Abstract
Pediatric acute kidney injury (AKI) represents a complex disease process for clinicians as it is multifactorial in cause and only limited treatment or preventatives are available. The renal microvasculature has recently been implicated in AKI as a strong therapeutic candidate involved in both injury and recovery. Significant progress has been made in the ability to study the renal microvasculature following ischemic AKI and its role in repair. Advances have also been made in elucidating cell–cell interactions and the molecular mechanisms involved in these interactions. The ability of the kidney to repair post AKI is closely linked to alterations in hypoxia, and these studies are elucidated in this review. Injury to the microvasculature following AKI plays an integral role in mediating the inflammatory response, thereby complicating potential therapeutics. However, recent work with experimental animal models suggests that the endothelium and its cellular and molecular interactions are attractive targets to prevent injury or hasten repair following AKI. Here, we review the cellular and molecular mechanisms of the renal endothelium in AKI, as well as repair and recovery, and potential therapeutics to prevent or ameliorate injury and hasten repair.
Keywords: Acute kidney injury, Microvasculature, Endothelium, Immune response, Tissue hypoxia, Ischemia reperfusion injury
Introduction
Acute kidney injury (AKI) is characterized as a rapid (hours to days) decrease in kidney function [1]. AKI is one of the most serious and common health complications, occurring in up to 20 % of all hospitalized patients and over 45 % of patients in critical care settings [2, 3]. While not well defined in children, the incidence of AKI in pediatric intensive care units is reported to range from 8 to 30 % and occurs in roughly 7 % of the general pediatric population [4–6]. The incidence of AKI in children appears to be increasing, and the etiology over the past decades has shifted from primary renal disease to multi-factorial causes, particularly in hospitalized children. An important cause of AKI in hospitalized children is the setting of post-cardiac surgery and stem cell transplantation [7]. Pharmacotherapy is also one of the major causes of AKI and may play a causative role in as many as 25 % of all pediatric cases [6, 8]. Drugs, including antimicrobials, chemotherapeutic agents, and non-steroidal anti-inflammatory drugs, among others, have all been implicated in drug-induced renal injury in children [5]. Severe sepsis with shock, use of vasopressors along with invasive ventilation, fluid overload, and tumor lysis syndrome also contribute to the numbers of AKI seen in children [9]. In newborns, the incidence of AKI in the USA is 3.9 per 1000 live births and 34.5 per 1000 newborns admitted to the neonatal unit [7, 10]. Several genetic factors may also predispose some children to AKI [7]. As the kidney is a highly vascularized organ, the complex vasculature is extremely sensitive to damage during AKI. The role that the complex microvasculature system plays during AKI events remains vastly understudied. Subtle malformations in kidney vasculature development could leave the kidneys at significant risk of further insults.
Microvascular development and the role of Foxd1 during AKI
Kidney development involves interactions between the metanephric mesenchyme and the ureteric epithelium [11–13]. The metanephric mesenchyme subdivides into the nephrogenic mesenchyme and the renal stroma [14]. As the kidney develops, the renal stroma interdigitates between the nephron progenitor caps, and the ureteric bud branches to form primary renal interstitium [11, 15]. The renal stroma eventually gives rise to many of the vascular supportive cells (including pericytes, fibroblasts, renin-producing cells, and mesangium), as well as to endothelial progenitors [11, 16, 17]. The kidney receives approximately 20 % of the cardiac output through its vasculature system [17, 18]. The vascular system begins with the growth and invagination (angiogenesis) of new blood vessels. At the same time, resident endothelial precursors, within the kidney mesenchyme, form primitive vascular structures (vasculogenesis) that eventually connect with the angiogenic vessels to form a patent vascular system. The exact combination of angiogenesis and vasculogenesis that contributes to the formation of the kidney vasculature is unclear. In an earlier study, our laboratory showed that a subset of the renal endothelium derived from the renal stroma (marked by the Foxd1 gene) gave rise to peritubular capillaries [16]. Foxd1 is a transcription factor in the stromal cells and is the earliest identifier of the renal stroma [11, 18]. Kidneys with a Foxd1 deletion display severe structural deformities [11, 19] as well as reduced branching of the ureteric bud, decreased number of nephrons, abnormalities of the renal capsule, misplaced vasculature in the renal capsule, and overall aberrant patterning of renal structures [11, 19, 20]. Foxd1 has recently been shown to participate in the proper orientation of the kidney vasculature [18]. When the kidney is subjected to injury models [unilateral ureteral obstruction or ischemia–reperfusion injury (IRI)], it is the Foxd1-derived cells that contribute to the fibrotic response [21–47] (Table 1). Our laboratory is currently investigating the role of the Foxd1-derived endothelium in determining susceptibility to AKI.
Table 1.
Important findings implicating renal endothelium in acute kidney injury
| Major findings | Implications | Authors |
|---|---|---|
| During AKI, peritubular capillary blood flow is reduced, and morphology is distorted, as well as loss of endothelial cell function | The vasculature of the kidney plays a role in injury and progression to chronic disease. The endothelium may be a potential target for therapeutics | Andreoli et al. (2009) [7]; Sutton et al. (2002) [22]; Basile et al. (2007) [23] |
