Abstract
Objective
To examine the link between cytokines in intervertebral disc (IVD) tissues and axial back pain.
Design
In vitro study with human IVD cells cultured from cadaveric donors and annulus fibrosus (AF) tissues from patients.
Results
Cultured nucleus pulposus (NP) and AF cells were stimulated with interleukin (IL)-1β. IL-8 and IL-7 gene expression was analyzed using real-time PCR. IL-8 protein was quantified by ELISA. Following IL-1β stimulation, IL-8 gene expression increased 26,541 fold in NP cells and 22,429 fold in AF cells, while protein released by the NP and AF cells increased 2,389 and 1,784 fold, respectively. IL-7 gene expression increased 3.3 fold in NP cells (p<0.05).
Cytokine profiles in AF tissues collected from patients undergoing surgery for back pain (painful group) or scoliosis (controls) were compared by cytokine array. IL-8 protein in the AF tissues from patients with back pain was 1.81 fold of that in controls. IL-7 and IL-10 in AF tissues from the painful group were, respectively, 6.87 and 4.63 times greater than the corresponding values in controls (p<0.05).
Conclusion
Inflammatory mediators found in AF tissues from patients with discogenic back pain are likely produced by IVD cells, and may play a key role in back pain.
Keywords: intervertebral disc, discogenic back pain, chemokine, cytokine, interleukin
BACKGROUND
Chronic low back pain is a major public health and economic problem leading to billions of dollars in healthcare expenditure1 in the United States. Chronic low back pain has been estimated to be discogenic in origin 40% of the time.2, 3 Currently, provocative lumbar discography is the most sensitive and specific tool for diagnosis of lumbar discogenic pain,4 although controversies exist.5
Discogenic low back pain is not well correlated with intervertebral disc (IVD) degeneration: most individuals with disc degeneration are not affected by chronic symptoms.6 The individuals that are affected by chronic, debilitating spinal pain due to symptomatic degenerative disc disease usually begin to suffer during their early adult years and rarely achieve complete relief from the available medical therapies. Current surgical treatments for severely symptomatic disc degeneration are limited to procedures that disrupt, rather than restore, the disc structure (e.g., spinal fusion or disc arthroplasty). Furthermore, the degree of symptomatic relief following surgery is unpredictable and often incomplete, mainly because the exact cause of pain is poorly understood. Degenerative disc disease may be responsive to biologic therapies;7 whether restoring disc structure will reduce back pain is unclear, however.
Kang and others have reported increased cytokine production in herniated disc tissues.8 However, axial discogenic pain is different in character from radicular pain resulting from disc herniation and is less responsive to current treatments. In this study, we aimed to identify biological markers that differentiate painful degenerative discs and asymptomatic discs with matching degrees of degeneration to understand why morphologically similar discs can show marked differences in clinical symptomatology.6 Cytokines that differentiate pain-generating IVDs from similar appearing degenerative IVDs that are not major sources of pain might help to predict treatment outcomes and serve as targets for biological therapies.
The source of cytokines in IVDs has been debatable. In herniated IVDs, inflammatory cells infiltrate the disc tissues and may produce cytokines. In this study, we aimed to demonstrate that cultured IVD cells are fully capable of secreting inflammatory mediators.
MATERIALS AND METHODS
Cytokine gene expression and protein production by cultured cells from cadaveric donors
Tissue collection
IVDs were obtained from human spine segments (donor age range 21–75 years) procured by the Gift of Hope Human Donor and Tissue Network of Illinois (approved by the Institutional Review Board; ORA #L01012604). IVDs were dissected within 24 hours from time of death, immediately after the spine segments were transported to the laboratory. AF and NP were separated. Cells were released by 0.4% Pronase (Calbiochem/Millipore) for 1 hour followed by 0.025% collagenase-P (Boehringer Ingelheim) digestion overnight. Cells were then plated in monolayer at 4×104 cells/cm2 in 12-well plates (Corning Life Sciences) with 1ml/well of DMEM/F12 medium with 20% FBS, until confluent. Cells were then serum-starved for 24 hours, and then stimulated with IL-1β (10 ng/mL; R&D systems, MN) in serum-free media for 24 hours. IL-8, IL-7 and IL-10 gene expression was analyzed using real-time PCR. IL-8 protein in the conditioned media was quantified using ELISA (Invitrogen, CA).
