Abstract
Rationale
Frequent exposure to methamphetamine has been reported to adversely influence cognitive behavior and, in particular, inhibitory control processes.
Objective
The present studies were conducted in squirrel monkeys to assess the effects of daily intravenous methamphetamine self-administration on touchscreen-based repeated acquisition and discrimination reversal tasks thought to reflect behavioral dimensions of, respectively, learning and response inhibition.
Methods
First, stable methamphetamine-maintained behavior was established (0.35-1.6 mg/kg/session) and, subsequently, a second daily session of discrimination learning was conducted (20 hr later). Subjects first learned to discriminate between two simultaneously presented stimuli (acquisition) and, subsequently, to re-learn the discrimination with the contingencies switched (reversal). The role of the interval between self-administration and touchscreen sessions was evaluated, as well as the effects of abrupt methamphetamine discontinuation.
Results
Results indicate that daily methamphetamine self-administration markedly disrupted the development of discrimination learning, initially requiring nearly twice the number of trials to master discriminations. The magnitude of adverse effects in individual subjects correlated to the level of daily methamphetamine intake. Importantly, however, behavioral disruption of discrimination learning was surmounted following remedial training. Once criterion levels of discrimination performance were achieved, subsequent development of reversal performance was largely unaffected except when the interval between self-administration and touchscreen session was short and, thus, likely vulnerable to methamphetamine’s direct effects. Discontinuation of methamphetamine produced no disruption in acquisition or reversal.
Conclusion
These results indicate that self-administered methamphetamine can markedly disrupt learning processes and, as well, highlights key differences in its effects on different aspects of discrimination learning.
Keywords: methamphetamine, self-administration, discrimination learning, inhibitory control, touchscreen, squirrel monkey
INTRODUCTION
The illicit use of methamphetamine remains a global health concern, with surveys estimating approximately 33.8 million people worldwide to have used it in the previous year (UNODC 2013). Repeated exposure to methamphetamine is thought to result in deficits in several domains of cognitive function, as revealed in assays of episodic memory, executive function, information processing speed in human subjects (reviewed in Scott et al. 2007) and, in particular, cognitive flexibility and response inhibition (reviewed in Baicy and London 2007). However, the magnitude and reliability of these deficits are unclear. For example, a recent review by Dean et al. (2013) that focused on cross-sectional, twin, and functional/structural neural studies offered mixed conclusions, with only limited support for a relationship between methamphetamine abuse and mild-to-moderate cognitive decline during early-to-middle adulthood and no indication whether such deficits are long-lived. An additional consideration in understanding the effects of methamphetamine is the determining factor of dose (Wood et al. 2014). Although large doses of methamphetamine may impair task performance, relatively low doses of methamphetamine, like other psychomotor stimulant drugs, can have attention-enhancing effects that benefit task performance. In fact, though not widely prescribed, methamphetamine is a Food and Drug Administration-approved medication for the treatment of attention-deficit/hyperactivity disorder (ADHD). Such complex considerations regarding the safety of chronically used or abused methamphetamine have led some investigators to question whether reported methamphetamine-related cognitive deficits are clinically or functionally significant (see Hart et al. 2012).
In view of ambiguities within the clinical literature, research with laboratory animals under controlled conditions has provided additional important information regarding the effects of methamphetamine on cognitive behavior. For example, in recent studies with nonhuman primates, Groman et al. (2012; 2013) examined the ability of vervet monkeys to acquire, retain, and reverse a 3-choice visual discrimination before and after a 31-day chronic escalating-methamphetamine dosage regimen and observed that discrimination reversal performance, but not acquisition or retention, was adversely affected, especially during the second half of the escalating regimen. These studies provide evidence that the loss of inhibitory control assayed by discrimination reversal performance may be a cardinal feature of methamphetamine abuse and, possibly, addictive behavior more generally (reviewed in Izquierdo and Jentsch 2012).
The present studies were designed to examine further the effects of methamphetamine on discrimination learning and inhibitory control in a primate species. To both complement and extend previous research in monkeys, four additional factors were investigated in the present studies. First, the effects of self-administered, rather than experimenter-administered, methamphetamine were studied, adjusting unit dose and session length to achieve high levels of drug intake. Second, the present studies included a focus on the development, rather than steady-state maintenance, of 2-choice discrimination learning and reversal performance. Third, the effects of self-administered methamphetamine on learning and reversal performance were evaluated at different time points following the daily self-administration session. Finally, the effects of abrupt methamphetamine discontinuation on learning and reversal performance were assessed to measure disturbances in ongoing cognitive behavior that might be consistent with an amphetamine-type stimulant withdrawal syndrome (see DSM-IV [APA 2000]; reviewed in Cruickshank and Dyer 2009; Meredith et al. 2005). Studies were conducted with squirrel monkeys in touchscreen-based variants of the repeated acquisition and discrimination reversal tasks thought to assay, respectively, dimensions of learning and inhibitory control (Kangas and Bergman 2014).
