Abstract
Objectives
To examine the relationships over time between dual trajectories of depressive symptoms and several cognitive domains.
Design
5-year longitudinal study.
Setting
Population-based cohort.
Participants
1978 randomly selected individuals aged 65+ years at recruitment and assessed annually.
Measurements
Repeated measures of (1) depressive symptoms on the modified Center for Epidemiologic Studies-Depression Scale; (2) composite scores in the cognitive domains of attention, executive function, memory, language, and visuospatial function. Latent class trajectories were identified for depression and for each cognitive domain, and their associations investigated using dual trajectory modeling. Cognitive trajectories with z scores below −1 were designated as persistently low.
Results
Five depressive symptom trajectories were observed: rarely depressed (60.5%); low-grade, decreasing symptoms (18.5%); low-grade, increasing symptoms (9.6%); moderate-grade symptoms (7.4%); and consistent higher-grade symptoms (4.0%). For each cognitive domain, six trajectories were observed. The rarely depressed and low-grade decreasing symptom groups were the least likely to have persistently low cognition. The symptom trajectory most strongly associated with persistently low functioning in each domain was not the higher-grade group, but rather the low-grade increasing group in the case of attention, and the moderate-grade trajectory in the other four domains.
Conclusions
Consistently higher-grade depressive symptoms are less strongly associated with poor cognitive functioning than with either moderate or low-grade increasing depressive symptom trajectories, over time and across different domains. Examining both depression and cognition longitudinally allows heterogeneity of both to be addressed, revealing latent groups with potential diagnostic and prognostic implications.
Keywords: aging, community, epidemiology, neuropsychological functioning, group-based trajectory model
OBJECTIVES
Depression and cognitive impairment are both common and comorbid in older adults (1,2,3), and have shown consistent cross-sectional associations (4, 5). Evidence is mixed as to whether depression at a given time predicts subsequent cognitive decline, and at least partly depends on study sample and longitudinal methods as well as the specific outcomes assessed (4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15). Depression has been associated with subsequent cognitive decline in studies with prospective, retrospective, and cross-lagged designs (5, 6, 7, 11, 14, 15). However, some prospective studies have not supported this relationship (9, 12), and in others, results have depended on whether the outcome was general cognitive function or specific cognitive domains (8, 10, 13).
A more dynamic view of the relationship is provided by studies which examine both depression and cognition longitudinally (4, 8, 10, 13, 16, 17, 18, 19, 20). In one study, a prominent association was found between depressive symptoms and cognitive decline amongst individuals who had already experienced cognitive decline, suggesting that depression was a possible reaction to worsening cognitive abilities (16). An association has also been shown between the chronicity of depressive symptoms and cognitive decline (8, 10, 17, 18). To our knowledge, sub-patterns of depressive symptomatology have not been examined longitudinally in relation to cognitive functioning.
Study results would also be expected to vary between study samples composed of patients with depressive disorders found in clinical settings and those comprising individuals in the community with varying degrees of depressive symptoms. There are selection factors that lead some, but not all, individuals with symptoms of depression and/or cognitive impairment to seek clinical services, and additional factors affecting their eligibility for clinic-based research. Clinical studies usually focus on mood disorders rather than on depressive symptoms. The majority of studies examining the relationship between depression and cognition longitudinally have been based on community samples (4, 8, 9, 13, 16, 17, 18, 19, 20). We built on this body of research by investigating trajectories of depressive symptoms and cognitive function in a prospective study of an aging population-based cohort, over five years of follow-up, with the aim of determining whether any particular depressive symptom trajectory was associated with poor cognitive outcome.
METHODS
Participants
As previously reported (21, 22), the Monongahela-Youghiogheny Healthy Aging Team (MYHAT) cohort is an age-stratified random sample drawn from publicly available voter registration lists based in a region of southwestern Pennsylvania. Recruitment criteria included (a) age 65 or older, (b) living within the selected area, and (c) not already living in long-term care institutions. Individuals were ineligible if they were (d) too ill to participate, (e) too severely impaired in vision or hearing, or (f) decisionally incapacitated. After initially recruiting 2036 individuals, we excluded those with moderate to severe cognitive impairment, defined as an age-education-corrected Mini-Mental State Examination (MMSE) (23, 24) score of less than 21 out of 30. The remaining 1982 individuals underwent the complete assessment at baseline and five subsequent annual visits, for a total of six assessments.
Assessment – Neuropsychological Testing
Cognitive functioning was assessed in five domains: (1) attention/processing speed (Trailmaking Test A, digit span), (2) executive function (Trailmaking Test B, initial letter fluency, clock drawing), (3) language (Boston Naming Test, category fluency, modified Token Test), (4) memory (immediate and delayed logical memory and visual reproduction), and (5) visuospatial skill (block design) (21, 22). A composite z-score was created in each cognitive domain by first standardizing each individual test score, i.e. subtracting off the baseline sample mean and dividing by the baseline sample standard deviation, and then taking the arithmetic mean of the standardized scores within each domain for each individual at each time point.
