Abstract
CpG oligodeoxynucleotides (CpG) potently activate the immune system by mimicking microbial DNA. Conjugation of CpG to chTNT-3, an antibody targeting the necrotic centers of tumors, enabled CpG to accumulate in tumors after systemic delivery, where it can activate the immune system in the presence of tumor antigens. CpG chemically conjugated to chTNT-3 (chTNT-3/CpG) were compared to free CpG in their ability to stimulate the immune system in vitro and reduce tumor burden in vivo. In subcutaneous Colon 26 adenocarcinoma and B16-F10 melanoma models in BALB/c and C57BL/6 mice, respectively, chTNT-3/CpG, free CpG, or several different control constructs were administered systemically. Intraperitoneal injections of chTNT-3/CpG delayed tumor growth and improved survival and were comparable to intratumorally administered CpG. Compared to saline-treated mice, chTNT-3/CpG-treated mice had smaller average tumor volumes by as much as 72 % in Colon 26-bearing mice and 79 % in B16-bearing mice. Systemically delivered free CpG and CpG conjugated to an isotype control antibody did not reduce tumor burden or improve survival. In this study, chTNT-3/CpG retained immunostimulatory activity of the CpG moiety and enabled delivery to tumors. Because systemically administered CpG rapidly clear the body and do not accumulate into tumors, chTNT-3/CpG provide a solution to the limitations observed in preclinical and clinical trials.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1813-x) contains supplementary material, which is available to authorized users.
Keywords: CpG oligodeoxynucleotides, Toll-like receptor agonist, Immunoconjugate, Immunotherapy, Solid tumors
Introduction
For several centuries, historical accounts describe the therapeutic effects of infectious disease processes on malignancy (reviewed in [1]). These observations led to the direct injection of attenuated pathogens or pathogen-associated molecular patterns (PAMPs) into the tumor space [2]. Pathogens and PAMPs promote the ability of immune cells to identify tumors as a diseased environment. Unlike TCRs or antibodies, which are antigen-specific, the receptors for PAMPs are germline encoded and critical to innate immunity. Because immunotherapies solely relying on T cell or antibody activity work poorly in tumors with low antigen expression [3], inclusion of the innate immune system offers the opportunity to combat a wider range of cases. With the recognition that innate immune cells can destroy tumor cells and that there is extensive cross talk between the innate and adaptive systems, targeting innate immunity is an intuitive approach to cancer immunotherapy (reviewed in [4]).
Toll-like receptor (TLR) agonists are a group of PAMPs that include CpG oligodeoxynucleotides (CpG), characterized by unmethylated cytosine guanine motifs found commonly in microbial but not in mammalian DNA [5]. As a TLR9 agonist, CpG rapidly activate plasmacytoid DC, NK cells, and B cells, to promote the production of TH1 cytokines and enhance cross-presentation of antigens by professional antigen-presenting cells [6]. Intratumoral injections of CpG elicited immune responses against tumors in murine models of melanoma [7–10], fibrosarcoma [7], renal cell carcinoma [8], colon adenocarcinoma [8, 9, 11], pancreatic adenocarcinoma [11], neuroblastomas [12], and lymphoma [13]. Following its success in preclinical models, CpG were used in clinical trials for a wide range of cancers (reviewed in [14, 15]). However, optimal therapeutic effects were generally limited to intratumoral injections. Disappointing clinical results [16, 17] were in part due to its poor efficacy when given systemically or in non-tumor sites [8, 10, 13, 14, 18]. In clinical trials where CpG resulted in systemic tumor regression, CpG were still given intratumorally [19, 20]. Rapid clearance of oligodeoxynucleotides and inadequate accumulation in tumors may have limited systemic delivery. Furthermore, non-targeted, systemic CpG can hamper immune responses by inducing immunosuppressive mechanisms [21]. Because multiple intratumoral injections over time are not feasible clinically and may not result in an abscopal effect [22], we explored the use of CpG as an antibody conjugate to allow tumor accumulation.
In addition to previously describing the chemical conjugation of CpG to antibodies trastuzumab and rituximab [23], we chemically linked the 3′-end of CpG to lysine residues on chimeric TNT-3 antibody. chTNT-3 [24] belongs to a series of antibodies, designated as tumor necrosis therapy (TNT), directed against ubiquitous and stable nucleic acid antigens retained in necrotic tissues [25]. By binding nucleic acid antigens that are universally present in solid tumors regardless of species, TNT antibodies can be used in both experimental animal models and clinical settings. Because degenerating tumor cells release tumor antigens, the necrotic cores of tumors are ideal sites for delivering immunostimulatory molecules. Several TNT antibodies are in clinical trials for the radiotherapy of lung and brain cancers [26–28] and for the immunotherapy of solid tumors as fusion proteins [29]. In preclinical tumor models, we demonstrated the tumor-targeting specificity of chTNT-3 and its derivatives in imaging and therapeutic studies [30–35].
Here, we describe the conjugation of two different classes of CpG (class A and B) to chTNT-3. Both classes of CpG are TLR9 agonists, but preferentially elicit distinct cytokine responses, likely due to structural differences. Class B CpG (or type K) principally acts as a monomer on monocytes and B cells to promote IL-6, IL-10, and IL-12 secretion, and antibody production [36]. In contrast, class A (or type D) CpG aggregate into higher-order structures due to their poly-G tail. Although a weak stimulator of B cells, class A CpG potently activate DC and promote the secretion of type I IFNs and IL-12 [37]. While most tumor studies and clinical trials utilized class B sequences, we compared the two classes as immunoconjugates for their ability to generate anti-tumor responses in highly (Colon 26) and poorly (B16) immunogenic tumor models. To our knowledge, this is the first in vivo study of antibody/CpG conjugates delivered systemically as a monotherapy in preclinical models of solid tumors.
Methods
Cell lines
J774A.1 murine monocytic cell line was acquired in 1997 as a gift from Dr. Stephen A. Stohlman (Cleveland Clinic Foundation, Cleveland, OH, USA). B16-F10 melanoma and Colon 26 adenocarcinoma were acquired in 1988 and 1999, respectively, from ATCC (Manassas, VA, USA). All cell lines were grown in RPMI-1640 supplemented with 10 % fetal bovine serum (Hyclone, Logan, UT, USA), non-essential amino acids, penicillin G, and streptomycin (Gemini Bio-Products, West Sacramento, CA, USA). Cell lines were authenticated by STR DNA profiling by DDC Medical (Fairfield, OH, USA) in 2015.
CpG sequences
All oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA, USA). CpG1585 is a class A murine CpG sequence, CpG1826 is a class B murine CpG sequence, and sc1585 and sc1826 are negative controls for CpG1585 and CpG1826, respectively. To enable conjugation, the 3′-ends contained a C3 thiol modifier. For sequences, see Table 1.
Table 1.