| TSP1 is found to bind to CD47 and acts to suppress the NO pathway following IRI | Supplementation of NO mitigates IRI | Isenburg et al. (2007) [21]; Martinez-Mier et al. (2000) [24]; Rodriguez-Pena et al. (2004) [25]; Liu et al. (2007) [26]; Lang et al. (2007) [27] |
| Limiting CD47 activation prevents TSP1 binding in mice. | Mitigates the complications of IRI providing a potential therapeutic | Rogers et al. (2012) [18] |
| A subset of the renal endothelium derived from the renal stroma (marked by Foxd1) gives rise to peritubular capillaries | Foxd1 gives rise to vasculogenic endothelium and contributes to a fibrotic response following AKI | Sims-Lucas et al. (2013) [28]; Hum et al. (2014) [29]; Sequeira-Lopez et al. (2015) [30]; Levinson et al. (2005) [31]; Hatini et al. (1996) [32] |
| Renal endothelial functions contributes to a reduction in renal blood flow following AKI. NOS3 has positive effects on renal endothelial function | Enhanced endothelial function may have protective effects on AKI | Brodsky et al. (2002) [33]; Basile et al. (2014) [34]; Arriero et al. (2004) [35] |
| Following AKI, hypoxic areas remain after normal function is restored. Furthermore, HIF-2α is critical for protection in AKI |
HIFs are implicated as taking part in the pathogenesis of AKI | Ergin et al. (2015) [36]; Kapitisinou et al. (2015) [37] |
| A fluorescence microangiography technique is established for visualization if the microvasculature | An invaluable technique to understand microvasculature alterations | Advani et al. (2011) [38]; Kramann et al. (2014) [11] |
| Inflammatory processes mediated by the immune system are crucial in mediating renal injury | Targeting inflammation may be a potential therapeutic for AKI | Basile et al. (2012) [39]; Bonventre et al. (2004) [40]; Gonclaves et al. (2010) [41]; Jang et al. (2015) [42] |
| Using retinoic acid, M1 macrophages can be suppressed, indirectly inducing M2 macrophages to enhance repair following AKI | Administration of retinoic acid following AKI enhances repair | Chiba et al. (2015) [43] |
| Endothelial cell dysfunction may contribute to the failure of blood to reperfuse an ischemic area | Further implicates endothelial cells as a possible target for therapeutic intervention | Brodsky et al. (2002) [33]; Sutton et al. (2003) [44]; Jang et al. (2015) [42] |
| CDK4/6 is activated early during AKI, and displays protective effects of CDK4/6 inhibitors in animal models of AKI | Rational for the clinical development of CDK4/6 inhibitors for the prevention and treatment of AKI | Flipski et al. (2009) [45]; Ciarimboli et al. (2010) [46]; Sprowl et al. (2014) [47] |
AKI, Acute kidney injury; IRI, ischemia–reperfusion injury; NO, nitric oxide; NOS3, nitric oxide synthase 3; HIF-2α, hypoxia inducible factor-2 alpha; CDK4/6, cyclin-dependent kinase 4/6
Ischemic reperfusion AKI
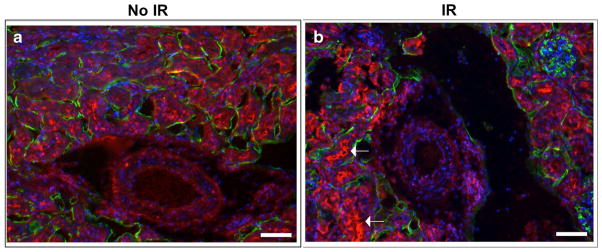
There are many different causes of pediatric AKI; however, hypoxic/ischemic AKI will be the focus of this review. IRI leading to AKI is characterized by early vasoconstriction followed by patchy tubular necrosis. Mild to moderate acute ischemic injury results in epithelial injury and death, although the renal tubules can repair following injury. However, severe or multiple ischemic injury events cause incomplete repair resulting in fibrogenesis [24] (Fig. 1). Part of the work carried out in our laboratory has focused on the relative tissue hypoxia following ischemic AKI in mice using pimonidazole hydrochloride (Hypoxyprobe™-1). Hypoxyprobe™-1 is activated in hypoxic cells and forms covalent adducts with sulphydryl groups within the tissue. Immunostaining of kidneys for hypoxyprobe, as well as for the endothelial marker endomucin 24 h post unilateral IRI was performed, and hypoxic tissues were visualized (Fig. 2). The kidney receives approximately 25 % of the cardiac output, however it is also one of the most naturally hypoxic organ systems. Oxygen tensions in the renal parenchyma are lower than that in most other organs, with the renal medulla considered an area with one of the lowest oxygen tensions [25, 26]. Twenty-four hours following IRI, a significant amount of hypoxic tissue was visible, and it was especially prevalent around the renal tubules compared to the contralateral control kidney (Fig. 2a, b). The pathophysiology of IRI in the kidney is very complex, with many pathological pathways implicated, including activation of neutrophils and the release of re-active oxygen species and other inflammatory mediators, including adhesion molecules and cytokines [27]. In response to hypoxia, inflammatory, renal tubular epithelial cells, and vascular cells secrete thrombospondin-1 (TSP1), which is a matricellular glycoprotein [22, 23, 48, 49]. TSP1 binds to the cell surface receptor CD47 to regulate the canonical nitric oxide (NO) pathway, which is suppressed in IRI [29, 49–51]. Several therapies have mitigated IRI through NO supplementation [49, 52–55]. Furthermore, Rogers et al. demonstrated that limiting CD47 activation prevents TSP1 binding and reduces complications of renal IRI in mice, providing a potential therapeutic intervention [49] (Table 1).
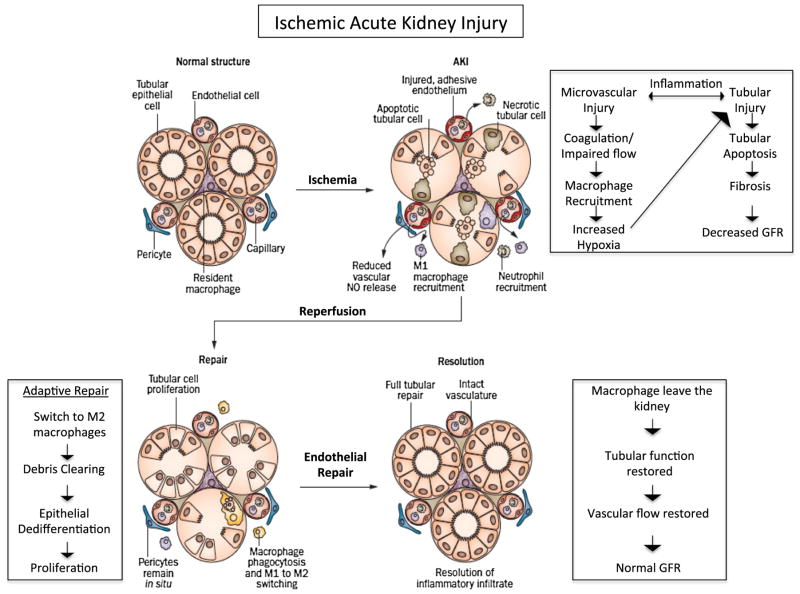
Fig. 1.
Injury, repair, and resolution during ischemic acute kidney injury (AKI). Following ischemia, there is substantial microvascular injury, leading to increased coagulation, reduced nitric oxide release, macrophage recruitment, and increased hypoxia. These events in turn lead to significant tubular injury and death, ultimately causing a decrease in glomerular filtration rate (GFR). Following reperfusion, the kidney enters adaptive repair in which inflammation and debris begins to resolve through a switch from M1 macrophages to M2 macrophages. Both endothelial repair and epithelial tubular proliferation begin, leading to resolution. Adapted from Fernenback and Bonventre [121] with permission from Macmillan Publishers Ltd
Fig. 2.