Assessment – Depressive Symptom Screen
Depression was assessed using the modified Center for Epidemiologic Studies-Depression scale (mCES-D) (25, 26). The participant rates 20 symptoms of depression as present or absent (score 1 or 0) over most of the preceding week, summed to a mCES-D total score between 0 and 20.
Statistical Analyses
To identify distinct trajectory patterns for depression symptoms and cognitive functioning and assess their relationships, we used a latent group-based dual trajectory modeling approach that estimates the probabilistic links between two sets of developmental trajectories (27). Model outputs include (1) determination of the optimal number of distinct trajectory groups and the shapes of the trajectories, (2) estimated proportion of the population belonging to each trajectory group, and (3) estimated joint and conditional probabilities of group membership linking the two sets of trajectories. The model determines a posterior probability of membership in each of the trajectory groups for each individual in the sample, and the maximum posterior probability assignment rule assigns each individual to the trajectory group with the highest probability.
Model-fitting proceeded in two stages: (1) The best univariate trajectory models were found for mCES-D and each of the five cognitive domains based on a combination of optimum Bayesian information criterion (BIC), Wald tests, and clinical plausibility. (2) The dual trajectory models were then fit using starting parameters from the final univariate models. In order to find unadjusted trajectory patterns, trajectory models were fit using mCES-D and cognitive z-scores alone; covariate information was not incorporated. We used a zero-inflated Poisson model (28) for mCES-D and normal models for each of the five cognitive domain z-scores. We examined model diagnostics for all of the univariate and dual trajectory models using three criteria (29): (1) average posterior probability of group membership at least 0.7 for all groups, (2) odds of correct classification greater than 5.0 for all groups, and (3) estimated group probabilities reasonably close to the proportion of the sample assigned to the group based on the maximum posterior probability assignment rule. The SAS procedure TRAJ (30) was used to fit the trajectory models in SAS 9.3, (Cary, North Carolina: SAS Institute Inc, 2011). All other analyses were carried out using R version 3.1.3 (See Supplemental Digital Content 1).
As we will show under Results, we found five unique depressive symptom trajectories. We named them relative to one another using the following terms: rarely depressed; low-grade, decreasing depressive symptoms; low-grade, increasing depressive symptoms; moderate-grade depressive symptoms; and higher-grade depressive symptoms. We emphasize that these are based on a symptom scale and not on clinical diagnoses of depressive disorders.
We calculated descriptive statistics to characterize the sample at baseline, years of follow-up, and antidepressant use during the course of the study, for the overall sample and stratified by depression trajectory group. We performed global tests of association of these variables with the depression trajectory groups and did all pairwise comparisons of proportion antidepressant use among the depression trajectory groups using Holm-adjusted p-values.
With regard to cognition, we defined as “persistently low” the cognitive trajectories with a composite z-score of −1 or lower during the majority of the study period. We performed pairwise comparisons of proportions of persistently low cognitive trajectory membership among the depression trajectory groups using Holm-adjusted p-values. We also fit logistic regression models for each cognitive domain to model the log odds of persistently low cognition as a function of depressive symptom trajectory group (as an unordered categorical variable), both with and without adjustment for baseline demographics.
Since the high-grade symptom group had a shorter median follow-up time (2 years) than the other groups (4 or 5 years), we refit the five depression group dual trajectory models using only the first four time points (3 year follow-up) in a post-hoc analysis. Also, to determine whether results were influenced by the small group size for the persistently low attention group, we investigated whether expanding it to incorporate the next lowest trajectory altered our conclusions.
See Supplemental Digital Content 1 for a full description of our statistical methods.
RESULTS
Of the 1982 eligible recruited subjects, we excluded four who did not respond to the mCES-D questions at any of the six assessments, resulting in a baseline sample size of 1978. Our sample had mean and standard deviation (SD) age at baseline of 77.7 (7.4) years; 61.1% were women, 94.7% were white, and 58.9% had high school or less education. Their mean (SD) MMSE at baseline was 26.9 (2.4) and mean (SD) mCES-D score at baseline was 0.94 (2.1). The overall median follow-up time based on the mCES-D data was 5 years (range 0–5 years) (Table 1).
TABLE 1.
