Abstract
To understand the role of the CCAAT-binding factor, CBF, in transcription, we developed a strategy to purify the heterotrimeric CBF complex from HeLa cell extracts using two successive immunoaffinity chromatography steps. Here we show that the p32 protein, previously identified as the ASF/SF2 splicing factor-associated protein, copurified with the CBF complex. Studies of protein–protein interaction demonstrated that p32 interacts specifically with CBF–B subunit and also associates with CBF–DNA complex. Cellular localization by immunofluorescence staining revealed that p32 is present in the cell throughout the cytosol and nucleus, whereas CBF is present primarily in the nucleus. A portion of the p32 colocalizes with CBF-B in the nucleus. Interestingly, reconstitution of p32 in an in vitro transcription reaction demonstrated that p32 specifically inhibits CBF-mediated transcription activation. Altogether, our study identified p32 as a novel and specific corepressor of CBF-mediated transcription activation in vitro.
INTRODUCTION
The mammalian CCAAT-binding factor (CBF), also called nuclear factor Y (NF-Y), consists of three different subunits, CBF-A, CBF-B and CBF-C, which together form a complex with promoter DNA containing the CCAAT motif, which is present in the promoters of many mammalian genes. The CCAAT motifs are often located within the few hundred base pairs of the proximal promoters for these genes. Mutations in the CCAAT motifs prevent CBF binding to these sites and decrease the transcriptional activity. The CBF binding site is one of the most common motifs present in cell-cycle-regulated promoters, endoplasmic reticulum stress-responsive promoters and some tissue-specific promoters such as major histocompatibility complex class II, type I collagen promoters (1,2).
Interestingly, many of the cell-cycle-regulated promoters, such as topoisomerase IIα, cyclin B1, cyclin B2, CDC25C, CDC2 and E2F1, contain multiple CBF binding sites, which play an important role in their cell-cycle-dependent activation (3–7). Our study showed that mutation of all the CBF binding sites in the topoisomerase promoter resulted in a complete inhibition of promoter activation during the cell cycle (3). Since the DNA binding activity of CBF is unchanged during the cell-cycle, the specific role of CBF during cell cycle-dependent activation of the promoter remains unclear.
Multiple CBF sites are also present in the promoters of a group of genes such as BiP/GRP78, GRP94, protein disulfide isomerase and calreticulin that are involved in the endoplasmic reticulum stress pathway (8–10). The accumulation of unfolded proteins in the endoplasmic reticulum is responsible for the unfolded protein response and the CBF binding sites in the above promoters play a critical role in transcription activation during such a response (11). In addition, a single CBF binding site is present in the promoters of various genes, such as type I collagen, major histocompatibility complex class II genes, which are expressed in specific cell types (12–13). The CBF-B and CBF-C subunits both possess transcription activation domains that activate transcription additively (14). Thus, CBF by itself can act as a strong transcription activator. Hence, it is not very clear how the specificity of CBF-dependent transcription activation of various tissue-specific promoters occurs since CBF expression is ubiquitous.
Altogether, these studies indicate that CBF is a common DNA-binding protein that plays an important role in the transcription of various cellular genes that are regulated by different cellular pathways. Thus, although CBF is constitutively expressed and its activity is not directly regulated by any of the above-mentioned pathways, it is critically involved in the transcription of genes that are regulated by various cellular responses or in specific cell types. Therefore, we hypothesized that the function of CBF as a transcription activator is regulated in vivo, possibly through interactions with other cellular proteins. To test this possibility and identify the cellular proteins that interact with CBF, we purified a CBF complex assembled in vivo from human HeLa cell extracts using two different immunoaffinity resins. The polypeptides present in the complex were identified using mass spectrometry. The analysis of these data showed that human protein p32, previously identified as a splicing factor ASF/SF2-associated protein, is present in the purified CBF complex. Our study also showed that the p32 protein specifically interacts with the CBF-B subunit, and inhibits CBF-mediated transcription in vitro.
