Abstract
There are compelling reasons to study the role of steroids and sex differences in the circadian timing system. A solid history of research demonstrates the ubiquity of circadian changes that impact virtually all behavioral and biological responses. Furthermore, steroid hormones can modulate every attribute of circadian responses including the period, amplitude and phase. Finally, desynchronization of circadian rhythmicity, and either enhancing or damping amplitude of various circadian responses can produce different effects in the sexes.
Studies of the neuroendocrine underpinnings of circadian timing systems and underlying sex differences have paralleled the overall development of the field as a whole. Early experimental studies established the ubiquity of circadian rhythms by cataloging daily and seasonal changes in whole organism responses. The next generation of experiments demonstrated that daily changes are not a result of environmental synchronizing cues, and are internally orchestrated, and that these differ in the sexes. This work was followed by the revelation of molecular circadian rhythms within individual cells. At present, there is a proliferation of work on the consequences of these daily oscillations in health and in disease, and awareness that these may differ in the sexes.
In the present discourse we describe the paradigms used to examine circadian oscillation, to characterize how these internal timing signals are synchronized to local environmental conditions, and how hormones of gonadal and/or adrenal origin modulate circadian responses. Evidence pointing to endocrinologically and genetically mediated sex differences in circadian timing systems can be seen at many levels of the neuroendocrine and endocrine systems, from the cell, the gland and organ, and to whole animal behavior, including sleep/wake or rest/activity cycles, responses to external stimuli, and responses to drugs. We review evidence indicating that the analysis of the circadian timing system is amenable to experimental analysis at many levels of the neuraxis, and on several different time scales, rendering it especially useful for the exploration of mechanisms associated with sex differences.
Keywords: circadian rhythms, steroids, sex difference, suprachiasmatic nucleus
1. Introduction and Background
While sex differences1 are best known in relation to reproduction and reproductive behaviors, they in fact occur in phenomena completely unrelated to reproductive processes. Given that each cell of the body contains copies of the sex chromosomes identifying the animal as male or female, it is to be expected that bodily functions unrelated to reproduction also differ between the sexes. One such example is seen in the circadian system and the mechanisms underlying these differences.
In general, we are acutely aware of the regular alternation between activity and rest, and we are especially aware of the daily need for sleep. Less salient are the multitudes of regularly recurring changes in endocrine and metabolic responses that accompany these regular rest-activity cycles. These changes are not externally driven by the light-dark cycle or our alarm clocks, but are orchestrated by a plethora of clocks within the body. The internally organized daily rhythms are termed “circadian rhythms”. In the absence of all external temporal cues, circadian rhythms persist under constant conditions, with a period (cycle duration) close to 24 hours. This is an important piece of evidence supporting the notion that circadian rhythms are indeed driven by internal clocks, rather than external stimuli.
Internally orchestrated circadian rhythms can be distinguished from daily rhythms (changes that are driven by signals from the environment). This is achieved by testing for rhythmicity in the absence of external cues, thereby distinguishing internally timed from externally driven responses. Circadian rhythms also differ from “masked” responses, which can influence behavior but do not entrain or phase shift the body clock. Rhythms can be measured by their amplitude, frequency and phase relationship to other markers of time. The tools and terms used by chrono-researchers to explore these aspects of circadian rhythms are described in Textbox 1.
The circadian timing system has the dual functions of enabling the anticipation of upcoming events and determining the time of maximal and minimal expression times of a multitude of responses. These two functions, anticipation and modulation of expression, can be seen in both behavioral and physiological responses. Thus, circadian timing system enables organisms to anticipate the daily fluctuations in light, temperature, food availability or predator activity, and prepare the body to meet the physical and cognitive demands that are essential for survival. This system also ensures that the peak activity time of individual cells, and of each behavioral and physiological activity, occurs at an optimal time of the day.
As will be discussed, virtually each cell in the body has a “circadian clock”. Furthermore, the secretions of gonadal hormones differ in males and females, and these sex-typical hormones circulate in the blood and act back on many sites in the body, thereby affecting circadian processes at the cellular level. Male-female differences in the circadian timing system range from the localization of steroid receptors of the master brain clock within the suprachiasmatic nucleus (SCN), to daily rhythms of hormone secretion. The sexual dimorphism in the circadian timing system derives in part from the diverse effects of steroid hormones in regulating physiological and neurological processes. The two major sources of steroids involved in modulating clock functions are the adrenal and gonadal glands, each of which influences circadian rhythmicity in unique and often sex-specific ways.
In the present review, we introduce the basic organization of circadian system. Next we discuss the reciprocal regulation of circadian clocks and steroid hormone effects, and then explore the sex differences in circadian system and the potential underlying mechanisms.
2. Overview of the circadian timing system
One of the advantages of studying the circadian timing system is that it is experimentally tractable at multiple levels of analysis including rhythmic changes in genes and proteins within cells, in electrical and metabolic responses, rhythmic responses in specific tissues and organs, and in the behavior of the organism as a whole. Sex differences have been described at each level of the neuraxis regulating circadian rhythms, from genes to behavior. We discuss this system using a bottom-up approach, first describing the timing system at a cellular/molecular level, then discussing the master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus, and then delineating the ways in which the SCN orchestrates the phase relationships of oscillation in clock bearing cells in the rest of the body, including extra-SCN brain regions and peripheral organs.