Characteristics of Participants, Overall and According to Depression Trajectory Group
| Depression Trajectory Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| All Participants (N=1978) |
Rarely (N=1248, 63.1%) |
Low, Decreasing (N=395, 20.0%) |
Low, Increasing (N=130, 6.6%) |
Moderate (N=126, 6.4%) |
High (N=79, 4.0%) |
Global Test | |||
| N (%) | Test statistic | df | p | ||||||
| Age group at baseline | 10.32a | 8 | 0.2430 | ||||||
| 65–74 | 679 (34.3) | 455 (36.5) | 125 (31.6) | 37 (28.5) | 36 (28.6) | 26 (32.9) | |||
| 74–85 | 914 (46.2) | 570 (45.7) | 184 (46.6) | 65 (50.0) | 60 (47.6) | 35 (44.3) | |||
| 85+ | 385 (19.5) | 223 (17.9) | 86 (21.8) | 28 (21.5) | 30 (23.8) | 18 (22.8) | |||
| Education | 20.51a | 4 | 0.0004 | ||||||
| High school or less | 1164 (58.9) | 690 (55.3) | 246 (62.3) | 86 (66.2) | 87 (69.0) | 55 (69.6) | |||
| More than high school | 814 (41.2) | 558 (44.7) | 149 (37.7) | 44 (33.8) | 39 (31.0) | 24 (30.4) | |||
| Sex | 50.21a | 4 | <0.0001 | ||||||
| Male | 770 (38.9) | 556 (44.6) | 116 (29.4) | 39 (30.0) | 44 (34.9) | 15 (19.0) | |||
| Female | 1208 (61.1) | 692 (55.4) | 279 (70.6) | 91 (70.0) | 82 (65.1) | 64 (81.0) | |||
| Race | NAb | NA | 0.0813 | ||||||
| White | 1873 (94.7) | 1189 (95.3) | 375 (94.9) | 117 (90.0) | 120 (95.2) | 72 (91.1) | |||
| Non-white | 105 (5.3) | 59 (4.7) | 20 (5.1) | 13 (10.0) | 6 (4.8) | 7 (8.9) | |||
| Antidepressant use | |||||||||
| At baselined | 267 (13.5) | 115 (9.2) | 80 (20.2) | 19 (14.6) | 27 (21.4) | 26 (32.9) | 67.47a | 4 | <0.0001 |
| At any timee | 392 (19.8) | 160 (12.8) | 108 (27.3) | 34 (26.2) | 54 (42.9) | 36 (45.6) | 130.87a | 4 | <0.0001 |
| Mean (SD)/Median | |||||||||
| Age at baseline | 77.7 (7.4)/78 | 77.3 (7.5)/78 | 78.1 (7.5)/79 | 78.6 (6.9)/80 | 78.4 (7.3)/79.5 | 78.8 (7.4)/80 | 11.87c | 4 | 0.0183 |
| MMSE at baseline | 26.9 (2.4)/27 | 27.1 (2.2)/27 | 26.8 (2.6)/27 | 27.0 (2.7)/28 | 26.1 (2.8)/26.5 | 26.1 (2.9)/27 | 24.87c | 4 | <0.0001 |
| mCES-D at baseline | 0.94 (2.1)/0 | 0.1 (0.3)/0 | 2.2 (1.9)/2 | 0.3 (0.7)/0 | 2.4 (2.6)/2 | 7.1 (4.0)/7 | 1241.89c | 4 | <0.0001 |
| Median (range) | |||||||||
| Years of follow-up | 5 (0, 5) | 5 (1, 5) | 4 (0, 5) | 5 (1, 5) | 4 (0, 5) | 2 (0, 5) | 18.31c | 4 | 0.0011 |
Notes: MMSE: Mini-Mental State Examination; mCES-D: modified Center for Epidemiologic Studies-Depression Scale; df: degrees of freedom; SD: standard deviation; KW: Kruskal-Wallis; NA: Not applicable
Chi-squared test
Fisher’s exact test
Kruskal-Wallis test
For antidepressant use at baseline, Holm-adjusted pairwise Fisher’s exact tests at familywise error rate of 0.05 resulted in significantly greater antidepressant use for all groups with respect to the rarely depressed group (Padjusted < 0.001 in each case) with the exception of the low, increasing group (Padjusted = 0.30). The high-grade depression group had significantly greater antidepressant use than the low-increasing group (Padjusted<0.02). The other comparisons were not significant.
For antidepressant use at any time during the course of the study, Holm-adjusted pairwise Fisher’s exact tests at familywise error rate of 0.05 resulted in significantly greater antidepressant use for all groups with respect to the rarely depressed group (Padjusted < 0.001 in each case). The moderate-grade and high-grade depression groups had significantly greater antidepressant use than the low-decreasing group (Padjusted<0.01 for both) and the low-increasing group (Padjusted=0.02 for both). The other comparisons were not significant.
