Abstract
BACKGROUND
Single-center analyses have suggested that the number of CF pulmonary exacerbations (PEx) treated with intravenous antibiotics an individual has experienced in the prior year is significantly associated with their future PEx hazard.
METHODS
We studied prior-year PEx association with future PEx hazard by Cox proportional hazards regression among CF Foundation Patient Registry patients who experienced PEx after Jan 1, 2010.
RESULTS
Among 13,579 patients, those with 1, 2, 3, or ≥4 prior-year PEx treated with intravenous antibiotics were at 1.8, 2.9, 4.8, and 8.7 higher PEx hazard vs those without (P<.0001). Adjustment with significant demographic and clinical covariates (univariate P ≤.0001) reduced prior-year PEx hazard ratios to 1.6, 2.4, 3.6, and 6.0 (P <.0001). No other covariates had adjusted hazard ratios >1.7.
CONCLUSIONS
Prior-year PEx strongly associate with future PEx hazard and should be accounted for in prospective trials where treatment-associated change in PEx hazard is an efficacy outcome.
Keywords: cystic fibrosis, pulmonary exacerbation, risk factors, modeling, exacerbation hazard
Graphical abstract
BACKGROUND
Individuals with cystic fibrosis (CF) are susceptible to pulmonary exacerbations (PEx), acute periods of intense increases in signs and symptoms of lung infection coupled with decreased lung function and other complications such as weight loss and/or malaise (1). Although a consensus prospective PEx definition remains elusive, PEx-associated health outcomes have been studied retrospectively using the surrogate marker of treatment with antibiotics for respiratory symptoms (2–15). Using this approach, investigators have reported associations between CF PEx treated with intravenous (IV) antibiotics and a variety of adverse health outcomes, ranging from reduced quality of life to an increased mortality risk (4,6,8–15). Because of the recognized clinical importance of IV-treated PEx, reduction in IV-treated PEx hazard and increase in median time-to-next antibiotic treatment for PEx have been used as efficacy endpoints in controlled trials of both chronic CF respiratory therapies as well as PEx treatments themselves (16,17).
Past IV-treatment of PEx has been identified as a significant risk factor for future IV-treatment of PEx in individuals with CF harboring multidrug-resistant bacterial airways infection (2), as well as in more diverse CF populations (5,18). In a recent analysis of time-to-next IV-treated PEx among 193 pediatric and adult patients followed at the Cleveland Ohio CF Care Center, we observed that the number of IV-treated PEx an individual had experienced in the prior calendar year (Prior-Year PEx) had the strongest association with their future PEx hazard among over 60 clinical, demographic, and treatment covariates analyzed (18). Adjustment with covariates previously reported to be associated with future PEx hazard had little effect on the magnitude and or statistical significance of this association. To test the hypothesis that this association exists for the broader US CF population, we have characterized prior-year PEx number as an independent risk factor for future IV-treated PEx among individuals followed in the US CF Foundation Patient Registry (CFFPR) between 2009 and 2013 (19).
METHODS
To be included in analyses, CFFPR patients had to have been treated with IV antibiotics for PEx (termed a Care Episode in the CFFPR) at least once on or after January 1, 2010, and to have had at least one additional encounter or a death recorded after completion of that Care Episode by December 31, 2013. Patients also had to have data fields related to clinical complications completed in the CFFPR case report form during the calendar year (January through December) of their Index PEx, as well as annualized data and at least one encounter recorded for each calendar year included in the analyses (i.e., patients with an Index PEx in 2010 and their next encounter recorded in 2012 were excluded for lacking 2011 data). Care Episodes and clinic visits occurring after lung transplantation were also excluded from analyses. Adults and guardians of children followed in the CFFPR provided consent for data analyses. CFFPR patient data were de-identified prior to analyses, which were approved prospectively by the CFFPR Registry Committee and the University Hospitals Case Medical Center Institutional Review Board (#01-15-15). Data management and manipulation were performed in R version 3.1 (R Foundation for Statistical Computing, Vienna, Austria). Statistical analyses were performed with R v3.1 and MedCalc Statistical Software version 14.10.2 (MedCalc Software bvba, Ostend, Belgium).