Molecular and cellular clocks
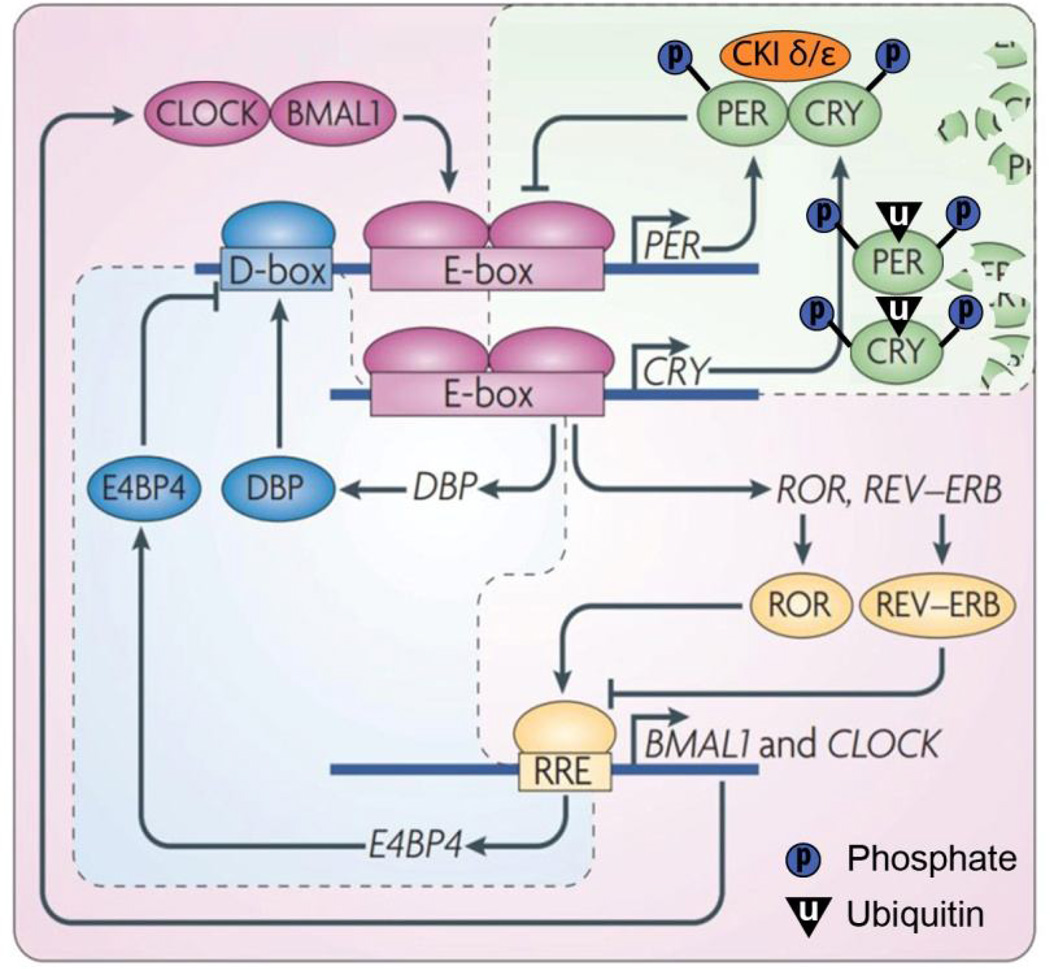
Individual cells are capable of self-sustained circadian oscillation in gene expression [1, 2]. The cellular-molecular clock entails a set of transcriptional modulators and their protein products, constituting interlocked transcriptional/translational feedback loops (reviewed in [3, 4]). In this molecular clock (Fig. 1), CLOCK and BMAL1 are two transcriptional factors that bind to the highly conserved E-box (sequence CACGTG) in the promoter region of numerous target genes, including the other set of core clock genes Per and Cry, and activate their transcription. The protein products PER and CRY form a dimer that translocates into the nucleus inhibiting the transcriptional activity of CLOCK/BMAL1. Posttranslational mechanisms such as phosphorylation and ubiquitination control the stability, or degradation rate, of PER and CRY proteins. The CLOCK/BMAL1 dimers also activate the transcription of another set of core clock genes Rev-erb and Ror. REV-ERB and ROR bind to the retinoic acid-related orphan receptor response element (RRE) in the promoter region of their target genes including clock and Bmal1, and regulate transcription. Additionally, an accessary loop involving transcriptional factors such as DBP and E4BP4 that bind to the D-box in the promoter region further contributes to the robustness of the molecular clock. The inter-connected transcriptional/translational feedback loops regulate each cycle of clock genes to last approximately 24 hours, thereby serving as the molecular basis for the autonomous circadian oscillation. The genes that are not directly involved in the feedback loop but contain the regulatory elements including E-box, RRE site or D-box, and are thus under the control of the molecular clock, are termed as clock-controlled genes (CCG).
Figure 1. Schematic of the molecular clock in mammals.
Readers are referred to the text for details. Modified from Zhang & Kay, 2010 [3, 4] with permission.
Suprachiasmatic nucleus
A master circadian clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus serves to signal time of day to the rest of the body and to process information from cues in the external and internal environment. Among the most salient of external cues are those derived from the light-dark cycle; the most salient of internal cues are those derived from daily oscillations in steroid hormones.
The evidence for the brain clock rests on decades of research in many laboratories using a variety of techniques [5]. While the existence of such a clock is no longer challenged, knowledge of how it works to encode time remains incomplete. In rodents, the SCN is comprised of a heterogeneous population of about 10,000 cells on each side of the third ventricle above the optic chiasm. Lesions of the SCN abolish circadian rhythms while transplants of perinatal SCN tissues restore behavioral rhythms, including locomotor activity, eating, gnawing, and drinking in host lesioned animals [6]. In contrast, rhythmic endocrine functions, such as secretion of luteinizing hormone (LH) or cortisol do not recover following transplantation [7–9]. SCN transplants contained within polymer capsules that permit diffusion but prevent neuronal outgrowth similarly restore behavioral, but not endocrine, rhythms [10]. These findings suggest humoral and axonal output signals are produced by the SCN. The humoral signals are sufficient to drive behavioral rhythms, but the circadian regulation of neuroendocrine function requires precise neuronal connections from the SCN. The SCN sends monosynaptic efferents locally, to nearby hypothalamic regions - both to neurosecretory cells and other extra-SCN brain regions [11–14]. From these sites, timing signals reach the rest of the brain and peripheral tissues [15, 16].
Extra-SCN brain clocks
While their phase is normally set by the SCN, neurons in extra-SCN regions also bear the molecular machinery of circadian clocks. For example, there are rhythms of PER2 expression in the forebrain, most of which peak near dawn or dusk [17]. As one indication of the important regional heterogeneity in the mammalian forebrain, a second study by this group showed that the expression of Per2, Bmal1 and Dbp mRNA oscillates, with the phase and amplitude of rhythms of each gene, varying across forebrain regions [18]. Presumably these regional differences ensure that the timing of specific behaviors occur at appropriate times of day.
Peripheral clocks
Cell-based clocks also occur in the periphery where they control molecular and cellular events related to tissue-specific functions and generate tissue-specific output rhythms [19–21]. In peripheral tissues, CCGs are estimated to represent 8–10 % of the transcripts. CCG’s regulate important physiological/biochemical processes within each tissue, including rate-limiting enzymes for the biosynthesis or metabolism of hormones and receptors responding to upstream hormones that are critical for neuroendocrine function [22, 23]. This hierarchical time-keeping system regulates circadian rhythms throughout the body [24–26]. Relevant in the present context, sex differences occur at several levels of the neuraxis.