Depression trajectory model
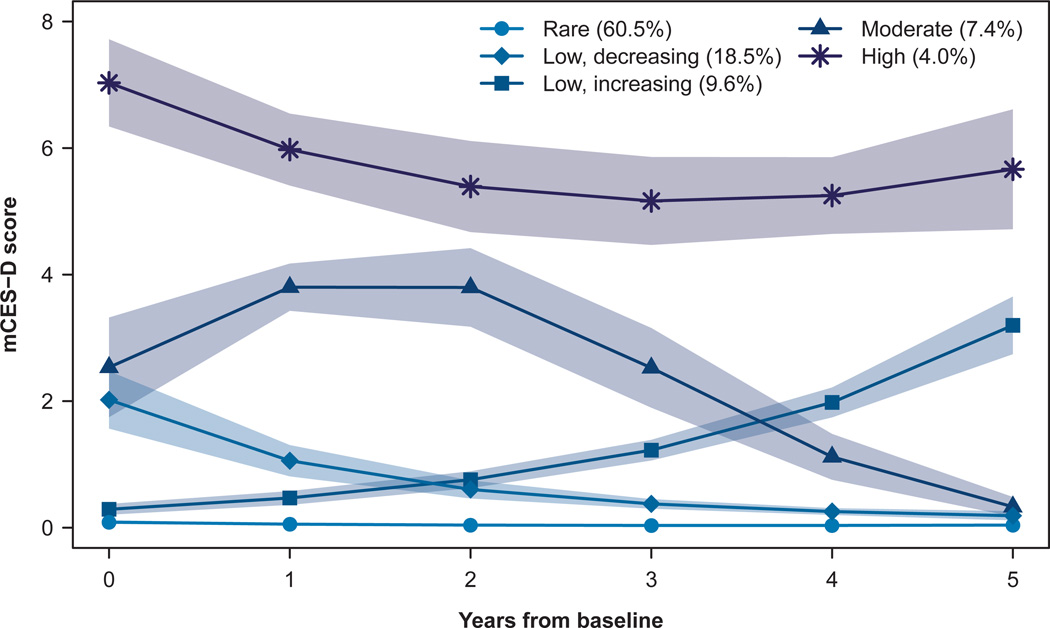
We report in this section the estimated values from the univariate model rather than from each of the five dual trajectory models; results were similar although not identical across models (data not shown). Each depressive symptom trajectory group had an estimated population percent group membership (Figure 1): rarely depressed (60.5%); low-grade, decreasing symptoms (18.5%); low-grade, increasing symptoms (9.6%); moderate-grade symptoms (7.4%); and higher-grade symptoms (4.0%).
FIGURE 1. Trajectories of depressive symptoms, with 95% confidence bands.
Note: mCES-D: modified Center for Epidemiologic Studies-Depression scale.
Baseline characteristics (Table 1). Women and those with lower education were significantly overrepresented among all depressive symptom trajectory groups other than the rarely depressed. The moderate- and higher-grade symptom groups had the lowest mean baseline MMSE scores. Baseline mCES-D scores and baseline antidepressant use also differed significantly across groups.
Follow-up duration differed significantly by symptom group, ranging from a median of 2 years in the higher-grade group to 5 years in the rarely depressed and low-grade, increasing groups (Table 1).
Antidepressant use. Overall, 392 (19.8%) of the 1978 participants reported daily antidepressant use at one or more of the assessments, and proportions differed significantly across symptom groups, ranging from 45.6% in the higher-grade group to 12.8% in the rarely depressed group (Table 1).
Cognitive domain trajectory models
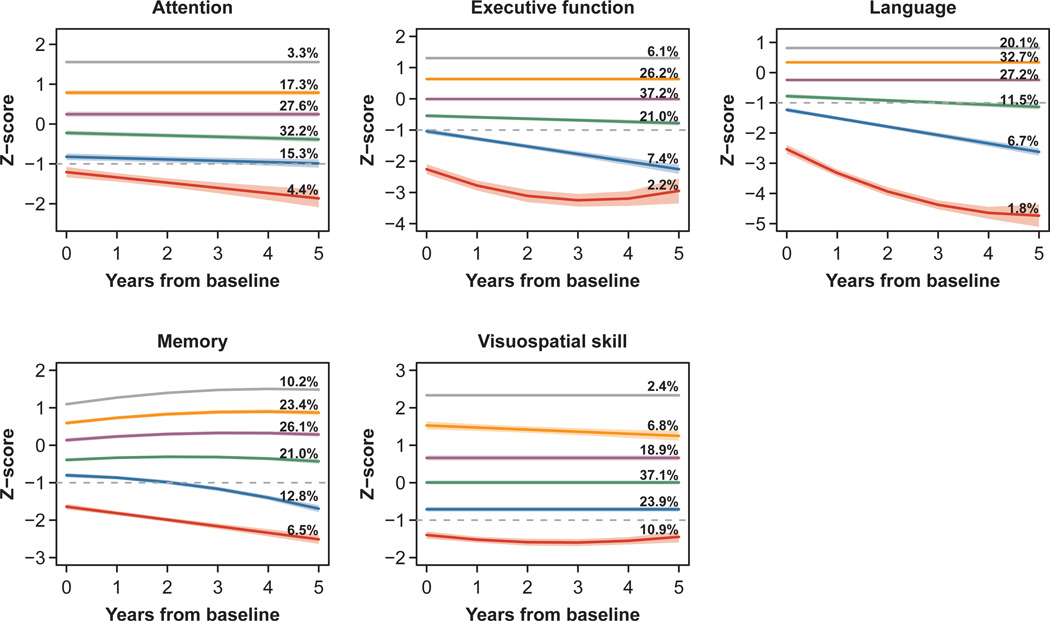
We identified six trajectories for each cognitive domain (Figure 2) which are ranked from lowest (1) to highest (6) composite z-score. The trajectory groups with the lowest mean z-scores tend to show most decline in all domains other than visuospatial skills, which had fairly stable trajectories overall. Memory increases slightly in the four highest trajectory groups, which is consistent with known practice effects associated with repeated memory tests (31).
FIGURE 2. Trajectories of cognitive functioning, with 95% confidence bands.
Note: Dotted gray lines indicate z-score of −1. Trajectories were designated as persistently low if they had a z-score of −1 or below for the majority of the study period.