Real-time PCR
RNA from cultured NP and AF cells was isolated using an RNeasy Kit (Qiagen). Total RNA (0.5–1.0 μg) was reverse transcribed into cDNA with random primers using a High Capacity RNA to cDNA kit (Applied Biosystems). The expression levels of IL-8, IL-7 and IL-10 were determined with real-time PCR using Taqman Gene Expression Assays (Applied Biosystems). Amplification was performed with Taqman PCR Master Mix (Applied Biosystems) and a spectrofluorometric thermal cycler (7300 Real-time PCR System, Applied Biosystems). To standardize mRNA levels, the GAPDH gene was used as an internal control.
ELISA
Conditioned media from cultured NP and AF cells were collected and stored at −80°C until assayed. IL-8 was quantified using the Human IL-8 CytoSet ELISA kit (Invitrogen) according to the manufacturer’s instructions.
Cytokine protein content in patient-derived tissues by cytokine array
Patient selection and tissue preparation
Lumbar IVD tissues were collected from patients undergoing spinal surgery at Thomas Jefferson University Hospital under an IRB-approved protocol (IRB Control# 05U.237), with written informed consent. This study included adult patients undergoing anterior lumbar interbody fusion (ALIF) surgery for discogenic pain or spinal deformity reconstruction. Larger and less fragmented IVD tissue can be isolated with anterior lumbar fusion surgery compared with the posterior approach. All discs in the painful group were evaluated with provocative discography prior to surgery (Figure 1A and 1B). Specifically, discography was performed on patients under conscious sedation, with a double needle technique. A 25G needle was introduced into the central region of the disc (confirmed on AP and lateral fluoroscopy; Figure 1). 0.5 ml of contrast solution was injected into the disc, and the patient’s objective response (grimace, wince, etc.) was recorded; the patients were asked for an immediate grading of the pain (0/10 to 10/10 scale) and whether the pain was concordant (identical to the clinical pain) or discordant (different from the clinical symptoms). Discography was graded as “positive,” when the pain was 8–10/10 and concordant to axial symptoms according to the patient, “indeterminate,” when the pain was 4–7/10 and concordant to clinical symptoms, and “negative,” when pain was discordant to clinical symptoms. The morphology of the contrast flow on fluoroscopy was noted and recorded. For a disc level to be considered “positive,” the discographer also had to confirm annular disruption by the fluoroscopic contrast pattern at the painful level, and the presence of a “negative” level within the lumbar spine of the same patient. “Indeterminate” or “negative” discs were excluded from the study. Lumbar discs were also obtained from patients undergoing anterior lumbar fusion for scoliosis (as controls). Scoliosis patients undergoing deformity correction surgeries have been selected as negative controls, since these patients have degenerative IVDs but back pain is not the primary reason for operation. This allows matching of tissues from discogenic back pain with controls of similar degeneration on MRI. However, we acknowledge the limitation of this approach, since patients with scoliosis often have low back pain. Patients with discitis, systemic infection, or systemic inflammatory diseases were excluded from the study. Each study subject was evaluated with MR scanning of the lumbar spine. All studied discs were graded by T2 weighted MRIs using the Pfirrmann grading scheme.9 Every effort was made to select patients with comparable degrees of IVD degeneration for the painful and control groups, in order to reduce disc degeneration as a major confounding factor (Table 1). Patient age and sex were also noted (Table 1). Patients undergoing discography are under conscious sedation, typically provided by Midazolam injection to cause drowsiness, relieve anxiety, and prevent any memory of the event. This medication is not known to affect local tissue inflammation, and is thus unlikely to affect the biomarkers in the intervertebral disc tissue collected after surgery.
Figure 1. Discography: contrast has been injected into the L3/4, L4/5, and L5/S1 intervertebral discs (IVD).