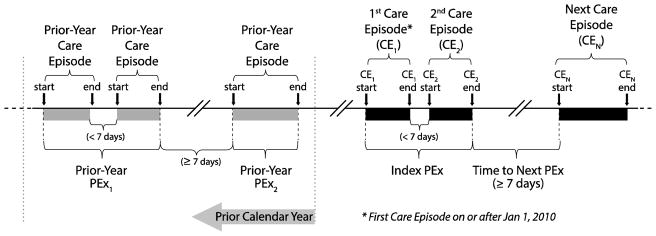
The start date of a patient’s first PEx Care Episode beginning on or after January 1, 2010 was defined as the beginning of their Index PEx. Methods for calculating Index PEx treatment duration and time to next PEx are described in Figure 1. PEx beginning in the calendar year (January to December) prior to the year of the Index PEx were counted for each patient to create the covariate Prior-Year PEx, combining adjacent Care Episodes separated by <7 days (as in Figure 1). Demographic and treatment covariates for each patient were collected from CFFPR Annual Review data for the calendar year (January to December) in which their Index PEx began and separately for the prior year. Categorical variables were created from selected continuous variables (Prior-Year PEx, age at Index PEx end, body mass index [BMI], percent predicted forced expiratory volume in 1 second [FEV1% predicted]), where all missing values were assigned to a unique category. Age and FEV1 categories were selected based on previous analyses (12,18), BMI categories were chosen based on distribution within the study population. For FEV1, missing values were divided into separate categories for those patients <6 years of age at the end of their Index PEx and those ≥6 years of age. The CFFPR uses the Global Lung Initiative normative equations (20) to calculate FEV1 % predicted. Duration of IV treatment associated with the Index PEx was divided into three categories: <9 days (‘short’), 9–14 days (‘usual’), and >14 days (‘extended’).
Figure 1. Temporal Relationships of Care Episodes, Index PEx, Treatment Duration, Time to Event, and Prior-Year PEx.
For each patient, the beginning of their first Care Episode for treatment of PEx recorded on or after Jan 1, 2010 during which at least one day of IV antibiotics were administered (CE1) was identified as the beginning of the Index PEx. Any subsequent PEx Care Episodes (CE2) that began shortly after the end of the previous episode (i.e., with a gap of <7 days from CE1 end to CE2 start) were considered continuations of the same PEx and combined for analyses. Index PEx treatment duration was defined as the entire elapsed time from the start of CE1 to the end of all combined Care Episodes. PEx Care Episodes beginning ≥7 days after the end of a previous PEx were considered the start of a next PEx, and time to next PEx was calculated accordingly. For patients who had no subsequent PEx Care Episodes, the elapsed time from the end of their Index PEx to the date of their last recorded clinic encounter was collected. Prior-Year PEx were counted using Care Episodes in the calendar year preceding the Index PEx using the same method, where adjacent Care Episodes with gaps of <7 days were combined. In the example shown, two Prior-Year PEx consisted of three Care Episodes occurring in the preceding year.
Association of Prior-Year PEx with time to next PEx was studied by Kaplan-Meier survival analysis to select appropriate categorical values of Prior-Year PEx for subsequent analyses. The potential for other demographic and clinical covariates (e.g., age, FEV1, pulmonary complications) to account for differences in time to next PEx associated with Prior-Year PEx was studied by Cox proportional hazard regression. Proportionality of the Prior-Year PEx covariate with respect to future PEx hazard over time was investigated graphically by plotting log-log survival and Schoenfeld residuals, and tested using a weighted residuals approach (21). Cox proportional hazard regression of Prior-Year PEx for future PEx hazard was first performed as univariate analysis and subsequently modeled with inclusion of demographic and clinical complication covariates with a univariate future PEx hazard ratio having P ≤.0001. Distributions of patient demographics and clinical covariates among Prior-Year PEx categories during the year of the Index PEx were also tabulated. Proportional hazards regressions were repeated using annualized clinical and demographic covariates from the calendar year prior to the Index PEx (i.e., the same year during which the covariate Prior-Year PEx was collected) as a sensitivity analysis.
In order to characterize associations of chronic pulmonary medications prescribed during the Index PEx calendar year and future PEx hazard, treatment covariates were tabulated by Prior-Year PEx category and characterized by univariate Cox proportional hazard modeling and with adjustment for Prior-Year PEx. Patients missing all chronic pulmonary medication data during their Index year were excluded from these analyses.
RESULTS
Among 16,410 CFFPR patients experiencing an IV-treated PEx on or after January 1, 2010, 13,579 met study inclusion criteria and contributed an Index PEx to the analysis (Supplemental Figure 5). A majority of these patients (8098; 59.6%) had not experienced a PEx treated with IV antibiotics in the prior calendar year, while 18.1% (2457) had experienced one Prior-Year PEx, 10.8% (1466) had experienced two, and the remaining 11.5% (1558) had experienced three or more (Table 1).
Table 1.