We designated as persistently low the trajectories with z-scores of −1 (dotted lines in Figure 2) or below for the majority of the study period. This included the lowest trajectory for attention and visuospatial function, and the lowest two trajectories for executive function, language, and memory.
Relationships between depression and cognitive function trajectories
We considered the proportion of individuals in each depressive symptom group who were assigned to the persistently low cognitive trajectories (defined above) for each cognitive domain (Table 2). In the domain of attention, the low-grade increasing group had the highest percent membership in the persistently low attention group. Specifically, 12% of the low-increasing symptom group was classified into the persistently low attention group, compared to 5% of the moderate and higher-grade symptom groups, 3% of the rarely depressed group, and 0% of the low-decreasing symptom group. For the other cognitive domains, the moderate-grade symptom group had the highest percent membership in the persistently low trajectory groups. Individuals in the rarely and low-grade decreasing symptom groups were least likely to be in the persistently low cognitive trajectory groups. Pairwise comparisons of proportions of all groups against the highest-ranked group revealed significant differences between rare and low-grade decreasing symptom groups and the highest-ranked group in each domain. The higher-grade symptom group differed significantly from the highest-ranked group (moderate-grade symptoms) only in executive function. These rank orderings (Table 2) were consistent with results of unadjusted logistic regression models for persistently low cognition among the symptom groups. After adjusting for baseline demographics, the odd ratios for the symptom groups and their rankings did not change substantially. The exception was the memory domain, in which the higher-grade group displaced the moderate-grade group for highest odds ratio for persistently low cognition; however, these odds ratios had overlapping confidence intervals (Supplemental Digital Content 2).
Table 2.
Rank-ordering of Depression Trajectory Groups by Proportion with Persistently Low Cognition
| Rank | Attention | Executive | Language | Memory | Visuospatial |
|---|---|---|---|---|---|
| 1 | Low, increasing (12%) | Moderate (28%) | Moderate (21%) | Moderate (32%) | Moderate (27%) |
| 2 | Moderate (5%); P=0.0908 | *Low, increasing (17%); P=0.0170 | High (16%); P=0.2406 | High (31%); P=0.4952 | High (21%); P=0.1683 |
| 3 | High (5%); P=0.0908 | *High (11%); P=0.0052 | Low, increasing (15%); P=0.2349 | Low, increasing (23%); P=0.1178 | *Low, increasing (13%); P=0.0034 |
| 4 | *Rare (3%); P<0.0001 | *Low, decreasing (9%); P<0.0001 | *Low, decreasing (10%); P=0.0042 | *Low, decreasing (20%); P=0.0088 | *Low, decreasing (11%); P<0.0001 |
| 5 | *Low, decreasing (0%); P<0.0001 | *Rare (6%); P<0.0001 | *Rare (6%); P<0.0001 | *Rare (16%); P<0.0001 | *Rare (6%); P<0.0001 |
Notes:
Significant difference (P<0.05) from the Rank 1 group; one-sided Fisher’s exact test of difference in proportions.
P-values within each cognitive domain were adjusted using Holm’s correction.
Population estimates of joint and conditional probabilities of depression and cognitive function trajectory group membership are tabulated in Supplemental Digital Content 3.
Model diagnostics
Model diagnostics (Supplemental Digital Content 4) indicated that all of the dual trajectory models satisfied the three diagnostic criteria, with the exception that group 3 of visuospatial had an odds of correct classification (OCC) of 4.69, which is less than the suggested minimum of 5 (29). Given that the other diagnostics for this model satisfied the criteria, and that the visuospatial domain had more missing data than the other cognitive domains, this is probably close to the best fitting model that can be achieved for the visuospatial data.
Post-hoc analyses
To address potential bias from the shorter median follow-up time for the higher-grade depressive symptom group (2 years) versus the other groups (4 or 5 years), we refit the five depression group dual trajectory models using only the first four rather than all six time points, i.e., restricting follow-up to three years. We found that a moderate depressive symptom group was again ranked highest in all unadjusted and adjusted models including in the attention domain (Supplemental Digital Content 5).
Finally, incorporating the second-lowest trajectory into the persistently low attention group did not change our major conclusions, suggesting that small group size does not explain the results. The low-increasing depression group was again ranked highest, and results of pairwise significance tests were unchanged.
CONCLUSIONS
Examining depressive symptoms over time in our population-based cohort, we identified five distinct trajectories: a rarely depressed group, a low-grade decreasing group; a low-grade increasing group, a moderate-grade group, and a group with consistently higher-grade symptoms. Those with the higher-grade symptoms were more likely to be women, while those rarely reporting depression tended to be men. The rarely depressed group had the largest proportion of individuals with greater than high school education; the reverse was true among individuals with moderate and higher grade depressive symptoms. These results are consistent with our previous study (32).
Our findings were somewhat unexpected regarding our main outcome. Persistently low cognitive function trajectories were not associated the most strongly with the high-grade depressive symptom trajectory. Rather, they were most strongly associated with the low-grade increasing trajectory in the attention domain and the moderate-grade trajectory in the domains of executive function, memory, language, and visuospatial function. This pattern held true even after accounting for the greater attrition in the higher-grade symptom group (by refitting the models using a shorter follow-up time).