METHODS
Subjects
Eight adult male squirrel monkeys (Saimiri sciureus) were used in the present studies (methamphetamine self-administration group, n=4; drug-free control group, n=4). All subjects previously served in behavioral studies of dopamine-related drugs or opioids, but had not received drug treatments for at least 6 months prior to the present studies. In addition, all subjects had no touchscreen experience prior to these studies. Portions of repeated acquisition and discrimination reversal baseline performance in drug-free control subjects were previously reported in Kangas and Bergman (2014). All subjects were individually housed in a temperature- and humidity-controlled vivarium with a 12-h light/dark cycle (7am-7pm), and had unlimited access to water in the home cage. Subjects were maintained at approximate free-feeding weights by post-session feedings of a nutritionally balanced diet of high protein banana-flavored biscuits (Purina Monkey Chow, St. Louis, MO). In addition, fresh fruit and environmental enrichment were provided daily. Experimental sessions were conducted 7 days a week. The protocol for the present studies was approved by the Institutional Animal Care and Use Committee at McLean Hospital. Subjects were maintained in a facility licensed by the U.S. Department of Agriculture and in accordance with guidelines provided by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animals Resources, Commission on Life Sciences, National Research Council (2011).
Procedures
Self-Administration
During self-administration sessions, subjects sat in a Plexiglas chair (Kelleher and Morse 1968) in a ventilated, sound-attenuating enclosure. Subjects faced a panel containing two response levers, colored stimulus lights, and a custom-designed Plexiglas receptacle (5×3.5×1.27 cm) mounted in front of the subject. Each press of the lever with a force greater than 0.25 N produced an audible click and was recorded as a response. An infusion pump (PHM-100-10, Med Associates, St. Albans, VT) outside the enclosure was used to deliver either i.v. drug infusions or, for initial training, 0.15 ml of a 30% sweetened condensed milk solution into the Plexiglas receptacle. All experimental events and data collection were controlled by Med Associates.
Four subjects comprising the methamphetamine self-administration group were first trained to respond on one lever under a 10-response fixed ratio (FR10) schedule of milk delivery. Responses on the other lever were recorded but had no programmed consequences. Lever assignment was counterbalanced across subjects. The completion of the FR10 on the active lever turned off the stimulus lights, delivered the reinforcer, and initiated a timeout (TO) period of 60-s during which all stimulus lights were off and responding had no scheduled consequences. Following the establishment of robust milk-maintained responding, each subject was prepared with an intravenous catheter for drug delivery, using procedures initially described by Herd et al. (1969). Under isoflurane anesthesia and in aseptic conditions, one end of a hydrophilically coated polyurethane catheter (inside diameter, 0.381 mm; outside diameter, 0.762 mm) was inserted and secured into a femoral or jugular vein. Catheters subcutaneously exited the subject’s back. Catheterized subjects wore nylon jackets at all times to protect the catheters. Next, i.v. infusions of methamphetamine (mg/kg; 0.1 ml/infusion) replaced milk deliveries as reinforcing events. Subjects first self-administered methamphetamine during 1-hr sessions. Once stable levels were observed via visual inspection, the methamphetamine dose-response function (0.0032-0.1 mg/kg/inj) was determined three times in each subject. Doses were studied in an irregular order and each unit dose was studied for a minimum of 5 days and until session-intake across 3 consecutive sessions was within ±20% of the 3-session mean. Subsequently, session length was increased to 2-hr to increase daily intake and methamphetamine dose-response functions were redetermined under otherwise identical conditions. The unit-dose that resulted in maximum average daily intake (peak unit dose) was used during daily self-administration sessions for the remainder of the study.