Hypoxic markers are present following ischemic reperfusion injury. a, b Hypoxyprobe- (red) and endomucin- (green) stained kidneys 1 day following 20 min of unilateral ischemic reperfusion injury (IR) (b), and the contralateral controls (a). One day following injury, ischemic kidneys (b) display markedly more hypoxic tubules (arrows) when compared with the contralateral control (a). Scale bars:100 μm
Recent studies suggest that the renal vasculature plays a role in acute and chronic injury. Furthermore, the endothelial cells have been identified as a target of injury and potential for therapeutics. The peritubular capillaries, which comprise the arterial portal system derived from the efferent arteriole, supply adjacent tubules in the cortex and renal medulla [28]. During AKI, peritubular capillary blood flow is abnormal during reperfusion, and this is accompanied by loss of endothelial cell function in association with distorted peritubular capillary morphology and function [7, 30, 56]. Moreover, pediatric patients following ischemic AKI have a high predisposition to progressive renal failure and hypertension, [30–32], while injury in the setting of transplantation (i.e., delayed graft function) represents an independent risk factor for graft survival and the development of post-transplant hypertension [31, 57]. These observations suggest that ischemic acute injuries to the kidney predispose to chronic complications.
Kidney microvasculature and AKI
The kidney contains one of the most diverse and rich populations of endothelial cells within the body [58]. Tubular epithelial cell injury is linked to AKI. However, unlike the renal tubules, the kidney microvasculature lacks regenerative capacity following severe or multiple AKI events [24, 31, 59]. Renal injury may directly damage the renal vasculature and alter its activity; such damage may influence vascular responsiveness, barrier function, coagulation cascades, and/or inflammatory processes [33, 56, 60]. Early alterations in peritubular capillary blood flow during reperfusion is linked with the loss of endothelial cell function [59]. Capillary loss, which alters renal function and predisposes patients to the development of chronic renal insufficiency, is due in part to hypoxia [30, 59]. Inflammation and procoagulant activity, which contribute to vascular congestion, are also induced by hypoxia/ischemia [59]. Several significant studies suggest that altered renal endothelial function contributes to a reduction in renal blood flow following AKI [34]. Arrerio and colleagues propagated endothelial like cells from mesenchymal stem cells; these cells expressed markers typical of endothelial cells such as Tie-2 (an angiopoietin receptor), vascular endothelial growth factor receptors (VEGFR) 1 and 2, and endothelial nitric oxide synthase 3 (eNOS3). Prophylactic injection of these cells to control rats generated short-term engraftment into the vasculature and short-term protection from AKI [35]. These studies suggest that endothelial function may have protective effects on AKI. Dimke and colleagues recently determined that VEGF-A is highly expressed in renal tubular epithelial cells, allowing tubulovascular cross-talk to its receptor (VEGFR2) which is located almost exclusively to peritubular capillary endothelial cells. Using a genetic approach to excise VEGF-A from the renal tubules, the authors demonstrated a substantial reduction in peritubular capillary density upon its removal. VEGF-A is deemed necessary and critical for maintenance of the peritubular microvasculature by directing tubulovascular cross-talk with the VEFGR2-expressing endothelial cells. This implicates a physiologic role of tubular VEGF-A in mediating cross-talk between the tubular system and the vasculature in the kidney [61]. Several studies have provided further evidence supporting endothelial cell/tubule cell cross-talk. It has been elegantly demonstrated that proximal tubule cells release cytokines and chemokines in response to cell injury and that these agents have direct effects on endothelial function [36, 37, 62, 63].
Adenosine plays an important role in the kidney by regulating renin release, glomerular filtration rate (GFR), and renal vascular tone [64, 65], while also playing a critical role in the regulation of tubular glomerular feedback [64–66]. During pathological insults to the kidney, adenosine levels increase due to renal ATP consumption, impaired renal perfusion, and hypoxia [65]. Grenz and colleagues demonstrated how adenosine provides protection against ischemic AKI in mouse models by preserving peritubular capillary blood flow during reperfusion. These authors showed that adenosine activation of endothelial Adora2b results in less tissue hypoxia and improved reperfusion [38, 44].
With damage to the microvasculature leading to capillary loss following AKI, fibrogenesis and capillary rarefaction progress, which induces focal hypoxia activating an injury, cascade leading to inflammation, and continued fibrosis [24, 67, 68]. The damaged or dysfunctional renal endothelium is often characterized by an impaired dilator capacity, which can be attributed to reduced production of NOS3 [39]. Unfortunately, it is unclear whether vasodilators can work to correct this impaired dilator capacity, as the endothelial tissue injury prevents vasodilator therapy from generating the desired effects [34]. Several studies have demonstrated that infusing endothelial cells with NOS3 gene expression constructs help to protect against early compromised blood flow in the peritubular capillaries caused by ischemic AKI, thereby supporting the positive effects of NOS3 on endothelial function [33–35] (Table 1). Following endothelial damage during AKI, the majority of normal renal function can be restored. However, hypoxic areas may remain, which can alter sodium reabsorption [69]. Furthermore, an increased expression of hypoxia inducible factor-1α (HIF-1α) and HIF-2α has been found in AKI. The role of these HIFs in the pathogenesis of AKI was unclear until recently [70]. Kapitsinou and colleagues utilized a genetic approach to inactivate both HIF-1α and HIF-2α in the renal endothelium, where they found that the HIF-2α isoform in the renal endothelium was critical for protection from AKI (Table 1) [70]. Until recently, microvascular damage following AKI was assessed through examination of the surface area of endothelial cells, or visualization of the capillaries [24, 40–42], through immunostaining and genetic labeling of the endothelium. Advani and colleagues were able to develop a fluorescence microangiography technique by renal artery injection in rats [71]. This technique was refined by Kramaan and colleagues and utilized in a mouse model of AKI to evaluate the microvasculature; they also generated a sophisticated MATLAB-based script for high-throughput analysis of the microvascular changes [24] (Table 1). This methodology to evaluate the renal endothelium will be invaluable to understand microvasculature alterations.
Renal ischemic injury alters the cytoskeletal organization of small arterioles and endothelial cells that may relate to the presentation of surface expression molecules. This disruption in cellular morphology may also disrupt endothelial cell tight junctions, resulting in endothelial leakiness. Endothelial leakiness can cause an increase in edema and compromise renal perfusion [56, 72, 73]. The loss of endothelial cell function may represent an important therapeutic target in which vascular trophic support and/or endothelial regeneration by progenitor cells ameliorate the acute and chronic effects of ischemic AKI [74].
Immune response to AKI is mediated by endothelial cells
In addition to the myriad of altered vascular functions that influence AKI progression, inflammation is mediated in part by the adhesion of leukocytes to damaged endothelial cells [75]. Following AKI, tissue damage initiates an inflammatory cascade including reactive oxygen species (ROS), cytokines, chemokines, and leukocytes [27, 43, 76].