A handful of previous studies have examined the relationship between depression and cognitive decline over time. One found an association between depressive symptoms and change in MMSE scores over 13 years; however, this association lost significance when adjusted for potential confounders (13). Other studies have found associations between persistent depressive scores over time and greater risk of cognitive decline (18, 20), specifically, with declines in verbal knowledge and fluency, attention, and memory (8, 10). Unlike ours, these previous studies either dichotomized depression scores from the CES-D or the Geriatric Depression Scale (8, 10) or examined measures of global cognitive function rather than specific cognitive domains (18, 20). Conversely, associations have been found between baseline levels of immediate and short-term verbal memory, verbal abilities, and mental tracking with persistent depression (17). Impairments in processing speed, but not general cognitive functioning, have also been found to be predictive of depressive symptoms (13).
Our results broadly demonstrate that the initial level of depressive symptoms reveals less about associated cognitive trajectories than the subsequent diverging paths these symptoms take. While depression measured on a given day provides a useful snapshot at a single point in time, it does not tell us where the individual’s depression on that day stands in relation to the course of his or her depression, i.e. whether a given depression score is part of a declining, stable, or increasing time course. The same holds true for cognition. By examining both depression and cognition annually for five years, we were able to first identify the latent trajectories of both and then to investigate their relationships with each other. Further, by examining the full range of depressive symptom scores in our cohort, rather than dichotomizing them as in previous studies (8, 10), we were able to identify five latent trajectories of depressive symptoms over time. By investigating – rather than burying – the inherent heterogeneity of depression and cognition, we were able to examine in finer grain the relationships of interest. This approach led us to the unexpected finding that it was the moderate symptom group and not the consistently higher-grade symptom group which was most associated with poor cognitive functioning over time. Notably, both the moderate and low-decreasing symptom groups had initial mCES-D mean scores around 2 (i.e., reported two depressive symptoms over the preceding week.). Similarly, while both the low-grade increasing group and the rarely depressed group started with mean scores around zero, the low-grade increasing group displayed worse subsequent cognitive functioning than the rarely depressed group.
It is plausible to speculate that the persistent higher-grade symptom group, which was our smallest trajectory group, of whom nearly half reported using antidepressant drugs, represents individuals with clinically significant depressive disorders. While such individuals are in the majority as patients seeking care for late life depression in clinical settings, they are in the minority at the population level. In such patients, depression has been associated with impairments in information processing speed, visuospatial functioning, and executive functioning; the slowed processing speed has been proposed to underlie the additional cognitive deficits seen in this group (33).
In addition, in our population-based sample, we are able to identify a larger group of individuals with likely subsyndromal depression, such as our moderate and low-increasing symptom groups, who might not be encountered in the clinical setting but have poor cognition. Potentially, one or more shared processes may underlie both their depressive symptoms and their poor cognitive functioning.
One possible shared process is that persistent depression may be in reaction to continued cognitive decline (16). In a three year prospective study, while depressive symptoms were unrelated to subsequent cognitive functioning, cognitive functioning was related to subsequent depressive symptoms (12). Other studies (34, 35) have shown an association of increasing mood and/or anxiety symptoms with decline in cognitive functioning. This association disappears at a certain point during the process of cognitive decline, perhaps because the individuals have lost awareness of their own decline.
Alternatively, a common neuropathological entity might drive both the depression and cognitive decline. Late onset depression has been associated with more severe cognitive and neurological changes than early onset depression, with late onset depression also associated with a faster rate of hippocampal volume loss than early onset (36). The vascular depression hypothesis argues for a significant relationship between cerebrovascular disease and depression, either through direct lesions or through an accumulation of white matter changes, leading or contributing to a depressive episode (37). Depressive symptoms have been associated with white matter hyperintensities in the frontal and temporal regions (38), although the impact of depressive symptoms on cognitive decline has been shown to be independent of the severity of white matter change and medial temporal lobe atrophy (14). Particular emphasis has also been placed on the role of the frontolimbic and frontostriatal pathways in the depression-executive dysfunction syndrome (37). Interactions between hypercortisolemia related to depression and its effects on hippocampal structure and function as well as the concomitant role of cerebrovascular disease may also help to explain the relationship between depression and cognitive impairment (39). Dementia is also much more strongly associated with depressive symptoms which have developed within a year of the diagnosis of Alzheimer’s disease than with depressive symptoms which had developed earlier (40).
Our study had a large, representative, population-based sample and a prospective design with repeated depression symptom screens and detailed cognitive assessments, allowing subtle effects to be detected using appropriate statistical modeling techniques. A sample, when drawn from the community at large, allows the identification of groups who would not be seen in clinical settings, such as those with subsyndromal depression and normal variations in mood. However, in such samples, the assessment of depression relies on self-reported depressive symptoms, rather than on expert diagnosis of depressive disorders as used in clinical research. The increasing proportions of antidepressant usage across increasing depressive symptom trajectories provide added face validity to our trajectory-based classification. Like previous studies (1) we found about 4% of our cohort was in our higher-grade depressive symptom trajectory group. Individuals with low and moderate-grade depressive symptoms may not fulfill entry criteria for clinical research; their exclusion from such research may lead significant interactions and nuanced symptom relationships to remain undetected in the preclinical stages. From a public health perspective these individuals with subsyndromal depressive symptoms warrant further investigation, as they are essential to our understanding of possible emerging disease processes within the population as a whole. Our findings demonstrate the importance of longitudinal population-based studies in understanding the fine-grained relationships between depression and cognitive decline.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the MYHAT staff and participants.
