Abstract
An increased serum alkaline phosphatase concentration is known to be associated with a negative prognosis in canine and human osteosarcoma. To expand upon previous studies regarding the biological relevance of increased serum alkaline phosphatase as a negative prognostic factor, xenogeneic heterotopic transplants were performed using six canine primary osteosarcoma cell lines generated from patients with differing serum alkaline phosphatase concentrations (three normal and three increased). Three of the six cell lines were capable of generating tumours and tumour formation was independent of the serum alkaline phosphatase status of the cell line. Microarray analysis identified 379 genes as being differentially expressed between the tumourigenic and non-tumourigenic cell lines. Frizzled-6 was upregulated to the greatest extent (7.78-fold) in tumourigenic cell lines compared with non-tumourigenic cell lines. Frizzled-6, a co-receptor for Wnt ligands has been associated with enhanced tumour-initiating cells and poor prognosis for other tumours. The increased expression of frizzled-6 was confirmed by quantitative reverse transcription polymerase chain reaction (QPCR) and Western blot analysis. Additionally, the tumourigenic cell lines also had an increase in the percentage of side population cells compared with non-tumourigenic cell lines (5.89% versus 1.58%, respectively). There were no differences in tumourigenicity, frizzled-6 or percentage of side population cells noted between osteosarcoma cell lines generated from patients of differing serum alkaline phosphatase concentration. However, to our knowledge this is the first study to identified frizzled-6 as a possible marker of osteosarcoma cell populations with enhanced tumourigenicity and side population cells. Future work will focus on defining the role of frizzled-6 in osteosarcoma tumourigenesis and tumour-initiating cells.
Keywords: cell signalling, comparative oncology, mouse models, oncology, tumour biology
Introduction
Osteosarcoma (OSA) is the most common primary bone malignancy of dogs, frequently affecting large and giant breed dogs. Osteosarcoma is a very aggressive disease and despite advances in surgery and chemotherapy the prognosis for patients with OSA remains unfavourable due the high frequency of micrometastatic disease at the time of diagnosis, which is estimated to be as high as 90%.1 The median survival time for canine patients ranges from 5 months with amputation alone to 10–14 months when combined with chemotherapy.2
Clinical features including anatomic location, tumour size, macroscopic metastasis at diagnosis and increased serum alkaline phosphatase (ALP) concentration have been correlated with OSA prognosis.2–4 While increased serum ALP is a widely accepted negative prognostic factor in patients with OSA, the biological relevance of this prognostic marker is poorly understood.2–5 Recent work by Sternberg et al. found ALP concentration to be associated with OSA tumour burden.6 This would suggest the negative prognostic value of serum ALP concentration is a reflection of tumour size, which is a known independent prognostic factor, and not necessarily indicative of intrinsic differences within the tumour cells. The lack of intrinsic differences between tumour cells from OSA associated with normal and increased serum ALP is consistent with findings from our previous study in which there were no differences in the in vitro biological behaviour of primary OSA cell lines from patients with different ALP status.7 Six new canine primary cell lines were generated from dogs with normal and high serum ALP concentration at diagnosis, and cell growth, migration, invasion and sensitivity to carboplatin and doxorubicin chemotherapy were found to be no different between cell lines based upon the patient's serum ALP concentration from which they were generated.7
However, as with all cancer modelling systems in vitro behavioural assays have limitations, specifically in the inability to recapitulate the in vivo micro-environment that is known to impact cancer behaviour. Therefore, the primary aim of this study was to determine whether there were differences in the tumourigenicity of primary cell lines generated from canine OSA patients with normal or increased serum ALP concentration. A secondary aim was to further characterize any differences noted between cell lines of differing tumourigenicity. To evaluate the tumourigenicity of these six cell lines in vivo, a xenogeneic, heterotopic transplant study in nude mice was performed. Tumour formation did not correlate with the cell lines original serum ALP concentration. The results of the transplant studies did reveal differences in the gene expression profile of tumourigenic and non-tumourigenic cell lines, in particular frizzled-6 (FZD-6), which may be involved with tumour-initiating cells. Additionally, differences were noted in percentage of side population (SP) cells between tumourigenic and non-tumourigenic cell lines.
Materials and methods
Cell culture and reagents
The generation and characterization of canine primary OSA cell lines (UWKOS1, UWKOS2, UWKOS3, UWKOS6, UWKOS7, UWKOS8) used in this study were described previously and were generated by procedures approved by the University of Wisconsin-Madison Animal Care and Use Committee.7 All cell lines were generated from primary OSA lesions. UWKOS1 was derived from a right humeral OSA in a 10-year-old FS Rottweiler; UWKOS2 is from a left humeral OSA in a 9.5-year-old M Irish Terrier; UWKOS3 is from a left tibial OSA in a 10-year-old MN Mastiff; UWKOS6 is from a right radial OSA in an 11-year-old Labrador retriever; UWKOS7 is from a left radial OSA in a 9-year-old FS German shepherd mix; and UWKOS8 is from a left radial OSA in a 9-year-old MN Border collie mix. With the exception of the patient from which UWKOS8 was derived, all patients were deemed free of metastatic disease based on thoracic radiographs, evaluation of regional lymph nodes and/or post-mortem evaluation. The patient from which UWKOS8 was derived had a soft tissue opacity in the right cranial lung lobe on thoracic radiographs. Therefore, cell lines UWKOS1, UWKOS2, UWKOS3, UWKOS6 and UWKOS7 were all derived from patients with stage IIB OSA; cell line UWKOS8 was derived from a patient suspected as having stage IIIB OSA.1 All cell lines were maintained in complete minimum essential medium (CMEM): minimum essential medium Eagle (MEM) supplemented with 10% cosmic calf serum, sodium pyruvate, l-glutamine, MEM vitamins, non-essential amino acids and 1% Pen/Strep (all products from Fisher Scientific, Fair Lawn, NJ, USA). Primary canine osteoblasts (k9Ob) were maintained in Canine Osteoblast Growth Medium, cultured using a Subculture Reagent Kit (cells and reagents from Cell Applications, San Diego, CA, USA). All cells were maintained at 37 °C in a humidified incubator with 5% CO2.
Tumour transplantation
Immunocompromised recipient mice (athymic nudes) were used for the xenogeneic, heterotopic transplantation model to reduce the likelihood of tumour-cell rejection. Six-to-eight week old, female athymic nude mice were purchased from a commercial vendor (Charles River, Wilmington, MA, USA) and maintained in a pathogen-free environment. Mice were allowed to acclimate to the housing environment for a minimum of 2weeks prior to transplantation. Mice were housed and maintained according to The Guide for the Care and Use of Laboratory Animals in an AAALAC-accredited facility. All experimental procedures were approved by the University of Wisconsin-Madison Animal Care and Use Committee.
On the day of transplantation, cell lines were trypsinized and added to sterile saline at a concentration of 1 × 105 or 1 × 106 tumour cells per 0.2 ml. To determine whether the tumourigenicity differs between cell lines, 1 × 105 or 1 × 106 tumour cells in 0.2 ml of saline were transplanted into the subcutaneous tissue in the left flank of all mice using a 26-gauge needle and leur-lock syringe. Each cell line was transplanted into five recipient mice at both cell concentrations. Murase et al. recently assessed the differences in tumourigenicity of a side-population of OSA cells, considered to be tumour-initiating cells, and an unsorted OSA cell population using a xenogeneic, heterotopic murine transplant model.8 The minimal number of unsorted OSA cells necessary for tumour formation was 1 × 105.