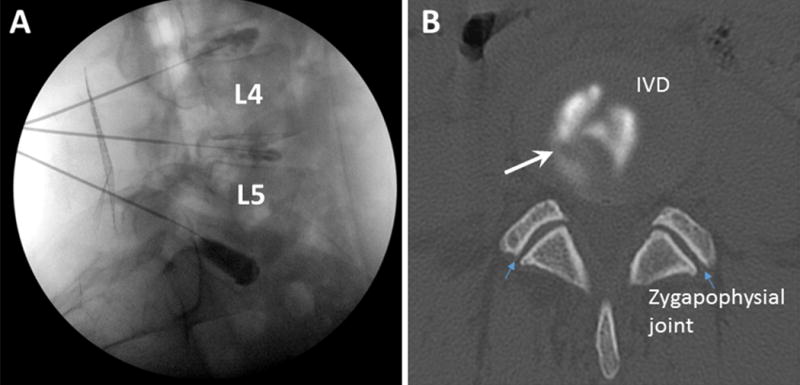
A. fluoroscopic lateral view of the lumbar spine; B: post discography CT scan axial view of the L4/5 disc. Large white arrow indicates an annular tear; small blue arrows indicate zygapophysial joints.
Table 1.
Patient characteristics.
| Patients | Control | Painful |
|---|---|---|
| Average years of age (range) | 53.5 (25–69) | 45.3 (26–54) |
| Sex (F/M) | 3/4 | 7/2 |
| MR grade | I (n=2) IV (n=3) V(n=2) |
III (n=3) IV (n=4) V (n=2) |
|
| ||
| Total | 7 | 9 |
The AF were isolated and snap frozen in liquid nitrogen and then stored in a −80°C freezer. Protein concentration was determined by BCA assay (Pierce). Quality of extracted proteins was verified by separation on a 4–12% polyacrylamide gel and staining with Coomassie blue. One hundred μg of protein was used for each cytokine array.
Cytokine array analysis
Human Cytokine Array 3.1 (RayBiotec, Norcross, GA), which detects 42 cytokines simultaneously, was used. The Array 3.1 utilizes the sandwich immunoassay principle, wherein a panel of capture antibodies is immobilized on a nitrocellulose membrane solid support. This array includes antibodies recognizing most of the cytokines known to be present in discs, or those that affect chondrocyte extracellular matrix metabolism. The membranes were blocked for 30 minutes before incubation with AF tissue lysates from patients who had undergone lumbar spine surgery for discogenic low back pain and for deformity corrections (as controls) at 4°C overnight. Then the array membranes were washed and incubated with biotin-conjugated detection antibodies for 2 hours. Following washing, membranes were incubated with horseradish peroxidase-conjugated streptavidin for 2 hours (RayBiotec). The membranes were washed, and the chemiluminescence was detected using Digital Imaging System (ImageQuant LAS-400, GE Healthcare). Each density was further normalized to the average of four positive controls within the same membrane and represented as % of positive control.
Pathway analysis for cytokine array
A pathway analysis was performed using Ingenuity Pathway Analysis (IPA) software (Qiagen, Valencia, CA). Specifically, the raw expression values were log-normal transformed, and a baseline transformation to the median of all values was then performed. The cytokines were subsequently filtered by independent sample Student’s t-test (p < 0.1) and fold change (≥ 1.5). Of the 42 cytokines, only 8 cytokines met these criteria (Figure 4). The data from these cytokines were then applied to hierarchical clustering, using Pearson-centered distance metric. Comparison between the painful and control groups was performed with two-sample unpooled t-test for unequal variances (also known as Welch’s t-test).
Figure 4. Inflammatory mediators identified in human annulus fibrosus (AF) tissues by cytokine array.