Sequences of constructs and oligo/antibody ratios
| Experiment | Construct | Sequence | Oligo:Ab ratio |
|---|---|---|---|
| Figure 1 | chTNT-3/CpG1826-biotin | 5′-biotin-tccatgacgttcctgacgtt-3′ | 3.5:1 |
| Figures 2, 3 | chTNT-3/CpG1585 | 5′-ggGGTCAACGTTGAgggggg-3′ | 8:1 |
| Figure 2 | chTNT-3/sc1585 | 5′-ggGGTCAAGCTTGAgggggg-3′ | 8:1 |
| Figure 3 | chTNT-3/CpG1826 | 5′-tccatgacgttcctgacgtt-3′ | 4.4:1 |
| Figures 2, 4, 5 | chTNT-3/CpG1826 | 5′-tccatgacgttcctgacgtt-3′ | 2.8:1 |
| Figures 2, 4 | chTNT-3/sc1826 | 5′-tccatgagcttcctgagctt-3′ | 3.5:1 |
| Figure 4 | Cetuximab/CpG1826 | 5′-tccatgacgttcctgacgtt-3′ | 4.4:1 |
C–G or G–C motifs are underlined. Bases in lower case have a phosphorothioate backbone, and bases in capital letters have a phosphodiester linkage
Conjugation of antibodies with CpG
The chemical conjugation of thiol-modified CpG1826 to primary amines on antibodies was previously described [23]. chTNT-3 or cetuximab was incubated with Sulfo-EMCS (Pierce, Rockford, IL, USA) at 1:6 antibody-to-sulfo-EMCS ratio in PBS containing 1 mM EDTA for 1 h at room temperature. The excess cross-linker was removed using Zeba™ Spin Desalting Columns (Pierce). Thiol-modified CpG1826 or sc1826 was reduced in 0.1 M DTT for 1 h at room temperature. Excess DTT was removed using PD-10 Desalting Columns (GE Healthcare, Little Chalfont, UK). Reduced thiol-modified CpG were mixed with antibody/EMCS at a 6:1 ratio overnight at 4 °C. Unconjugated CpG were separated from chTNT-3/CpG by gel filtration using a Sephacryl S-100 column (GE Healthcare). See Supplementary Fig. S1 for conjugation reactions.
Because of their poly(G) motif, CpG1585 and sc1585 self-assemble into higher-order structures known as G-tetrads [38, 39]. To prevent precipitation during conjugation, thiol-modified CpG1585 (or sc1585) was mixed with native CpG1585 (or sc1585) at a 1:3 ratio in 10 mM MOPS buffer. This mixture was heated at 95 °C for 5 min and cooled to room temperature to allow reannealing. This thiol-modified/native CpG mixture was reduced in DTT and desalted prior to conjugation with chTNT-3/EMCS (8:1 ratio) as described above. The oligo/antibody ratios for the final products are listed in Table 1.
Fluorescence microscopy
J774A.1 cells seeded on poly-d-lysine-coated coverslips (Neuvitro, Vancouver, WA, USA) were incubated with CpG-biotin, chTNT-3, or chTNT-3/CpG-biotin in medium at 37 °C for 60 min. Cells were fixed in 2 % paraformaldehyde and permeabilized with 0.5 % Triton-X100. Non-specific binding sites were blocked with 5 % BSA/PBS-Tween 20 (PBST). Permeabilized cells were incubated with polyclonal rabbit anti-mouse TLR9 antibody (Pierce) in 1 % BSA/PBST overnight at 4 °C. Cells were washed in PBST and stained with streptavidin-Alexa Fluor®488 or goat anti-human IgG-Alexa Fluor®488, and goat anti-rabbit IgG-Alexa Fluor®568 (Life Technologies, Carlsbad, CA, USA) in 1 % BSA/PBST for 1 h. Coverslips were washed and mounted on slides using Fluoroshield Mounting Medium with DAPI (Abcam, Cambridge, MA, USA). Images were captured on a PerkinElmer UltraVIEW spinning disk confocal microscope (Waltham, MA, USA).
Mice
Six-week-old female BALB/c and C57BL/6 mice were used in this study. All mice were purchased from Harlan Laboratories (Indianapolis, IN, USA). All protocols using mice were approved by University of Southern California’s Institutional Animal Care and Use Committee (IACUC).
Radiolabeling and biodistribution studies of chTNT-3/CpG1826
To radiolabel chTNT-3/CpG186 with iodine-125, 1 mCi (37 MBq) of I-125 was added to 100 µg of chTNT-3/CpG1826 in the presence of chloramine-T [35]. The solution was quenched after 2 min with sodium metabisulfite. The reaction was purified using a Sephadex G-25 column (GE Healthcare) and administered within 2 h after labeling.
Colon 26 tumors were grown subcutaneously until reaching approximately 0.5 cm in diameter. Mice were given potassium iodide in their drinking water for 3 days to block thyroid uptake of free radioiodide. Mice were injected with 100 µCi (10 µg) of 125I-chTNT-3/CpG1826. Groups of mice were euthanized 1, 3, and 5 days post-injection, and organs, blood, and tumors were removed and weighed. Radioactivity was measured with a gamma counter.
In vitro assay for cytokine production
Splenocytes from naïve BALB/c mice (n = 3 mice) were acquired by flushing spleens with medium and passing cells through a 70-µm filter followed by red blood cell lysis with BD Pharm Lyse™ (BD Biosciences, San Jose, CA, USA). Leukocytes were then washed and incubated in low serum medium (2.5 % FBS in RPMI supplemented with NEAA) (2.5 × 105 cells/200 µL) with or without plated anti-CD3 stimulation, in the presence of chTNT-3, chTNT-3/sc1585, chTNT-3/sc1826, CpG1585, CpG1826, chTNT-3/CpG1585, or chTNT-3/CpG1826 (corresponding to 0.1, 1.0, or 10 µg/mL of oligo, or 83 µg/mL antibody) for 4 days at 37 °C in a humidified incubator. Anti-CD3 antibody (clone 145–2C11, eBioscience, San Diego, CA, USA) was plated by incubating 5 µg/mL antibody in PBS on non-treated tissue culture plates at 4 °C overnight, followed by multiples washes with PBS.
Supernatants were collected and measured for IL-2, IL-4, IL-6, IL-10, IL-12p40, IL-12p70, IL-17(F), IL-23p19, and IFN-γ using Bio-Plex® Multiplex System (Bio-Rad, Hercules, CA, USA). Data were collected on the Bio-Rad Bio-Plex Suspension Array System at the USC Immune Monitoring Core. IFN-α was analyzed using the mouse IFN-α Platinum ELISA kit (eBioscience).
Tumor treatment studies
Two million B16 cells and Colon 26 cells were injected subcutaneously into the left flank of C57BL/6 and BALB/c mice, respectively. When average tumor sizes reached 75–100 mm3, all mice were randomized into treatment groups. Mice then received treatments diluted in PBS by intraperitoneal (i.p.) or intratumoral (i.t.) injection daily for 5 days. Tumor volumes were determined by length, width, and height measurements using a caliper (volume = length × width × height). For survival analysis, an event was considered to have occurred if a mouse was deceased, or had to be euthanized due to conditions previously specified in IACUC protocols.
Treatments included antibodies (chTNT-3 or cetuximab), free CpG (CpG1826), immunoconjugates (chTNT-3/CpG1826, chTNT-3/CpG1585, and cetuximab/CpG1826), or free CpG with parental chTNT-3 (chTNT-3+CpG1826). As a negative control, chTNT-3/sc1826 was used. Each dose corresponded to either 18 or 10 µg of oligo (50–100 µg antibody), as specified in the figure legends.