3. Sex differences in circadian timing system
Sex differences in the SCN
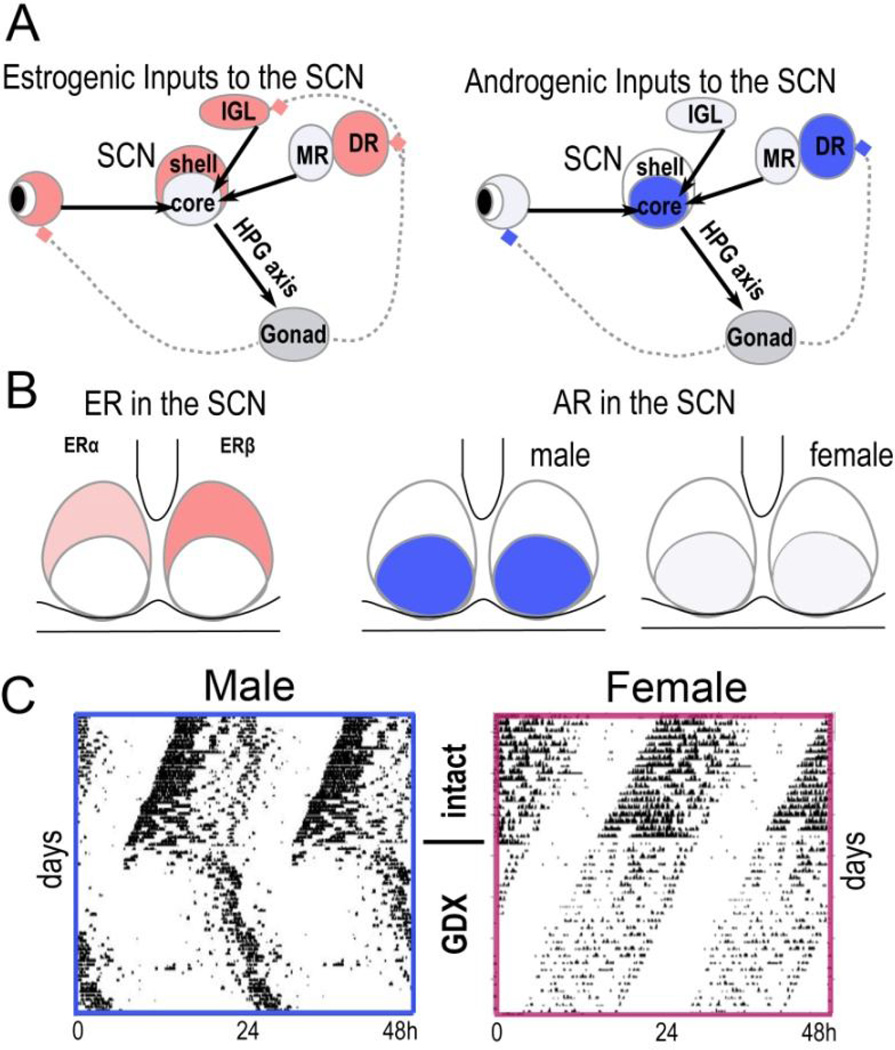
Sex differences in SCN morphology, including the volume, number of cells and synapse density as well as in neuropeptide expression have long been known [27]. SCN receives both estrogenic and androgenic inputs [28] (Fig. 2A). There are also sex differences in the receptor population in the SCN (Fig. 2B). Androgen receptors (ARs) are expressed in the core SCN region in intact male mice, but not in castrated males [29]. Treatment of gonadectomized male and female mice with androgens results in expression levels comparable to intact males, indicating that activating effects of the hormone determine sex-typical receptor expression. In contrast, estrogen receptors (ER) are primarily expressed in the shell SCN with greater numbers being found in ovariectomized females, than in orchidectomizeed male mice [30]. Estrogen treatment of the gonadectomized mouse had no effects on ERα but produced a five-fold reduction in ERβ in females, but only a two-fold reduction in males. In humans, there is a significantly stronger nuclear ERα expression in female SCN neurons but no significant sexual dimorphism in nuclear ERβ or progesterone receptors [31]. Electrophysiological recordings in rodent SCN indicate that daytime firing rates are higher than nighttime in both males and females, but interesting in the present context, daytime firing rates are higher in male than female mice under baseline conditions [32]. The effects of gonadal hormones in modulating circadian rhythms have been investigated in animals following gonadectomy and hormone replacement. Orchidectomy, but not ovariectomy results in a marked lengthening of the free-running period in mice [33] (Fig. 2C).
Figure 2. Gonadal hormones modulate the SCN and circadian rhythms.
A. Schematic representation of differences between the estrogenic versus androgenic inputs to the SCN. The SCN receives inputs from ER-rich (red) and AR-rich (blue) nuclei, including the retina, intergeniculate leaflet (IGL), and dorsal raphe (DR). DR projects to the SCN via the median raphe (left). B. Schematic showing the distribution of ER and AR in the SCN (see text for details). C. Representative actograms show free running locomotor activity of male and female mice housed in constant darkness before and after gonadectomy (GDX). The horizontal axis is 48 h and the vertical axis is consecutive days. Adapted from [33] with permission.
Sex differences in pathways from SCN to HPG axis
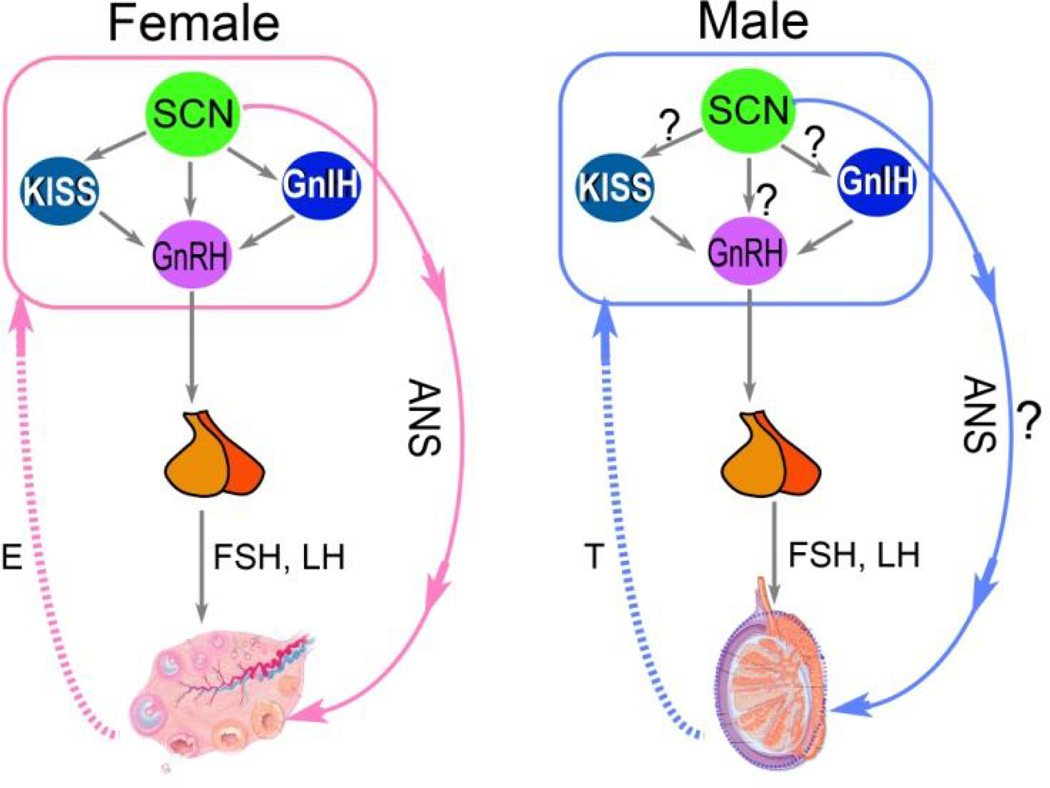
The SCN plays a critical role in female reproduction by regulating the LH surge and subsequent ovulation during the estrous cycle (reviewed in [34, 35]). Although normally the LH surge only occurs once in each estrous cycle, in ovariectomized, estrogen-treated rats, daily LH surges occur at the precisely the same time of day, every day [36, 37]. This precise temporal control is achieved by neural signals produced by the SCN, which create a temporal window during which the LH surge can occur [35]. The key neuroendocrine cell populations involved in this event are those containing gonadotropin inhibiting hormone (GnIH), gonadotropic releasing hormone (GnRH) and kisspeptin (Kiss1). These neurosecretory cells receive monosynaptic projections from the SCN, but studies of these pathways have focused on females, with a dearth of evidence in males [27, 38] (Fig. 3).