Patient Demographics during their Index PEx Year Stratified by Prior-Year PEx.
| Prior-Year PEx | ||||||
|---|---|---|---|---|---|---|
| None (N=8,098) | One (N=2,457) | Two (N=1,466) | Three (N=762) | ≥ Four (N=796) | All (N=13,579) | |
| Age Group, N (%)* | ||||||
| <6 yrs | 1123 (13.9%) | 219 (8.9%) | 85 (5.8%) | 31 (4.1%) | 30 (3.8%) | 1488 (11.0%) |
| 6–12 yrs | 1840 (22.7%) | 460 (18.7%) | 215 (14.7%) | 106 (13.9%) | 83 (10.4%) | 2704 (19.9%) |
| 13–17 yrs | 1512 (18.7%) | 464 (18.9%) | 292 (19.9%) | 166 (21.8%) | 171 (21.5%) | 2605 (19.2%) |
| 18–24 yrs | 1462 (18.1%) | 580 (23.6%) | 407 (27.8%) | 223 (29.3%) | 281 (35.3%) | 2953 (21.7%) |
| 25–50 yrs | 1569 (19.4%) | 565 (23.6%) | 345 (23.5%) | 193 (25.3%) | 196 (24.6%) | 2868 (21.1%) |
| >50 yrs | 592 (7.3%) | 169 (6.9%) | 122 (8.3%) | 43 (5.6%) | 35 (4.4%) | 961 (7.1%) |
| FEV1 % Predicted Group, N (%)* | ||||||
| ≥100 | 1128 (13.9%) | 198 (8.1%) | 87 (6.0%) | 25 (3.3%) | 15 (1.9%) | 1453 (10.7%) |
| 70 to <100 | 3339 (41.2%) | 865 (35.2%) | 471 (32.1%) | 197 (25.9%) | 155 (19.5%) | 5027 (37.0%) |
| 40 to <70 | 1910 (23.6%) | 869 (35.4%) | 554 (37.8%) | 351 (46.1%) | 354 (44.5%) | 4038 (29.7%) |
| <40% | 610 (7.5%) | 299 (12.2%) | 255 (17.4%) | 151 (19.8%) | 227 (28.5%) | 1542 (11.4%) |
| NA, age <6 yrs | 984 (12.2%) | 191 (7.8%) | 71 (4.8%) | 28 (3.7%) | 25 (3.1%) | 1299 (9.6%) |
| NA, age ≥6 yrs | 127 (1.6%) | 35 (1.4%) | 28 (1.9%) | 10 (1.3%) | 20 (2.5%) | 220 (1.6%) |
| BMI kg/m2 | ||||||
| Mean (SD) | 20.1 (4.2) | 20.1 (4.0) | 20.5 (4.0) | 20.4 (3.7) | 20.1 (3.8%) | 20.1 (4.1) |
| N (%) | 8090 (99.9%) | 2452 (99.8%) | 1465 (99.9%) | 761 (99.9%) | 794 (99.7%) | 13562 (99.9%) |
| F508del Genotype, N (%)* | ||||||
| Homozygous | 4043 (49.9%) | 1243 (50.6%) | 724 (49.4%) | 381 (50.0%) | 401 (50.4%) | 6792 (50.0%) |
| Heterozygous | 2945 (36.4%) | 900 (36.6%) | 534 (36.4%) | 281 (36.9%) | 276 (34.7%) | 4936 (36.4%) |
| No F508del | 895 (11.1%) | 227 (9.2%) | 166 (11.3%) | 69 (9.1%) | 77 (9.7%) | 1434 (10.6%) |
| Genotype NA | 215 (2.7%) | 87 (3.5%) | 42 (2.9%) | 31 (4.1%) | 42 (5.3%) | 417 (3.1%) |
| Female Sex, N (%) | ||||||
| 4001 (49.4%) | 1314 (53.5%) | 826 (56.3%) | 442 (58.0%) | 464 (58.3%) | 7047 (51.9%) | |
| Medicaid Insurance, N (%)* | ||||||
| No | 5195 (64.2%) | 1428 (58.1%) | 818 (55.8%) | 386 (50.7%) | 328 (41.2%) | 8155 (60.1%) |
| Yes | 2840 (35.1%) | 1001 (40.7%) | 627 (42.8%) | 365 (47.9%) | 445 (55.9%) | 5278 (38.9%) |
| NA | 63 (0.8%) | 28 (1.1%) | 21 (1.4%) | 11 (1.4%) | 23 (2.9%) | 146 (1.1%) |
| Supplemental Ventilation, N (%)* | ||||||
| No | 7786 (96.1%) | 2325 (94.6%) | 1377 (93.9%) | 680 (89.2%) | 666 (83.7%) | 12834 (94.5%) |
| Yes | 137 (1.7%) | 72 (2.9%) | 49 (3.3%) | 57 (7.5%) | 92 (11.6%) | 407 (3%) |
| NA | 175 (2.2%) | 60 (2.4%) | 40 (2.7%) | 25 (3.3%) | 38 (4.8%) | 338 (2.5%) |
| Supplemental Feeding, N (%)* | ||||||