Touchscreen Assays
After the peak unit dose was identified, daily self-administration sessions with that unit dose continued for 30 days. On day 31, touchscreen sessions were introduced, also daily, 20 hr following each self-administration session. This interval was chosen to minimize the direct effects of methamphetamine during touchscreen sessions. Thus, subjects were exposed daily (7 days/week) to an approximately 1-hr touchscreen session at 1 pm, followed by a 1-hr break (spent in a holding chamber), and then a 2-hr self-administration session from 3-5 pm. Details, schematics, and photographs of the chamber used for touchscreen studies can be found in Kangas and Bergman (2012). All experimental events and data collection were programmed in E-Prime Professional 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA).
Repeated Acquisition
Previously-established methods were used to train subjects to repeatedly discriminate novel visual discriminations (Kangas and Bergman 2014). Each session began with concurrent presentation of two 7×7 cm digital photographs, each in a different randomly-selected quadrant of the screen. A touch response on one stimulus initiated the delivery of 0.15 ml of a 30% sweetened condensed milk solution into the reservoir (S+) paired with an 880 ms yellow screen flash, and followed by a 10 s intertrial interval (ITI) blackout; a touch response to the other stimulus immediately initiated the 10 s ITI (S−). The same two stimuli were presented during each of 200 trials comprising the day’s session. Photographs for each session were randomly selected from a laboratory bank of >10,000 images. Thus, the subject was required to learn a new S+/S− discrimination based on distinguishing features of two visual stimuli that had not been previously viewed. If the subject failed to master the discrimination within the 200-trial session (see below for mastery criterion), the same stimuli were presented during the next day’s 200-trial session. Subjects in the drug-free control group were exposed to the repeated acquisition task until 30 discriminations were mastered, after which a discrimination reversal task was introduced into the daily session. Subjects in the methamphetamine self-administration group required additional training (mastery of 60 discriminations) to achieve rates of within-session acquisition comparable to those of the control group (see Results for additional details).
Discrimination Reversal
In the discrimination reversal task, a variant of discrimination learning for examining response inhibition (Easton 2005; Mackintosh et al. 1968), the programmed consequences of responding reliably to the S+, and not S− stimulus are reversed after the subject learns the initial discrimination in daily sessions. The first 100 trials in the daily discrimination reversal session were conducted as described above, i.e., subjects learned a novel discrimination each session. However, on Trial 101 the relationship between S+ and S− was reversed without signal, i.e., during Trials 101-200, the stimulus that was initially S+ was made S−, and vice versa. If the subject failed to master the reversal within 100 trials, the same stimuli and contingencies were presented during a 100-trial session the following day. Subjects in both groups were exposed to the reversal task until 30 reversals were mastered prior to time course assessments.
Direct effects of methamphetamine self-administration on repeated acquisition and discrimination reversal
Following the characterization of daily methamphetamine self-administration on the development of acquisition and reversal performance, the 20-hr interval between self-administration and touchscreen session was reduced in order to evaluate more immediate effects of self-administered methamphetamine on performance. Acquisition and reversal performance were assessed following 12, 6, 3, and 1 hr, and 10 min. Each interval was evaluated once in an irregular order in each subject with 3-4 sessions conducted at the standard 20-hr interval between each test. All other experimental details remained the same.
Assessment of withdrawal via abrupt methamphetamine discontinuation
Following time course assessments, baseline control sessions with a 20-hr interval were conducted for an additional 7 sessions. Then, methamphetamine was replaced with saline for the next 4 daily self-administration sessions to determine whether abrupt discontinuation of methamphetamine self-administration would affect acquisition or reversal performance.
Data Analysis
In i.v. methamphetamine self-administration studies, the primary dependent measure was session intake of methamphetamine (mg/kg). Session intake was calculated by multiplying the total number of injections in a session by the self-administered unit dose of methamphetamine (mg/kg/inj). Dose-response functions were constructed by averaging intake for each subject during the last 3 sessions of each unit dose tested and then presenting group average unit dose intake (±SEM). The primary dependent measure used to evaluate repeated acquisition and discrimination reversal performance was trials-to-mastery. Mastery was defined as 9 of 10 consecutive trials correct (i.e., 90% correct). Therefore, this criterion quantified the number of trials required for the subject to either learn a novel discrimination in the case of repeated acquisition, or reverse response allocation in the case of discrimination reversal. A repeated measures two-way analysis of variance (ANOVA) was conducted to evaluate group differences in the development of acquisition and reversal performance. A repeated measures one-way ANOVA followed by a Dunnett’s test was conducted to evaluate whether acquisition and reversal performance at different intervals in time course studies was statistically different from the 20-hr control values. The criterion for significance was set at p<.05.