MATERIALS AND METHODS
Plasmids
The pTRE-flag-CBF-B was constructed as described previously (3,15). The pTRE-flag-ha plasmid was constructed from the above pTRE-flag plasmid by insertion of a double-stranded oligonucleotide, coding for the HA peptide sequence (TAC CCA TAC GAC GTC CCA GAC TAC GCT CAT) at the NdeI–XbaI site. The final construct contained a single NdeI site at the end of the HA-tag. The CBF-B DNA was inserted between the NdeI and the XbaI sites to construct pTRE-flag-ha-CBF-B. The pTRE-flag-CBF-A and pTRE-flag-CBF-C plasmids used to express flag-CBF-A and flag-CBF-C polypeptides were constructed by inserting CBF-A or CBF-C DNA at NdeI and XbaI sites of the pTRE-flag vector. The full-length p32-myc-His DNA in pCDNA3.1 was a kind gift from O'Hare (16). This construct has been referred to as the mycp32 (1–282). The shorter mycp32 (corresponding to amino acids 75 to 282 of the full-length protein) was generated by PCR from the above plasmid and subsequently inserted in the pTRE vector for expression in the HeLa cells. The pRSETA-Hisp32 plasmid was a kind gift from Matthews (17). The glutathione S transferase (GST)-CBF-B, GST-CBF-Bdbd and GST-CBF-A/CBF-C plasmids have been described before (14,18).
Expression of recombinant CBF and recombinant p32 in bacterial cells
His-p32 protein was expressed in bacteria and purified using Ni-NTA Agarose (QIAGEN, Valencia, CA). The purification of GST-CBF polypeptides is described before (14,18).
Cell lines and transfection
The tetracycline-responsive, HeLa tet-off cell line (HtTA) was obtained from Clontech (BD Biosciences, Clontech, Palo Alto, CA). This cell line was used to select clones stably expressing flag-ha-CBF-B polypeptide in the presence of hygromycin, as described before (3). The expression of flag-ha-CBF-B in each such cell clone was checked by immunoblotting the cell extracts with anti-FLAG-HRP conjugated (Sigma-Aldrich, St Louis, MO) and anti-HA antibodies (Roche, Indianapolis, IN). For both the stable and the transient transfection experiments, plasmid DNAs were transfected in HeLa cells using FuGENE 6 Transfection Reagent (Roche).
Affinity purification of CBF-B complex
Expression of flag-ha-CBF-B in the stable clone was induced in the absence of tetracycline for 72 h. These cells were then used to prepare cell extracts by incubation in ice-cold buffer A (200 mM NaCl, 1% NP40, 50 mM Tris, pH 7.9) for 30 min. The extract was loaded over anti-FLAG-M2 affinity resin (Sigma-Aldrich) and incubated at 4°C for 3 h in a rotary shaker. The unbound proteins were removed by centrifugation, and the resin was washed first with 10 bed volumes of buffer A and subsequently with 5 bed volumes of buffer B (150 mM NaCl, 10% glycerol, 0.05% NP40, 50 mM HEPES, pH 7.9). The bound proteins were eluted by incubation with 200 ng/μl of 3 × FLAG peptide (Sigma-Aldrich) in buffer B for 30 min at 4°C. The eluted material was then fractionated over an anti-HA affinity resin (Roche) and the bound proteins were eluted with 1 mg/ml of HA-peptide (Roche) for 30 min at 30°C.
Preparation of samples for mass spectrometry
The proteins eluted from the anti-HA resin were precipitated by acetone, electrophoreses in a 10% sodium dodecyl sulphate (SDS)–polyacrylamide gel and stained with Colloidal Coomassie blue (Sigma-Aldrich). The stained bands were excised from the gel, subjected to in-gel tryptic digestion, and then analyzed using mass spectrometry. Briefly, the gel slice was washed with water, 50% acetonitrile–water containing 0.01% trifluoroacetic acid, reduced and alkylated with iodoacetamide, washed again, dried, and then incubated 18 h at 37°C with 100 ng of modified porcine trypsin (Promega, Madison, WI) in 30 mM ammonium bicarbonate. The supernatant and two washings of 50% acetonirile–water containing 0.01% trifluoroacetic acid were combined, reduced in volume and the tryptic peptides were separated on a 0.3 × 50 mm2 Targa-C18 column (Higgins Analytical, Mountain View, CA), loaded in 0.01% trifluoroacetic acid in 5% acetonitrile and eluted over a 40 min gradient to 32% acetonitrile (0.01% trifluoroacetic acid) delivered by splitting the flow from an inert Ettan HPLC (high-pressure liquid chromatography) (Amersham Pharmacia, Parsippany NJ) to ∼1 μl/min. The flow was electrified with a gold wire installed in a micro-tee using a nanospray source (Protana, Odense, Denmark) mounted to an LCQ-DECA-XP ion trap mass spectrometer (Thermo-Finnigan, San Jose, CA). Mass spectra were acquired in the data- dependent mode, with one survey and two fragment spectra of the two most intense ions, with dynamic exclusion to prevent resampling of the abundant signals. Spectra were searched using Sonar (Genomic Solutions, Madison WI) and Mascot (Matrix Science, London, UK) against the NCBI non-redundant database (NIH, Bethesda, MD) and the MSDB (ICRF, London, UK).