Flow cytometry analysis of inguinal lymph nodes, splenocytes, and peripheral blood
Colon 26 tumor-bearing BALB/c mice were treated with PBS or chTNT-3/CpG1826 (doses corresponding to 10 µg of CpG) daily for 5 days by i.p. injections. Three days following the last dose, contralateral, tumor-draining inguinal lymph nodes (TDLN), spleens, and peripheral blood were acquired. Cells were flushed from lymph nodes and spleens and passed through a 70-µm filter. Red blood cells were lysed with BD Pharm Lyse™. Fc receptors (FcR) were blocked with a mouse FcR blocking agent (Miltenyi Biotec, Bergisch Gladbach, Germany) prior to staining. Cells were stained with anti-CD4-FITC, anti-CD8-APC, anti-CD3-APC-Cy7, anti-CD25-FITC, and/or anti-CD4-PE. For IFN-γ staining, cells were fixed in 2 % paraformaldehyde, followed by permeabilization in 1X Permeabilization Buffer (eBioscience) and staining with anti-IFN-γ-PE. For FoxP3 detection, cells were fixed and permeabilized in FoxP3 fixation/permeabilization solutions (eBioscience) prior to staining with anti-FoxP3-APC. All antibody clones and manufacturers are listed in Supplementary Table S1. Cells were washed three times prior to collection on the Attune Flow Cytometer (Life Technologies). Data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Statistical analysis
Biodistribution and tumor/organ ratio of chTNT-3/CpG uptake were analyzed by two-way ANOVA with time and organ as the independent variables, followed by Tukey’s test for pairwise comparisons after significance was found in the primary analysis. In vitro cytokine concentrations were analyzed by one-way ANOVA followed by Dunnett’s test to compare every treatment with PBS or PBS with CD3 stimulation (control groups). In the Colon 26 model, tumor volume curves over time were compared using two-way repeated-measures ANOVA followed by Tukey’s test for pairwise comparisons between each treatment group after significance was found in the primary analysis. Due to the loss of mice in the B16 tumor model prior to 30 days, group comparisons on tumor volumes were analyzed up to day 13. To account for repeatedly measured tumor volumes over days, treatment groups were compared using generalized estimating equations (GEE), followed by pairwise comparisons by day with p values adjusted for false discovery rate. Survival curves were compared using a log-rank test. The percentages of T regulatory cells in TDLN were compared using a two-tailed unpaired t test. Statistical analyses were carried out using GraphPad Prism 6 software (La Jolla, CA, USA). GEE analysis was carried out using SASv9.4 (SAS Institute, Cary, NC, USA).
Results
Intact localization of chTNT-3/CpG in vitro and in vivo
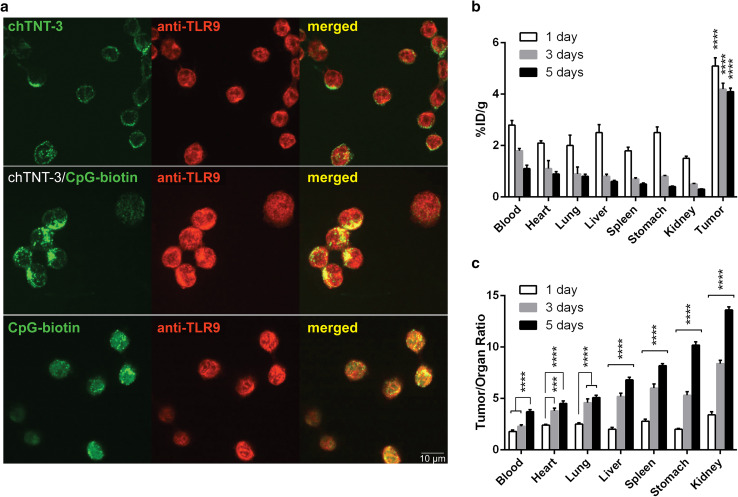
Because CpG engage TLR9 in endosomes to stimulate immune cells, we compared the internalization and localization of free CpG and chTNT-3/CpG in murine monocytic cell line J774A.1 (Fig. 1a). Both free CpG and chTNT-3/CpG co-localized with TLR9. Although J774A.1 cells bound unconjugated chTNT-3, likely through Fc/FcR interactions as demonstrated by membranous staining patterns (Fig. 1a), cells did not internalize chTNT-3, even after 2 h incubation (data not shown). This finding suggested that co-localization with TLR9 required the presence of CpG. Because chTNT-3/CpG were chemically conjugated using a non-cleavable linker, the chTNT-3 moiety of the conjugate also co-localized with TLR9 and the CpG moiety (Supplementary Fig. S2).
Fig. 1.
chTNT-3/CpG co-localized with TLR9 in vitro and targeted tumor in vivo. a Fluorescent microscopy demonstrating internalization of chTNT-3/CpG-biotin and CpG-biotin and co-localization with TLR9 (×600 magnification). chTNT-3 was detected using anti-huIgG-AF488 antibodies (top panel, green). CpG-biotin was detected using streptavidin-AF488 (bottom two panels, green). TLR9 was detected using anti-TLR9 and anti-rabbit IgG-AF568 antibodies (red). Images are representative of 3 independent experiments. b Biodistribution of 125I-chTNT-3/CpG1826 over 1, 3, and 5 days expressed as %ID/g. Statistical significances are shown for tumor compared to each of the normal organs. c Biodistribution expressed as tumor/organ ratio of %ID/g. Statistical significances are shown for comparisons between time points. Error bars represent standard error of the mean (SEM). ***p < 0.001; ****p < 0.0001, n = 5 mice/group
In addition to preserving the ability of CpG to co-localize with TLR9, chemical conjugation permitted chTNT-3 to bind its target antigens in vivo. Biodistribution studies in Colon 26 tumor-bearing mice demonstrated tumor uptake and retention of 125I-chTNT-3/CpG. At each time point, tumor uptake of 125I-chTNT-3/CpG was greater than uptake in normal organs and blood (p < 0.0001, Fig. 1b) and comparable to tumor uptake of parental chTNT-3 [34, 40]. Whereas chTNT-3/CpG cleared rapidly from normal tissues and blood, 125I-chTNT-3/CpG were retained by tumors after five days. The rapid clearance of 125I-chTNT-3/CpG in blood and normal organs resulted in increasing tumor/organ uptake ratios for every organ over time (p < 0.0001, Fig. 1c). Using whole-body radioactivity to estimate half-life, 125I-chTNT-3/CpG had a half-life of 48.0 ± 1.2 h (mean ± SD), which was shorter than the previously reported half-life of 125I-chTNT-3, 134.2 ± 4.0 h [34, 40].