Recipient mice were monitored daily for the first 4 days post-transplant. Thereafter, mice were monitored at least every 2–3 days for tumour growth until the time of euthanasia. The time from tumour cell transplantation until evidence of tumour formation was recorded. Tumour measurements (mm) were taken with digital callipers along longest axis of the tumour and the perpendicular axis. Measurements were converted to tumour volume using the formula: volume = (length × [width]2)/2.
Transplant recipients were euthanized prior to the tumour affecting animal well-being as set forth by the University of Wisconsin-Madison All Campus Animal Care and Use Committee Police dated March 2007. Euthanasia was indicated for transplant recipients if tumour burden causes an impediment to normal movement or normal body function, or if the animal was showing signs of adverse effects secondary to tumour formation. Adverse signs would include loss of body condition, hunched posture, dehydration, reluctance to move or unkempt haircoat. At the time of euthanasia all mice were weighed and examined grossly, with information regarding lesion size, multiplicity and presence of metastatic lesions being recorded.
Microarray experiments
Array analysis with GeneChip Canine 2.0 Genome Arrays (Affymetrix, Santa Clara, CA, USA) was performed for six cell line samples. Each sample was represented by a single array. The oneCycle Target Labeling and Control Reagents package (Afftmetrix) was used to synthesize cDNA from total RNA spiked with prokaryotic Poly-A RNA as a control. The Sample Cleanup Module (Affymetrix) was used purify the cDNA which was then used for synthesis of biotin-labelled cRNA. cRNA was purified, quantified and fragmented before hybridization with the GeneChips. Hybridized chips were washed, stained using streptavidin-conjugated phycoerythrin fye (Invitrogen, Carlsbad, CA, USA) and enhanced with biotinylated goat anti-streptavidin antibody (Vector Laboratories, Burlingame, CA, USA) using an Affymetrix GeneChip Fluidics Station 450 and GeneChip Operating Software. The Affymetrix GeneChip scanner 3000 at the University of Wisconsin-Madison Biotechnology Center was used to acquire images.
Hoechst assay
The ability of cells to efflux a Hoechst 33342 dye was assessed in all six cell lines. The Hoechst dye is effluxed by cells via ABC membrane transporter proteins and is often used to identify subpopulations of cancer cells that have enhanced resistance to chemotherapy or are enriched for tumour-initiating cells, termed SP cells. For this assay, all cell lines were grown in a monolayer culture until reaching 80–90% confluency, at which point cell lines were trypsinized and resuspended at 1.0–2.0 × 106 cells mL−1 in pre-warmed MEM culture medium and divided into two populations. The first population was treated with 50 μM of verapamil and the second population remained untreated. Hoechst 33342 was added to both cell populations at 2.5 μg mL−1 and cells were incubated for 90 min at 37 °C. Cells were gently agitated every 15 min. Following Hoechst-staining, the cells were centrifuged at 4 °C during 7 min at 12 000 rpm and resuspended in cold Hanks. The Hoechst 33342 dye was excited at 350 nm ultraviolet and resultant fluorescence was measured at two wavelengths using 424/44 BP and 675 LP filters for detection of blue and red respectively using the LSRII Flow Cytometer System (BD Biosciences, San Jose, CA, USA).
qPCR for FZD-6
Total RNA was isolated from cell lines using Trizol (Invitrogen), and purified by PureLink RNA Mini Kit (Ambion; Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions. cDNA was synthesized from 250 ng of total RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Life Technologies) according to the manufactures protocol. qPCR was performed using TaqMan Gene Expression Master Mix with TaaMan Gene Expression Assays (Applied Biosystems, Life Technologies) according to the manufactures' protocol on a Bio-Rad iCycler machine with a Bio-Rad iQ5 Multicolor Real-Time PCR Detection System. The canine FZD-6 (Cf02625614_m1) assay was utilized (Applied Biosystems, Life Technologies). Ct values were normalized to 18S expression (4352930E). Normal canine osteoblasts were used to determine relative expression of FZD-6 in canine OSA cell lines using the ΔΔCt method.9 Gene expression from each cell line was measured in triplicate.
Western blot analysis with FZD-6 antibody
Western blot analysis was performed on cell lysates from canine OSA cell lines (UWKOS1, UWKOS2, UWKOS3, UWKOS6, UWKOS7 and UWKOS8) to determine the protein expression of FZD-6. Cells were lysed using a mammalian protein extraction reagent (Pierce, Rockford, IL, USA) and protein lysates collected. Protein lysates were separated by electrophoresis on 7.5% Mini-PROTEAN TGX Gel (BioRad, Hercules, CA, USA) at 150 V for 45 min, transferred to nitrocellulose membranes (Whatman, Dassel, Germany) at 100 V for 1 h, then blocked with tris-buffered saline/0.05% Tween 20 (TBST) containing 5% non-fat dry milk and 1% bovine serum albumin for 1 h (all reagent from Fisher Scientific). The membranes were probed overnight at 4 °C with either (1) rabbit anti-FZD-6 antibody (ab98180, Abcam Inc., Cambridge, MA, USA) diluted 1:500 in blocking solution or (2) goat anti-Actin (sc-1616, Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:200 in blocking solution. Excess primary antibody was removed by washing three times for 5 min with TBST. Membranes were incubated with 50 ng mL−1 horseradish peroxidase-conjugated anti-rabbit secondary antibody (Termo Scientific, Waltham, MA, USA) diluted in blocking solution for 1 h at room temperature, washed three times for 5 min with TBST, and treated with SuperSignal West Dura Chemiluminescent Substrate (Thermo Scientific). Blots were exposed to film, developed, and then imaged using a Gel Logic 100 Imaging System (Kodak, Rochester, NY, USA).
Statistical analyses
Microarrays were checked for outliers and preprocessed using robust multiarray averaging (RMA). Differential expression between two groups was tested using gene-specific two-sample t-tests coupled with Benjamini-Hochberg p-value adjustment. Genes showing both small adjusted p-value and large fold change were reported. The gene-set enrichment utilized GO/KEGG and the random-set enrichment method.10
QPCR for evaluation of FZD-6 expression and Hoechst dye exclusion assays were performed in triplicate for each cell line. For statistical analysis, the results of each cell line were combined according to their tumourigenicity (i.e. tumourigenic versus non-tumourigenic). A Mann–Whitney t-test was performed to determine whether the averages differed between tumourigenic versus non-tumourigenic cell lines using a commercially available software package (Prism5, GraphPad Software, La Jolla, CA, USA) with p <0.05 was considered significant.
Results
Tumourigenicity of primary canine osteosarcoma cell lines in a xenogeneic, heterotopic transplant model
To assess the tumourigenicity of OSA cell lines generated from tumours of patients with differing serum ALP concentration, we used six primary canine OSA cell lines.7 The UWKOS1, UWKOS2 and UWKOS3 are from OSA tissue associated with normal serum ALP concentration, while UWKOS6, UWKOS7 and UWKOS8 were derived from the OSA tissue of patients with increased serum ALP concentration. A total of 1 × 105 or 1 × 106 cells were injected subcutaneously in immunocompromised mice. When implanting 1 × 105 cells, only the UWKOS1 (one tumour/five mice) resulted in tumour formation by 90 days post-injection (Table 1). The tumour formation was noted 44 days post-injection. However, when 1 × 106 cells were implanted, UWKOS1 (2/5), UWKOS3 (1/5) and UWKOS7 (5/5) cell lines were all capable of generating subcutaneous tumours by 46 days post-transplant (Table 1). When tumours were evaluated histologically, they were consistent with the original diagnosis of osteosarcoma (Fig. 1A,B). The UWKOS2, UWKOS6 and UWKOS8 cell lines did not generate subcutaneous tumours by up to 90 days post-transplant with either amount of transplanted cells. There were no differences in the tumour-forming ability of cell lines based on the serum ALP concentration. While there were no differences noted in the tumourigenicity of cell lines based on serum ALP concentration, there was a distinct difference between cell lines capable of forming tumours at the 1 × 106 cell concentration. Therefore, we aimed to further characterize any differences in the gene expression profile and phenotype of these cell lines that may contribute to the differences in tumourigenicity.