| No | 3272 (40.4%) | 956 (38.9%) | 536 (36.6%) | 270 (35.4%) | 205 (25.8%) | 5239 (38.6%) |
| Yes | 4641 (57.3%) | 1433 (58.3%) | 892 (60.8%) | 475 (62.3%) | 561 (70.5%) | 8002 (58.9%) |
| NA | 185 (2.3%) | 68 (2.8%) | 38 (2.6%) | 17 (2.2%) | 30 (3.8%) | 338 (2.5%) |
| Supplemental Oxygen, N (%)* | ||||||
| No | 7076 (87.4%) | 1998 (81.3%) | 1066 (72.7%) | 483 (63.4%) | 380 (47.7%) | 11003 (81.0%) |
| Yes | 866 (10.7%) | 407 (16.6%) | 360 (24.6%) | 254 (33.3%) | 386 (48.5%) | 2273 (16.7%) |
| NA | 156 (1.9%) | 52 (2.1%) | 40 (2.7%) | 25 (3.3%) | 30 (3.8%) | 303 (2.2%) |
| Smoker, N (%)* | ||||||
| No | 7482 (92.4%) | 2224 (90.5%) | 1290 (88.0%) | 663 (87.0%) | 684 (85.9%) | 12343 (90.9%) |
| Yes | 114 (1.4%) | 54 (2.2%) | 43 (2.9%) | 28 (3.7%) | 28 (3.5%) | 267 (2.0%) |
| NA | 502 (6.2%) | 179 (7.3%) | 133 (9.1%) | 71 (9.3%) | 84 (10.6%) | 969 (7.1%) |
| Second-Hand Smoke Exposure, N (%)* | ||||||
| No | 3879 (47.9%) | 1094 (44.5%) | 632 (43.1%) | 320 (42.0%) | 305 (38.3%) | 6230 (45.9%) |
| Yes | 1264 (15.6%) | 447 (18.2%) | 251 (17.1%) | 151 (19.8%) | 164 (20.6%) | 2277 (16.8%) |
| NA | 2955 (36.5%) | 916 (37.3%) | 583 (39.8%) | 291 (38.2%) | 327 (41.1%) | 5072 (37.4%) |
-column percentages; yrs = years; NA= covariate status not available
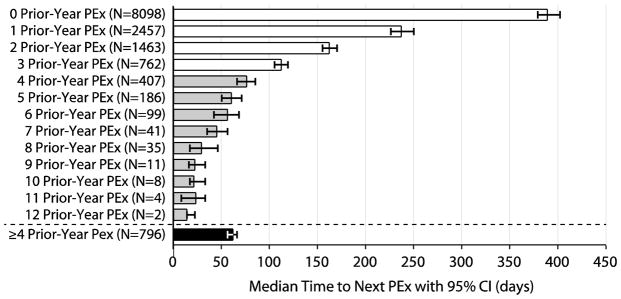
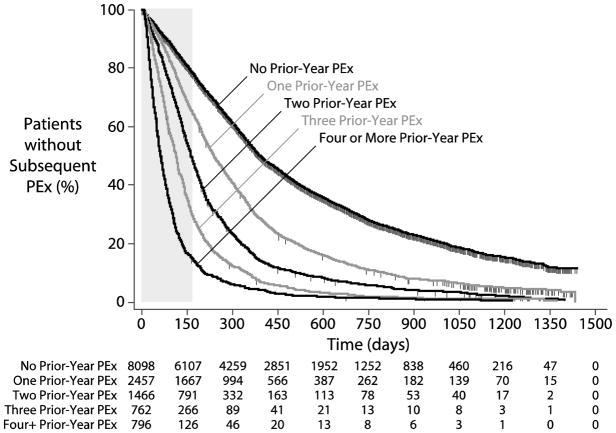
A total of 10,694 patients (78.8%) experienced a second Care Episode during which IV antibiotics were administered for PEx after their Index PEx and prior to December 31, 2013. Median time to next PEx from the end of the Index PEx (accounting for censored patients) was 273 days [95% CI 267, 281]. The number of IV-treated PEx a patient had experienced in the calendar year prior to their Index PEx (Prior-Year PEx) was strongly associated with time-to-next PEx by Kaplan-Meier survival analysis. Median times to next PEx were significantly different for patients with 0, 1, 2, 3, and 4 Prior-Year PEx, while median times to next PEx for patients with greater Prior-Year PEx values were less well separated (Figure 2). Based on this observation, 796 patients with a Prior-Year PEx value ≥4 were combined into a single category for Prior-Year PEx categorical analyses. Median time to next PEx among patients with no Prior-Year PEx was 389 days [379, 402], compared with 237 [226,250], 162 [155,170], 112 [105, 119], and 62 [56, 66] days among those with 1, 2, 3, and ≥4 Prior-Year PEx (Figures 2 and 3).
Figure 2. Kaplan-Meier Median Time-to-PEx by Number of Prior-Year PEx.
White bars highlight Prior-Year PEx values where 95% CI for associated median times to next PEx do not overlap with adjacent values. Gray bars show Prior-Year PEx values where 95% CI for associated median times to next PEx overlap with adjacent values. The black bar shows the median time to PEx for the group of pooled patients with Prior-Year PEx ≥4. CI=confidence interval.