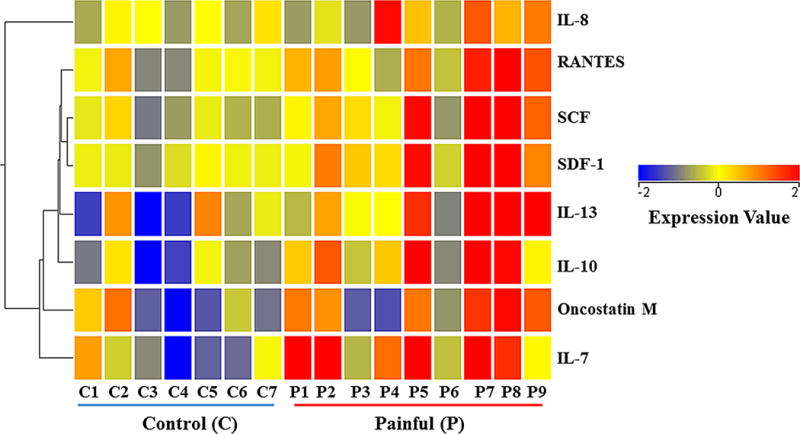
Tissues in the painful group (P1-9) are from patients undergoing surgery for axial back pain. Control tissues (C1-7) are from patients undergoing surgery for scoliosis deformity. SCF: Stem Cell Factor; SDF-1: chemokine stromal-derived factor-1; OSM: Oncostatin M.
RESULTS
IL-8 gene expression and protein secretion were upregulated by IL-1β treatment in cultured primary human NP and AF cells
IL-8 gene expression increased 29,664 fold (n=4, p=0.0083) in NP cells after stimulation with 10 ng/ml of IL-1β for 24 hours (Figure 2A). Similarly, IL-8 gene expression increased 22,429 fold in AF cells (n=4; p=0.0105; Figure 2B).
Figure 2. IL-8 gene expression and protein secreted by the annulus fibrosus (AF) and nucleus pulposus (NP) cells increase in response to IL-1β.
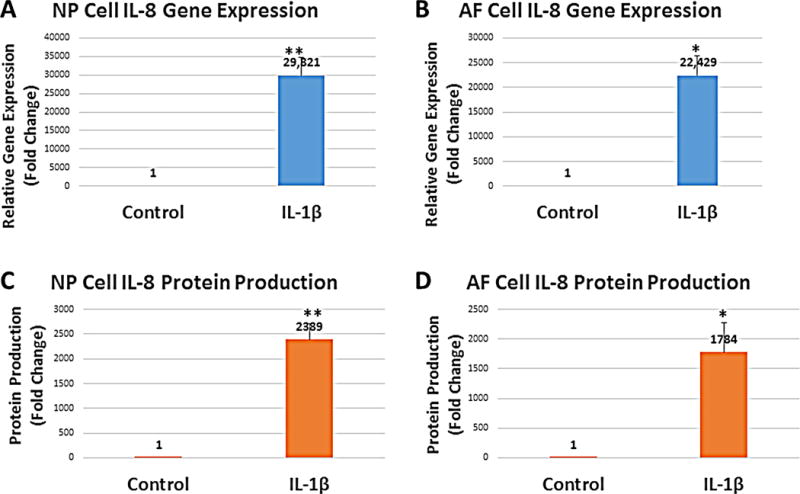
**p<0.01; *p<0.05. Error bars represent standard error of the mean; n=4.
IL-8 protein concentration in the culture media also increased in response to IL-1β stimulation (Figure 2C and 2D). NP cells secreted an average of 31 pg/ml of IL-8 into the culture media in the absence of IL-1β (n=4). The IL-8 concentration increased to 74,056 pg/ml after incubation with IL-1β for 24 hours (n=4). This represents a 2,389 fold increase (n=4; p=0.0055; Figure 2C). Also, IL-8 protein secreted by the AF cells into the culture media increased from an average of 53 pg/ml to 94,540 pg/ml in response to IL-1β treatment. This represents a 1,784 fold increase (n=4; p=0.0532; Figure 2D).
Expression of IL-7 and IL-10 genes was induced by IL-1β
Interestingly, gene expression for IL-7 (known as a hematopoietic growth factor) in NP cells also showed a moderate increase in response to IL-1β stimulation (3.28 fold increase, n=4; p=0.0145; Figure 3A). IL-7 gene expression did not increase significantly in AF cells after IL-1β stimulation (4.35 fold of control, n=4; p=0.0760; Figure 3B). IL-10 gene expression also showed a trend to increase following IL-1β stimulation in both NP and AF cells, but owing to the large individual variation, this was not statistically significant (NP: 1,489 fold induction, n=4; p=0.1330; AF: 98 fold induction, n=4; p=0.0648; Figure 3C&D).