Table 1. Number of mice with tumour formation and day of tumour detection after the canine osteosarcoma cell line transplantation for each cell line.
| UWKOS1 | UWKOS3 | UWKOS7 | UWKOS2 | UWKOS6 | UWKOS8 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| Cell amount | Mice | Time | Mice | Time | Mice | Time | Mice | Time | Mice | Time | Mice | Time |
| 1 × 105 | 1 | 44 days | 0 | 68 days | 0 | 68 days | 0 | 68 days | 0 | 68 days | 0 | 68 days |
| 1 × 106 | 2 | 46 days | 1 | 43 days | 5 | 10 days | 0 | 89 days | 0 | 66 days | 0 | 68 days |
Figure 1.
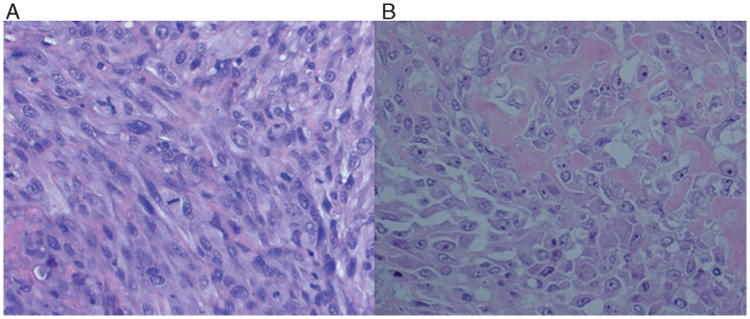
(A) Photomicrograph of tumour histology from mouse receiving UWKOS3. The lesion is representative of the three tumour-forming cell lines. (B) Representative histopathology section of the canine primary osteosarcoma lesion from which UWKOS3 was generated.
Gene expression analysis shows differential gene expression pattern in tumourigenic and non-tumourigenic cell lines
Microarray analysis was performed to evaluate differences in gene expression of cell lines noted to be tumourigenic and non-tumourigenic in the xenogeneic, heterotopic murine transplant model. There were 379 genes identified that were differentially regulated between the tumourigenic and non-tumourigenic cell lines with a false discovery rate (FDR) of 10%. In the tumourigenic group, 210 genes were found to be upregulated, of which 29 genes were upregulated with greater than a 2-fold log change (Table 2). Among the upregulated genes, FZD-6 has the greatest degree of change at 7.78 log-fold in the tumourigenic compared with non-tumourigenic cell lines. Similarly, when an unsupervised analysis approach was utilized FZD-6 retained a very high gene-specific standard deviation, the sixth highest, amongst the 43 035 probe sets examined. Conversely, 169 genes were downregulated in the tumourigenic compared with non-tumourigenic osteosarcoma cell lines. Of the 169 downregulated genes, 42 genes had at least a 2-fold log change, with the asporin gene having the greatest fold reduction at 8.71 log-fold. The data from a subset of the genes in Table 2 are plotted in a heat map in Fig. 2 allowing the visualization of two distinct clusters according to similarities in gene expression patterns, the tumourigenic and non-tumourigenic cell lines.
Table 2. Genes found to be upregulated and downregulated with more than 2.0 log-fold change in tumourigenic cell lines compared with non-tumourigenic cell lines.
| Gene symbol | Gene description | Tumourigenic | Non-tumourigenic | Log2 fold | Fold change | Q-value (%) |
|---|---|---|---|---|---|---|
| FZD6 | Frizzled family receptor 6 | 11.258 | 3.478 | 7.780 | 219.79 | 0.03 |
| NA | Cfa.9807.1.A1_at | 8.047 | 3.047 | 5.001 | 32.02 | 0.08 |
| EPDR1 | Ependymin-related protein 1 (zebrafish) | 9.952 | 5.163 | 4.789 | 27.65 | 0.087 |
| FZD6 | Frizzled family receptor 6 | 8.469 | 3.978 | 4.491 | 22.49 | 0.055 |
| SLC1A1 | Solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1 | 8.213 | 3.806 | 4.406 | 21.20 | 0.092 |
| TKTL1 | Transketolase-like 1 | 8.476 | 4.127 | 4.349 | 20.38 | 0.061 |
| RAB39 | RAB39A, member RAS oncogene family | 8.015 | 3.703 | 4.312 | 19.86 | 0.061 |
| CDCA7 | Cell division cycle associated 7 | 10.031 | 6.377 | 3.654 | 12.59 | 0.098 |
| NA | Cfa.1647.1.A1_at | 8.005 | 4.652 | 3.353 | 10.22 | 0.092 |
| CDC6 | Cell division cycle 6 homologue (S. cerevisiae) | 8.197 | 4.963 | 3.234 | 9.41 | 0.061 |
| LMNB1 | Lamin B1 | 8.840 | 5.825 | 3.015 | 8.08 | 0.078 |
| EPDR1 | Ependymin related protein 1 (zebrafish) | 8.719 | 5.726 | 2.994 | 7.97 | 0.068 |
| GRIP1 | Glutamate receptor interacting protein 1 | 7.429 | 4.513 | 2.917 | 7.55 | 0.09 |
| LMNB1 | Lamin B1 | 9.754 | 6.889 | 2.865 | 7.29 | 0.043 |
| HS3ST3B1 | Heparan sulphate (glucosamine) 3-O-sulfotransferase 3B1 | 7.647 | 4.820 | 2.828 | 7.10 | 0.096 |
| CHD7 | Chromodomain helicase DNA binding protein 7 | 7.254 | 4.519 | 2.735 | 6.66 | 0.043 |
| IL7R | Interleukin 7 receptor | 5.514 | 3.019 | 2.495 | 5.64 | 0.084 |
| CHD7 | Chromodomain helicase DNA binding protein 7 | 6.635 | 4.151 | 2.484 | 5.59 | 0.09 |
| AKAP12 | A kinase (PRKA) anchor protein 12 | 10.857 | 8.374 | 2.483 | 5.59 | 0.087 |
| LOC100855917 | Origin recognition complex subunit 1-like | 8.599 | 6.164 | 2.436 | 5.41 | 0.098 |
| LMNB1 | Lamin B1 | 10.485 | 8.060 | 2.425 | 5.37 | 0.061 |
| DKC1 | Dyskeratosis congenita 1, dyskerin | 9.287 | 6.920 | 2.367 | 5.16 | 0.077 |