Figure 3. Schematics showing the pathways from the SCN to HPG axis in female and male.
In females, direct projections from the SCN to GnRH, GnIH and Kiss1 cells, as well as a multi-synaptic pathway form the SCN to ovary have been identified (left panel). In males, it remains to be determined whether similar pathways from the SCN also exist.
Neurons in the core SCN containing vasoactive intestinal peptide project directly to GnRH neurons in the medial preoptic area (MPOA), while those in the shell SCN containing vasopressin project to Kiss1 neurons in the anteroventral paraventricular nucleus (AVPV) [38]. Although the neuropeptidergic phenotype remains to be discovered, direct projection from the SCN to GnIH neurons in the dorsomedial hypothalamus (DMH) has been reported [39]. Both Kiss1 and GnIH neurons project to GnRH neurons. Through these neural pathways, the SCN regulates the timing of LH surge and ovulation in females. Although there are no known sex differences in GnRH neurons, the Kiss1 cells in AVPV are more abundant in females [40]. The sex differences in Kiss1 cells are thought to be due to the organizational programming by steroids: this is evidenced in the fact that following testosterone treatment of females during a critical period of development, there is no difference from males in Kiss1 cells in AVPV. The sexually-dimorphic expression of Kiss1 cells in AVPV has been proposed as a neural mechanism underlying the sexually-dimorphic LH surge, which occurs in females but not in males [41].
Sex differences in peripheral clocks
Pituitary
GnRH stimulates the baseline release and the surge of gonadotropins (FSH, LH) from the anterior pituitary, and the question of interest is whether the pituitary itself expresses circadian rhythms and sex differences therein. The pituitary cells possess the core molecular clock mechanism described above in Fig. 1. A primary culture of a cell line generated from rat anterior pituitary revealed a circadian expression pattern of the core-clock genes, indicating the presence of a functional circadian oscillator [42]. By sampling pituitary tissue every hour over two days, a microarray study identified more than 250 genes, including the genes encoding the core clock components BMAL1 and PER2, that are expressed in a circadian fashion [43]. Expression of pituitary PER2 has a circadian rhythm in both sexes, but it is phasedelayed in females compared to males [32]. The functional significance of the intrinsic circadian clock in the pituitary has been tested in female mice that specifically lack BMAL1 in their gonadotropes [44]. Although the animals showed increased variability in estrous cycle length, they were otherwise reproductively normal, suggesting that the intrinsic clock in gonadotropes is not necessary for generating LH surge but rather contributes to estrous cycle robustness.
Ovaries
Circadian rhythms in clock gene expression have been observed in the ovaries of numerous mammalian species [45–47]. In vitro studies have shown that follicular cells contain a cell autonomous circadian oscillator that can be affected by gonadotrophins [48–50]. In accordance with this finding, it has been shown that in rats, hypophysectomy diminished the circadian oscillation of clock genes Per2 and Bmal1, while replacement with human chorionic gonadotrophin, a placenta analog to LH, reinitiated the rhythms [51]. The results together suggest that gonadotrophins play a critical role in entraining the individual cellular oscillators in the ovaries to produce overt circadian rhythms.
Testes
In contrast to the ovaries, most data from various mammalian species suggest that the testes are not intrinsically rhythmic and that the expression of clock genes in the testes does not show circadian rhythmicity [45, 52]. Furthermore, StAR, the rate-limiting enzyme for steroidogenesis is not rhythmically expressed in mouse testes [53]. However, circadian clock mechanisms have been found in accessory structures such as the epididymis, vas deferens and seminal vesicles [54].
Sex differences in behavioral rhythms
As discussed above, gonadectomy results in a marked lengthening of the free-running period in male mice [33] (Fig. 2C). Treatment of gonadectomized males with testosterone reinstates the shorter period typical of the intact animal [55]. Impressively, the change in period does not require extra-SCN brain regions or peripheral effectors as testosterone implants directly in the brain, near the SCN, also produce a shortening of the free-running period in gonadectomized male mice [56]. In addition to modulating SCN timekeeping and locomotor activity, testosterone also modulates SCN responsiveness to light [57]. ARs are located in the core SCN (Fig. 2B), the subregion that receives direct retinal inputs in mice [58]. The colocalization of AR and retinal innervation suggests that information from testicular hormones and that of photic cues is integrated within this region of the SCN. In accordance, Butler et al has shown that in mice, gonadectomy potentiates the parametric effects of light that lengthen circadian period. Furthermore, the precision of activity onset was positively correlated with T concentration following hormone replacement in gonadectomized mice [57]. In mice, ovariectomy has no effect on the period of free-running rhythms [33] (Fig. 2C). This is consistent with the findings that in mice lacking ERα, the free-running period in constant dark is not different from wild type controls in both males and females [59]. However, in other rodent species, ovariectomy can lead to a lengthening of the free-running period, pointing to a species difference in estrogenic regulation of circadian rhythms [60–62].
The role of ERs in regulating circadian rhythms has been investigated using animals with deficiency of either ERα or ERβ. It appears that ERα plays a dominant role in the estradiolinduced increase in locomotor activity [63]. However, in this paradigm, the potential developmental effects of the absence of estrogenic signaling may confound the interpretation of the results in global knockouts. This limitation can be circumvented: evaluation of the role of ER in circadian regulation using selective receptor agonists in mice following ovariectomy shows that ERα modulates the magnitude of activity and rhythms, while ERβ regulates phase and temporal distribution of the activity [64]. Such evidence points to a distinct role of the two receptors in circadian regulation.
The 4 core genotypes (FCG) mouse model, in which sex chromosome complement is independent of gonadal phenotype has been useful in exploring whether sex differences in behavior are sex chromosome or gonadal sex dependent [65]. In this model, the genotype may be XX or XY, and each genotype may develop a testis or an ovary allowing assessment of whether sex differences are caused by the activational effects of gonadal hormones, organizational effects of gonadal hormones, or by sex chromosome complement. Gonadal males (XX and XY with testes) had more androgen receptor expression in the SCN and behaviorally reduced photic phase shift response compared with gonadal female (XY and XX with ovaries) FCG mice [32]. Removal of circulating gonadal hormones in adults, to test the activational versus organizational effects of sex, revealed that XX animals have longer activity duration than XY animals regardless of gonadal phenotype pointing to a chromosomal effect. The activational effects of gonadal hormones were more important for regulating activity levels in gonadal male mice than in gonadal female FCG mice.