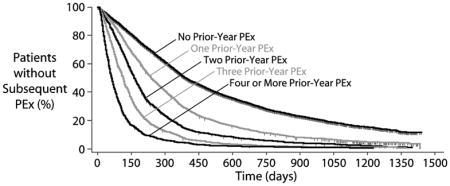
Figure 3. Kaplan-Meier Time-To-PEx by Prior-Year PEx Category.
Vertical dashed lines represent censored patients. Patients at risk at different time points are shown for each category below the plot. The 24 week period studied by Cox proportional hazards regression is highlighted with a gray box
Some categorical demographic covariates showed consistent increases in prevalence with increasing Prior-Year PEx, including female sex, Medicaid Insurance coverage, smoking, and exposure to second hand smoke, as well as supplemental feeding, ventilation, and oxygen utilization. In contrast, covariates such as F508del genotype and mean BMI did not appear to differ substantially across Prior-Year PEx categories (Table 1). Among individuals with an FEV1 recorded for the year of their Index PEx, lower FEV1 % predicted was associated with increased Prior-Year PEx, and patients 6 years of age or older with an unknown FEV1 were more likely to have higher Prior-Year PEx values than those <6 years of age. Increasing age was associated with higher Prior-Year PEx values up to age 25, beyond which there was a decrease in the proportion of patients with higher Prior-Year PEx values, suggestive of a survival effect.
The prevalence of clinical complications associated with CF, including ABPA, massive hemoptysis, pneumothorax, sinus disease, hepatic steatosis, GERD, CF-related diabetes, cirrhotic and non-cirrhotic liver disease, pancreatitis, and hypertension tended to increase as Prior-Year PEx increased (Table 2). Similarly, P. aeruginosa, MRSA, and Burkholderia cepacia complex isolation from respiratory secretions during the Index PEx calendar year was more common in patients with higher Prior-Year PEx (Supplemental Table 3), as were the prescription of most chronic pulmonary medications (Supplemental Table 4).
Table 2.
CF-Related Complications during the Index PEx Year Stratified by Prior-Year PEx.
| Prior-Year PEx | ||||||
|---|---|---|---|---|---|---|
| None (N=8,098) | One (N=2,457) | Two (N=1,466) | Three (N=762) | ≥ Four (N=796) | All (N=13,579) | |
| Allergic Bronchopulmonary Aspergillosis (ABPA), N (%) | ||||||
| 455 (5.6%) | 190 (7.7%) | 135 (9.2%) | 76 (10.0%) | 95 (11.9%) | 951 (7.0%) | |
| Asthma, N (%) | ||||||
| 2185 (27.0%) | 739 (30.1%) | 499 (34.0%) | 241 (31.6%) | 251 (31.5%) | 3915 (28.8%) | |
| Massive Hemoptysis, N (%) | ||||||
| 99 (1.2%) | 42 (1.7%) | 37 (2.5%) | 29 (3.8%) | 33 (4.1%) | 240 (1.8%) | |
| Pneumothorax, N (%) | ||||||
| 34 (0.4%) | 15 (0.6%) | 15 (1.0%) | 7 (0.9%) | 12 (1.5%) | 83 (0.6%) | |
| Sinus Disease, N (%) | ||||||
| 2553 (31.5%) | 825 (33.6%) | 548 (37.4%) | 296 (38.8%) | 294 (36.8%) | 4516 (33.3%) | |
| Hepatic Steatosis, N (%) | ||||||
| 33 (0.4%) | 8 (0.3%) | 5 (0.3%) | 4 (0.5%) | 7 (0.9%) | 57 (0.4%) | |
| Gastroesophageal Reflux Disease (GERD), N (%) | ||||||
| 2386 (29.5%) | 848 (34.5%) | 548 (37.4%) | 319 (41.9%) | 350 (44.0%) | 4451 (32.8%) | |
| Glucose Tolerance, N (%)* | ||||||
| Normal | 5838 (72.1%) | 1596 (65.0%) | 878 (59.9%) | 410 (53.8%) | 346 (43.5%) | 9068 (66.8%) |
| Impaired | 736 (9.1%) | 251 (10.2%) | 133 (9.1%) | 94 (12.3%) | 87 (10.9%) | 1301 (9.6%) |
| Diabetes | 1461 (18.0%) | 582 (23.7%) | 434 (29.6%) | 247 (32.4%) | 340 (42.7%) | 3064 (22.6%) |
| NA | 63 (0.8%) | 28 (1.1%) | 21 (1.4%) | 11 (1.4%) | 23 (2.9%) | 146 (1.1%) |
| Liver Disease, Cirrhosis, N (%) | ||||||
| 184 (2.3%) | 76 (3.1%) | 50 (3.4%) | 34 (4.5%) | 46 (5.8%) | 390 (2.9%) | |
| Liver Disease, Non-Cirrhosis, N (%) | ||||||
| 426 (5.3%) | 172 (7.0%) | 106 (7.2%) | 66 (8.7%) | 60 (7.5%) | 830 (6.1%) | |
| Liver Disease, Other, N (%) | ||||||
| 192 (2.4%) | 39 (1.6%) | 24 (1.6%) | 16 (2.1%) | 14 (1.8%) | 285 (2.1%) | |
| Pancreatitis, N (%) | ||||||
| 121 (1.5%) | 39 (1.6%) | 28 (1.9%) | 16 (2.1%) | 34 (4.3%) | 238 (1.8%) | |
| Hypertension, N (%) | ||||||
| 176 (2.2%) | 67 (2.7%) | 51 (3.5%) | 39 (5.1%) | 41 (5.2%) | 374 (2.8%) | |
-column percentages; NA= covariate status not available
Proportionality of Prior-Year PEx with respect to future PEx hazard was observed to change with increasing observation time, being fairly consistent through the first 6 months after the end of Index PEx treatment and becoming increasingly less so with extended observation. For this reason, Cox proportional hazard regressions were limited to the 24-week period immediately following the end of Index PEx IV treatment (gray box, Figure 2).