Drugs
Methamphetamine (d-methamphetamine hydrochloride) was purchased from Sigma Pharmaceuticals (St. Louis, MO), prepared for administration in a saline solution, and refrigerated and protected from light. Drug concentrations were prepared for each subject so as to deliver the unit dose in a 0.1 ml infusion. Doses are expressed in terms of the free base.
Plasma Analysis of Methamphetamine and Metabolite
In order to determine drug levels at the time of touchscreen sessions (i.e., 20 hr after self-administration), blood was drawn from each subject in the methamphetamine self-administration group. Samples were collected toward the end of the discrimination reversal condition and analyzed to determine plasma levels of both d-methamphetamine and its major active metabolite d-amphetamine. Approximately 1 ml of blood was withdrawn from the saphenous vein into a heparin-coated vacutainer. Plasma was separated and analysis was provided by NIDA Drug Supply Program Analytical Services and performed at the Center for Human Toxicology at the University of Utah (Salt Lake City, UT) using liquid chromatography/tandem mass spectrometry with a 0.1 ng/ml lower limit of quantitation of analyte (Slawson et al. 2002).
RESULTS
Figure 1 presents dose-response functions for total i.v. methamphetamine intake during 1-hr (circles) and 2-hr (triangles) self-administration sessions. The rate of intake increased in a dose- and session length-dependent manner in all subjects and, on average, was greatest when the unit dose of 0.032 mg/kg was self-administered in 2-hr sessions (0.85 [±0.25 SEM] mg/kg/day). Averaged results reflect individual data during the availability of 0.032 mg/kg/inj during 2-hr sessions in 3 of 4 subjects (0.1 mg/kg/inj was the peak unit dose in the 4th subject). Consequently, the unit dose of 0.032 mg/kg/inj methamphetamine was made available for self-administration in subsequent experiments (0.032 mg/kg/inj). Over the next thirty consecutive daily 2-hr sessions, average intake remained highly stable throughout this time, ranging from 0.35-1.6 mg/kg/session across individual subjects. Next, daily touchscreen training sessions were introduced 20 hr following daily self-administration sessions. Over the course of touchscreen training, group average intake increased from 0.81 (±0.3 SEM) mg/kg/day to 1.04 (±0.15 SEM) and, thereafter, remained stable throughout the remainder of the present experiments.
Figure 1.
Average (±SEM) methamphetamine (MA) intake (mg/kg) during sessions in which different unit doses of methamphetamine (mg/kg/inj) and saline (S) were available to self-administer during 1-hr (circles) and 2-hr (triangles) sessions.
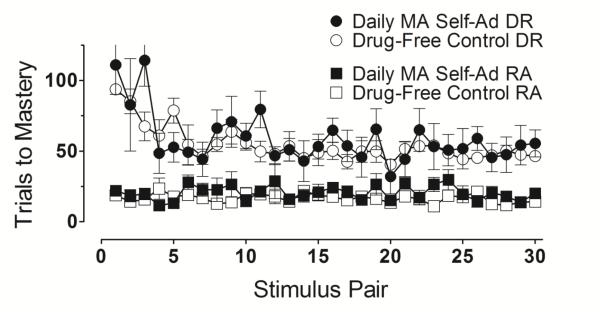
After touchscreen sessions involving the response-contingent delivery of sweetened condensed milk were introduced, training of the repeated acquisition task was undertaken as described in Methods. Figure 2 presents the mean number of trials required to master each successive discrimination in drug-free control subjects (unfilled circles) and in subjects that self-administered methamphetamine daily (filled circled). Drug-free control subjects took an average of approximately 150 (±28 SEM) trials to meet criteria in their first discrimination task. The number of trials to master subsequent discriminations decreased steadily over the course of training and, after 20 successful discriminations, very few trials (<20) were required to master novel discriminations daily. Subjects that self-administered methamphetamine daily required an average of almost 400 (±87 SEM) trials across multiple daily sessions to meet criteria in their first discrimination task. As in drug-free control subjects, the number of trials to master subsequent discriminations decreased steadily over the course of training in methamphetamine-exposed subjects. However, the position of the function describing trials to mastery across the first 30 discriminations was consistently and significantly higher for the methamphetamine self-administration group than drug-free controls (f=6.18, p<.05).
Figure 2.
Average number of trials (±SEM) to master discriminations for the methamphetamine (MA) self-administration group (filled circles) and the drug-free control group (unfilled circles).