A review of sex differences in rodent circadian behaviors, including period, responses to external stimuli, and anticipatory behaviors, indicates that the most apparent sex difference in behavior is in the timing of activity onset [66]. Daily variation of activity onset is closely related to the phase of estrous cycle in females; males are similarly variable in activity onset when tested on 4 consecutive days though the causes of male variability are not known [67]. There are also reports of sex differences in the behavioral responses of circadian system to photic cues, such that females re-entrain faster following a shift of the light/dark cycle [68] and show bigger phase shifts to a light pulse [32].
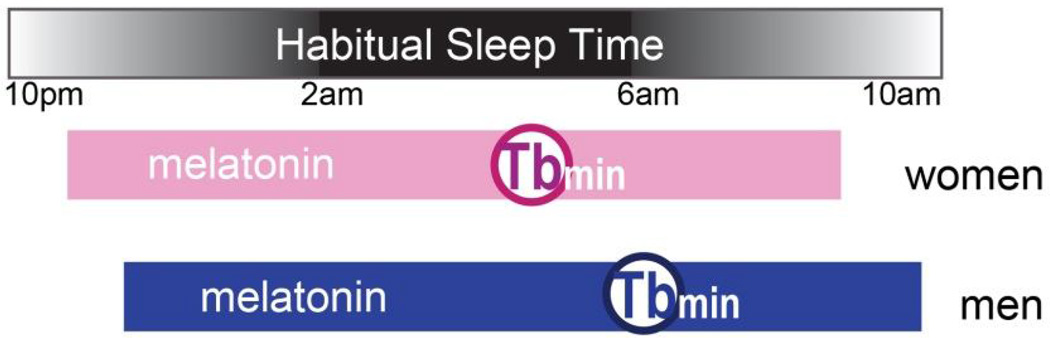
In humans, the best documented sex differences in circadian rhythms lies in the chronotype or phase of the rhythms/sleep [69, 70] (Fig. 4). Although the phase of sleep changes over lifespan, during adulthood, between 20–50 years of age, women tend to have earlier sleep phase compared to men at the same age, and this difference disappears only at the time of menopause/andropause. The earlier sleep phase is consistent with the shorter circadian period observed in women [71, 72]. Interestingly, there appears to be a sex-by-race interaction, such that the circadian period is shorter in women relative to men in European-Americans, but longer in women in African-Americans [73].
Figure 4. Timing of the circadian phase markers relative to sleep time in women and men.
Black/white bar on top indicates the timing of the habitual sleep episode with the onset range between 10pm to 2pm and offset range between 6am to 10am in both men and women. Pink and blue bars indicate the timing of melatonin secretion, while the open circles indicate the average timing of core body temperature minimum (Tbmin). Adapted from [64].
Impact of sex differences in circadian phase/period
The sex differences in circadian phase and/or period may have implications in mental health, e.g. insomnia [72] or seasonal affective disorder (SAD) [74], which are closely related to circadian rhythms and more prevalent in females. The sex difference in the prevalence of SAD emerges during puberty, is highest during the reproductive years, and tends to fade away after menopause [75, 76]. This time window appears to coincide with the developmental stages during which the greatest sex differences are observed in the chronotype or phase of circadian rhythms [69, 70]. Whether or not there is a causal link between the two phenomena remains to be determined: the sex differences seen in the circadian chronotype and SAD prevalence could be derived from a common source, such as cycling ovarian hormones.
4. Sex differences in the circadian modulation of HPA axis
In addition to the circadian phenotypes that are directly regulated by the principal brain clock, sex differences have also been observed in downstream physiological circadian processes, i.e. the HPA axis. The sex differences in HPA function are in part due to differences in the circulating gonadal steroid hormones, with testosterone acting to inhibit HPA function, whereas estrogen enhances HPA function.
Connectomics: The pathways from the SCN to adrenal
There are two distinct neural pathways through which the SCN regulates the function of HPA axis: projections to the hypothalamic CRH neurons and multi-synaptic innervation to the adrenal cortex via autonomic nervous system [77]. Projections from the SCN to the PVN mediate the diurnal rise in glucocorticoids [78–80]. Lesions of the SCN eliminate the daily rhythm in corticosterone [81, 82], and the afternoon increase in corticosterone is absent in CRF-deficient mice [83], indicating that CRF plays a role in the diurnal rhythm of corticosterone. Pituitary ACTH is also rhythmic and [80, 84, 85] there is an increased adrenal sensitivity to ACTH, driven by autonomic inputs via the splanchnic nerve, which enhances corticosterone secretion [86–88].
Sex differences in HPA axis
Sex differences in the activation of the stress-responsive HPA axis are very well established in rodent studies, with females reacting more than males [89, 90]. Rhythmic GC levels differ between males and females: in laboratory rats and mice, the peak level and the amplitude of the GC rhythm are much higher in females [91, 92]. The rhythmic secretion of GC is an important output signal of the circadian system that conveys timing information generated in the SCN to the extra-SCN brain clocks and/or peripheral oscillators through its widely distributed receptors. It has been shown that GC can reset extra-SCN cellular clocks through GR signaling, synchronizing the peripheral clocks [93, 94]. Thus, sex differences in GC rhythm may contribute to a divergence in the hierarchical organization of the circadian timing system.
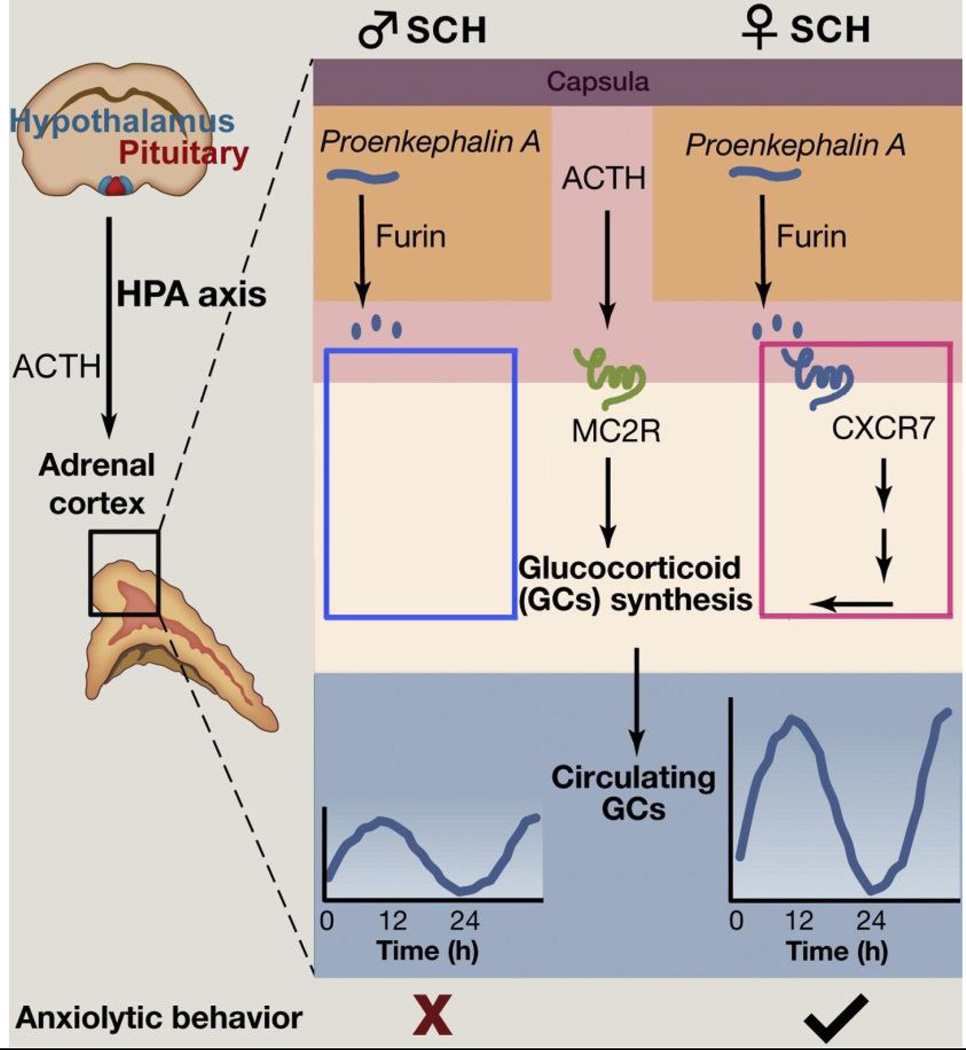
The functional consequences of sex differences at various points in the circadian hierarchy are well demonstrated in a naturally occurring condition known as subcapsular cell hyperplasia (SCH), where the GC amplitude is augmented in females but not in males [92]. The female specific increase in GC amplitude has been linked to the activation of CXCR7, a β-arrestin-biased G-protein-coupled receptor mediated signaling pathway. Interestingly, the CXCR7 gene is selectively expressed in the adrenal cortex of females, but not sufficiently in males. Thus, the sex-dependent expression of genes that modulate GC rhythms represents a mechanism through which the sex differences arise in circadian regulation and emotional responses (Fig. 5).