In combination with endothelial adhesiveness, inflammatory mediators are synthesized and released by both tubular epithelial cells (one of the primary sites of damage) and activated leukocytes. Tubular epithelial cells produce tumor necrosis factor-alpha, interleukin (IL)-1, IL-6, IL-8, transforming growth factor beta, monocyte chemotactic protein 1 (MCP-1), ENA78, RANTES and fractalkines, while leukocytes produce IL-1, IL-8, MCP-1, ROS, and eicosanoids. These factors act in concert to promote inflammation in a positive feedback loop, promoting further kidney injury [75, 77]. The endothelium is also a source of chemoattractant factors, such as fractalkine (CX3CL1), which is expressed following renal injury and promotes macrophage infiltration [75]. Numerous studies conducted over the past two decades have revealed that inflammatory processes mediated by the immune system are crucial in mediating renal injury [78, 79]. Both innate and adaptive immune systems are directly involved in the pathogenesis of ischemic AKI. Various cellular and humeral immune system components contribute to AKI, some of which are also thought to be involved in the repair process following AKI [76, 79, 80]. Resident macrophages in normal kidneys are few, while in post-ischemic kidneys (especially in the outer medulla), directly following IRI, their number markedly increases [79, 81]. Monocytes then adhere to the vasa recta 2 h after reperfusion, while most macrophage recruitment occurs around post-capillary venules in the outer medulla [79, 82]. Chemokines are also direct mediators of chemotaxis and activation of immune cells; specifically, they guide neutrophils and pro-inflammatory (M1) macrophages to the injury site [83, 84]. M1 macrophages amplify the inflammatory response and promote tissue damage following AKI. Over time (days) M1 macrophages are replaced by alternatively activated M2 macrophages that promote repair [85–87]. The mechanisms that regulate these macrophage phenotypes remain poorly understood. Chiba and colleague’s recently demonstrated that retinoic acid (RA) is able to regulate macrophage activation following AKI through suppressing M1 macrophages and indirectly inducing M2 macrophages, thereby enhancing post-AKI repair [86] (Table 1). Neutrophils, which are important effector cells of the innate immune system, then phagocytose pathogens and particles, generate reactive oxygen and nitrogen species, and release antimicrobial peptides. Neutrophil infiltration has been detected in post-ischemic mouse kidneys [88, 89] and in biopsy samples from patients with early AKI [90, 91]. Neutrophils are, therefore, expected to play an important role in the pathogenesis of IRI [79]. Intra-renal activation of HIFs following AKI occurs in tubular, interstitial, and endothelial cells. Upregulation of HIF-1α occurs within 1 h and is sustained for up to 7 days; it induces the infiltration of macrophages following IRI [79, 92]. Mechanical interruption of renal vascular endothelial integrity caused by IRI, and the consequent increase in vascular permeability is another factor that facilitates infiltration of immune cells into the post-ischemic kidney [33, 72, 79]. Furthermore, endothelial cell dysfunction is thought to contribute to the failure of blood to re-perfuse an ischemic area after removal of any physical obstruction (termed the ‘no-reflow’ phenomenon) in post-ischemic kidneys [79].
Therapeutic intervention
As the number of AKI patients continues to grow, so has the interest in therapeutic interventions. However, due to the complex and multifaceted nature of AKI, successful treatment or prevention will involve cellular, molecular, and immune processes. Appropriate therapeutics for AKI will most likely involve targeting the endothelium and renal tubule cells to minimize injury, as well as mediating the immune response [37]. Studies are currently being conducted to modulate the involvement of immune infiltrating cells in AKI in order to limit their associated damage [37, 93]. Most immune invading cells are detrimental, but some, such as regulatory T cells (Tregs), are beneficial [37, 94–96], and enhancing Tregs using IL-2 complexes has been shown to reduce histologic injury and improve function in mice [37, 97].
Endothelial dysfunction has been shown to be one of the earliest pathological sequences following AKI [98, 99]. When endothelial cells are damaged during AKI, they undergo apoptosis, which further amplifies the coagulation cascade [37, 100]. This cascade leads to enhanced microvascular coagulation and endothelial cell dysfunction. Ultimately, microvascular function is compromised, and local tissue perfusion is decreased. Pretreatment or post-injury treatment with soluble thrombomodulin (TM) attenuates ischemic AKI by reducing vascular permeability defects and minimizing white blood cell–endothelial interactions, and thus improves microvascular perfusion [37, 101]. Ischemic injury leads to the release of many cytokines that downregulate the expression of TM, hence causing a state of relative TM deficiency, leaving the microvasculature in a pro-coagulant state [43, 102]. TM has now been well established to possess beneficial roles in inflammation, fibrinolysis, apoptosis, cell adhesion, and cellular proliferation [45, 46, 101, 103].
Selective inhibition, depletion, or deletion of inducible NOS (iNOS) has also clearly shown renoprotective effects during ischemia [37, 47, 104]. It has been proposed that with a relative decrease in endothelial NOS (eNOS), secondary to endothelial dysfunction and damage, there is a loss of antithrombogenic properties of the endothelium, leading to increased susceptibility to microvascular thrombosis [37, 105]. Administration of the L-arginine nitric oxide (NO) donor molsidomine or the eNOS cofactor tetrahydrobiopterin can preserve medullary perfusion and attenuate IRI-induced AKI; conversely, the administration of N-nitro-L-arginine methyl ester, an NO blocker, has been reported to aggravate the course of AKI following IRI [37, 106, 107].
It is not clear yet whether apoptosis and necrosis play a major role in endothelial cell dropout. Ischemia has been shown to inhibit the angiogenic protein vascular endothelial growth factor (VEGF), while inducing the putative VEGF inhibitor ADAMTS-1 [37, 108]. It has been postulated that the lack of vascular repair could be due to VEGF deficiency, as shown by experiments where the administration of VEGF-121 preserved microvascular density [37, 109].
In a recent study, Pabla and colleagues provided evidence that the CDK4/6 (cyclin-dependent kinases) pathway is activated early during AKI and demonstrated significant protective effects of CDK4/6 inhibitors in animal models of cisplatin-induced AKI. In addition, these authors found that the CDK4/6 inhibitors palbociclib and LEE011 are potent inhibitors of organic cation transporter 2 (OCT2), a cisplatin uptake transporter highly expressed in renal tubular cells [110–113]. Their findings provide a rationale for the clinical development of palbociclib and LEE011 for the prevention and treatment of AKI (Table 1).
Fairly recently, micoRNAs (miRNAs) have emerged as potential biomarkers useful in AKI risk assessment, diagnosis, prognosis, and severity of injury [114]. miRNAs are highly conserved and are essential for normal development and physiology [114]. Lorenzen and colleagues identified 13 miRNAs with differential regulation between AKI and healthy control patients [115]. The authors proceed to describe miR-210, a miRNA known to be upregulated in endothelial cells in association with tissue hypoxia and upregulated in patients with AKI [116, 117], and miR-320 and miR-16, both downregulated, as potential biomarkers. Cantaluppi and colleagues suggest that miRNAs such as miR-126 and miR-296, which are derived from microparticles in circulating endothelial progenitor cells, may ameliorate the effects of AKI [118, 119]. While the research on miRNAs in AKI is still limited, these molecules hold much promise as a potential therapeutic application.