Future PEx hazard ratios associated with Prior-Year PEx categories over the 24 weeks following Index PEx treatment were large and highly significant (P <.0001) by Cox proportional hazards modeling, with hazard ratios among patients with Prior-Year PEx ranging from 1.8 to 8.7 compared to patients who did not have an IV-treated PEx in the prior year (Figure 4). Adjustment of Prior-Year PEx hazard ratios with demographic and clinical covariates with significant (P ≤.0001) univariate associations with future PEx hazard modestly reduced observed Prior-Year PEx hazard ratios to a range of 1.6 to 6.0 (Figure 4). By comparison, the largest adjusted future PEx hazard ratio among all other covariates included in the model was 1.7 (oxygen supplementation versus none, Figure 4). Sensitivity analyses in which Prior-Year PEx hazard ratios were adjusted with clinical and demographic covariates collected during the calendar year prior to the Index PEx had essentially no effect on this observation.
Figure 4. Unadjusted and Adjusted Cox Proportional Hazards Modeling of PEx Hazard within 24 weeks of Index PEx Treatment.
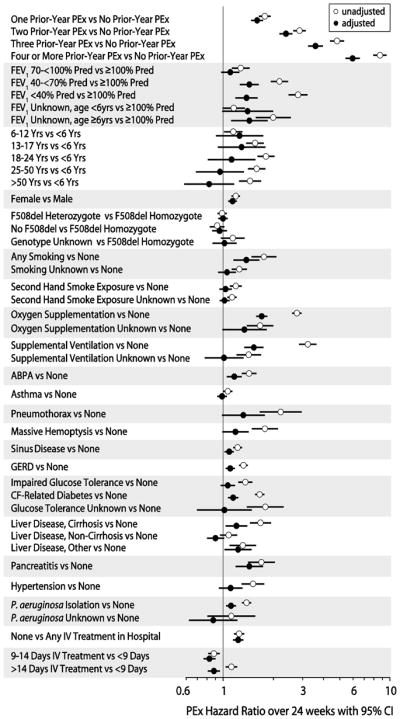
Hazard ratios for demographic and clinical complication covariates with univariate P≤.0001, as well as PEx treatment and P. aeruginosa isolation status covariates, are shown unadjusted (open circles) and adjusted in a single Cox proportional hazards regression model (closed circles). Yrs= years, ABPA=allergic bronchopulmonary aspergillosis, GERD=gastroesophageal reflux disease.
A total of 48 patients (0.4%) were missing all chronic pulmonary medication data during their Index PEx year and were excluded from modeling of associations between chronic therapies and future PEx hazard ratios (Supplemental Table 4). Only three chronic therapies (hypertonic saline, high-dose ibuprofen, and inhaled corticosteroids) did not have a highly significant (P <.0001) univariate association with increased future PEx hazard within 6 months of the Index PEx; hazard ratios for other therapies ranged from 1.18 for leukotriene modifiers to 1.92 for inhaled colistimethate (Supplemental Table 4). With the exception of hypertonic saline, addition of Prior-Year PEx to modeling as a categorical variable reduced future PEx hazard associated with all other chronic therapies over the 24 weeks following Index PEx treatment.
DISCUSSION
History of IV-treated PEx has been shown in previous single-center analyses to be an important covariate associated with future IV-treated PEx hazard among CF patients (2,18). The current analysis of >13,500 CFFPR patients confirms and expands upon these previous observations. The sample size of our previous analysis of Cleveland CF Care Center patients (N=193) precluded distinction in future PEx hazard between patients with one versus two IV-treated PEx in the prior year, or for patients with three versus more than three PEx in the prior year. With this much larger sample, we have observed that as Prior-Year PEx rises from zero through nine events, median time to next PEx drops correspondingly (Figure 3).