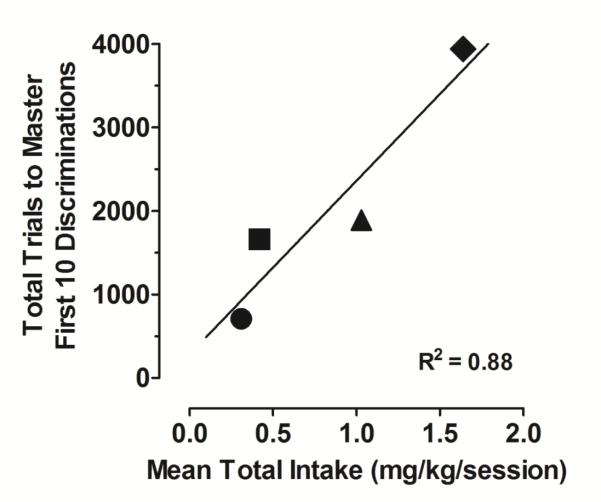
The disruption of repeated acquisition performance was evident across the first 30 discriminations, but was most pronounced during the first 10 discriminations. Data from the first 10 discriminations are noteworthy for at least 2 reasons. First, much of the development of discrimination learning skills appears to occur early in training. Second, as the error bars of the filled circles in Figure 2 indicate, relatively high levels of variability in trials-to-mastery was evident among individual subjects in the methamphetamine group. Likewise, high levels of variability in the number of injections taken also were observed among individual subjects (see the 2-hr 0.032 mg/kg/inj data point in Figure 1). To examine more closely the relationship between intake and trials-to-mastery, Figure 3 presents data from individual subjects showing the total number of trials required to master the first 10 discriminations as a function of average daily methamphetamine intake during the sessions in which those 10 discriminations were mastered. Each data point represents that relationship for an individual subject. Although the number of subjects is small, a high correlation (R2=.88) is apparent between methamphetamine intake and the number of trials to master the first 10 discriminations.
Figure 3.
Total number of trials required to master the first 10 discriminations as a function of average daily methamphetamine intake during the sessions those 10 discriminations were mastered. Each data point represents that relationship for an individual subject. The solid line represents a standard linear regression fit to the data.
To determine whether, following extended training, subjects that self-administered methamphetamine daily could approximate the learning rate of drug-free control subjects, all monkeys in the methamphetamine group were given the opportunity to learn 30 additional discriminations. As Figure 2 shows, subjects continued to show improvement and required fewer and fewer trials to master novel discriminations during additional training. In fact, the last 10 discriminations (i.e., stimulus pairs 51-60) were mastered by the group that self-administered methamphetamine at a rate that did not differ significantly from the rate at which drug-free control subjects mastered their last 10 discriminations (i.e., stimulus pairs 21-30) (f=1.42, p=.28).
Figure 4 shows data from the discrimination reversal condition that was introduced in the drug-free control and methamphetamine self-administration groups of monkeys after, respectively, 30 and 60 successful discriminations. The mean number of trials required to master novel discriminations (prior to reversal) is represented by the filled squares (methamphetamine self-administration group) and open squares (drug-free control group). Both groups rapidly mastered novel discriminations daily following introduction of the discrimination reversal condition. The filled and open circles in Figure 4 show mean number of trials required to master each successive discrimination reversal for the methamphetamine self-administration and drug-free control group, respectively. Fewer and fewer trials were required to master each successive discrimination reversal until a relatively stable rate of reversal learning occurred. Interestingly, unlike the development of repeated acquisition performance, the development of discrimination reversal performance did not differ significantly between the two groups (f=0.22, p=.66).
Figure 4.
Average number of trials (±SEM) to master reversals for the methamphetamine (MA) self-administration group (filled circles) and the drug-free control group (unfilled circles) conducted during Trials 101-200 following discrimination mastery for the methamphetamine self-administration group (filled squares) and the drug-free control group (unfilled squares) acquired during Trials 1-100.
Table 1 presents plasma concentrations (ng/ml) of methamphetamine and its major metabolite, amphetamine, in the 4 self-administration subjects collected immediately prior to a discrimination reversal session (i.e., 20 hrs following methamphetamine self-administration). Subjects that had been self-administering methamphetamine daily for >100 consecutive days showed no detectable levels of methamphetamine at the 20-hr time point. However, 3 of the 4 subjects did register detectable levels of amphetamine (4.0-12.0 ng/ml).
Table 1.