Figure 5. CXCR7 pathway underlies the sex differences in glucocorticoid amplitudes and anxiolytic behavior in subcapsular hyperplasic (SCH).
SCH cells express proenkephalin A, which can be converted to active opioid peptides by the processing enzyme furin. Once in the portal plexus, opioid peptides activate the chemochine receptor CXCR7, which is expressed mainly in females, and promote glucocorticoid (GC) synthesis and anxiolytic behaviors. Adapted from [95] with permission.
5. Summary
The circadian timing system evolved over millions of years under stable and predictable environmental lighting conditions resulting from the rotation of our planet. It is only in the last two centuries, as artificial lighting and rapid transmeridian travel became possible, that this ancient time keeping system in our body has been challenged. Numerous studies have focused on the impact of circadian disruption caused by aberrant lighting condition and the resulting changes in our work and play habits as well as the negative consequences for bodily health, i.e. cancer, cardiovascular disease, and the disorders of the sleep, metabolism and mood associated with shift work and modern life styles [96–103]. However, sex differences in these responses are not well established. Secretion of sex steroids is affected by shift work, sleep or circadian disturbance, which may modulate the vulnerability to negative outcomes [104, 105]. An especially salient consideration, given the available information on sex differences in immune responses, is the effect of circadian disruptions on this system [106]. The intrinsic sex differences in the circadian timing system and the different responses of gonadal steroids following circadian disturbance should be considered in order to achieve a better understanding of the role of the circadian system in health and disease in males and females.
Highlights.
Circadian rhythms can be observed at multiple levels of analysis from gene expression to the behavior of the organism as a whole.
Sex differences have been described at each level of the neuraxis regulating circadian rhythms.
Sex differences have also been observed in downstream physiological circadian processes, i.e. the HPA axis.
The intrinsic sex differences and the different responses of gonadal steroids should be considered to better understand the role of the circadian system in health and disease in males and females.
Acknowledgements
We thank Dr. Antonio Nunez for help comments on the manuscript, Drs. Lance Kriegsfeld and Ilia Karatsoreos for discussion during the preparation of this review, and Ms Erica Mezias for editing assistance. Some of the work in this manuscript and research described herein was supported by grants NSF 25605 and NINDS NS37919 to RS.
Definitions of terms used in circadian biology
- Circadian rhythms
endogenous biological rhythms with a near 24-hour period that persist in the absence of environmental cues, such as constant darkness.
- Free-running rhythm
the circadian rhythm that is not entrained/synchronized to any external timing cue.
- Entrainment
the process by which a circadian rhythm synchronizes to an external signal, such as the local daily 24 hr light/dark (LD) cycle.
- Subjective day & subjective night
the organism’s own internal day or night, irrespective or time in the external world.
- Masking
acts through acute inhibition or stimulation, usually by the light-dark cycle to transiently perturb the level of the rhythm being measured, without leading to a persistent shift in the rhythm after the masking signal is removed
- Period
the duration of time to complete one cycle. It is typically measured from peak to peak, but it can also be measured from any specific position on the curve.
- Phase
the relative position on the curve (e.g., the peak) in reference to a particular time, such as time placed in constant darkness. Positive phase shifts are termed advances, and negative phase shifts are termed delays.
- Amplitude
the measurement of the recorded output from the midline of the curve to either the peak or trough.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper focuses on sex differences in the circadian timing system. We follow the convention of distinguishing between sex and gender. Sex differences refer to the biological sex as it occurs in normal development with concordance between genetic, gonadal and hormonal sex. Gender, gender role and gender identity refer to the subjective experience of being a man or a woman, consisting primarily of the acceptance of membership into a category of people such as male or female. The latter topic is outside the domain of this review.