Notably, despite the high prevalence and mortality rates of AKI, no clinically proven therapeutic interventions are available to prevent it [120]. While many laboratories are working on possible treatments or preventatives for AKI, a significant amount of pre-clinical studies are required to test efficacy.
Conclusion
In conclusion, this review highlights the role of the renal microvasculature in AKI. We have highlighted the many causes of AKI while focusing on those related to ischemic injury (as described in Fig. 1; [121]). Furthermore, this review examines the molecular and cellular mechanisms that currently exist and have been elegantly studied in murine models. The study of the renal microvasculature during AKI may provide a critical therapeutic window that ultimately will enable prevention of this widespread disease.
Acknowledgments
This work was supported by an NIH K01 DK096996 (SSL) and an NIH T32 DK061296 (KM).
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- 1.Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol. 2011;7:201–208. doi: 10.1038/nrneph.2011.14. [DOI] [PubMed] [Google Scholar]
- 2.Korkeila M, Ruokonen E, Takala J. Costs of care, long-term prognosis and quality of life in patients requiring renal replacement therapy during intensive care. Intensive Care Med. 2000;26:1824–1831. doi: 10.1007/s001340000726. [DOI] [PubMed] [Google Scholar]
- 3.Bagshaw SM. The long-term outcome after acute renal failure. Curr Opin Crit Care. 2006;12:561–566. doi: 10.1097/01.ccx.0000247445.71381.72. [DOI] [PubMed] [Google Scholar]
- 4.Chan JC, Williams DM, Roth KS. Kidney failure in infants and children. Pediatr Rev. 2002;23:47–60. doi: 10.1542/pir.23-2-47. [DOI] [PubMed] [Google Scholar]
- 5.Patzer L. Nephrotoxicity as a cause of acute kidney injury in children. Pediatr Nephrol. 2008;23:2159–2173. doi: 10.1007/s00467-007-0721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faught LN, Greff MJ, Rieder MJ, Koren G. Drug-induced acute kidney injury in children. Br J Clin Pharmacol. 2015;80(4):901–909. doi: 10.1111/bcp.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreoli SP. Acute kidney injury in children. Pediatr Nephrol. 2009;24:253–263. doi: 10.1007/s00467-008-1074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentley ML, Corwin HL, Dasta J. Drug-induced acute kidney injury in the critically ill adult: recognition and prevention strategies. Crit Care Med. 2010;38:S169–S174. doi: 10.1097/CCM.0b013e3181de0c60. [DOI] [PubMed] [Google Scholar]
- 9.Ashraf M, Shahzad N, Irshad M, Hussain SQ, Ahmed P. Pediatric acute kidney injury: a syndrome under paradigm shift. Indian J Crit Care Med. 2014;18:518–526. doi: 10.4103/0972-5229.138156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal A, Kumar P, Chowdhary G, Majumdar S, Narang A. Evaluation of renal functions in asphyxiated newborns. J Trop Pediatr. 2005;51:295–299. doi: 10.1093/tropej/fmi017. [DOI] [PubMed] [Google Scholar]
- 11.Hum S, Rymer C, Schaefer C, Bushnell D, Sims-Lucas S. Ablation of the renal stroma defines its critical role in nephron progenitor and vasculature patterning. PLoS One. 2014;9:e88400. doi: 10.1371/journal.pone.0088400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sariola H. Nephron induction. Nephrol Dial Transplant. 2002;17(Suppl 9):88–90. doi: 10.1093/ndt/17.suppl_9.88. [DOI] [PubMed] [Google Scholar]
- 13.Saxen L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987;1:385–392. doi: 10.1007/BF00849241. [DOI] [PubMed] [Google Scholar]
- 14.Kanwar YS, Carone FA, Kumar A, Wada J, Ota K, Wallner EI. Role of extracellular matrix, growth factors and proto-oncogenes in metanephric development. Kidney Int. 1997;52:589–606. doi: 10.1038/ki.1997.372. [DOI] [PubMed] [Google Scholar]
- 15.Alcorn D, Maric C, McCausland J. Development of the renal interstitium. Pediatr Nephrol. 1999;13:347–354. doi: 10.1007/s004670050624. [DOI] [PubMed] [Google Scholar]
- 16.Sims-Lucas S, Schaefer C, Bushnell D, Ho J, Logar A, Prochownik E, Gittes G, Bates CM. Endothelial progenitors exist within the kidney and lung mesenchyme. PLoS One. 2013;8:e65993. doi: 10.1371/journal.pone.0065993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sequeira Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol. 2011;22:2156–2165. doi: 10.1681/ASN.2011080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sequeira-Lopez ML, Lin EE, Li M, Hu Y, Sigmund CD, Gomez RA. The earliest metanephric arteriolar progenitors and their role in kidney vascular development. Am J Physiol Regul Integr Comp Physiol. 2015;308:R138–R149. doi: 10.1152/ajpregu.00428.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132:529–539. doi: 10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- 20.Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10:1467–1478. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- 21.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smadja DM, d’Audigier C, Bieche I, Evrard S, Mauge L, Dias JV, Labreuche J, Laurendeau I, Marsac B, Dizier B, Wagner-Ballon O, Boisson-Vidal C, Morandi V, Duong-Van-Huyen JP, Bruneval P, Dignat-George F, Emmerich J, Gaussem P. Thrombospondin-1 is a plasmatic marker of peripheral arterial disease that modulates endothelial progenitor cell angiogenic properties. Arterioscler Thromb Vasc Biol. 2011;31:551–559. doi: 10.1161/ATVBAHA.110.220624. [DOI] [PubMed] [Google Scholar]
- 23.Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramann R, Tanaka M, Humphreys BD. Fluorescence microangiography for quantitative assessment of peritubular capillary changes after AKI in mice. J Am Soc Nephrol. 2014;25:1924–1931. doi: 10.1681/ASN.2013101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubbers DW, Baumgartl H. Heterogeneities and profiles of oxygen pressure in brain and kidney as examples of the pO2 distribution in the living tissue. Kidney Int. 1997;51:372–380. doi: 10.1038/ki.1997.49. [DOI] [PubMed] [Google Scholar]
- 26.Eckardt KU, Bernhardt WM, Weidemann A, Warnecke C, Rosenberger C, Wiesener MS, Willam C. Role of hypoxia in the pathogenesis of renal disease. Kidney Int Suppl. 2005;(99):S46–51. doi: 10.1111/j.1523-1755.2005.09909.x. [DOI] [PubMed] [Google Scholar]