Immunostimulatory activity of chTNT-3/CpG in vitro
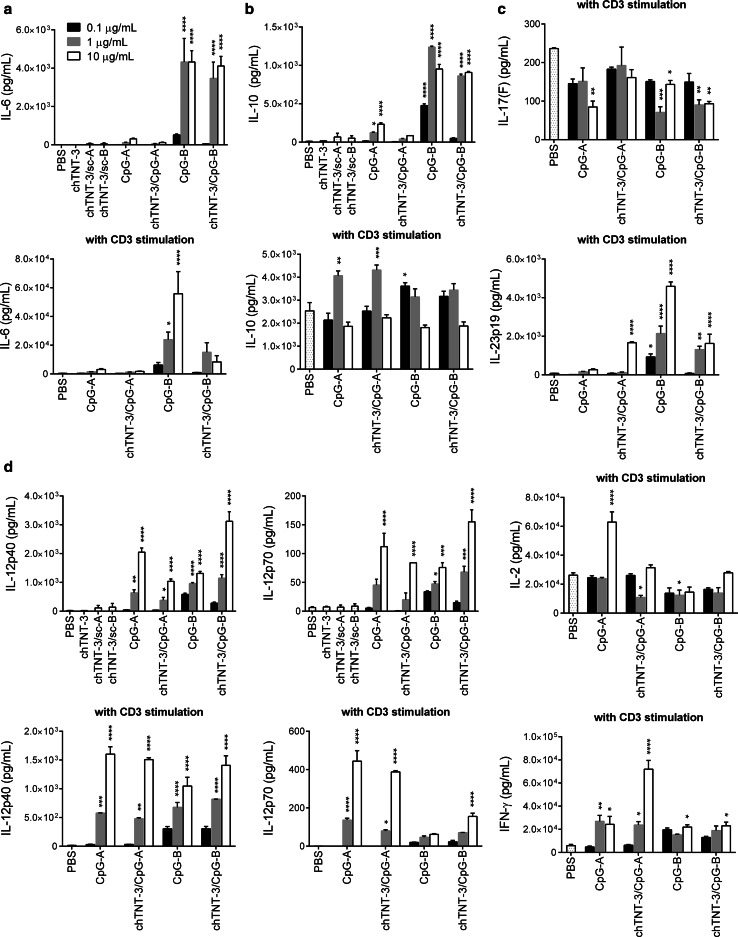
Because chemical modifications can change the physical properties of CpG, especially higher-ordered structures of class A CpG, we compared cytokine responses to chTNT-3/CpG and free CpG in vitro (Fig. 2). BALB/c splenocytes treated with chTNT-3/CpG1826 or free CpG1826 (class B) produced IL-6, IL-10, IL-12p40, and IL-12p70 in a dose-dependent manner (p < 0.0001). chTNT-3/CpG1585 and free CpG1585 (class A) did not produce statistically significant amounts of IL-6, but produced significant amounts of IL-12p40 and IL-12p70 (p < 0.0001) compared to negative controls. In the absence of CD3 stimulation, IL-2, IL-4, IL-17(F), IL-23p19, IFN-α, and IFN-γ production were below 100 pg/mL in all treatment groups (Supplementary Fig. S3, S4).
Fig. 2.
Cytokine production in response to chTNT-3/CpG1585 and chTNT-3/CpG1826. a Pro-inflammatory (IL-6), b anti-inflammatory (IL-10), c TH17 (IL-17(F) and IL-23p19), and d TH1 (IL-12p40, IL-12p70, IL-2, and IFN-γ) cytokines released from murine splenocytes treated in vitro and measured using Luminex xMAP technology. Means were compared to the means of PBS-treated splenocytes with or without CD3 stimulation and adjusted for multiple comparisons using Dunnett’s method. chTNT-3, chTNT-3/sc-A, chTNT-3/sc-B corresponded to antibody concentrations of 83 µg/mL or oligo concentrations of 10 µg/mL. Error bars represent SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001, n = 3, except IL-17(F) where n = 2 mice. CpG-A = CpG1585, CpG-B = CpG1826
In combination with T cell stimulation using an agonistic antibody to CD3, CpG1826 and chTNT-3/CpG1826 increased IL-23p19 (p < 0.0001) and IFN-γ secretion (p < 0.05) and decreased IL-17(F) (p < 0.01). In the absence or presence of CD3 stimulation, cytokine profiles were similar for chTNT-3/CpG1826 and free CpG1826 and demonstrated induction of pro-inflammatory (Fig. 2a) and TH1 cytokines (Fig. 2d).
While both CpG1585 and its immunoconjugate similarly induced IL-12, only free CpG1585 significantly increased secretion of IL-2 when combined with CD3 stimulation (p < 0.0001, Fig. 2d). In contrast, with CD3 stimulation, chTNT-3/CpG1585 significantly increased IFN-γ (12-fold compared to PBS control, p < 0.0001, Fig. 2d) and IL-23p19 (p < 0.0001, Fig. 2c) secretion. Unlike CpG1826 and its immunoconjugate, CpG1585 and chTNT-3/CpG1585 induced IFN-α production (p < 0.0001, Supplementary Fig. S4), as expected [37].
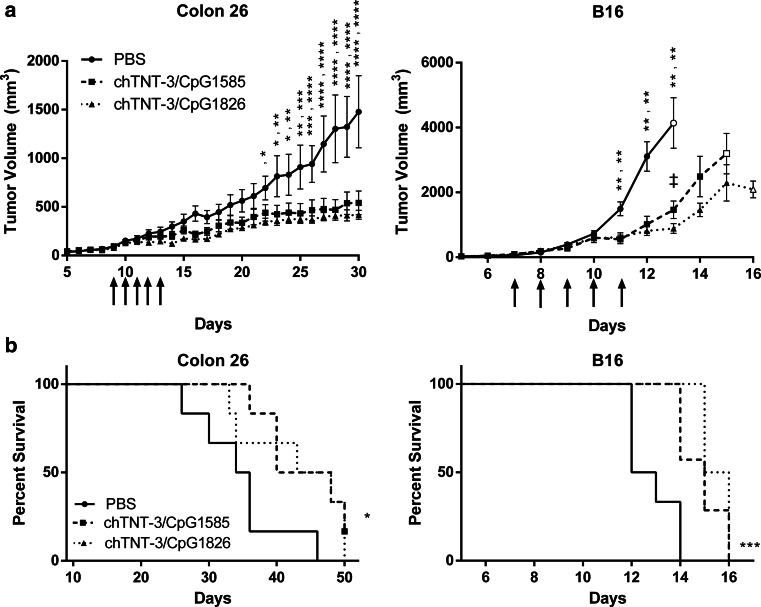
chTNT-3/CpG delays tumor growth in vivo
By delivering CpG to the tumor microenvironment, systemically administered chTNT-3/CpG can stimulate the immune system in the presence of in situ tumor antigens. In Colon 26 adenocarcinoma and B16 melanoma tumor models, chTNT-3/CpG delayed growth (Fig. 3a) following five daily i.p. injections. By day 30 in the Colon 26 tumor model, mean tumor volumes were smaller by 63 % in the chTNT-3/CpG1585 (class A) group (541 ± 297 mm3; p < 0.0001) and by 72 % in the chTNT-3/CpG1826 (class B) group (418 ± 112 mm3; p < 0.0001) compared to the PBS-treated group (1478 ± 829 mm3). By day 13 in the B16 melanoma model, chTNT-3/CpG1585 reduced mean tumor volumes by 65 % (1463 ± 718 mm3; p < 0.01) and chTNT-3/CpG1826 by 79 % (888 ± 152 mm3; p < 0.01) compared to the PBS-treated group (4139 ± 1346 mm3). These results highlight the therapeutic potential of chTNT-3/CpG in two divergent types of tumors.
Fig. 3.