Figure 3. IL-7 and IL-10 gene expression in NP and AF cells in response to IL-1β stimulation.
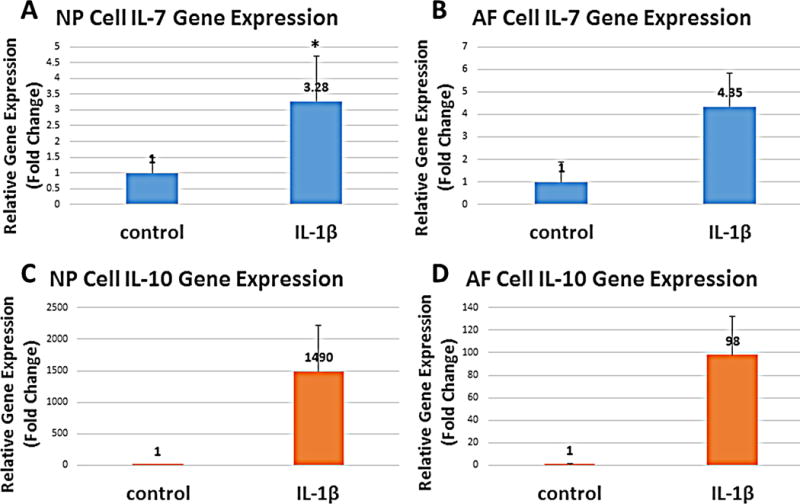
*p<0.05. Error bars represent standard error of the mean; n=4.
Levels of multiple cytokine proteins in AF tissues from patients with discogenic low back pain were higher than in tissues from control patients
Multiple chemokines/cytokines were elevated in AF tissues from patients with discogenic pain, compared with control tissues from patients with comparable degree(s) of degeneration (Figure 4). The color coding corresponds to fold changes compared with baseline (median of all values). Only cytokines showing a 1.5 fold or more difference between the painful and control groups are shown. Cytokines are clustered according to known pathways/gene ontology, indicated with the dendrogram on the left. The branches show the degree of similarity between different molecules: the shorter the branch, the more similarity in the ontology of cytokines.
To further illustrate individual variations, IL-8, IL-7 and IL-10 densities of each patient were plotted (Figure 5). IL-8 level in painful discs was 1.81 fold that of controls (p=0.0513), while IL-7 increased 6.87 fold (p=0.0241). IL-10 showed a 4.63 fold increase (p=0.0171; Figures 4 and 5).
Figure 5. Inflammatory mediators identified in human annulus fibrosus (AF) tissues by cytokine array.
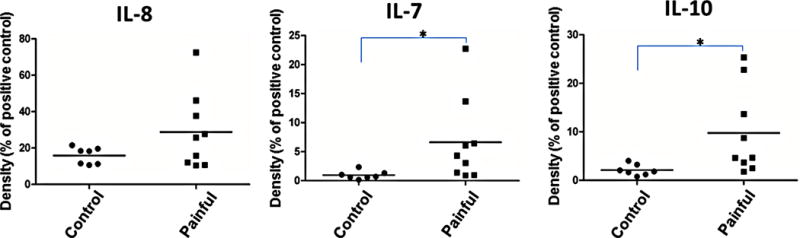
Tissues in the painful group are from patients undergoing surgery for axial back pain. Control tissues are from patients undergoing surgery for scoliosis deformity correction; *p<0.05.
Five other factors showed a significant increase in discogenic pain patients (Figure 4): RANTES (regulated and normal T cell expressed and secreted, also known as Chemokine C-C motif ligand 5 (CCL5)), showed a 2.29 fold increase (p=0.0218). Stem Cell Factor (SCF) increased 3.13 fold (p=0.0142), while chemokine stromal-derived factor-1 (SDF-1) increased 3.20 fold (p=0.0217). IL-13 showed a 3.18 fold increase (p=0.0329), while oncostatin M (OSM) showed a 2.31 fold increase in the painful group compared with controls (p=0.0247).