Plasma concentrations (ng/ml) of methamphetamine and the amphetamine metabolite 20 hr following i.v. methamphetamine self-administration (upper values).
| Plasma Concentration (ng/ml) | ||
|---|---|---|
| 20 hr Post Self-ad Session | ||
| Methamphetamine | Amphetamine | |
| Ss81 | < 4.0 | < 2.0 |
| Ss87 | < 4.0 | 11.3 |
| Ss124 | < 4.0 | 4.0 |
| Ss125 | < 4.0 | 12.0 |
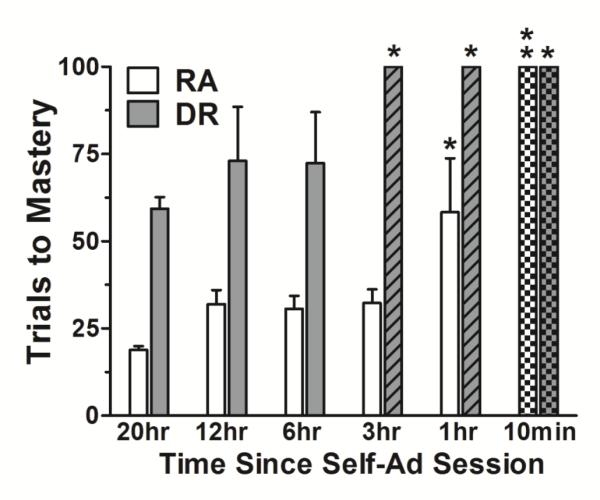
Figure 5 presents data for the number of trials to master a novel discrimination (open bars) and a discrimination reversal (shaded bars) at differing times following the i.v. methamphetamine self-administration session (10 min to 20 hrs). When the daily interval was reduced from 20 hr to 12 hr, the number of trials-to-mastery increased moderately and, on average, approximately 15 additional trials were required to master both a novel discrimination (±3 SEM) and reversal (±15 SEM). Similar values were obtained when the touchscreen session occurred 6-hr after the i.v. methamphetamine self-administration session. When the interval was reduced further to 3 hr, no additional trials were required to master a novel discrimination; however, all subjects failed to master the discrimination reversal. All 4 subjects performed at better than chance levels by the end of the session, but failed to meet the 90% criterion within the 100-trial reversal component (signified by the hashed shaded bar). Reducing the interval between sessions further to 1-hr significantly increased the number of trials to master the novel discrimination (f=3.89, p<.05) requiring, on average, approximately 30 (±15 SEM) additional trials. As with the 3 hr interval, all subjects failed to master the discrimination reversal with a 1-hr interval between sessions. When the time between self-administration and touchscreen session was further reduced to 10-min, none of the subjects were able to engage in the touchscreen task within the first 30 min; therefore, the session was terminated (signified by the checkered bars).
Figure 5.
Average trials (±SEM) to master a novel discrimination (open bars) and a discrimination reversal (shaded bars) following several intervals between the daily self-administration session and the touchscreen session. Hashed bars indicate the reversal was not mastered within the 100-trial session. Checkered bars indicate the inability of any subject to engage in the touchscreen task. (*p<.05; **p<.01)
The effects of abrupt methamphetamine discontinuation on repeated acquisition (filled squares) and discrimination reversal (filled circles) are presented to the right of the abscissa break in Figure 6. For comparison, group average control values for both measures under methamphetamine self-administration conditions are presented left of the abscissa axis break. Average trials-to-mastery for both measures were unaffected and remained within the range of control values during this 4-session saline condition.
Figure 6.
Average trials (±SEM) to master a novel discrimination (squares) and reversal (circles) during drug discontinuation sessions in which methamphetamine was replaced with saline. Data to the left of the abscissa break indicate average (±SEM) control values immediately prior to discontinuation condition.
DISCUSSION
The present studies were designed to assess the effects of daily self-administered methamphetamine on discrimination learning and reversal. The results of these studies indicate that daily self-administration of methamphetamine significantly disrupted the ability to develop repeated acquisition performance. The high correlation observed between methamphetamine intake and the number of trials to master initial discriminations among individual subjects (Figure 3) suggests that the degree of impairment may be related to the level of daily methamphetamine intake and, more generally, further supports the view that methamphetamine can disrupt the development of learning. Another possibility, however, is that individual differences in learning capabilities may relate to the effectiveness of methamphetamine reinforcers. Interestingly, providing additional opportunities for the methamphetamine self-administration group to master novel discriminations eventually resulted in a learning rate that reliably approximated that of drug-free control subjects. This indicates that under the present conditions, methamphetamine’s adverse effects on learning can be surmounted by remedial training.