| WISP3 | WNT1 inducible signalling pathway protein 3 | 7.336 | 4.979 | 2.357 | 5.12 | 0.055 |
| H2AFY | H2A histone family, member Y | 8.667 | 6.329 | 2.338 | 5.06 | 0.093 |
| DCTPP1 | dCTP pyrophosphatase 1-like | 9.553 | 7.280 | 2.273 | 4.83 | 0.085 |
| ILF3 | Interleukin enhancer binding factor 3, 90 kDa | 8.581 | 6.427 | 2.153 | 4.45 | 0.099 |
| SHMT1 | Serine hydroxymethyltransferase 1 (soluble) | 7.631 | 5.486 | 2.145 | 4.42 | 0.087 |
| NSD1 | Nuclear receptor binding SET domain protein 1 | 8.452 | 6.321 | 2.131 | 4.38 | 0.071 |
| NA | Cfa.11200.1.A1_at | 8.339 | 6.302 | 2.037 | 4.10 | 0.075 |
| NA | CfaAffx.21067.1.S1_at | 3.984 | 6.120 | −2.136 | 4.40↓ | 0.098 |
| NA | Cfa.3162.1.A1_at | 7.507 | 9.648 | −2.142 | 4.41↓ | 0.071 |
| LOC100856132 | Uncharacterized LOC100856132 | 5.381 | 7.582 | −2.201 | 4.60↓ | 0.071 |
| CYBRD1 | Cytochrome b reductase 1 | 8.652 | 10.919 | −2.266 | 4.81↓ | 0.1 |
| LOC100688140 | Protein BEX5-like | 3.937 | 6.212 | −2.274 | 4.84↓ | 0.071 |
| NA | Cfa.16197.1.A1_at | 9.119 | 11.424 | −2.305 | 4.94↓ | 0.099 |
| FMOD | Fibromodulin | 3.351 | 5.661 | −2.309 | 4.96↓ | 0.081 |
| CERCAM | Cerebral endothelial cell adhesion molecule | 6.900 | 9.357 | −2.457 | 5.49↓ | 0.075 |
| NA | Cfa.10651.1.A1_at | 10.342 | 12.807 | −2.465 | 5.52↓ | 0.09 |
| CYBRD1 | Cytochrome b reductase 1 | 7.942 | 10.426 | −2.484 | 5.59↓ | 0.093 |
| S100A16 | S100 calcium binding protein A16 | 5.642 | 8.235 | −2.593 | 6.03↓ | 0.08 |
| CD81 | CD81 molecule | 10.641 | 13.280 | −2.639 | 6.23↓ | 0.099 |
| CLEC11A | C-type lectin domain family 11, member A | 6.355 | 9.160 | −2.804 | 6.98↓ | 0.035 |
| LOC100688140 | Protein BEX5-like | 3.402 | 6.229 | −2.827 | 7.10↓ | 0.069 |
| CCPG1 | Cell cycle progression 1 | 8.253 | 11.108 | −2.854 | 7.23↓ | 0.096 |
| CERCAM | Cerebral endothelial cell adhesion molecule | 7.063 | 10.099 | −3.036 | 8.20↓ | 0.098 |
| HYAL1 | Hyaluronoglucosaminidase 1 | 5.795 | 8.864 | −3.069 | 8.39↓ | 0.09 |
| GAS6 | Growth arrest-specific 6 | 7.701 | 10.894 | −3.192 | 9.14↓ | 0.085 |
| FAM26E | Family with sequence similarity 26, member E | 6.170 | 9.402 | −3.232 | 9.40↓ | 0.093 |
| SORBS2 | Sorbin and SH3 domain containing 2 | 3.715 | 7.141 | −3.426 | 10.75↓ | 0.092 |
| NA | CfaAffx.3418.1.S1_at | 4.457 | 8.059 | −3.602 | 12.14↓ | 0.09 |
| HNMT | Histamine N-methyltransferase | 4.907 | 8.648 | −3.741 | 13.37↓ | 0.084 |
| ECM2 | Extracellular matrix protein 2, female organ and adipocyte specific | 3.315 | 7.129 | −3.814 | 14.06↓ | 0.096 |
| NA | Cfa.6163.1.A1_at | 4.392 | 8.546 | −4.154 | 17.80↓ | 0.09 |
| NA | Cfa.2703.1.S1_at | 3.876 | 8.079 | −4.202 | 18.40↓ | 0.098 |
| S100A16 | S100 calcium binding protein A16 | 6.709 | 10.945 | −4.237 | 18.86↓ | 0.061 |
| NA | Cfa.15527.1.A1_at | 5.025 | 9.417 | −4.392 | 20.98↓ | 0.09 |
| SGCD | Sarcoglycan, delta (35 kDa dystrophin-associated glycoprotein) | 5.238 | 9.840 | −4.602 | 24.29↓ | 0.094 |
| EFEMP2 | EGF containing fibulin-like extracellular matrix protein 2 | 6.677 | 11.409 | −4.732 | 26.58↓ | 0.083 |
| HNMT | Histamine N-methyltransferase | 3.697 | 8.569 | −4.872 | 29.28↓ | 0.061 |
| GAS6 | Growth arrest-specific 6 | 5.793 | 11.271 | −5.478 | 44.57↓ | 0.075 |
| FXYD1 | FXYD domain containing ion transport regulator 1 | 5.896 | 11.771 | −5.875 | 58.69↓ | 0.03 |
| FXYD1 | FXYD domain containing ion transport regulator 1 | 5.798 | 11.702 | −5.904 | 59.88↓ | 0.03 |
| MTAP | Methylthioadenosine phosphorylase | 3.619 | 9.839 | −6.219 | 74.49↓ | 0.098 |
| EFEMP2 | EGF containing fibulin-like extracellular matrix protein 2 | 4.725 | 11.042 | −6.317 | 79.73↓ | 0.061 |
| NA | Cfa.15772.1.A1_at | 4.164 | 10.656 | −6.492 | 90.01↓ | 0.09 |
| FGF7 | Fibroblast growth factor 7 | 3.720 | 10.397 | −6.677 | 102.32↓ | 0.071 |
| LOC100685375 | Uncharacterized LOC100685375 | 4.369 | 11.095 | −6.727 | 105.93↓ | 0.052 |
| ASPN | Asporin | 4.756 | 11.628 | −6.872 | 117.13↓ | 0.078 |
| NA | Cfa.5860.1.A1_at | 4.174 | 11.240 | −7.066 | 133.99↓ | 0.041 |
| EIF2S3 | Eukaryotic translation initiation factor 2, subunit 3 gamma, 52 kDa | 2.954 | 10.820 | −7.865 | 233.13↓ | 0.003 |
| ASPN | Asporin | 4.822 | 12.893 | −8.071 | 268.91↓ | 0.098 |
↓Fold reduction.
Figure 2.
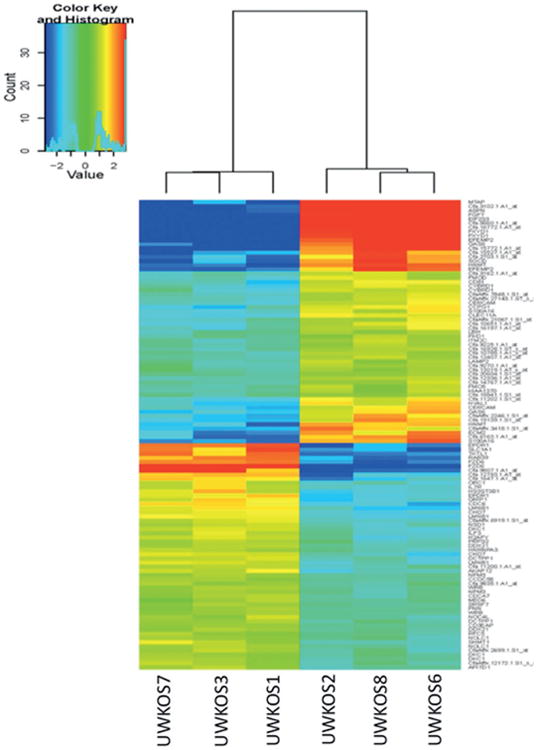
Hierarchical clustering of six canine osteosarcoma cell lines on the top-112 significant genes differentially expressed between tumourigenic and non-tumourigenic lines.
