner N, Evyapan D, Nalbantgil S. Microembolic signal detection in patients with symptomatic and asymptomatic lone atrial fibrillation. Cerebrovasc Dis. 2001;12:192–6. doi: 10.1159/000047703. [DOI] [PubMed] [Google Scholar]
- 42.Gaita F, Corsinovi L, Anselmino M, Raimondo C, Pianelli M, Toso E, et al. Prevalence of silent cerebral ischemia in paroxysmal and persistent atrial fibrillation and correlation with cognitive function. J Am Coll Cardiol. 2013;62:1990–7. doi: 10.1016/j.jacc.2013.05.074. [DOI] [PubMed] [Google Scholar]
- 43.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. Jama. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 44.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 45.Cha MJ, Park HE, Lee MH, Cho Y, Choi EK, Oh S. Prevalence of and risk factors for silent ischemic stroke in patients with atrial fibrillation as determined by brain magnetic resonance imaging. Am J Cardiol. 2014;113:655–61. doi: 10.1016/j.amjcard.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–10. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 47.Jacobs V, Woller SC, Stevens S, May HT, Bair TL, Anderson JL, et al. Time outside of therapeutic range in atrial fibrillation patients is associated with long-term risk of dementia. Heart Rhythm. 2014;11:2206–13. doi: 10.1016/j.hrthm.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Rabinstein AA. Prolonged cardiac monitoring for detection of paroxysmal atrial fibrillation after cerebral ischemia. Stroke. 2014;45:1208–14. doi: 10.1161/STROKEAHA.113.003389. [DOI] [PubMed] [Google Scholar]
- 49.Friberg L, Hammar N, Rosenqvist M. Stroke in paroxysmal atrial fibrillation: report from the Stockholm Cohort of Atrial Fibrillation. Eur Heart J. 2010;31:967–75. doi: 10.1093/eurheartj/ehn599. [DOI] [PubMed] [Google Scholar]