As with all observational registry studies, there are inherent limitations to these analyses, as they are dependent on accurate and complete capture of patient data by CF care centers, and we were unable to randomly audit for data accuracy or submit queries for missing data fields. In order to accurately assess time to next PEx from the end of an Index PEx treatment, we necessarily discarded >10% of patients who had apparently useful Index PEx but who were lacking data between the calendar year of their Index PEx and the calendar year of either their next recorded PEx or censor date (Supplemental Figure 5). There is no method to determine whether the future PEx hazards of these discarded patients were comparable to those of retained patients. In addition, our analyses have been necessarily limited to PEx treated with IV antibiotics, and it is not possible to extrapolate our findings to risk of PEx treated with antibiotics by other routes, which may not share this relationship. Finally, the use of annualized data to adjust for future PEx hazard is challenging, as clinical complications may have arisen after both the Index PEx and subsequent PEx but within the Index year. However, use of clinical complications recorded in the prior year (which avoid this possible problem) provided essentially identical results.
Interestingly, associations of PEx treatment decisions (duration of IV treatment and location of treatment) with future PEx risk that we had previously observed in our single center analysis (18) were also observed in this more definitive study. Very short IV treatments and those without hospital admission were associated with increased hazard of future PEx, results that run contrary to what might be expected from indication bias (where ‘healthier’ patients might be expected to be treated for shorter durations and/or without hospital admission and also have reduced hazard) and warrant further attention.
That past behavior is predictive of future performance with respect to PEx treatment with IV antibiotics is not a particularly novel or unexpected finding (2,5,18), although it is not a covariate that is uniformly included in Cox proportional hazard analyses (22). However, the remarkable strength of these relationships runs counter to some aspects of current thinking regarding CF exacerbation. PEx have been considered to be somewhat ‘random’ events (although it has been recognized that some patients are more ‘prone’ to these events than others [2,3,5,18,22]) that can be precipitated by stochastic ‘insults’ such as viral infection or air pollution (1,23,24), as well as delayed or avoided by increased intervention (1). These are important assumptions when change in PEx hazard is employed as an efficacy endpoint in randomized interventional studies (16). Our current results suggest that risk of treatment with IV antibiotics among individuals with apparently ‘similar’ clinical and demographic profiles may differ by as much as 6-fold within 6 months of their last PEx based solely on their prior treatment history, although we cannot infer that this covariate is also associated with risk of antibiotic treatment by other routes. This observation has important ramifications for design of randomized controlled CF trials that employ treatment-associated change in IV-treated PEx hazard as an efficacy endpoint: 1) stratification of subject randomization by Prior-Year PEx is more likely to balance baseline PEx hazard across treatment groups than stratification by FEV1 % predicted and/or age, and 2) adjustment for Prior-Year PEx when estimating treatment-associated reductions in PEx hazard should reduce variance and increase study power accordingly.
An individual’s frequency of IV treatment for PEx in the prior year is an ‘effect’ (as opposed to a cause) that has strong association with future PEx hazard, but the underlying bases for differences in PEx frequency and risk between apparently similar patients remain uncharacterized. Differences may result from (as yet uncaptured) biological differences between patients, unique behavioral interactions between patients and caregivers (note that this is a longitudinal phenomenon: in most instances the same care team/patient pair will have participated in past and future PEx treatments), or a mixture of both. Regardless of the reason(s), an individual’s propensity for IV treatment of PEx in the near term (time periods commonly covered by randomized clinical trials) appears to be less well predicted by their age, lung function, sex, or the myriad of other covariates followed in CF patient registries, as by their recent treatment history. Further, although chronic pulmonary medications may attenuate future PEx hazard, these effects would appear to be relatively modest compared to the magnitude of hazard associated with PEx treatment history.
Supplementary Material
Highlights.