To better appreciate the functional significance of gene expression differences we utilized two different databases, the Gene Ontology (GO) and the Kyoto Encyclopaedia of Genes and Genomes (KEGG) to arrange genes according to specific informative groups. The GO database is divided into three different ontologies called Molecular Function (MF), Cellular Comportment (CC) and Biological Process (BP). The structure of GO is an acyclic direct graph with a set of genes being annotated to each GO term, although each gene may be assigned within more than one GO term.11 Within the BP ontology, the terms associated with the greatest number of differentially expressed genes included mRNA processing (291 genes), followed by RNA Splicing (260 genes), ncRNA Metabolic Process (231 genes) and DNA replication (226 genes) (Table 3). To better define pathways associated with the differentially expressed genes, we used the KEGG pathway analysis. The KEGG database provides a sorting of genes according to the biological pathway they belong to. The KEGG database is the major resource for pathway analysis and provides a reference knowledge base for linking genomes to biological system.11,12 Using the KEGG database, the differential expression of genes between the tumourigenic and non-tumourigenic cell lines were enriched for three pathways including mismatch repair, ribosome biogenesis in eukaryotes and spliceosome pathway (Table 4).
Table 3. Gene ontology terms enriched for genes differently expressed between tumourigenic and non-tumourigenic cell lines.
| Term | Ontology | Proportion of set that is differentially expressed | # Genes in set |
|---|---|---|---|
| mRNA cis splicing, via spliceosome | BP | 0.500 | 8 |
| Negative regulation of mRNA splicing, via spliceosome | BP | 0.364 | 11 |
| Somatic diversification of immune receptors via somatic mutation | BP | 0.333 | 6 |
| Somatic hypermutation of immunoglobulin genes | BP | 0.333 | 6 |
| Negative regulation of mRNA processing | BP | 0.308 | 13 |
| Establishment of mitochondrion localization, microtubule-mediated | BP | 0.286 | 7 |
| Mitochondrion transport along microtubule | BP | 0.286 | 7 |
| mRNA splice site selection | BP | 0.278 | 18 |
| Negative regulation of RNA splicing | BP | 0.267 | 15 |
| Aspartate transport | BP | 0.250 | 8 |
| C4-dicarboxylate transport | BP | 0.222 | 9 |
| Establishment of mitochondrion localization | BP | 0.222 | 9 |
| Regulation of transcription involved in G1/S phase of mitotic cell cycle | BP | 0.176 | 17 |
| Spliceosomal complex assembly | BP | 0.167 | 36 |
| Regulation of mRNA splicing, via spliceosome | BP | 0.129 | 31 |
| Spliceosomal snRNP assembly | BP | 0.125 | 32 |
| rRNA processing | BP | 0.123 | 73 |
| RNA export from nucleus | BP | 0.123 | 65 |
| Ribosome biogenesis | BP | 0.121 | 99 |
| mRNA transport | BP | 0.121 | 58 |
| Nucleic acid transport | BP | 0.120 | 75 |
| RNA transport | BP | 0.120 | 75 |
| Establishment of RNA localization | BP | 0.120 | 75 |
| rRNA metabolic process | BP | 0.114 | 79 |
| Termination of RNA polymerase II transcription | BP | 0.114 | 44 |
| Nuclear export | BP | 0.110 | 100 |
| RNA localization | BP | 0.110 | 82 |
| Ribonucleoprotein complex biogenesis | BP | 0.109 | 175 |
| mRNA export from nucleus | BP | 0.105 | 57 |
| Nucleobase-containing compound transport | BP | 0.095 | 95 |
| Ribonucleoprotein complex assembly | BP | 0.094 | 96 |
| Ribonucleoprotein complex subunit organization | BP | 0.088 | 102 |
| RNA splicing, via transesterification reactions with bulged adenosine as nucleophile | BP | 0.083 | 206 |
| mRNA splicing, via spliceosome | BP | 0.083 | 206 |
| RNA splicing, via transesterification reactions | BP | 0.081 | 211 |
| RNA splicing | BP | 0.069 | 260 |
| ncRNA processing | BP | 0.069 | 145 |
| ncRNA metabolic process | BP | 0.065 | 231 |
| mRNA processing | BP | 0.058 | 291 |
| DNA replication | BP | 0.058 | 226 |
BP, biological process.
Table 4. Tree most significant Kegg pathways in the tumourigenic cell lines comparing in a non-tumourigenic cell lines, showing the proportion of set that is differentially expressed.
| Pathways | Proportion of set that is differentially expressed |
|---|---|
| Mismatch repair | 16 |
| Ribosome biogenesis in eukaryotes | 67 |
| Spliceosome | 110 |
Evaluation of FZD-6 expression in OSA tumourigenic and non-tumourigenic cell lines
Given the interest in the Wnt signalling pathway in OSA biology and to verify the microarray analysis findings qPCR was performed on the gene with the greatest degree of modulation in both groups, FZD-6. FZD-6 is a co-receptor for Wnt ligands and aids in modulating canonical Wnt pathway activity.13 Similar to the results of the microarray analysis, the relative expression of FZD-6 mRNA was significantly increased in the tumourigenic cell lines compared with the non-tumourigenic cell lines. Tumourigenic cell lines had FZD6 mRNA relative gene expression of 4.209518 and the non-tumourigenic 0.001419 (P = 0.039) (Fig. 3 and Table 5).
Figure 3.
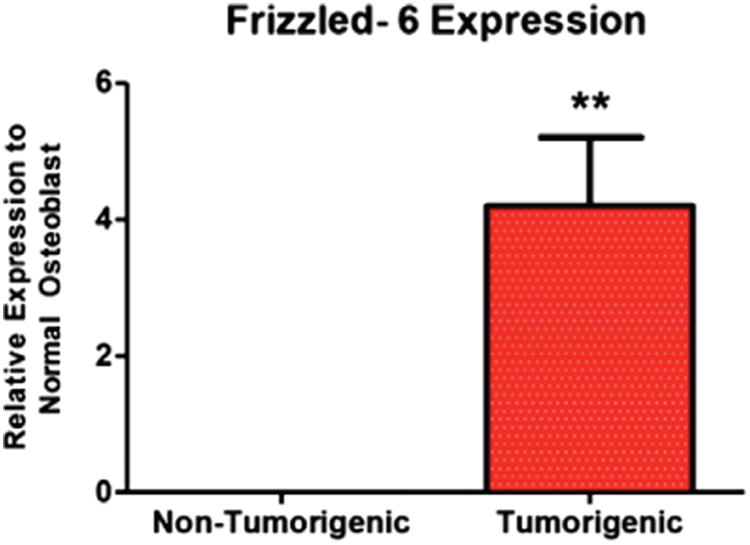
Expression of frizzled-6 mRNA in canine osteosarcoma cell lines relative to a canine normal osteoblast cell line.
Table 5.
Characteristics of frizzled-6 expression and side population cells in six canine primary osteosarcoma cell lines (three tumourigenic and three non-tumourigenic). Frizzled-6 mRNA expression is relative to normal canine osteoblast cell line. All experiments were performed in triplicate and the data is presented as the mean ± SD
| Tumourigenic cell lines | Non-tumourigenic cell lines | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Cell line | Frizzled-6 expression | Side population | Cell line | Frizzled-6 expression | Side population |
| UWKOS1 | 2.406037 ±0.3879 | 9.90% ±3.46554 | UWKOS2 | 0.001801 ±0.0018 | 0.20% ± 0.1 |
| UWKOS3 | 3.582369 ±0.75623 | 5.93% ± 3.61156 | UWKOS6 | Not detectable | 0.97% ±0.41633 |
| UWKOS7 | 6.640148 ±4.56758 | 1.83% ± 1.76163 | UWKOS8 | 0.002455 ±0.00032 | 3.60% ± 0.96437 |
| Combined† | 4.209518 ± 2.99649* | 5.89% ± 4.38** | Combined† | 0.001419 ± 0.00118* | 1.58% ±1.63** |
p = 0.039, non-tumourigenic cell lines compared with tumourigenic cell lines using a Mann Whitney two-tail test.
p = 0.0192, non-tumourigenic cell lines compared with tumourigenic cell lines using a Mann Whitney two-tail test.