DISCUSSION
We have demonstrated that cultured IVD cells produced IL-8, and expressed IL-7 and IL-10 genes in this study. Kang et al. have reported cytokines including IL-6 in herniated cervical and lumbar discs,8, 10 and infiltrating inflammatory cells are thought to be the source of these inflammatory mediators. We have shown in the current study that contained, non-herniated IVD tissues express multiple cytokines; the origin of these mediators has been debated, because the IVD tissues have limited blood supply and access of inflammatory cells to IVDs is limited. Cultured IVD cells have been shown to produce TNF-α and IL-1β.11 Our current and previous12 studies have demonstrated that IVD cells, isolated in culture, are fully capable of producing a variety of cytokines and chemokines.
In this study, we have shown that cultured IVD cells produce large amounts of IL-8 in response to an inflammatory stimulus (e.g., IL-1β). IL-8, also known as CXCL8, is a chemokine produced by macrophages and other cell types, including epithelial cells, airway smooth muscle cells and endothelial cells.13 There are many receptors on the surface membrane of various cell types capable of binding IL-8; the most frequently studied types are the G protein-coupled serpentine receptors CXCR1 and CXCR2.14 Expression and affinity for IL-8 differ between the two receptors (CXCR1 > CXCR2).14 IL-8 is also known to be a potent promoter of angiogenesis15 and a chemoattractant for neutrophils.16 In target cells, IL-8 induces a series of physiological responses required for migration and phagocytosis, including increases in intracellular Ca2+, exocytosis (e.g. histamine release), and the respiratory burst.
We have shown for the first time that human IVD cells express the IL-7 and IL-10 genes. IL-7 is a hematopoietic growth factor secreted by stromal cells in the bone marrow and thymus.17 IL-10, also known as human cytokine synthesis inhibitory factor, is an anti-inflammatory cytokine. IL-10 itself can repress proinflammatory responses and limit excessive tissue disruption caused by inflammation,18 perhaps representing part of the repair mechanism. Intriguingly, elevated levels of IL-8, along with several other chemokines, correlate with acute inflammatory pain after tissue injury.19 IL-8 was found to be positively associated with pain in patients with fibromyalgia, a chronic pain syndrome refractory to treatments.20 The role of IL-8 in discogenic back pain was explored by Burke et al.21 They showed increased IL-8 and IL-6 production by cultured lumbar IVD explants from patients with discogenic pain compared with patients with sciatica.21 Consistent with their findings, we have shown that IL-8 gene expression and protein production by cultured primary human IVD cells increased by a thousand fold or more after exposure to IL1β. Based on our findings, we hypothesize that a high level of IL-8 and a cluster of additional inflammatory mediators may, at least in part, mediate back pain. Future studies will be conducted to uncover the role of IL-8 in discogenic pain.
We have shown elevated levels of chemokines, cytokines and growth factors in AF tissues isolated from patients with discogenic low back pain. Our group has previously reported increased RANTES and IL-1β levels in patients with discogenic low back pain.22 The current study extends our previous findings to include IL-8, IL7, IL-10, SCF, SDF-1, IL-13, and OSM (Figure 4). As seen in other studies using patient samples, we found a large individual variation among patients in both the painful and control groups (Figure 5). These variations may represent different stages of IVD degeneration, time from onset of disease to surgery, and age/genetics.
It is worth noting that patients undergoing surgery for discogenic pain are primarily female (Table 1), and about 10 years younger on average compared with patients undergoing surgery for deformity corrections. IVD degeneration is a slowly progressive cascade mediated in part by inflammation.23, 24 Inflammatory stimulation directly alters the mechanobiology of NP cells25 and inhibits cell extracellular matrix (ECM) production.26, 27 In this study, we made every effort to include morphologically similar IVD tissues by selecting similar degrees of degeneration by matching the same MRI grade for the discogenic pain group and the control group (Table 1), in order to tease out the etiology of pain. Although clinically we have observed that older patients are less likely to have discogenic axial back pain and are more likely to suffer neurogenic claudication due to spinal stenosis than are younger individuals, there is not enough power in the current study to confirm this. The average age in the painful group (45.3 years) is, however, lower than that of the control group (53.5 years).