Class A and B CpG sequences decreased tumor burden and improved survival in highly (Colon 26) and poorly (B16) immunogenic tumor models when conjugated to chTNT-3. a Tumor volume curves in BALB/c mice-bearing Colon 26 adenocarcinoma and C57BL6 mice-bearing B16 melanoma. Doses of chTNT-3/CpG1585 and chTNT-3/CpG1826 corresponded to 18 µg of CpG. Open symbols represent data points where n = 3–4 mice due to death of mice prior to that time point. Arrows indicate when treatments were administered. Error bars represent SEM. b Kaplan–Meier survival curves, with data symbols representing mice alive at the end of study. Statistically significant differences between chTNT-3/CpG1585 or chTNT-3/CpG1826 and PBS treatment are represented by *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Statistically significant difference between chTNT-3/CpG1585 and chTNT-3/CpG1826 is represented by ‡ p < 0.05. N = 5–7 mice/group
While different classes of CpG induced distinct sets of cytokines (Fig. 2, Supplementary Fig. S4), chTNT-3/CpG1585 and chTNT-3/CpG1826 had similar reductions in tumor growth for both tumor models. Mice treated with chTNT-3/CpG1826 had smaller mean tumor volumes than mice treated with chTNT-3/CpG1585, but differences only reached statistical significance on day 13 in the B16 model (p < 0.05). For individual tumor volume curves of each mice, see Supplementary Fig. S5.
By delaying tumor growth, chTNT-3/CpG1585 and chTNT-3/1826 improved survival in Colon 26 (p < 0.05) and B16 (p < 0.001) models (Fig. 3b). One of the six Colon 26 tumor-bearing mice treated with chTNT-3/CpG1585 had no palpable tumor by day 25 (Supplementary Fig. S5). This mouse achieved a memory response against Colon 26 cells, as indicated by the lack of tumor growth following tumor rechallenge on the opposite flank (data not shown).
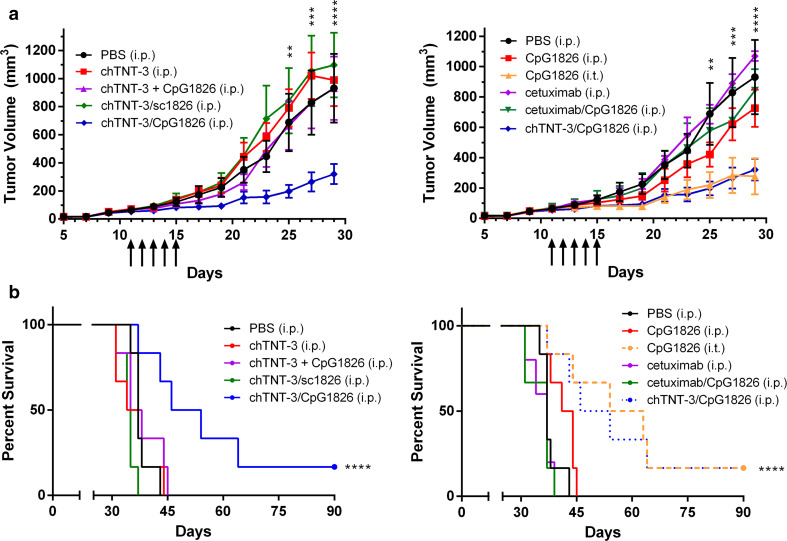
Effect of chTNT-3/CpG on tumor growth depends on its ability to localize to tumor
Except as a vaccine adjuvant, numerous studies established that systemic delivery of free CpG is ineffective in eliciting anti-tumor immunity [8, 10, 13, 14, 18]. chTNT-3/CpG1585 and chTNT-3/1826 monotherapies likely delayed tumor growth and improved survival by delivering the CpG moiety to the tumor and may have increased CpG half-life. To demonstrate that tumor accumulation is critical, we compared systemically delivered chTNT-3/CpG1826 to free CpG1826 (i.p.), CpG1826 (i.t.), CpG1826 conjugated to cetuximab (cetuximab/CpG1826, i.p.), and co-administered unconjugated CpG1826 and chTNT-3 (chTNT-3+CpG1826, i.p.) in the Colon 26 tumor model. Cetuximab (Erbitux) is a chimeric anti-human EGFR antibody that does not cross-react with murine EGFR and does not bind to Colon 26 cells. For this study, cetuximab served as a negative isotype control for chTNT-3.
Systemically delivered chTNT-3/CpG1826 and intratumoral free CpG1826 had statistically significantly smaller tumor volumes compared to every other treatment group and were the only two treatment groups to be statistically different than PBS treatment (p < 0.0001, Fig. 4a). By day 29 of the study, tumor volumes were smaller by 66 % in chTNT-3/CpG1826-treated mice (320 ± 174 mm3; p < 0.0001) and by 70 % in CpG1826 (i.t.)-treated mice (277 ± 291 mm3; p < 0.0001) compared to PBS treatment (931 ± 599 mm3). Cetuximab/CpG1826 treatment (842 ± 141 mm3) did not result in statistically significant smaller tumor volumes than PBS treatment, in spite of demonstrating immunostimulatory activity in vitro (Supplementary Fig. S6).
Fig. 4.
Optimal effect on tumor growth required delivery of CpG to the tumor. a Tumor volume curves in BALB/c mice-bearing Colon 26 adenocarcinoma. Treatment groups are divided into two graphs for easier visualization. All doses corresponded to 10 µg of oligo (CpG1826 or sc1826). Arrows indicate when treatments were administered. Error bars represent SEM. b Kaplan–Meier survival curves, with data symbols representing mice alive at the end of the study. Statistically significant differences between chTNT-3/CpG1826 or CpG (i.t.) and PBS treatment are represented by ***p < 0.001; ****p < 0.0001, n = 6 mice/group
Corresponding survival data demonstrated an improvement in survival with chTNT-3/CpG1826 (i.p.) and CpG1826 (i.t.) (p < 0.0001, Fig. 4b). As expected, co-administration of unconjugated chTNT-3 and CpG1826 did not delay tumor growth or improve survival (Fig. 4), indicating that the beneficial effects of chTNT-3/CpG1826 required their chemical conjugation. In addition, this study demonstrated that CpG-specific responses mediated the anti-tumor effects of chTNT-3/CpG1826, as no reduction in tumor volume or improvement in survival was seen with chTNT-3 conjugated to a negative control oligo (chTNT-3/sc1826) (Fig. 4).