FZD-6 protein expression in the tumourigenic and non-tumourigenic cell lines was assessed using Western blot analysis. Similar to the FZD6 mRNA results of the microarray analysis and qPCR, the expression of FZD6 protein appeared greater in the tumourigenic cell lines (UWKSO1 and UWKOS7) than in the non-tumourigenic cell lines (UWKOS2, UWKOS6 and UWKOS8) (Fig. 4).
Figure 4.
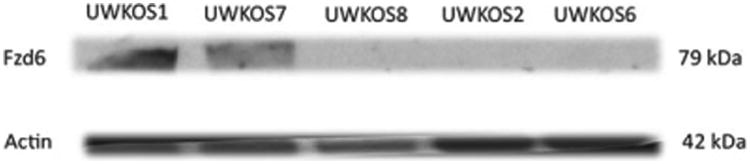
Western blot analysis for frizzled-6 expression in tumourigenic (UWKOS1 and UWKOS7) and non-tumourigenic (UWKSO2, UWKOS6 and UWKOS8) cell lines. The tumourigenic UWKOS3 cell line is not included in this image, however, frizzled-6 expression has been confirmed via Western blot analysis in this cell line as well.
Functional assays for canine OSA cell lines
Finally, the Hoechst efflux capacity of cell lines was assessed in all cell lines to determine whether there was an increase in the SP cells in the tumourigenic cell lines compared with the non-tumourigenic cell lines. The SP portion of cell lines has been proposed as being enriched for tumour-initiating cells, which may impact the tumourigenicity of cell lines. While all cell lines displayed the capacity to efflux Hoechst dye, there were dramatic differences in the proportion of cells capable of Hoechst-exclusion between cell lines, with UWKOS1 having the greatest (9.9%) and UWKOS2 the least (0.2%) (Table 5). When the cell lines are grouped according to their ability to form tumours in the transplant model a significant difference was noted with the non-tumourigenic group (UWKOS2, UWKOS6 and UWKOS8) having an average SP of 1.58% compared with 5.89% for the tumourigenic cell lines (p = 0.0192) (Fig. 5 and Table 5). There was no significant difference when the proportion of SP cells was compared according to serum ALP concentration (2.14% versus 5.35%, p = 0.44).
Figure 5.
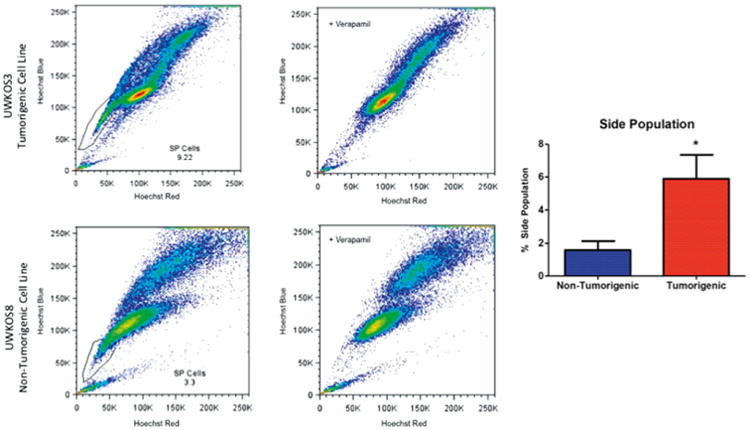
Frequency of side population cells as determined by Hoechst-exclusion assay. The flow cytometry images depict representative findings from tumourigenic (top row) and non-tumourigenic (bottom row) cell lines without (left column) and with verapamil treatment (right column). Bar graph to far right represents the frequency of side population cells (average ± SEM) in non-tumourigenic (n = 3) and tumourigenic (n = 3) canine primary osteosarcoma cell lines.
Discussion
Osteosarcoma is the most common primary bone malignancy of dogs and humans. In dogs and humans both, OSA is highly aggressive in its ability to incite local tissue destruction as well as in its ability to metastasize. Despite efforts to improve outcomes associated with OSA, minimal improvements have been made over the past two decades for either dogs or people with this disease. The negative prognostic value of increased serum ALP concentration in OSA patients is well described, yet poorly understood.2–5 This study initially aimed only to complete our characterization of canine primary OSA cell lines derived from patients of differing serum ALP concentration. However, detailed evaluation of cell lines differing in tumourigenicity, independent of serum ALP concentration, has identified FZD-6 as a molecule of interest in OSA, especially as it may relate to OSA tumour-initiating cells.
The lack of differences in tumourigenicity between cell lines derived from OSA patients of differing serum ALP concentration is not surprising and is consistent with the results of our previous study.7 The lack of differences in cell proliferation, migration, invasion and chemosensitivity noted in the previous study and tumourigenicity noted in the current study would support the theory that the negative prognostic value of increased serum ALP concentration is likely a reflection increased tumour burden, as showed recently by Sternberg et al.6 Unfortunately, in the current study we were unable to evaluate the significance of tumour burden on serum ALP concentration in recipient mice.
Although tumourigenicity was not associated with ALP status of cell lines, our transplant study did identify two distinct phenotypes within these newly established primary cell lines, cell lines that were tumourigenic (UWKOS1, UWKSO3 and UWKOS7) and non-tumourigenic (UWKOS2, UWKOS6 and UWKOS8) upon subcutaneous transplant into immunocompromised mice. Caution must be exercised when interpreting these findings, as there are limitations with the use of immunocompromised recipients and heterotopic transplants. Limitations of this model system include the inability to assess the immune system's impact on OSA nor the complex, natural tumour microenvironment in which OSA develops to be assessed. Additionally, the number of mice used to evaluate the tumourigenicity of each cell line at the two cell concentrations is relatively small (n = 5). The use of such a small number of recipient mice increases the likelihood for either a type I or type II error to be made in the assessment of tumourigenicity. It is worth noting that in a follow-up study that is on-going at the time of this manuscript's publication, the use of both non-modified UWK03 and UWKOS7 cell lines has resulted in similar tumour formation frequency when transplanted into 10 mice apiece (Stein, unpublished data). The remainder of the cell lines were not used in these studies. Despite these limitations, the difference in ability to form tumours between the cell lines indicates underlying differences within these cell lines. The differences in these cell lines were used in this study, and may be further exploited in future studies, to address mechanistic questions regarding tumourigenicity. Interestingly, when in vitro behaviour of these cell lines was examined according to serum ALP status there were no differences in proliferation, migration, invasion or chemosensitivity.7 However, when this data was re-examined according to tumourigenicity of cell lines there were significant differences in proliferation, doxorubicin-sensitivity and carboplatin-sensitivity of the tumourigenic cell lines compared with non-tumourigenic cell lines (data not shown). Therefore, we sought to characterize potential reasons for the differences in tumourigenicity by performing microarray analysis.