- 27.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev. 2015;4:20–27. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura N, Kimura H, Takahashi N, Hamada T, Maegawa H, Mori M, Imamura Y, Kusaka Y, Yoshida H, Iwano M. Renal resistive index correlates with peritubular capillary loss and arteriosclerosis in biopsy tissues from patients with chronic kidney disease. Clin Exp Nephrol. 2015 doi: 10.1007/s10157-015-1116-0. [DOI] [PubMed] [Google Scholar]
- 29.Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109:1945–1952. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 31.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens. 2004;13:1–7. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006;69:184–189. doi: 10.1038/sj.ki.5000032. [DOI] [PubMed] [Google Scholar]
- 33.Brodsky SV, Yamamoto T, Tada T, Kim B, Chen J, Kajiya F, Goligorsky MS. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am J Physiol Renal Physiol. 2002;282:F1140–F1149. doi: 10.1152/ajprenal.00329.2001. [DOI] [PubMed] [Google Scholar]
- 34.Basile DP, Yoder MC. Renal endothelial dysfunction in acute kidney ischemia reperfusion injury. Cardiovasc Hematol Disord Drug Targets. 2014;14:3–14. doi: 10.2174/1871529x1401140724093505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arriero M, Brodsky SV, Gealekman O, Lucas PA, Goligorsky MS. Adult skeletal muscle stem cells differentiate into endothelial lineage and ameliorate renal dysfunction after acute ischemia. Am J Physiol Renal Physiol. 2004;287:F621–F627. doi: 10.1152/ajprenal.00126.2004. [DOI] [PubMed] [Google Scholar]
- 36.Choong FX, Sandoval RM, Molitoris BA, Richter-Dahlfors A. Multiphoton microscopy applied for real-time intravital imaging of bacterial infections in vivo. Methods Enzymol. 2012;506:35–61. doi: 10.1016/B978-0-12-391856-7.00027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest. 2014;124:2355–2363. doi: 10.1172/JCI72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grenz A, Bauerle JD, Dalton JH, Ridyard D, Badulak A, Tak E, McNamee EN, Clambey E, Moldovan R, Reyes G, Klawitter J, Ambler K, Magee K, Christians U, Brodsky KS, Ravid K, Choi DS, Wen J, Lukashev D, Blackburn MR, Osswald H, Coe IR, Nurnberg B, Haase VH, Xia Y, Sitkovsky M, Eltzschig HK. Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J Clin Invest. 2012;122:693–710. doi: 10.1172/JCI60214. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Mattson DL, Lu S, Cowley AW., Jr Role of nitric oxide in the control of the renal medullary circulation. Clin Exp Pharmacol Physiol. 1997;24:587–590. doi: 10.1111/j.1440-1681.1997.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 40.Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, Mazzali M, Jefferson JA, Hughes J, Madsen KM, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12:1434–1447. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- 41.O’Riordan E, Mendelev N, Patschan S, Patschan D, Eskander J, Cohen-Gould L, Chander P, Goligorsky MS. Chronic NOS inhibition actuates endothelial-mesenchymal transformation. Am J Physiol Heart Circ Physiol. 2007;292:H285–H294. doi: 10.1152/ajpheart.00560.2006. [DOI] [PubMed] [Google Scholar]
- 42.Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, Chiang WC, Kuhnert F, Kuo CJ, Chen YM, Wu KD, Tsai TJ, Duffield JS. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol. 2011;178:911–923. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 44.Weinberg JM, Venkatachalam MA. Preserving postischemic reperfusion in the kidney: a role for extracellular adenosine. J Clin Invest. 2012;122:493–496. doi: 10.1172/JCI60957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang HC, Shi GY, Jiang SJ, Shi CS, Wu CM, Yang HY, Wu HL. Thrombomodulin-mediated cell adhesion: involvement of its lectin-like domain. J Biol Chem. 2003;278:46750–46759. doi: 10.1074/jbc.M305216200. [DOI] [PubMed] [Google Scholar]
- 46.Esmon CT, Owen WG. The discovery of thrombomodulin. J Thromb Haemost. 2004;2:209–213. doi: 10.1046/j.1538-7933.2003.00537.x. [DOI] [PubMed] [Google Scholar]
- 47.Ling H, Edelstein C, Gengaro P, Meng X, Lucia S, Knotek M, Wangsiripaisan A, Shi Y, Schrier R. Attenuation of renal ischemia-reperfusion injury in inducible nitric oxide synthase knockout mice. Am J Physiol. 1999;277:F383–F390. doi: 10.1152/ajprenal.1999.277.3.F383. [DOI] [PubMed] [Google Scholar]
- 48.Thakar CV, Zahedi K, Revelo MP, Wang Z, Burnham CE, Barone S, Bevans S, Lentsch AB, Rabb H, Soleimani M. Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J Clin Invest. 2005;115:3451–3459. doi: 10.1172/JCI25461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers NM, Thomson AW, Isenberg JS. Activation of parenchymal CD47 promotes renal ischemia–reperfusion injury. J Am Soc Nephrol. 2012;23:1538–1550. doi: 10.1681/ASN.2012020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci USA. 2005;102:13141–13146. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- 52.Martinez-Mier G, Toledo-Pereyra LH, Bussell S, Gauvin J, Vercruysse G, Arab A, Harkema JR, Jordan JA, Ward PA. Nitric oxide diminishes apoptosis and p53 gene expression after renal ischemia and reperfusion injury. Transplantation. 2000;70:1431–1437. doi: 10.1097/00007890-200011270-00006. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez-Pena A, Garcia-Criado FJ, Eleno N, Arevalo M, Lopez-Novoa JM. Intrarenal administration of molsidomine, a molecule releasing nitric oxide, reduces renal ischemia–reperfusion injury in rats. Am J Transplant. 2004;4:1605–1613. doi: 10.1111/j.1600-6143.2004.00560.x. [DOI] [PubMed] [Google Scholar]
- 54.Liu X, Huang Y, Pokreisz P, Vermeersch P, Marsboom G, Swinnen M, Verbeken E, Santos J, Pellens M, Gillijns H, Van de Werf F, Bloch KD, Janssens S. Nitric oxide inhalation improves microvascular flow and decreases infarction size after myocardial ischemia and reperfusion. J Am Coll Cardiol. 2007;50:808–817. doi: 10.1016/j.jacc.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 55.Lang JD, Jr, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, Cross RC, Frenette L, Kelley EE, Wilhite DW, Hall CR, Page GP, Fallon MB, Bynon JS, Eckhoff DE, Patel RP. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 57.Perez Fontan M, Rodriguez-Carmona A, Bouza P, Valdes F. The prognostic significance of acute renal failure after renal transplantation in patients treated with cyclosporin. QJM. 1998;91:27–40. doi: 10.1093/qjmed/91.1.27. [DOI] [PubMed] [Google Scholar]