The work reported here was supported in part by grants # R01 AG023651, K07 AG044395, and K23AG038479 from the National Institute on Aging, NIH, US DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous Presentation: This work was previously presented as a poster at the American Association for Geriatric Psychiatry 2015 Annual Meeting (March 28, 2015)
The authors have no disclosures to report.
The authors report no conflicts of interest.
LIST OF SUPPLEMENTAL DIGITAL CONTENT
Supplemental Digital Content 1. pdf
Supplemental Digital Content 2. pdf
Supplemental Digital Content 3. pdf
Supplemental Digital Content 4. pdf
Supplemental Digital Content 5. pdf
Contributor Information
Julie A. Graziane, University of Pittsburgh School of Medicine, Department of Psychiatry
Joanne C. Beer, University of Pittsburgh School of Medicine, Department of Psychiatry
Beth E. Snitz, University of Pittsburgh School of Medicine, Department of Neurology
Chung-Chou H. Chang, University of Pittsburgh School of Medicine, Department of Medicine University of Pittsburgh Graduate School of Public Health, Department of Biostatistics.
Mary Ganguli, University of Pittsburgh School of Medicine, Department of Psychiatry University of Pittsburgh School of Medicine, Department of Neurology; University of Pittsburgh Graduate School of Public Health, Department of Epidemiology.
REFERENCES
- 1.Committee on the Mental Health Workforce for Geriatric Populations, Board on Health Care Services, Institute of Medicine. The mental health and substance use workforce for older adults: in whose hands? In: Eden J, Maslow K, Le M, Blazer D, editors. Washington DC: National Academies Press (US); 2012. [Accessed April 20, 2015]. Available at: http://www-ncbi-nlm-nih-gov.pitt.idm.oclc.org/books/NBK201410/ [PubMed] [Google Scholar]
- 2.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steffens DC, Fisher GG, Langa KM, et al. Prevalence of depression among older Americans: the Aging, Demographics and Memory Study. Int Psychogeriatr. 2009;21:879–888. doi: 10.1017/S1041610209990044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dufouil C, Fuhrer R, Dartigues J, et al. Longitudinal analysis of the association between depressive symptomatology and cognitive deterioration. Am J Epidemiol. 1996;144:634–641. doi: 10.1093/oxfordjournals.aje.a008974. [DOI] [PubMed] [Google Scholar]
- 5.Panza F, D’Introno A, Colacicco AM, et al. Temporal relationship between depressive symptoms and cognitive impairment: the Italian Longitudinal Study on Aging. J Alzheimers Dis. 2009;17:899–911. doi: 10.3233/JAD-2009-1111. [DOI] [PubMed] [Google Scholar]
- 6.Barnes DE, Alexopoulos GS, Lopez OL, et al. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006;63:273–280. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 7.Bunce D, Batterham PJ, Christensen, et al. Causal associations between depression symptoms and cognition in a community-based cohort of older adults. Am J Geriatr Psychiatry. 2014;22:1583–1591. doi: 10.1016/j.jagp.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Dotson VM, Resnick SM, Zonderman AB. Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. Am J Geriatr Psychiatry. 2008;16:318–330. doi: 10.1097/JGP.0b013e3181662a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson AS, Korten AE, Jacomb PA, et al. The course of depression in the elderly: a longitudinal community-based study in Australia. Psychol Med. 1997;27:119–129. doi: 10.1017/s0033291796004199. [DOI] [PubMed] [Google Scholar]
- 10.Goveas JS, Espeland MA, Hogan PE, et al. Depressive symptoms and longitudinal changes in cognition: Women’s Health Initiative Study of Cognitive Aging. J Geriatr Psychiatry Neurol. 2014;27:94–102. doi: 10.1177/0891988714522697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Köhler S, van Boxtel MP, van Os J, et al. Depressive symptoms and cognitive decline in community-dwelling older adults. J Am Geriatr Soc. 2010;58:873–879. doi: 10.1111/j.1532-5415.2010.02807.x. [DOI] [PubMed] [Google Scholar]
- 12.Perrino T, Mason CA, Brown SC, et al. Longitudinal relationships between cognitive functioning and depressive symptoms among Hispanic older adults. J Gerontol B Psychol Sci Soc Sci. 2008;63:309–317. doi: 10.1093/geronb/63.5.p309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Kommer TN, Comijs HC, Aartsen MJ, et al. Depression and cognition: how do they interrelate in old age? Am J Geriatr Psychiatry. 2013;21(4):398–410. doi: 10.1016/j.jagp.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Verdelho A, Madureira S, Moleiro S, et al. Depressive symptoms predict cognitive decline and dementia in older people independently of cerebral white matter changes: the LADIS study. J Neurol Neurosurg Psychiatry. 2013;84(11):1250–1254. doi: 10.1136/jnnp-2012-304191. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RS, Mendes de Leon CF, Bennett DA, et al. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry. 2004;75:126–129. [PMC free article] [PubMed] [Google Scholar]