The anatomical basis for the IVD to generate pain is well documented, although there is still debate on whether the endplate or even the vertebral bodies are the main pain generators in the spine. In the normal disc, the NP is devoid of blood supply or nerve fibers, while the posterior 1/3 of the AF contains an extensive network of sensory nerve fibers. Pathologic findings, such as increased nerve and blood vessel ingrowth into the disc, have been associated with proteoglycan depletion.28 The cytokines described in this study may stimulate nerve endings within the IVD, resulting in discogenic pain. These cytokines may also diffuse to nearby tissues and stimulate nerve endings in the adjacent endplate, ligaments, or bone, contributing to the initiation and maintenance of spinal pain syndromes. Thus, these cytokines might serve as targets for future biological therapies to treat patients with discogenic pain. In addition to the IVD, other tissues may be sources of cytokines since biomarkers in serum have recently been observed to correlate to low back pain.29 In this study, all IVD tissues isolated are from lumbar spine. Thus, our findings are most applicable to low back pain. Finally, more research to characterize the role of cytokines in discogenic back pain is needed to assist in developing novel therapies for patients with this ubiquitous disease. As future clinical innovations of intradiscal therapies evolve, including bone morphogenetic protein, stem cells, and other biologic agents,7 understanding the mechanism of pain may further guide these exciting developments.
Acknowledgments
Author Disclosures: Yejia Zhang was supported by NICHD (K08 HD049598). This work was supported, in part, by a grant from the North American Spine Society (NASS) and Scott F. Nadler, D.O. Research Grant Award by the Physiatric Association of Spine, Sports and Occupational Rehabilitation (PASSOR).
The authors gratefully acknowledge Dr. Martin Heyworth for critically editing the manuscript and Dr. Carla Scanzello for valuable discussions.
Footnotes
This work has been presented as a poster presentation at the 2014 Philadelphia Spine Research Society Meeting.
AUTHORS’ CONTRIBUTIONS
AC performed tissue culture studies, real-time PCR and ELISA; PS performed human tissue dissections; DM performed human cytokine array; DGA and PC provided tissues from surgery and clinical information; YD performed cytokine pathway array and related data analysis; YZ, DGA and HSA were responsible for the conception, design, and data interpretation, and for obtaining grant funding. All authors critically reviewed and approved the manuscript.
References
- 1.United states bone and joint initiative: The burden of musculoskeletal diseases in the united states. 2011;2:21. [Google Scholar]
- 2.DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011;12:224–233. doi: 10.1111/j.1526-4637.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- 3.Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine (Phila Pa 1976) 1995;20:1878–1883. doi: 10.1097/00007632-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Wolfer LR, Derby R, Lee JE, Lee SH. Systematic review of lumbar provocation discography in asymptomatic subjects with a meta-analysis of false-positive rates. Pain Physician. 2008;11:513–538. [PubMed] [Google Scholar]
- 5.Carragee EJ, Lincoln T, Parmar VS, Alamin T. A gold standard evaluation of the “discogenic pain” diagnosis as determined by provocative discography. Spine (Phila Pa 1976) 2006;31:2115–2123. doi: 10.1097/01.brs.0000231436.30262.dd. [DOI] [PubMed] [Google Scholar]
- 6.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 7.Zhang Y, Chee A, Thonar EJ, An HS. Intervertebral disk repair by protein, gene, or cell injection: A framework for rehabilitation-focused biologics in the spine. PM R. 2011;3:S88–94. doi: 10.1016/j.pmrj.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF, 3rd, Evans CH. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976) 1996;21:271–277. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 10.Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Evans CH. Herniated cervical intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976) 1995;20:2373–2378. doi: 10.1097/00007632-199511001-00001. [DOI] [PubMed] [Google Scholar]