The results of the microarray analyses indicated 379 genes were differentially expressed in the tumourigenic cell lines compared with non-tumourigenic cell lines. Of the 379 differentially expressed genes, 210 genes had increased expression and 169 genes had decreased expression in tumourigenic cell lines relative to non-tumourigenic cell lines. Among the upreg-ulated genes, FZD-6 had the greatest increase, with a 7.78-fold increase followed by EPDR1 (ependymin-related protein 1) with 4.78-fold increase, and SLC1A1 (solute carrier family 1) with 4.04-fold increase. FZD-6 is a co-receptor for Wnt ligands and aids in modulating canonical Wnt pathway activity.13
Using GO and KEGG analysis, the functional significance of the genes differentially expressed between the tumourigenic and non-tumourigenic cell lines was able to more clearly defined. When utilizing the KEGG database, three pathways were enriched for based upon the differential expression of genes between the tumourigenic and non-tumourigenic cell lines including mismatch repair, ribosome biogenesis in eukaryotes and spliceosome pathway. The mismatch repair pathway is responsible for the recognition and repair of errors in DNA synthesis. Similar to our findings of mismatch repair pathway, Honoki et al. identified the mismatch repair pathway to be enriched for in chemically induced rat OSA compared with mesenchymal stem cells.14 The spliceosome pathway is responsible for post-transcriptional splicing of mRNA precursors. This process involves the excision of intronic sequences followed by the joining together of exons by a macromolecular.15 The comparison of gene expression patterns between tumours and cell lines with different behaviour may assist in identifying pathways contributing to the recurrence, metastasis, and poor prognosis for OSA. Previous studies utilizing microarray techniques to study canine OSA have shown significant differences between normal bone and OSA tissue and a striking similarity to human OSA16. Additionally, gene expression profiling has been used to identify differentially expressed genes and pathways associated with outcomes in dogs with OSA.17,18 In the study by Selvarajah et al. 37 genes were identified as being differentially expressed between dogs with short (less than 6 months) and long (6 months or longer) survival time. Of the 37 genes differentially expressed in that study, HSP90 (heat shock protein 90 kDa alpha, class B member 1) was the only one found in common with our study.17 Studies have also been performed comparing gene expression between human and murine metastatic OSA cell lines with their corresponding parental cell lines, allowing the identification of several genes related with metastases.19–25 The GO biology processes identified in these studies indicated the metastatic cell lines were enriched for immunological processes, including the T cell and B cell activation, chemokine and cytokine signalling pathway.24 In our microarray analysis, 40 GO biology processes were differentially regulated between the tumourigenic and non-tumourigenic cell lines. The biological processes involving mRNA processing, RNA splicing, ncRNA metabolic process and DNA replication had the largest number of gene in each category. The lack of similarities between multiple studies performing gene expression profiling on OSA cell lines or tissues is not surprising given the lack of standardization in the performance, platforms and statistical analyses used for microarrays as well as differences in programs used in pathway analysis.
The increased expression of FZD-6 in the tumourigenic cell lines compared with non-tumourigenic cell lines in the microarray analysis was confirmed by QPCR and Western blot analysis. To the authors' knowledge, FZD-6 expression has not been reported on in previous OSA studies. This finding is very exciting in light of FZD-6 function and recent studies describing FZD-6 involvement in neuroblastoma and leukaemia.26,27 FZD-6 is known to inhibit canonical Wnt signalling through its interaction with β-catenin, thereby preventing β-catenin from interacting with transcriptional co-factors.13 We have previously reported on the relatively low activity of the canonical Wnt signalling pathway in canine OSA despite the presence of cytoplasmic β-catenin in a high percentage of samples and FZD-6 may be one mechanism through which with the pathway is suppressed.28,29 However, FZD-6 is unlikely to be the only mechanism involved silencing the canonical signalling pathway, as the pathway seems to be suppressed in the majority of samples and FZD-6 appears to be over-expressed in only a subset of samples, although further investigation of this in clinical samples is necessary. Of additional interest is the role of FZD-6 in neuroblastoma, in which FZD-6 marks a subset of highly tumourigenic stem-like cells in both mouse and human tumours.26 Subsequently, Cantilena et al. showed the inhibition of FZD-6 reduced the tumourigenicity of these cell lines.26 Given the increased expression of FZD-6 in the tumourigenic cell lines in our study, we were interested in determining whether these cell lines were associated with any other characteristics of tumour-initiating cells (TICs). Finally, we are intrigued by the recent report of microarray analysis on canine OSA sarcospheres, adherent cells, and non-tumourigenic mesenchymal stem cells by Pang et al.30 Using the expression data in the publicly accessible, Gene Expression Omnibus (GEO accession GSE52063), we noted that FZD-6 expression was differentially expressed in the OSA cell lines (adherent and sarcospheres) relative to normal canine mesenchymal stem cells, with the OSA cell lines having increased expression of FZD-6.30 While there was no significant difference between the adherent OSA cells and sarcospheres, it is interesting to note the differential expression in tumour and non-tumour cell cells. Additionally, it is possible that there was no differential expression of FZD-6 between the adherent cells and sarcosphere due to both cell types being derived from the same cell line.
To determine whether the tumourigenic and non-tumourigenic cell lines differed in any other characteristics of TICs, the ability of cells to efflux the Hoechst-33342 was evaluated. Hoechst-3342 is a cell permeable DNA-specific bisbenzimidazole dye that is effluxed via ABC transporters, which can be inhibited by verapamil treatment of cell lines.8 The Hoechst efflux assay is considered a functional assay capable of identifying sub-populations of cells enriched for TICs. Cells capable of effluxing the dye have been termed SP cells and are biologically distinct from non-SP cells in regards to their stem-cell associated gene expression, chemotherapeutic resistance and tumourigenicity.8,31,32 This is consistent with our findings of the tumourigenic cell lines having an increased percentage of SP cells (5.89%) compared with the non-tumourigenic cell lines (1.58%). However, a recent study showed the reproducibility of the Hoechst-assay with canine cancer cell lines is inconsistent.33 Therefore, additional studies are necessary to assess other characteristics of TICs including stem-cell gene expression, aldehyde dehydrogenase (ALD) activity and sarcosphere formation.
In summary, we have found no difference in the tumourigenicity of canine primary OSA cell lines derived from patients with differing serum ALP concentration. This is consistent with our previous findings regarding the lack of in vitro behavioural differences between the cell lines associated with normal and increased serum ALP concentration. Of interest is the finding of increased FZD-6 expression in tumourigenic cell lines compared with non-tumourigenic cell lines. To the authors' knowledge FZD-6 expression has not been reported on in OSA previously. Given the requirement of Wnt signalling activation for osteoblast differentiation, it is conceivable the enhanced expression of FZD-6 may suppress the canonical Wnt signalling pathway thereby preventing the differentiation of cells and contribute to the maintenance of osteosarcoma TICs. This would be consistent with our findings that tumourigenic cell lines, having increased FZD-6 expression, are associated with an increased percentage of SP cells. The finding of tumourigenic cell lines having an increase in SP cells is consistent with previous studies on TICs.8,28 Further studies are necessary to clarify the role of FZD-6 in OSA and determine its utility as a marker for OSA TICs or its value as a therapeutic target.