- 16.Bassuk SS, Berkman LF, Wypij D. Depressive symptomatology and incident cognitive decline in an elderly community sample. Arch Gen Psychiatry. 1998;55:1073–1081. doi: 10.1001/archpsyc.55.12.1073. [DOI] [PubMed] [Google Scholar]
- 17.Mojtabai R, Olfson M. Cognitive deficits and the course of major depression in a cohort of middle-aged and older community-dwelling adults. J Am Geriatr Soc. 2004;52:1060–1069. doi: 10.1111/j.1532-5415.2004.52302.x. [DOI] [PubMed] [Google Scholar]
- 18.Paterniti S, Verdier-Taillefer MH, Dufouil C, et al. Depressive symptoms and cognitive decline in elderly people: Longitudinal study. Br J Psychiatry. 2002;181:406–410. doi: 10.1192/bjp.181.5.406. [DOI] [PubMed] [Google Scholar]
- 19.Zahodne LB, Stern Y, Manly JJ. Depressive symptoms precede memory decline, but not vice versa, in non-demented older adults. J Am Geriatr Soc. 2014;62:130–134. doi: 10.1111/jgs.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeki Al Hazzouri A, Vittinghoff E, Byers A, et al. Long-term cumulative depressive symptom burden and risk of cognitive decline and dementia among very old women. J Gerontol A Biol Sci Med Sci. 2014;69:595–601. doi: 10.1093/gerona/glt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganguli M, Snitz B, Vander Bilt J, et al. How much do depressive symptoms affect cognition at the population level? The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) study. Int J Geriatr Psychiatry. 2009;24:1277–1284. doi: 10.1002/gps.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganguli M, Fu B, Snitz BE, et al. Vascular risk factors and cognitive decline in a population sample. Alzheimer Dis Assoc Disord. 2014;28:9–15. doi: 10.1097/WAD.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Mungas D, Marshall SC, Weldon M, et al. Age and education correction of Mini-Mental State Examination for English and Spanish-speaking elderly. Neurology. 1996;46:700–706. doi: 10.1212/wnl.46.3.700. [DOI] [PubMed] [Google Scholar]
- 25.Ganguli M, Gilby J, Seaberg E, et al. Depressive symptoms and associated factors in a rural elderly population: The MoVIES Project. Am J Geriatr Psychiatry. 1995;3:144–160. doi: 10.1097/00019442-199500320-00006. [DOI] [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 27.Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychol Methods. 2001;6:18–34. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 28.Lambert D. Zero-Inflated Poisson Regression, with an Application to Defects in Manufacturing. Technometrics. 1992;34:1–14. [Google Scholar]
- 29.Nagin DS. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 30.Jones BL, Nagin DS. Advances in Group-Based Trajectory Modeling and an SAS procedure for estimating them. Sociol Methods Res. 2007;35:542–571. [Google Scholar]
- 31.Dodge HH, Wang CN, Chang CC, et al. Terminal decline and practice effects in older adults without dementia: The MoVIES project. Neurology. 2011;77:722–730. doi: 10.1212/WNL.0b013e31822b0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreescu C, Chang CH, Mulsant BH, et al. Twelve-year depressive symptom trajectories and their predictors in a community sample of older adults. Int Psychogeriatr. 2008;20:221–236. doi: 10.1017/S1041610207006667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 34.Forsell Y, Jorm AF, Winblad B. Association of age, sex, cognitive dysfunction, and disability with major depressive symptoms in an elderly sample. Am J Psychiatry. 1994;151:1600–1604. doi: 10.1176/ajp.151.11.1600. [DOI] [PubMed] [Google Scholar]
- 35.Bierman EJM, Comijs HC, Jonker C, et al. Symptoms of anxiety and depression in the course of cognitive decline. Dement Geriatr Cogn Disord. 2007;24:213–219. doi: 10.1159/000107083. [DOI] [PubMed] [Google Scholar]
- 36.Sachs-Ericsson N, Corsentino E, Moxley J, et al. A longitudinal study of differences in late and early onset geriatric depression: depressive symptoms and psychosocial, cognitive and neurological functioning. Aging Ment Health. 2013;17:1–11. doi: 10.1080/13607863.2012.717253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60:1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien JT, Firbank MJ, Krishnan MS, et al. White matter hyperintensities rather than lacunar infarcts are associated with depressive symptoms in older people: the LADIS study. Am J Geriatr Psychiatry. 2006;14:834–841. doi: 10.1097/01.JGP.0000214558.63358.94. [DOI] [PubMed] [Google Scholar]
- 39.Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10:345–367. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green RC, Cupples LA, Kurz A, et al. Depression as a risk factor for Alzheimer Disease: the MIRAGE Study. Arch Neurol. 2003;60:753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.