References
- 1.Welsh DK, et al. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14(24):2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagoshi E, et al. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119(5):693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24(2):90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nat Rev Mol Cell Biol. 2010;11(11):764–776. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- 5.Klein DC, Moore RY, Reppert SM, editors. The Mind's Clock. Oxford University Press; New York: 1991. Suprachiasmatic Nucleus; p. 467. [Google Scholar]
- 6.Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J. Biol. Rhythm. 1998;13:110–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 7.Lehman MN, et al. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J. Neirosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeSauter J, Lehman MN, Silver R. Restoration of circadian rhythmicity by transplants of SCN "micropunches". J Biol Rhythms. 1996;11(2):163–171. doi: 10.1177/074873049601100208. [DOI] [PubMed] [Google Scholar]
- 9.Meyer-Bernstein EL, et al. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology. 1999;140(1):207–218. doi: 10.1210/endo.140.1.6428. [DOI] [PubMed] [Google Scholar]
- 10.Silver R, et al. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- 11.Kriegsfeld LJ, et al. Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J Comp Neurol. 2004;468(3):361–379. doi: 10.1002/cne.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol. 2001;433(3):312–334. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- 13.van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J. Comp. Neurol. 1980;191:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- 14.van den Pol AN. The suprachiasmatic nucleus: morphological and cytochemical substrates for cellular interaction. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus The Mind's Clock. New York: Oxford University Press; 1991. pp. 17–50. [Google Scholar]
- 15.Herzog ED, et al. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J Biol Rhythms. 2004;19(1):35–46. doi: 10.1177/0748730403260776. [DOI] [PubMed] [Google Scholar]
- 16.Schaap J, et al. Heterogeneity of rhythmic suprachiasmatic nucleus neurons: Implications for circadian waveform and photoperiodic encoding. Proc Natl Acad Sci U S A. 2003;100(26):15994–15999. doi: 10.1073/pnas.2436298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harbour VL, et al. Comprehensive mapping of regional expression of the clock protein PERIOD2 in rat forebrain across the 24-h day. PLoS One. 2013;8(10):e76391. doi: 10.1371/journal.pone.0076391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harbour VL, et al. Phase differences in expression of circadian clock genes in the central nucleus of the amygdala, dentate gyrus, and suprachiasmatic nucleus in the rat. PLoS One. 2014;9(7):e103309. doi: 10.1371/journal.pone.0103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu AC, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129(3):605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schibler U, Naef F. Cellular oscillators: rhythmic gene expression and metabolism. Curr Opin Cell Biol. 2005;17(2):223–229. doi: 10.1016/j.ceb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Yoo SH, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101(15):5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panda S, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 23.Storch KF, et al. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130(4):730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson AJ, Yamazaki S, Menaker M. SCN: ringmaster of the circadian circus or conductor of the circadian orchestra? Novartis Found Symp. 2003;253:110–121. discussion 121-5, 281-4. [PubMed] [Google Scholar]
- 25.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4(8):649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 26.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey M, Silver R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol. 2014;35(1):111–139. doi: 10.1016/j.yfrne.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mong JA, et al. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31(45):16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karatsoreos IN, Silver R. Minireview: The neuroendocrinology of the suprachiasmatic nucleus as a conductor of body time in mammals. Endocrinology. 2007;148(12):5640–5647. doi: 10.1210/en.2007-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vida B, et al. Oestrogen receptor alpha and beta immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J Neuroendocrinol. 2008;20(11):1270–1277. doi: 10.1111/j.1365-2826.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- 31.Kruijver FP, Swaab DF. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology. 2002;75(5):296–305. doi: 10.1159/000057339. [DOI] [PubMed] [Google Scholar]
- 32.Kuljis DA, et al. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology. 2013;154(4):1501–1512. doi: 10.1210/en.2012-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwahana E, et al. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav. 2008;53(3):422–430. doi: 10.1016/j.yhbeh.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller BH, Takahashi JS. Central circadian control of female reproductive function. Front Endocrinol (Lausanne) 2014;4:195. doi: 10.3389/fendo.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams WP, 3rd, Kriegsfeld LJ. Circadian control of neuroendocrine circuits regulating female reproductive function. Front Endocrinol (Lausanne) 2012;3:60. doi: 10.3389/fendo.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96(1):50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- 37.Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96(1):57–62. doi: 10.1210/endo-96-1-57. [DOI] [PubMed] [Google Scholar]
- 38.Urlep Z, Rozman D. The Interplay between Circadian System, Cholesterol Synthesis, and Steroidogenesis Affects Various Aspects of Female Reproduction. Front Endocrinol (Lausanne) 2013;4:111. doi: 10.3389/fendo.2013.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibson EM, et al. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149(10):4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kauffman AS, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 41.Semaan SJ, Kauffman AS. Sexual differentiation and development of forebrain reproductive circuits. Curr Opin Neurobiol. 2010;20(4):424–431. doi: 10.1016/j.conb.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becquet D, et al. Evidence for an internal and functional circadian clock in rat pituitary cells. Mol Cell Endocrinol. 2014;382(2):888–898. doi: 10.1016/j.mce.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Hughes M, et al. High-resolution time course analysis of gene expression from pituitary. Cold Spring Harb Symp Quant Biol. 2007;72:381–386. doi: 10.1101/sqb.2007.72.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu A, et al. Global but not gonadotrope-specific disruption of Bmal1 abolishes the luteinizing hormone surge without affecting ovulation. Endocrinology. 2013;154(8):2924–2935. doi: 10.1210/en.2013-1080. [DOI] [PubMed] [Google Scholar]
- 45.Kennaway DJ, Boden MJ, Varcoe TJ. Circadian rhythms and fertility. Mol Cell Endocrinol. 2012;349(1):56–61. doi: 10.1016/j.mce.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Sellix MT. Circadian clock function in the mammalian ovary. J Biol Rhythms. 2015;30(1):7–19. doi: 10.1177/0748730414554222. [DOI] [PubMed] [Google Scholar]
- 47.Sellix MT, Menaker M. Circadian clocks in the ovary. Trends Endocrinol Metab. 2010;21(10):628–636. doi: 10.1016/j.tem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He PJ, et al. Gonadotropic regulation of circadian clockwork in rat granulosa cells. Mol Cell Biochem. 2007;302(1–2):111–118. doi: 10.1007/s11010-007-9432-7. [DOI] [PubMed] [Google Scholar]
- 49.He PJ, et al. The disruption of circadian clockwork in differentiating cells from rat reproductive tissues as identified by in vitro real-time monitoring system. J Endocrinol. 2007;193(3):413–420. doi: 10.1677/JOE-07-0044. [DOI] [PubMed] [Google Scholar]
- 50.Yoshikawa T, et al. Timing of the ovarian circadian clock is regulated by gonadotropins. Endocrinology. 2009;150(9):4338–4347. doi: 10.1210/en.2008-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: Effects of luteinizing hormone. Biol Reprod. 2006;75(4):624–632. doi: 10.1095/biolreprod.106.050732. [DOI] [PubMed] [Google Scholar]
- 52.Sellix MT, Menaker M. Circadian clocks in mammalian reproductive physiology: effects of the "other" biological clock on fertility. Discov Med. 2011;11(59):273–281. [PubMed] [Google Scholar]