References
- 1.Ehrhart NP, Stewart SD, Fan TM. Tumors of the skeletal system. In: Withrow SJ, Vail DM, Page RL, editors. Withrow and MacEwen's Small Animal Oncology. 3rd. St Louis: Elsevier Inc; 2013. pp. 463–503. [Google Scholar]
- 2.Phillips B, Powers BE, Dernell WS, Straw RC, Khanna C, Hogge GS, et al. Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. Journal of the American Animal Hospital Association. 2009;45:33–38. doi: 10.5326/0450033. [DOI] [PubMed] [Google Scholar]
- 3.Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 4.Ehrhart N, Dernell WS, Hoffmann WE, Weigel RM, Powers BE, Withrow SJ. Prognostic importance of alkaline phosphatase activity in serum from dogs with appendicular osteosarcoma: 75 cases (1990–1996) Journal of the American Veterinary Medical Association. 1998;213:1002–1006. [PubMed] [Google Scholar]
- 5.Garzotto CK, Berg J, Hoffmann WE, Rand WM. Prognostic significance of serum alkaline phosphatase activity in canine appendicular osteosarcoma. Journal of Veterinary Internal Medicine. 2000;14:587–592. doi: 10.1892/0891-6640(2000)014<0587:psosap>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg RA, Pondenis HC, Yang X, Mitchell MA, O'Brien RT, Garrett LD, et al. Association between absolute tumor burden and serum bone-specific alkaline phosphatase in canine osteosarcoma. Journal of Veterinary Internal Medicine. 2013;27:955–963. doi: 10.1111/jvim.12121. [DOI] [PubMed] [Google Scholar]
- 7.Holmes KE, Thompson V, Piskun CM, Kohnken RA, Huelsmeyer MK, Fan TM, et al. Canine osteosarcoma cell lines from patients with differing serum alkaline phosphatase concentration display no behavioural differences in vitro. Veterinary and Comparative Oncology. 2013 doi: 10.1111/vco.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murase M, Kano M, Tsukahara T, Takahashi A, Torigoe T, Kaawaguchi S, et al. Side population cells have the characteristics of cancer stem-like cells/cancer-initiating cells in bone sarcomas. British Journal of Cancer. 2009;101:1425–1432. doi: 10.1038/sj.bjc.6605330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2011;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 10.Newton MA, Quintana FA, den Boon JA, Sengupta S, Ahlquist P. Random-set methods identify distinct aspect of the enrichment signal in gene-set analysis. Annals of Applied Statistics. 2007;1:85–106. [Google Scholar]
- 11.Engeitner T. Enrichment analysis of GO terms, KEGG pathways and miRNAs by using the hypergeometrical distribution. 1–4. Oxford University Press; Oxford, UK: 2005. [Google Scholar]
- 12.Kanehisa M, Goto S, Hattori M, Aoki-Kinoshita KF, Itoh M, Kawashima S, et al. From genomics to chemical genomic: new developments in KEGG. Nucleic Acids Research. 2006;34:354–357. doi: 10.1093/nar/gkj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golan T, Yaniv A, Bafico A, Liu G, Gazit A. The human frizzled-6 (HFz6) acts as a negative regulator of the canonical Wnt/β-catenin signaling cascade. Journal of Biological Chemistry. 2004;279:14879–14888. doi: 10.1074/jbc.M306421200. [DOI] [PubMed] [Google Scholar]
- 14.Honoki K, Fujii H, Tohma Y, Tsujiuchi T, Kido T, Tsukamoto S, et al. Comparison of gene expression profiling in sarcomas and mesenchymal stem cells identifies tumorigenic pathways in chemically induced rat sarcoma model. International Scholarly Research Network Oncology. 2012;2012:1–8. doi: 10.5402/2012/909453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahl MC, Will CL, Luhrmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Paoloni M, Davis S, Lana S, Withrow S, Sangiorgi L, Picci P, et al. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genomics. 2009;10:625. doi: 10.1186/1471-2164-10-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selvarajah GT, Kirpensteijn J, van Wolferen ME, Rao NA, Fieten H, Mol JA. Gene expression profiling of canine osteosarcoma reveals genes associated with short and long survival times. Molecular Cancer. 2009;7:72. doi: 10.1186/1476-4598-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Donoghue LE, Ptitsyn AA, Kamstock DA, Siebert J, Thomas RS, Duval DL. Expression profiling in canine osteosarcoma: identification of biomarkers and pathways associated with outcome. BMC Cancer. 2010;22:506. doi: 10.1186/1471-2407-10-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khanna C, Khan J, Nguyen P, Prehn J, Caylor J, Yeung C, et al. Metastasis-associated differences in gene expression in a murine model of osteosarcoma. Cancer Research. 2001;61:3750–3759. [PubMed] [Google Scholar]
- 20.Flores RJ, Li Y, Yu A, Shen J, Rao PH, Lau SS, et al. A systems biology approach reveals common metastatic pathways in osteosarcoma. BMC Systems Biology. 2012;6:50. doi: 10.1186/1752-0509-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajkumar T, Yamuna M. Multiple pathways are involved in drug resistance to doxorubicin in an osteosarcoma cell line. Anti-Cancer Drugs. 2008;19:257–265. doi: 10.1097/cad.0b013e3282f435b6. [DOI] [PubMed] [Google Scholar]
- 22.Muff R, Ram Kumar RM, Botter SM, Born W, Fuchs B. Genes regulated in metastatic osteosarcoma: evaluation by microarray analysis in four human and two mouse cell line systems. Sarcoma. 2012;2012:937506. doi: 10.1155/2012/937506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano T, Tani M, Ishibashi Y, Kimura K, Park YB, Imaizumi N, et al. Biological properties and gene expression associated with metastatic potential of human osteosarcoma. Clinical and Experimental Metastasis. 2003;20:665–674. doi: 10.1023/a:1027355610603. [DOI] [PubMed] [Google Scholar]
- 24.Namløs HM, Kresse SH, Müller CR, Henriksen J, Holdhus R, Sæter G, et al. Global gene expression profiling of human osteosarcomas reveals metastasis-associated chemokine pattern. Sarcoma. 2012;2012:639038. doi: 10.1155/2012/639038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srivastava A, Fuchs B, Zhang K, Ruan M, Halder C, Mahlum E, et al. High WT1 expression is associated with very poor survival of patients with osteogenic sarcoma metastasis. Clinical Cancer Research. 2006;12:4237–4243. doi: 10.1158/1078-0432.CCR-05-2307. [DOI] [PubMed] [Google Scholar]
- 26.Cantilena S, Pastorino F, Pezzolo A, Chayka O, Pistoia V, Ponzoni M, et al. Frizzled receptor 6 marks rare, highly tumorigenic stem-like cells in mouse and human neuroblastomas. Oncotarget. 2011;2:976–983. doi: 10.18632/oncotarget.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu QL, Zierold C, Ranheim EA. Dysregulation of Frizzled 6 is a critical component of B-cell leukemogenesis in a mouse model of chronic lymphocytic leukemia. Blood. 2009;113:3031–3039. doi: 10.1182/blood-2008-06-163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piskun CM, Stein TJ. β-catenin transcriptional activity is minimal in canine osteosarcoma and its targeted inhibition results in minimal changes to cell line behavior. Veterinary and Comparative Oncology. 2013 doi: 10.1111/vco.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein TJ, Holmes KE, Muthuswamy A, Thompson V, Heulsmeyer MK. Characterization of β-catenin in canine osteosarcoma. Veterinary and Comparative Oncology. 2011;9:65–73. doi: 10.1111/j.1476-5829.2010.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang LY, Gatenby EL, Kamida A, Whitelaw BA, Hupp TR, Argyle DJ. Global gene expression analysis of canine osteosarcoma stem cells reveals a novel role for COX-2 in tumor initiation. PLoS ONE. 2014;9:e83144. doi: 10.1371/journal.pone.0083144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M, Yan M, Zhang R, Li J, Luo Z. Side population cells isolated from human osteosarcoma are enriched with tumor-initiating cells. Cancer Science. 2011;102:1774–1781. doi: 10.1111/j.1349-7006.2011.02028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martins-Neves SR, Lopes ÁO, do Carmo A, Paiva AA, Simões PC, Abrunhosa AJ, et al. Therapeutic implications of an enriched cancer stem-like cell population in a human osteosarcoma cell line. BMC Cancer. 2012;4:139. doi: 10.1186/1471-2407-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blacking TM, Waterfall M, Samuel K, Argyle DJ. Flow cytometric techniques for detection of candidate cancer stem cell subpopulations in canine tumor models. Veterinary and Comparative Oncology. 2012;10:252–273. doi: 10.1111/j.1476-5829.2011.00293.x. [DOI] [PubMed] [Google Scholar]