The plasma analyses in the present studies provide some indication of the role of methamphetamine pharmacokinetics in its effects on discrimination learning. For example, even following extended daily exposure to 2-hr self-administration sessions (>100 consecutive days), levels of methamphetamine and its metabolite amphetamine were, respectively, not detectable and quite low (<2-12 ng/ml) during touchscreen sessions 20 hr later. These results comport well with pharmacokinetic data from Yuan et al. (2006) in squirrel monkeys and Melega et al. (2008) in vervet monkeys. For example, Yuan et al. (2006) found that plasma levels of methamphetamine peaked approximately 30 min after the p.o. administration of 1.25 mg/kg methamphetamine, and declined to near-zero levels 6 hours later. On the other hand, plasma levels of amphetamine peaked approximately 3 hrs post-administration, and declined to approximately 70% of peak values 6 hrs after administration. Thus, it is possible that the longer-acting metabolite, amphetamine, may have played a role in the effects of methamphetamine on repeated acquisition 20 hr following its self-administration in the present study.
Surprisingly, daily methamphetamine self-administration had no significant effects on the development of discrimination reversal performance. Possibly, the cognitive flexibility required to successfully adjust response allocation was not vulnerable to methamphetamine self-administration in the present studies. This seems unlikely in the view of recent findings documenting methamphetamine-related deficits in response inhibition in vervet monkeys (Groman et al. 2012; 2013). Alternatively, it is possible that the subject’s extended history of learning novel discriminations under conditions of methamphetamine self-administration successfully created a learning framework or state dependency that protected or blunted the disruptive impact of methamphetamine on further learning, including reversal learning. In this regard, it is important to note that remedial training was necessary in the methamphetamine self-administration group to achieve control levels of performance and terminal rates of discrimination learning. These group differences in history are an important consideration and deficits in reversal learning may have been observed if acquisition rates had not been comparable prior to introducing the reversal condition. However, if acquisition rates were not equivalent prior to introduction of the discrimination reversal condition, group comparisons of reversal performance would have been particularly problematic.
Despite not observing disruptions in the development of discrimination reversal, the time course data (Figure 5) show that its performance was more vulnerable than initial discrimination learning to the direct effects of self-administered methamphetamine. Despite differences in route/contingency of drug administration, the greater vulnerability of reversal learning agrees well with the findings by Groman et al. (2012; 2013) using escalating experimenter-administered methamphetamine of a dose-range (0.35-1.0 mg/kg) highly similar to that self-administered among subjects in the present study. Here, self-administration conditions were arranged to maximize daily intake and yielded daily dosages considerably larger than therapeutic doses of methamphetamine (Desoxyn) administered orally in ADHD-diagnosed humans (5-25 mg/day; Berman et al. 2009). However, commonly abused doses of methamphetamine can range from 100-1000 mg/day (NHTSA, 2015), making the dosages self-administered in the present studies comparable to the lower end of that range for a typical 70 kg human.
The stability of both acquisition and reversal performance following abrupt discontinuation of methamphetamine is not particularly surprising, given that methamphetamine does not produce overt physiological signs of withdrawal in nonhuman animals. Rather, as described in the amphetamine-type stimulant withdrawal syndrome that is defined in the DSM-IV (APA 2000), abrupt methamphetamine discontinuation in human clinical populations can result in disturbed sleep, mood, and anxiety (reviewed in Cruickshank and Dyer 2009; Meredith et al. 2005). Assessing whether abrupt abstinence disrupts organized behavioral performance can provide important clues related to more subtle forms of drug withdrawal (e.g., Beardsley and Martin 2000). The present results indicate that following >100 daily sessions of subjects self-administering relatively large dosages of methamphetamine, no signs of withdrawal, either overtly physical or cognitive, were observed following abrupt cessation.
In summary, daily methamphetamine self-administration resulted in marked disruption in the development of discrimination learning assayed by the repeated acquisition task; however, those deleterious effects could be surmounted following extended exposure to the task. Once discrimination learning was mastered, the development of reversal performance did not significantly differ from drug-free control subjects. However, reversal performance was much more vulnerable to methamphetamine’s direct effects. Taken together, these results highlight interesting differences in the direct effects of methamphetamine and the consequences of chronic exposure of dosages comparable to those abused.