We studied association of past pulmonary exacerbations and future exacerbation risk
More prior-year exacerbations were associated with greater exacerbation risk
Adjustment with other exacerbation risk covariates had little effect on observations
Prior-year exacerbations are important independent predictors of exacerbation
Acknowledgments
The authors thank the patients, families, CF care providers, and support staff associated with the Cystic Fibrosis Foundation Patient Registry, as well as Dr. Shawn Aaron for critical review of this manuscript and David Pasta for helpful discussions of hazard proportionality. This project was supported by the Cystic Fibrosis Foundation (KONSTA09Y0) and the National Institutes of Health through the Clinical and Translational Science Collaborative of Cleveland (UL1TR000439) and P30 DK027651.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr. 2006 Feb;148(2):259–64. doi: 10.1016/j.jpeds.2005.10.019. Review. [DOI] [PubMed] [Google Scholar]
- 2.Block JK, Vandemheen KL, Tullis E, Fergusson D, Doucette S, Haase D, Berthiaume Y, Brown N, Wilcox P, Bye P, Bell S, Noseworthy M, Pedder L, Freitag A, Paterson N, Aaron SD. Predictors of pulmonary exacerbations in patients with cystic fibrosis infected with multi-resistant bacteria. Thorax. 2006;61(11):969–74. doi: 10.1136/thx.2006.061366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007;62(4):360–7. doi: 10.1136/thx.2006.060889. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.VanDevanter DR, Wagener JS, Pasta DJ, Elkin E, Jacobs JR, Morgan WJ, Konstan MW. Pulmonary outcome prediction (POP) tools for cystic fibrosis patients. Pediatr Pulmonol. 2010;45:1156–66. doi: 10.1002/ppul.21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.VanDevanter DR, Yegin A, Morgan WJ, Millar SJ, Pasta DJ, Konstan MW. Design and powering of cystic fibrosis clinical trials using pulmonary exacerbation as an efficacy endpoint. J Cyst Fibros. 2011;10:453–9. doi: 10.1016/j.jcf.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, Paterson N, Jackson M, Lougheed MD, Kumar V, Aaron SD. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax. 2011;66(8):680–5. doi: 10.1136/thx.2011.161117. [DOI] [PubMed] [Google Scholar]
- 7.VanDevanter DR, Elkin EP, Pasta DJ, Morgan WJ, Konstan MW for the Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Changing thresholds and incidence of antibiotic treatment of cystic fibrosis pulmonary exacerbations, 1995–2005. J Cyst Fibros. 2013;12(4):332–337. doi: 10.1016/j.jcf.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Lieu T, Ray G, Farmer G, Shay G. The cost of medical care for patients with cystic fibrosis in a health maintenance organization. Pediatrics. 1999;103:e72. doi: 10.1542/peds.103.6.e72. [DOI] [PubMed] [Google Scholar]
- 9.Liou T, Adler F, Fitzsimmons S, Cahill B, Hibbs J, Marshall B. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153:345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Britto M, Kotagal U, Hornung R, Atherton H, Tsevat J, Wilmott R. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121:64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- 11.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss C, Aitken M. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002;166:1550–1555. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 12.Konstan M, Morgan W, Butler S, Pasta D, Craib M, Silva S, Stokes D, Wohl M, Wagener J, Regelmann W, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151:134–139. 139. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182(5):627–32. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders DB, Bittner RC, Rosenfeld M, Redding GJ, Goss CH. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonol. 2011;46(4):393–400. doi: 10.1002/ppul.21374. [DOI] [PubMed] [Google Scholar]
- 15.Byrnes CA, Vidmar S, Cheney JL, Carlin JB, Armstrong DS, Cooper PJ, Grimwood K, Moodie M, Robertson CF, Rosenfeld M, Tiddens HA, Wainwright CE ACFBAL Study Investigators. Prospective evaluation of respiratory exacerbations in children with cystic fibrosis from newborn screening to 5 years of age. Thorax. 2013;68(7):643–51. doi: 10.1136/thoraxjnl-2012-202342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.VanDevanter DR, Konstan MW. Outcome measures for clinical trials assessing treatment of cystic fibrosis lung disease. Clin Invest. 2012;2(2):163–175. doi: 10.4155/cli.11.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith AL, Doershuk C, Goldmann D, Gore E, Hilman B, Marks M, Moss R, Ramsey B, Redding G, Rubio T, Williams-Warren J, Wilmott R, Wilson HD, Yogev R. Comparison of a beta-lactam alone versus beta-lactam and an aminoglycoside for pulmonary exacerbation in cystic fibrosis. J Pediatr. 1999;134(4):413–21. doi: 10.1016/s0022-3476(99)70197-6. [DOI] [PubMed] [Google Scholar]
- 18.VanDevanter DR, Pasta DJ, Konstan MW. Treatment and demographic factors affecting time to next pulmonary exacerbation in cystic fibrosis. J Cyst Fibros. 2015 Mar 5; doi: 10.1016/j.jcf.2015.02.007. pii: S1569–1993(15)00047–8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cystic Fibrosis Foundation Patient Registry. 2013 Annual data report to the Center Directors. Bethesda Maryland: Cystic Fibrosis Foundation; 2014. [Google Scholar]
- 20.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 22.Sequeiros IM, Jarad N. Factors associated with a shorter time until the next pulmonary exacerbation in adult patients with cystic fibrosis. Chron Respir Dis. 2012;9(1):9–16. doi: 10.1177/1479972311433575. [DOI] [PubMed] [Google Scholar]
- 23.Farhat SC, Almeida MB, Silva-Filho LV, Farhat J, Rodrigues JC, Braga AL. Ozone is associated with an increased risk of respiratory exacerbations in patients with cystic fibrosis. Chest. 2013;144(4):1186–92. doi: 10.1378/chest.12-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goeminne PC, Kiciński M, Vermeulen F, Fierens F, De Boeck K, Nemery B, Nawrot TS, Dupont LJ. Impact of air pollution on cystic fibrosis pulmonary exacerbations: a case-crossover analysis. Chest. 2013;143(4):946–54. doi: 10.1378/chest.12-1005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.