- 58.Verma SK, Molitoris BA. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol. 2015;35:96–107. doi: 10.1016/j.semnephrol.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto T, Tada T, Brodsky SV, Tanaka H, Noiri E, Kajiya F, Goligorsky MS. Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am J Physiol Renal Physiol. 2002;282:F1150–F1155. doi: 10.1152/ajprenal.00310.2001. [DOI] [PubMed] [Google Scholar]
- 61.Dimke H, Sparks MA, Thomson BR, Frische S, Coffman TM, Quaggin SE. Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. J Am Soc Nephrol. 2015;26:1027–1038. doi: 10.1681/ASN.2014010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mansson LE, Melican K, Boekel J, Sandoval RM, Hautefort I, Tanner GA, Molitoris BA, Richter-Dahlfors A. Real-time studies of the progression of bacterial infections and immediate tissue responses in live animals. Cell Microbiol. 2007;9:413–424. doi: 10.1111/j.1462-5822.2006.00799.x. [DOI] [PubMed] [Google Scholar]
- 63.Melican K, Boekel J, Mansson LE, Sandoval RM, Tanner GA, Kallskog O, Palm F, Molitoris BA, Richter-Dahlfors A. Bacterial infection-mediated mucosal signalling induces local renal ischaemia as a defence against sepsis. Cell Microbiol. 2008;10:1987–1998. doi: 10.1111/j.1462-5822.2008.01182.x. [DOI] [PubMed] [Google Scholar]
- 64.Vallon V, Osswald H. Adenosine receptors and the kidney. Handb Exp Pharmacol. 2009;2009:443–470. doi: 10.1007/978-3-540-89615-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yap SC, Lee HT. Adenosine and protection from acute kidney injury. Curr Opin Nephrol Hypertens. 2012;21:24–32. doi: 10.1097/MNH.0b013e32834d2ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 67.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 68.Tanaka T, Nangaku M. Angiogenesis and hypoxia in the kidney. Nat Rev Nephrol. 2013;9:211–222. doi: 10.1038/nrneph.2013.35. [DOI] [PubMed] [Google Scholar]
- 69.Ergin B, Kapucu A, Demirci-Tansel C, Ince C. The renal microcirculation in sepsis. Nephrol Dial Transplant. 2015;30:169–177. doi: 10.1093/ndt/gfu105. [DOI] [PubMed] [Google Scholar]
- 70.Kapitsinou PP, Sano H, Michael M, Kobayashi H, Davidoff O, Bian A, Yao B, Zhang MZ, Harris RC, Duffy KJ, Erickson-Miller CL, Sutton TA, Haase VH. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest. 2014;124:2396–2409. doi: 10.1172/JCI69073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Advani A, Connelly KA, Yuen DA, Zhang Y, Advani SL, Trogadis J, Kabir MG, Shachar E, Kuliszewski MA, Leong-Poi H, Stewart DJ, Gilbert RE. Fluorescent microangiography is a novel and widely applicable technique for delineating the renal microvasculature. PLoS One. 2011;6:e24695. doi: 10.1371/journal.pone.0024695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol. 2003;285:F191–F198. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- 73.Kwon O, Phillips CL, Molitoris BA. Ischemia induces alterations in actin filaments in renal vascular smooth muscle cells. Am J Physiol Renal Physiol. 2002;282:F1012–F1019. doi: 10.1152/ajprenal.00294.2001. [DOI] [PubMed] [Google Scholar]
- 74.Becherucci F, Mazzinghi B, Ronconi E, Peired A, Lazzeri E, Sagrinati C, Romagnani P, Lasagni L. The role of endothelial progenitor cells in acute kidney injury. Blood Purif. 2009;27:261–270. doi: 10.1159/000202005. [DOI] [PubMed] [Google Scholar]
- 75.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2:1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol. 2009;130:41–50. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 78.Goncalves GM, Zamboni DS, Camara NO. The role of innate immunity in septic acute kidney injuries. Shock. 2010;34(Suppl 1):22–26. doi: 10.1097/SHK.0b013e3181e7e69e. [DOI] [PubMed] [Google Scholar]
- 79.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol. 2015;11:88–101. doi: 10.1038/nrneph.2014.180. [DOI] [PubMed] [Google Scholar]
- 80.Jang HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med (Berl) 2009;87:859–864. doi: 10.1007/s00109-009-0491-y. [DOI] [PubMed] [Google Scholar]
- 81.Ysebaert DK, De Greef KE, Vercauteren SR, Ghielli M, Verpooten GA, Eyskens EJ, De Broe ME. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant. 2000;15:1562–1574. doi: 10.1093/ndt/15.10.1562. [DOI] [PubMed] [Google Scholar]
- 82.Celie JW, Rutjes NW, Keuning ED, Soininen R, Heljasvaara R, Pihlajaniemi T, Drager AM, Zweegman S, Kessler FL, Beelen RH, Florquin S, Aten J, van den Born J. Subendothelial heparan sulfate proteoglycans become major L-selectin and monocyte chemoattractant protein-1 ligands upon renal ischemia/reperfusion. Am J Pathol. 2007;170:1865–1878. doi: 10.2353/ajpath.2007.070061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anders HJ, Vielhauer V, Schlondorff D. Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int. 2003;63:401–415. doi: 10.1046/j.1523-1755.2003.00750.x. [DOI] [PubMed] [Google Scholar]
- 84.Swaminathan S, Griffin MD. First responders: understanding monocyte-lineage traffic in the acutely injured kidney. Kidney Int. 2008;74:1509–1511. doi: 10.1038/ki.2008.555. [DOI] [PubMed] [Google Scholar]
- 85.Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol. 2010;30:234–254. doi: 10.1016/j.semnephrol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chiba T, Skrypnyk NI, Skvarca LB, Penchev R, Zhang KX, Rochon ER, Fall JL, Paueksakon P, Yang H, Alford CE, Roman BL, Zhang MZ, Harris R, Hukriede NA, de Caestecker MP. Retinoic acid signaling coordinates macrophage-dependent injury and repair after AKI. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014111108. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huen SC, Huynh L, Marlier A, Lee Y, Moeckel GW, Cantley LG. GM-CSF promotes macrophage alternative activation after renal ischemia/reperfusion injury. J Am Soc Nephrol. 2015;26:1334–1345. doi: 10.1681/ASN.2014060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chiao H, Kohda Y, McLeroy P, Craig L, Housini I, Star RA. Alpha-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats. J Clin Invest. 1997;99:1165–1172. doi: 10.1172/JCI119272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nemoto T, Burne MJ, Daniels F, O’Donnell MP, Crosson J, Berens K, Issekutz A, Kasiske BL, Keane WF, Rabb H. Small molecule selectin ligand inhibition improves outcome in ischemic acute renal failure. Kidney Int. 2001;60:2205–2214. doi: 10.1046/j.1523-1755.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 90.Solez K, Morel-Maroger L, Sraer JD. The morphology of “acute tubular necrosis” in man: analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore) 1979;58:362–376. [PubMed] [Google Scholar]