Characterization of immune cells by flow cytometry
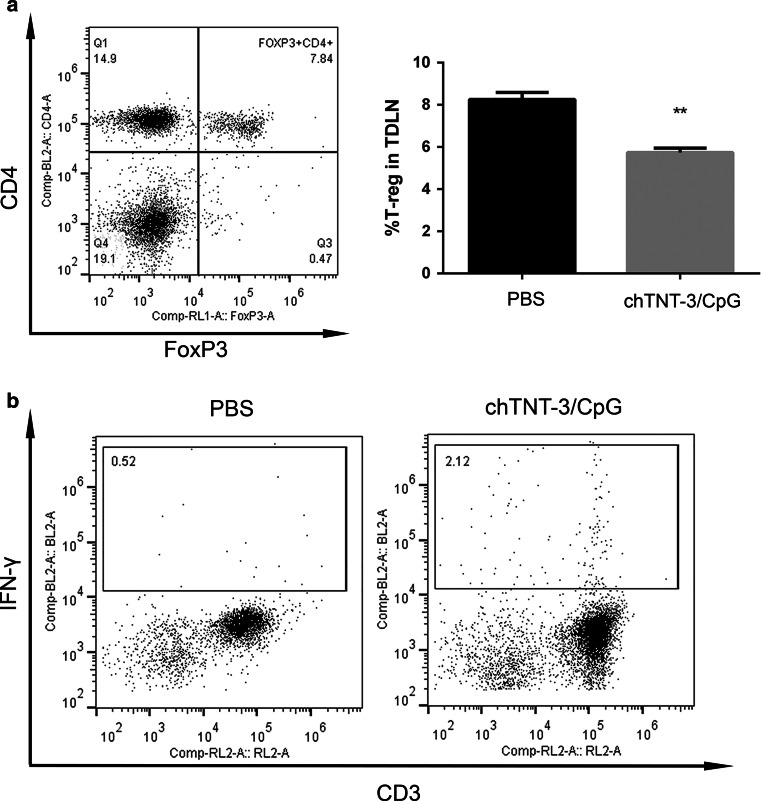
To characterize immune responses to chTNT-3/CpG1826 in vivo, we analyzed the inguinal lymph nodes (TDLN and contralateral), spleens, and peripheral blood from Colon 26 tumor-bearing mice three days following the last dose of chTNT-3/CpG1826 and PBS. There was a statistically significant reduction in the percentage of T regulatory (Treg) cells in the TDLN of mice treated with chTNT-3/CpG1826 (5.7 ± 0.4 %) compared to PBS (8.3 ± 0.6 %; p < 0.01, Fig. 5a). In two of three mice treated with chTNT-3/CpG1826, TDLN contained IFN-γ-producing cells, whereas no mice treated with PBS had detectable IFN-γ production (Fig. 5b). According to FACS analysis, CD3+ T cells comprised most of the IFN-γ-producing cells (Fig. 5b), indicating that chTNT-3/CpG1826 treatment elicited an adaptive immune response in addition to an innate immune response. In contrast, contralateral lymph nodes, spleens, and peripheral blood were similar between chTNT-3/CpG1826 and PBS treatment groups (data not shown), suggesting that immunomodulatory effects were localized to the tumor environment.
Fig. 5.
FACS analysis of TDLN in tumor-bearing mice treated with chTNT-3/CpG1826. a Treg was defined as CD3+CD4+FoxP3+. Error bars represent SEM. **p < 0.01, n = 3 mice/group. b Intracellular staining for IFN-γ
Discussion
The immunomodulatory activity of CpG and their ability to generate anti-tumor responses are well characterized [7–11, 13]. Clinical trials use intratumoral and systemic CpG as a monotherapy, vaccine adjuvant, and in combination with chemotherapy or radiotherapy with mixed results (reviewed in [14, 15]). While systemic delivery of CpG decreased tumor volumes in a small subset of patients’ tumors, optimal anti-tumor effects are seen when CpG are in the tumor microenvironment [8, 10, 13, 14, 18]. To meet this requirement, we conjugated CpG to solid tumor-targeting antibody chTNT-3. The conjugation methods we describe retained the ability of CpG to mount immune responses and affect tumor growth.
Other groups conjugated CpG to tumor-targeting antibodies, anti-Mucin1 [11] and anti-Her-2/neu [41], and demonstrated anti-tumor responses in murine models [41]. Systemic administration of anti-neu/CpG was evaluated only when given in combination with Treg-depleting agents [41]. In our present work, we evaluated systemically delivered chTNT-3/CpG as a monotherapy in two different models. In addition to these differences, chTNT-3/CpG conjugation employed a non-cleavable linker at the 3′-end, whereas anti-neu was conjugated to the 5′-end of CpG using a cleavable linker. The use of a non-cleavable linker negated activity of anti-neu/CpG [41]. Future studies need to compare pharmacokinetics and therapeutic effects between cleavable and non-cleavable linkers used in antibody/CpG conjugates.
Other groups also modified CpG to improve its in vivo stability and circulating half-life [42]. By conjugating CpG to dinitrophenyl haptens (DNP-CpG) and immunizing mice to DNP, DNP-CpG complexed with endogenous antibodies. Although this method increased CpG uptake into tumors, uptake was not tumor-specific as normal organs also increased DNP-CpG uptake. Because systemic delivery of DNP-CpG delayed growth of Colon 26 tumors, we expected cetuximab/CpG to delay tumor growth. However, cetuximab/CpG did not decrease tumor burden or improve survival (Fig. 4). These observations differentiate CpG from other immunotherapeutic agents (B7.1-Fc, B7.2-Fc, Fc-OX40L, and antibody/IL-2 fusion proteins) that do not require specific uptake into tumors to affect tumor growth [43, 44].
Like other immunoconjugates, only a portion of systemically delivered chTNT-3/CpG reached tumors (Fig. 1), yet systemic delivery delayed tumor growth almost equivalently to intratumoral CpG (Fig. 4). This observation was repeated in four independent mouse experiments (data not shown). In theory, the entire intratumoral CpG dose reaches the tumor. However, intratumoral injections do not prevent CpG from leaking at the injection site or into systemic circulation. This explanation is supported by findings demonstrating that intratumoral injections of anti-Mucin1/CpG conjugates exhibited superior therapeutic effects over intratumoral injections of free CpG [11]. In our biodistribution experiments, Colon 26 tumors retained chTNT-3/CpG over several days (Fig. 1b), and this retention of chTNT-3/CpG may explain our observations.
A unique aspect of this study was the inclusion of class A CpG immunoconjugates. In spite of their different activities in vitro (Fig. 2), chTNT-3/CpG1585 and chTNT-3/CpG1826 behaved similarly in vivo. Because they both strongly induced IL-12 secretion (Fig. 2d), this would suggest that IL-12 may be the critical mediator for in vivo responses against tumors. However, because IL-12 shares a subunit (IL-12p40) with IL-23, we also looked at IL-23p19 and IL-12p70 and showed that chTNT-3/CpG1585 and chTNT-3/CpG1826 induced both IL-12 and IL-23 secretion (Fig. 2c). Although IL-23 may promote tumor growth through IL-17 and STAT-3 pathways [45], there is mounting evidence that IL-23 can support TH1-mediated anti-tumor immune responses [46, 47]. Additional studies on these cytokines and tumor-infiltrating immune cells are currently being investigated.
In addition to characterizing activity, progress of chTNT-3/CpG into the clinic will require improving its therapeutic effect. A limitation to our study was the lack of a dose titration, which may suggest our study was underdosed. Because tumor size affects the amount of necrotic antigens available to chTNT-3, the relationship between starting tumor size and optimal chTNT-3/CpG dose needs further evaluation. Combining chTNT-3/CpG with cytoreductive chemotherapies may also increase necrotic antigens available to chTNT-3 [35]. Additional approaches to improve efficacy may include combining chTNT-3/CpG with agents reversing immunosuppressive mechanisms (e.g., anti-CTLA4, anti-PD-1, or anti-PD-L1 antibodies), as shown in studies with intratumoral CpG [48–50].