ACKNOWLEDGEMENTS
The authors thank Michael Z. Leonard for assistance conducting these studies and Drs. David Moody and David Andrenyak for analyzing plasma samples. This research was supported by grants K01-DA035974 (BDK) and R01-DA035857 (JB) from the National Institute on Drug Abuse.
Footnotes
The authors have no conflicts of interest to report.
REFERENCES
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th American Psychiatric Association; Washington DC: 2000. [Google Scholar]
- Baicy K, London ED. Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction. 2007;102:5–15. doi: 10.1111/j.1360-0443.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Martin BR. Effects of the cannabinoid CB(1) receptor antagonist, SR141716A, after Delta(9)-tetrahydrocannabinol withdrawal. Eur J Pharmacol. 2000;387:47–53. doi: 10.1016/s0014-2999(99)00792-x. [DOI] [PubMed] [Google Scholar]
- Berman SM, Kuczenski R, McCracken JT, London ED. Potential adverse effects of amphetamine treatment on brain and behavior: a review. Mol Psychiatry. 2009;14:123–142. doi: 10.1038/mp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2013;38:259–274. doi: 10.1038/npp.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A. Behavioural flexibility, social learning, and the frontal cortex. In: Easton A, Emery NJ, editors. The cognitive neuroscience of social behavior. Psychology Press; New York: 2005. pp. 59–80. [Google Scholar]
- Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, London ED, Jentsch JD. Dysregulation of D2-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci. 2012;32:5843–5852. doi: 10.1523/JNEUROSCI.0029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Morales AM, Lee B, London ED, Jentsch JD. Methamphetamine-induced increases in putamen gray matter associate with inhibitory control. Psychopharmacology (Berl) 2013;229:527–538. doi: 10.1007/s00213-013-3159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Marvin CB, Silver R, Smith EE. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology. 2012;37:586–608. doi: 10.1038/npp.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd JA, Morse WH, Kelleher RT, Jones LG. Arterial hypertension in the squirrel monkey during behavioral experiments. Am J Physiol. 1969;217:24–29. doi: 10.1152/ajplegacy.1969.217.1.24. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl) 2012;219:607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Bergman J. A novel touch-sensitive apparatus for behavioral studies in unrestrained squirrel monkeys. J Neurosci Meth. 2012;209:331–336. doi: 10.1016/j.jneumeth.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Bergman J. Repeated acquisition and discrimination reversal in the squirrel monkey (Saimiri sciureus) Anim Cogn. 2014;17:221–228. doi: 10.1007/s10071-013-0654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol Biol Chem Exp Pharmakol. 1968;60:1–56. doi: 10.1007/BFb0107250. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ, McGonigle B, Holgate V, Vanderver V. Factors underlying improvement in serial reversal learning. Can J Psychology. 1968;22:85–95. doi: 10.1037/h0082753. [DOI] [PubMed] [Google Scholar]
- Melega WP, Jorgensen MJ, Laćan G, Way BM, Pham J, Morton G, Cho AK, Fairbanks LA. Long-term methamphetamine administration in the vervet monkey models aspects of a human exposure: brain neurotoxicity and behavioral profiles. Neuropsychopharmacology. 2008;33:1441–1452. doi: 10.1038/sj.npp.1301502. [DOI] [PubMed] [Google Scholar]
- Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronicmethamphetamine use: a literature review. Harv Rev Psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- National Highway Traffic Safety Administration 2015 http://www.nhtsa.gov/people/injury/research/job185drugs/methamphetamine.htm Retrieved August 25, 2015.
- National Research Council . Guide for the care and use of laboratory animals. eighth National Academy Press; Washington DC: 2011. [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Slawson MH, Taccogno JL, Foltz RL, Moody DE. Quantitative analysis of selegiline and three metabolites (N-desmethylselegiline, methamphetamine, and amphetamine) by high-performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J Anal Toxicol. 2002;26:430–437. doi: 10.1093/jat/26.7.430. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime . World Drug Report. United Nations; Vienna: 2013. [Google Scholar]
- Wood S, Sage JR, Shuman T, Anagnostaras SG. Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol Rev. 2013;66:193–221. doi: 10.1124/pr.112.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Hatzidimitriou G, Suthar P, Mueller M, McCann U, Ricaurte G. Relationship between temperature, dopaminergic neurotoxicity, and plasma drug concentrations in methamphetamine-treated squirrel monkeys. J Pharmacol Exp Ther. 2006;316:1210–1218. doi: 10.1124/jpet.105.096503. [DOI] [PubMed] [Google Scholar]