- 11.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kepler CK, Markova DZ, Hilibrand AS, et al. Substance P stimulates production of inflammatory cytokines in human disc cells. Spine (Phila Pa 1976) 2013;38:E1291–9. doi: 10.1097/BRS.0b013e3182a42bc2. [DOI] [PubMed] [Google Scholar]
- 13.Modi WS, Dean M, Seuanez HN, Mukaida N, Matsushima K, O’Brien SJ. Monocyte-derived neutrophil chemotactic factor (MDNCF/IL-8) resides in a gene cluster along with several other members of the platelet factor 4 gene superfamily. Hum Genet. 1990;84:185–187. doi: 10.1007/BF00208938. [DOI] [PubMed] [Google Scholar]
- 14.Bacon K, Baggiolini M, Broxmeyer H, et al. Chemokine/chemokine receptor nomenclature. J Interferon Cytokine Res. 2002;22:1067–1068. doi: 10.1089/107999002760624305. [DOI] [PubMed] [Google Scholar]
- 15.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 16.Oka M, Norose K, Matsushima K, Nishigori C, Herlyn M. Overexpression of IL-8 in the cornea induces ulcer formation in the SCID mouse. Br J Ophthalmol. 2006;90:612–615. doi: 10.1136/bjo.2005.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niu N, Qin X. New insights into IL-7 signaling pathways during early and late T cell development. Cell Mol Immunol. 2013;10:187–189. doi: 10.1038/cmi.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 19.Wang XM, Hamza M, Wu TX, Dionne RA. Upregulation of IL-6, IL-8 and CCL2 gene expression after acute inflammation: Correlation to clinical pain. Pain. 2009;142:275–283. doi: 10.1016/j.pain.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Buchner M, Moser MT, Daniel V, Schiltenwolf M. The role of IL-8 in patients with fibromyalgia: A prospective longitudinal study of 6 months. Clin J Pain. 2009;25:1–4. doi: 10.1097/AJP.0b013e31817e13a3. [DOI] [PubMed] [Google Scholar]
- 21.Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 22.Kepler CK, Markova DZ, Dibra F, et al. Expression and relationship of proinflammatory chemokine RANTES/CCL5 and cytokine IL-1beta in painful human intervertebral discs. Spine (Phila Pa 1976) 2013;38:873–880. doi: 10.1097/BRS.0b013e318285ae08. [DOI] [PubMed] [Google Scholar]
- 23.Smith LJ, Nerurkar NL, Choi KS, Harfe BD, Elliott DM. Degeneration and regeneration of the intervertebral disc: Lessons from development. Dis Model Mech. 2011;4:31–41. doi: 10.1242/dmm.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 25.Maidhof R, Jacobsen T, Papatheodorou A, Chahine NO. Inflammation induces irreversible biophysical changes in isolated nucleus pulposus cells. PLoS One. 2014;9:e99621. doi: 10.1371/journal.pone.0099621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seguin CA, Pilliar RM, Madri JA, Kandel RA. TNF-alpha induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine (Phila Pa 1976) 2008;33:356–365. doi: 10.1097/BRS.0b013e3181642a5e. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, An HS, Toofanfard M, Li Z, Andersson GB, Thonar EJ. Low-dose interleukin-1 partially counteracts osteogenic protein-1-induced proteoglycan synthesis by adult bovine intervertebral disk cells. Am J Phys Med Rehabil. 2005;84:322–329. doi: 10.1097/01.phm.0000159972.85053.7e. [DOI] [PubMed] [Google Scholar]
- 28.Melrose J, Roberts S, Smith S, Menage J, Ghosh P. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine (Phila Pa 1976) 2002;27:1278–1285. doi: 10.1097/00007632-200206150-00007. [DOI] [PubMed] [Google Scholar]
- 29.Sowa GA, Perera S, Bechara B, et al. Associations between serum biomarkers and pain and pain-related function in older adults with low back pain: A pilot study. J Am Geriatr Soc. 2014;62:2047–2055. doi: 10.1111/jgs.13102. [DOI] [PubMed] [Google Scholar]