Conclusions
We demonstrated the feasibility of using chTNT-3 to deliver CpG and delay tumor growth. Unlike previously studied immunotherapies that produced therapeutic effects in immunogenic tumor models (Colon 26, RENCA, 4T1) but not poorly immunogenic models (MAD109, Lewis lung carcinoma, B16) [3], chTNT-3/CpG delayed tumor growth and improved survival in tumors of varying immunogenicities. In light of these results, the systemic use of CpG immunoconjugates is a step forward in the employment of innate immunity for cancer treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to acknowledge Dr. Wendy Jean Mack (University of Southern California) for statistical assistance. The project described was supported in part by National Center for Advancing Translational Sciences (NCATS award UL1TR00013) and National Cancer Institute (NCI award P30CA014089). Julie K. Jang and Brian W. Wu are TL1 trainees under Southern California Clinical and Translational Science Institute (SC CTSI) (NCATS award TL1TR000132).
Abbreviations
- ANOVA
Analysis of variance
- cetuximab/CpG
CpG conjugated to cetuximab
- chTNT-3
Chimeric tumor necrosis therapy-3 antibody
- chTNT-3/CpG
CpG conjugated to chTNT-3
- chTNT-3+CpG
Non-conjugated chTNT-3 and CpG
- CpG
Cytosine guanine dinucleotide
- CpG1585
CpG sequence 1585
- CpG1826
CpG sequence 1826
- DC
Dendritic cell
- DNP
Dinitrophenyl haptens
- EGFR
Epidermal growth factor receptor
- GEE
Generalized estimating equations
- 125I-chTNT-3/CpG
125-Iodinated chTNT-3/CpG
- IFN
Interferon
- i.p.
Intraperitoneal
- i.t.
Intratumoral
- NK cells
Natural killer cells
- PAMPs
Pathogen-associated molecular patterns
- sc1585
Negative control sequence for CpG1585
- sc1826
Negative control sequence for CpG1826
- TCR
T cell receptors
- TDLN
Tumor-draining lymph node
- TH1
T-helper 1
- TLR
Toll-like receptors
- Treg
T regulatory cells
Compliance with ethical standards
Conflict of interest
Peisheng Hu and Alan L. Epstein are co-founders of Cancer Therapeutics Laboratories, Inc. This study was partially funded by Cancer Therapeutics Laboratories, Inc. All other authors declare no conflict of interest.
References
- 1.Wiemann B, Starnes CO. Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64:529–564. doi: 10.1016/0163-7258(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 2.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res. 1991;262:3–11. [PubMed] [Google Scholar]
- 3.Lechner MG, Karimi SS, Barry-Holson K, Angell TE, Murphy KA, Church CH, Ohlfest JR, Hu P, Epstein AL. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J Immunother. 2013;36:477–489. doi: 10.1097/01.cji.0000436722.46675.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Zeng G. Cancer and innate immune system interactions: translational potentials for cancer immunotherapy. J Immunother. 2012;35:299–308. doi: 10.1097/CJI.0b013e3182518e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tokunaga T, Yamamoto H, Shimada S, et al. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J Natl Cancer Inst. 1984;72:955–962. [PubMed] [Google Scholar]
- 6.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawarada Y, Ganss R, Garbi N, Sacher T, Arnold B, Hammerling GJ. NK- and CD8(+) T cell-mediated eradication of established tumors by peritumoral injection of CpG-containing oligodeoxynucleotides. J Immunol. 2001;167:5247–5253. doi: 10.4049/jimmunol.167.9.5247. [DOI] [PubMed] [Google Scholar]
- 8.Heckelsmiller K, Rall K, Beck S, et al. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J Immunol. 2002;169:3892–3899. doi: 10.4049/jimmunol.169.7.3892. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Karakousis CP, Takita H, Shin K, Brooks SP. Intra-tumoral injection of CpG results in the inhibition of tumor growth in murine Colon-26 and B-16 tumors. Biotechnol Lett. 2003;25:149–153. doi: 10.1023/A:1021927621813. [DOI] [PubMed] [Google Scholar]
- 10.Amos SM, Pegram HJ, Westwood JA, et al. Adoptive immunotherapy combined with intratumoral TLR agonist delivery eradicates established melanoma in mice. Cancer Immunol Immunother. 2011;60:671–683. doi: 10.1007/s00262-011-0984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schettini J, Kidiyoor A, Besmer DM, Tinder TL, Roy LD, Lustgarten J, Gendler SJ, Mukherjee P. Intratumoral delivery of CpG-conjugated anti-MUC1 antibody enhances NK cell anti-tumor activity. Cancer Immunol Immunother. 2012;61:2055–2065. doi: 10.1007/s00262-012-1264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auf G, Chen L, Fornes P, Le Clanche C, Delattre JY, Carpentier AF. CpG-oligodeoxynucleotide rejection of a neuroblastoma in A/J mice does not induce a paraneoplastic disease. Neurosci Lett. 2002;327:189–192. doi: 10.1016/S0304-3940(02)00422-6. [DOI] [PubMed] [Google Scholar]
- 13.Betting DJ, Yamada RE, Kafi K, Said J, van Rooijen N, Timmerman JM. Intratumoral but not systemic delivery of CpG oligodeoxynucleotide augments the efficacy of anti-CD20 monoclonal antibody therapy against B cell lymphoma. J Immunother. 2009;32:622–631. doi: 10.1097/CJI.0b013e3181ab23f1. [DOI] [PubMed] [Google Scholar]
- 14.Murad YM, Clay TM. CpG oligodeoxynucleotides as TLR9 agonists: therapeutic applications in cancer. BioDrugs. 2009;23:361–375. doi: 10.2165/11316930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Holtick U, Scheulen ME, von Bergwelt-Baildon MS, Weihrauch MR. Toll-like receptor 9 agonists as cancer therapeutics. Expert Opin Investig Drugs. 2011;20:361–372. doi: 10.1517/13543784.2011.553187. [DOI] [PubMed] [Google Scholar]
- 16.Link BK, Ballas ZK, Weisdorf D, Wooldridge JE, Bossler AD, Shannon M, Rasmussen WL, Krieg AM, Weiner GJ. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother. 2006;29:558–568. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- 17.Weber JS, Zarour H, Redman B, Trefzer U, O’Day S, van den Eertwegh AJ, Marshall E, Wagner S. Randomized phase 2/3 trial of CpG oligodeoxynucleotide PF-3512676 alone or with dacarbazine for patients with unresectable stage III and IV melanoma. Cancer. 2009;115:3944–3954. doi: 10.1002/cncr.24473. [DOI] [PubMed] [Google Scholar]
- 18.Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188:1592–1599. doi: 10.4049/jimmunol.1101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brody JD, Ai WZ, Czerwinski DK, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–4332. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YH, Gratzinger D, Harrison C, et al. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood. 2012;119:355–363. doi: 10.1182/blood-2011-05-355222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wingender G, Garbi N, Schumak B, et al. Systemic application of CpG-rich DNA suppresses adaptive T cell immunity via induction of IDO. Eur J Immunol. 2006;36:12–20. doi: 10.1002/eji.200535602. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann MA, Kors C, Audring H, Walden P, Sterry W, Trefzer U. Phase 1 evaluation of intralesionally injected TLR9-agonist PF-3512676 in patients with basal cell carcinoma or metastatic melanoma. J Immunother. 2008;31:520–527. doi: 10.1097/CJI.0b013e318174a4df. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Jang JK, Lechner MG, Hu P, Khawli L, Scannell CA, Epstein AL. Generation of tumor-targeted antibody-CpG conjugates. J Immunol Methods. 2013;389:45–51. doi: 10.1016/j.jim.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Hornick JL, Sharifi J, Khawli LA, Hu P, Biela BH, Mizokami MM, Yun A, Taylor CR, Epstein AL. A new chemically modified chimeric TNT-3 monoclonal antibody directed against DNA for the radioimmunotherapy of solid tumors. Cancer Biother Radiopharm. 1998;13:255–268. doi: 10.1089/cbr.1998.13.255. [DOI] [PubMed] [Google Scholar]