- 91.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 92.Rosenberger C, Griethe W, Gruber G, Wiesener M, Frei U, Bachmann S, Eckardt KU. Cellular responses to hypoxia after renal segmental infarction. Kidney Int. 2003;64:874–886. doi: 10.1046/j.1523-1755.2003.00159.x. [DOI] [PubMed] [Google Scholar]
- 93.Lichtnekert J, Kawakami T, Parks WC, Duffield JS. Changes in macrophage phenotype as the immune response evolves. Curr Opin Pharmacol. 2013;13:555–564. doi: 10.1016/j.coph.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 95.Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, Crow MT, King LS, Rabb H. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009;76:717–729. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- 96.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20:1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim MG, Koo TY, Yan JJ, Lee E, Han KH, Jeong JC, Ro H, Kim BS, Jo SK, Oh KH, Surh CD, Ahn C, Yang J. IL-2/anti-IL-2 complex attenuates renal ischemia-reperfusion injury through expansion of regulatory T cells. J Am Soc Nephrol. 2013;24:1529–1536. doi: 10.1681/ASN.2012080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gupta A, Berg DT, Gerlitz B, Sharma GR, Syed S, Richardson MA, Sandusky G, Heuer JG, Galbreath EJ, Grinnell BW. Role of protein C in renal dysfunction after polymicrobial sepsis. J Am Soc Nephrol. 2007;18:860–867. doi: 10.1681/ASN.2006101167. [DOI] [PubMed] [Google Scholar]
- 99.Bouchard J, Malhotra R, Shah S, Kao YT, Vaida F, Gupta A, Berg DT, Grinnell BW, Stofan B, Tolwani AJ, Mehta RL. Levels of protein C and soluble thrombomodulin in critically ill patients with acute kidney injury: a multicenter prospective observational study. PLoS One. 2015;10:e0120770. doi: 10.1371/journal.pone.0120770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mizutani A, Okajima K, Uchiba M, Noguchi T. Activated protein C reduces ischemia/reperfusion-induced renal injury in rats by inhibiting leukocyte activation. Blood. 2000;95:3781–3787. [PubMed] [Google Scholar]
- 101.Sharfuddin AA, Sandoval RM, Berg DT, McDougal GE, Campos SB, Phillips CL, Jones BE, Gupta A, Grinnell BW, Molitoris BA. Soluble thrombomodulin protects ischemic kidneys. J Am Soc Nephrol. 2009;20:524–534. doi: 10.1681/ASN.2008060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ikeguchi H, Maruyama S, Morita Y, Fujita Y, Kato T, Natori Y, Akatsu H, Campbell W, Okada N, Okada H, Yuzawa Y, Matsuo S. Effects of human soluble thrombomodulin on experimental glomerulonephritis. Kidney Int. 2002;61:490–501. doi: 10.1046/j.1523-1755.2002.00160.x. [DOI] [PubMed] [Google Scholar]
- 103.Conway EM, Van de Wouwer M, Pollefeyt S, Jurk K, Van Aken H, De Vriese A, Weitz JI, Weiler H, Hellings PW, Schaeffer P, Herbert JM, Collen D, Theilmeier G. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J Exp Med. 2002;196:565–577. doi: 10.1084/jem.20020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001;281:F948–F957. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 105.Goligorsky MS, Brodsky SV, Noiri E. NO bioavailability, endothelial dysfunction, and acute renal failure: new insights into pathophysiology. Semin Nephrol. 2004;24:316–323. doi: 10.1016/j.semnephrol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 106.Mattson DL, Wu F. Control of arterial blood pressure and renal sodium excretion by nitric oxide synthase in the renal medulla. Acta Physiol Scand. 2000;168:149–154. doi: 10.1046/j.1365-201x.2000.00647.x. [DOI] [PubMed] [Google Scholar]
- 107.Chander V, Chopra K. Renal protective effect of molsidomine and L-arginine in ischemia-reperfusion induced injury in rats. J Surg Res. 2005;128:132–139. doi: 10.1016/j.jss.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 108.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol. 2008;294:F928–F936. doi: 10.1152/ajprenal.00596.2007. [DOI] [PubMed] [Google Scholar]
- 109.Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol. 2008;295:F1648–F1657. doi: 10.1152/ajprenal.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86:396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, Pavenstadt H, Lanvers-Kaminsky C, am Zehnhoff-Dinnesen A, Schinkel AH, Koepsell H, Jurgens H, Schlatter E. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol. 2010;176:1169–1180. doi: 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sprowl JA, Lancaster CS, Pabla N, Hermann E, Kosloske AM, Gibson AA, Li L, Zeeh D, Schlatter E, Janke LJ, Ciarimboli G, Sparreboom A. Cisplatin-induced renal injury is independently mediated by OCT2 and p53. Clin Cancer Res. 2014;20:4026–4035. doi: 10.1158/1078-0432.CCR-14-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pabla N, Gibson AA, Buege M, Ong SS, Li L, Hu S, Du G, Sprowl JA, Vasilyeva A, Janke LJ, Schlatter E, Chen T, Ciarimboli G, Sparreboom A. Mitigation of acute kidney injury by cell-cycle inhibitors that suppress both CDK4/6 and OCT2 functions. Proc Natl Acad Sci USA. 2015;112:5231–5236. doi: 10.1073/pnas.1424313112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Molitoris BA, Melnikov VY, Okusa MD, Himmelfarb J. Technology Insight: biomarker development in acute kidney injury–what can we anticipate? Nat Clin Pract Nephrol. 2008;4:154–165. doi: 10.1038/ncpneph0723. [DOI] [PubMed] [Google Scholar]
- 115.Lorenzen JM, Kielstein JT, Hafer C, Gupta SK, Kumpers P, Faulhaber-Walter R, Haller H, Fliser D, Thum T. Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2011;6:1540–1546. doi: 10.2215/CJN.00430111. [DOI] [PubMed] [Google Scholar]
- 116.Fasanaro P, D’Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Molitoris JK, Molitoris BA. Circulating micro-RNAs in acute kidney injury: early observations. Clin J Am Soc Nephrol. 2011;6:1517–1519. doi: 10.2215/CJN.04930511. [DOI] [PubMed] [Google Scholar]
- 118.Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, Sordi A, Biancone L, Tetta C, Camussi G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82:412–427. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- 119.Bitzer M, Ben-Dov IZ, Thum T. Microparticles and microRNAs of endothelial progenitor cells ameliorate acute kidney injury. Kidney Int. 2012;82:375–377. doi: 10.1038/ki.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 121.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11:264–276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]