- 53.Son GH, et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci U S A. 2008;105(52):20970–20975. doi: 10.1073/pnas.0806962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bebas P, et al. Circadian clock and output genes are rhythmically expressed in extratesticular ducts and accessory organs of mice. FASEB J. 2009;23(2):523–533. doi: 10.1096/fj.08-113191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karatsoreos IN, et al. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology. 2007;148(11):5487–5495. doi: 10.1210/en.2007-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Model Z, et al. Suprachiasmatic Nucleus as the Site of Androgen Action on Circadian Rhythms. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Butler MP, et al. Dose-dependent effects of androgens on the circadian timing system and its response to light. Endocrinology. 2012;153(5):2344–2352. doi: 10.1210/en.2011-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916(1–2):172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 59.Blattner MS, Mahoney MM. Circadian parameters are altered in two strains of mice with transgenic modifications of estrogen receptor subtype 1. Genes Brain Behav. 2012;11(7):828–836. doi: 10.1111/j.1601-183X.2012.00831.x. [DOI] [PubMed] [Google Scholar]
- 60.Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol. 1981;241(1):R62–R66. doi: 10.1152/ajpregu.1981.241.1.R62. [DOI] [PubMed] [Google Scholar]
- 61.Labyak SE, Lee TM. Estrus- and steroid-induced changes in circadian rhythms in a diurnal rodent, Octodon degus. Physiol Behav. 1995;58(3):573–585. doi: 10.1016/0031-9384(95)00096-2. [DOI] [PubMed] [Google Scholar]
- 62.Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196(4287):305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- 63.Ogawa S, et al. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144(1):230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- 64.Royston SE, et al. ESR1 and ESR2 differentially regulate daily and circadian activity rhythms in female mice. Endocrinology. 2014;155(7):2613–2623. doi: 10.1210/en.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnold AP, Chen X. What does the "four core genotypes" mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30(1):1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krizo JA, Mintz EM. Sex differences in behavioral circadian rhythms in laboratory rodents. Front Endocrinol (Lausanne) 2015;5:234. doi: 10.3389/fendo.2014.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 68.Goel N, Lee TM. Sex differences and effects of social cues on daily rhythms following phase advances in Octodon degus. Physiol Behav. 1995;58(2):205–213. doi: 10.1016/0031-9384(95)00051-j. [DOI] [PubMed] [Google Scholar]
- 69.Roenneberg T, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11(6):429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 70.Roenneberg T, et al. A marker for the end of adolescence. Curr Biol. 2004;14(24):R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 71.Cain SW, et al. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 2010;25(4):288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duffy JF, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A. 2011;108(Suppl 3):15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eastman CI, et al. Circadian rhythm phase shifts and endogenous free-running circadian period differ between African-Americans and European-Americans. Sci Rep. 2015;5:8381. doi: 10.1038/srep08381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenthal NE, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41(1):72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 75.Kasper S, et al. Epidemiological findings of seasonal changes in mood and behavior. A telephone survey of Montgomery County, Maryland. Arch Gen Psychiatry. 1989;46(9):823–833. doi: 10.1001/archpsyc.1989.01810090065010. [DOI] [PubMed] [Google Scholar]
- 76.Tonetti L, et al. Mood seasonality: a cross-sectional study of subjects aged between 10 and 25 years. J Affect Disord. 2007;97(1–3):155–160. doi: 10.1016/j.jad.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 77.Leliavski A, et al. Adrenal clocks and the role of adrenal hormones in the regulation of circadian physiology. J Biol Rhythms. 2015;30(1):20–34. doi: 10.1177/0748730414553971. [DOI] [PubMed] [Google Scholar]
- 78.Szafarczyk A, et al. CNS control of the circadian adrenocortical rhythm. J Steroid Biochem. 1983;19(1C):1009–1015. doi: 10.1016/0022-4731(83)90047-x. [DOI] [PubMed] [Google Scholar]
- 79.Kalsbeek A, et al. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Mol Cell Endocrinol. 2012;349(1):20–29. doi: 10.1016/j.mce.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 80.Kalsbeek A, et al. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci. 1996;16(17):5555–5565. doi: 10.1523/JNEUROSCI.16-17-05555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cascio CS, Shinsako J, Dallman MF. The suprachiasmatic nuclei stimulate evening ACTH secretion in the rat. Brain Res. 1987;423(1–2):173–178. doi: 10.1016/0006-8993(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 82.Ibuka N, Kawamura H. Loss of circadian sleep-wakefulness cycle in rats by suprachiasmatic nucleus lesion. Brain Res. 1975;96:76–81. doi: 10.1016/0006-8993(75)90574-0. [DOI] [PubMed] [Google Scholar]
- 83.Muglia LJ, et al. Impaired diurnal adrenal rhythmicity restored by constant infusion of corticotropin-releasing hormone in corticotropin-releasing hormone-deficient mice. J Clin Invest. 1997;99(12):2923–2929. doi: 10.1172/JCI119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akana SF, et al. Reset of feedback in the adrenocortical system: an apparent shift in sensitivity of adrenocorticotropin to inhibition by corticosterone between morning and evening. Endocrinology. 1986;119(5):2325–2332. doi: 10.1210/endo-119-5-2325. [DOI] [PubMed] [Google Scholar]
- 85.Dallman MF, et al. Nycthemeral rhythm in adrenal responsiveness to ACTH. Am J Physiol. 1978;235(5):R210–R218. doi: 10.1152/ajpregu.1978.235.5.R210. [DOI] [PubMed] [Google Scholar]
- 86.Oster H, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4(2):163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Jasper MS, Engeland WC. Splanchnicotomy increases adrenal sensitivity to ACTH in nonstressed rats. Am J Physiol. 1997;273(2 Pt 1):E363–E368. doi: 10.1152/ajpendo.1997.273.2.E363. [DOI] [PubMed] [Google Scholar]
- 88.Ulrich-Lai YM, Engeland WC. Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology. 2002;76(2):79–92. doi: 10.1159/000064426. [DOI] [PubMed] [Google Scholar]
- 89.Handa RJ, et al. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28(4):464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 90.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 91.Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138(9):3842–3848. doi: 10.1210/endo.138.9.5395. [DOI] [PubMed] [Google Scholar]
- 92.Ikeda Y, et al. Modulation of circadian glucocorticoid oscillation via adrenal opioid-CXCR7 signaling alters emotional behavior. Cell. 2013;155(6):1323–1336. doi: 10.1016/j.cell.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Balsalobre A, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 94.Lamia KA, et al. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480(7378):552–556. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rando G, Schibler U. Glucocorticoid rhythm renders female mice more daring. Cell. 2013;155(6):1211–1212. doi: 10.1016/j.cell.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 96.Foster RG, et al. Sleep and circadian rhythm disruption in social jetlag and mental illness. Prog Mol Biol Transl Sci. 2013;119:325–346. doi: 10.1016/B978-0-12-396971-2.00011-7. [DOI] [PubMed] [Google Scholar]
- 97.Knutsson A. Health disorders of shift workers. Occup Med (Lond) 2003;53(2):103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi JS, et al. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9(10):764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wirz-Justice A. Chronobiology and mood disorders. Dialogues Clin Neurosci. 2003;5(4):315–325. doi: 10.31887/DCNS.2003.5.4/awirzjustice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bailey SM, Udoh US, Young ME. Circadian regulation of metabolism. J Endocrinol. 2014;222(2):R75–R96. doi: 10.1530/JOE-14-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Depner CM, Stothard ER, Wright KP., Jr Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14(7):507. doi: 10.1007/s11892-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haus EL, Smolensky MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 2013;17(4):273–284. doi: 10.1016/j.smrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 103.Innominato PF, et al. The circadian timing system in clinical oncology. Ann Med. 2014;46(4):191–207. doi: 10.3109/07853890.2014.916990. [DOI] [PubMed] [Google Scholar]
- 104.Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry. 2014;26(2):139–154. doi: 10.3109/09540261.2014.911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Papantoniou K, et al. Increased and mistimed sex hormone production in night shift workers. Cancer Epidemiol Biomarkers Prev. 2015;24(5):854–863. doi: 10.1158/1055-9965.EPI-14-1271. [DOI] [PubMed] [Google Scholar]
- 106.Labrecque N, Cermakian N. Circadian Clocks in the Immune System. J Biol Rhythms. 2015 doi: 10.1177/0748730415577723. [DOI] [PubMed] [Google Scholar]