- 25.Epstein AL, Chen FM, Taylor CR. A novel method for the detection of necrotic lesions in human cancers. Cancer Res. 1988;48:5842–5848. [PubMed] [Google Scholar]
- 26.Chen S, Yu L, Jiang C, et al. Pivotal study of iodine-131-labeled chimeric tumor necrosis treatment radioimmunotherapy in patients with advanced lung cancer. J Clin Oncol. 2005;23:1538–1547. doi: 10.1200/JCO.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 27.Hdeib A, Sloan A. Targeted radioimmunotherapy: the role of (1)(3)(1)I-chTNT-1/B mAb (Cotara) for treatment of high-grade gliomas. Future Oncol. 2012;8:659–669. doi: 10.2217/fon.12.58. [DOI] [PubMed] [Google Scholar]
- 28.Yu L, Ju DW, Chen W, et al. 131I-chTNT radioimmunotherapy of 43 patients with advanced lung cancer. Cancer Biother Radiopharm. 2006;21:5–14. doi: 10.1089/cbr.2006.21.5. [DOI] [PubMed] [Google Scholar]
- 29.van den Heuvel MM, Verheij M, Boshuizen R, et al. NHS-IL2 combined with radiotherapy: preclinical rationale and phase Ib trial results in metastatic non-small cell lung cancer following first-line chemotherapy. J Transl Med. 2015;13:32. doi: 10.1186/s12967-015-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Hu P, Khawli LA, Epstein AL. Complete regression of experimental solid tumors by combination LEC/chTNT-3 immunotherapy and CD25(+) T-cell depletion. Cancer Res. 2003;63:8384–8392. [PubMed] [Google Scholar]
- 31.Khawli LA, Biela B, Hu P, Epstein AL. Comparison of recombinant derivatives of chimeric TNT-3 antibody for the radioimaging of solid tumors. Hybrid Hybridomics. 2003;22:1–9. doi: 10.1089/153685903321538026. [DOI] [PubMed] [Google Scholar]
- 32.Flanagan ML, Khawli LA, Hu P, Epstein AL. H60/TNT-3 fusion protein activates NK cells in vitro and improves immunotherapeutic outcome in murine syngeneic tumor models. J Immunother. 2006;29:274–283. doi: 10.1097/01.cji.0000199194.90222.1a. [DOI] [PubMed] [Google Scholar]
- 33.Mizokami MM, Hu P, Khawli LA, Li J, Epstein AL. Chimeric TNT-3 antibody/murine interferon-gamma fusion protein for the immunotherapy of solid malignancies. Hybrid Hybridomics. 2003;22:197–207. doi: 10.1089/153685903322328929. [DOI] [PubMed] [Google Scholar]
- 34.Hornick JL, Sharifi J, Khawli LA, Hu P, Bai WG, Alauddin MM, Mizokami MM, Epstein AL. Single amino acid substitution in the Fc region of chimeric TNT-3 antibody accelerates clearance and improves immunoscintigraphy of solid tumors. J Nucl Med. 2000;41:355–362. [PubMed] [Google Scholar]
- 35.Jang JK, Khawli LA, Park R, Wu BW, Li Z, Canter D, Conti PS, Epstein AL. Cytoreductive chemotherapy improves the biodistribution of antibodies directed against tumor necrosis in murine solid tumor models. Mol Cancer Ther. 2013;12:2827–2836. doi: 10.1158/1535-7163.MCT-13-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 37.Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::AID-IMMU2154>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 38.Sen D, Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988;334:364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- 39.Kerkmann M, Costa LT, Richter C, et al. Spontaneous formation of nucleic acid-based nanoparticles is responsible for high interferon-alpha induction by CpG-A in plasmacytoid dendritic cells. J Biol Chem. 2005;280:8086–8093. doi: 10.1074/jbc.M410868200. [DOI] [PubMed] [Google Scholar]
- 40.Khawli LA, Mizokami MM, Sharifi J, Hu P, Epstein AL. Pharmacokinetic characteristics and biodistribution of radioiodinated chimeric TNT-1, -2, and -3 monoclonal antibodies after chemical modification with biotin. Cancer Biother Radiopharm. 2002;17:359–370. doi: 10.1089/108497802760363150. [DOI] [PubMed] [Google Scholar]
- 41.Sharma S, Dominguez AL, Manrique SZ, Cavallo F, Sakaguchi S, Lustgarten J. Systemic targeting of CpG-ODN to the tumor microenvironment with anti-neu-CpG hybrid molecule and T regulatory cell depletion induces memory responses in BALB-neuT tolerant mice. Cancer Res. 2008;68:7530–7540. doi: 10.1158/0008-5472.CAN-08-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palma E, Cho MJ. Improved systemic pharmacokinetics, biodistribution, and antitumor activity of CpG oligodeoxynucleotides complexed to endogenous antibodies in vivo. J Control Release. 2007;120:95–103. doi: 10.1016/j.jconrel.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lechner MG, Russell SM, Bass RS, Epstein AL. Chemokines, costimulatory molecules and fusion proteins for the immunotherapy of solid tumors. Immunotherapy. 2011;3:1317–1340. doi: 10.2217/imt.11.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tzeng A, Kwan BH, Opel CF, Navaratna T, Wittrup KD. Antigen specificity can be irrelevant to immunocytokine efficacy and biodistribution. Proc Natl Acad Sci USA. 2015;112:3320–3325. doi: 10.1073/pnas.1416159112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teng MW, Andrews DM, McLaughlin N, et al. IL-23 suppresses innate immune response independently of IL-17A during carcinogenesis and metastasis. Proc Natl Acad Sci USA. 2010;107:8328–8333. doi: 10.1073/pnas.1003251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo CH, Lee SC, Wu PY, et al. Antitumor and antimetastatic activity of IL-23. J Immunol. 2003;171:600–607. doi: 10.4049/jimmunol.171.2.600. [DOI] [PubMed] [Google Scholar]
- 47.Kaiga T, Sato M, Kaneda H, Iwakura Y, Takayama T, Tahara H. Systemic administration of IL-23 induces potent antitumor immunity primarily mediated through Th1-type response in association with the endogenously expressed IL-12. J Immunol. 2007;178:7571–7580. doi: 10.4049/jimmunol.178.12.7571. [DOI] [PubMed] [Google Scholar]
- 48.Houot R, Levy R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood. 2009;113:3546–3552. doi: 10.1182/blood-2008-07-170274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mangsbo SM, Sandin LC, Anger K, Korman AJ, Loskog A, Totterman TH. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J Immunother. 2010;33:225–235. doi: 10.1097/CJI.0b013e3181c01fcb. [DOI] [PubMed] [Google Scholar]
- 50.Marabelle A, Kohrt H, Sagiv-Barfi I, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123:2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.