Detection of CBF-B-associated polypeptides
The HeLa cell extracts were fractionated with an anti-FLAG-M2 resin, and the bound proteins were eluted with 3 × FLAG peptide, as described earlier. The eluted material was separated using SDS–PAGE and then analyzed by western blotting using specific antibodies against each polypeptide. The anti-FLAG-M2 antibody conjugated to HRP (Sigma-Aldrich) was used to detect the FLAG-tagged proteins. The mouse monoclonal anti-p32 antibody (Covance, Princeton, NJ) was used to identify the endogenous p32 protein. The cellular CBF polypeptides were detected using rabbit polyclonal antibodies raised against each of the three CBF subunits.
Study of endogenous CBF-p32 interactions using coimmunoprecipitation reactions and DNA affinity resin
HeLa cells were used to prepare whole cell extracts in buffer A as described earlier. The extract was first precleared by incubation with protein A/G agarose for 4 h at 4°C and then incubated with either a rabbit polyclonal anti-CBF-B antibody or rabbit IgG at 4°C overnight. Next day each set was incubated with Protein A/G agarose for 2 h and then the supernatant removed after spin down. The resin was washed in each case with buffer A twice and the bound proteins were extracted with SDS–PAGE loading buffer. These were then used for SDS–PAGE and subsequent western blot analysis.
The DNA affinity resin for CBF was made with a 45 bp double-stranded oligonucleotide containing CBF binding site of the mouse α2(1) collagen promoter. The mutant resin was made with a similar size double stranded oligonucleotide containing single base pair mutation that inhibits CBF binding (14). Cell extracts were adjusted to 100 mM salt and were incubated with each DNA affinity resin at 4°C for an hour. After incubation the resins were washed with buffer A containing 250 mM salt, and the bound proteins were extracted with SDS–PAGE loading buffer for western blot analysis.
Transcription assays
The in vitro transcription reactions were done with nuclear extracts from the HeLa cells. Preparation of nuclear extracts, conditions of the in vitro transcription reactions, and the subsequent analysis of the RNAs using the primer extension method were described previously (18). The luciferase reporter gene assays were also performed as described previously (19). Each experiment was repeated a minimum of three times.
GST-pull down assays
Recombinant p32 (75 ng) was incubated with 150 ng of GST-CBF-B or GST-CBB-A/CBF-C or GST-CBF-Bdbd or GST in 100 μl buffer A in cold for 30 min. This reaction mixture was then precipitated with 20 μl of Glutathione Sepharose 4B resin (Amersham Biosciences, Piscataway, NJ) using standard procedures. The resin-bound proteins were then analyzed by subsequent western blots.
Immunofluorescent staining
HeLa cells were plated on cover slips and staining was done after a short PMA treatment of the cells for better visualization of the p32 protein in the nucleus as described by Majumdar et al. (20). The cells were washed twice with ice-cold PBS, fixed in 4% formaldehyde in PEM buffer (80 mM potassium–PIPES, 5 mM EGTA pH7, 2 mM MgCl2) for 30 min in ice, washed three times with PEM buffer, and incubated with 0.5% Triton-X in the PEM buffer for 30 mins for permeabilization. The cells were then washed three times with PEM buffer, placed in a blocking solution (1% BSA in Tris-buffered saline (TBS)–Tween) for another 30 min, and incubated for 1 h with anti-CBF-B antibody (rabbit polyclonal) and anti-p32 antibody (mouse monoclonal). The cells were washed four to five times with the blocking solution, incubated for another hour with anti-rabbit IgG coupled to Alexa Fluor 488 as well as anti-mouse IgG coupled to Alexa Fluor 594. The cells were washed next with blocking buffer and stained by 4′, 6′-diamidin-2-phenylindone (DAPI) (20 μg/ml) in blocking solution and then washed three times in TBS–Tween buffer. The cells were mounted using a ProLong Antifade kit from Molecular Probes (Eugene, OR). The images were acquired with a Zeiss Axioplan2 microscope using the Metamorph software (Universal Imaging Corp., Downingtown, PA) and then deconvolved using AutoDeBlur software (AutoQuant Imaging Inc., Watervilet, NY).
RESULTS
Purification of CBF complex by immunoaffinity resins
To purify and characterize the cellular CBF complex, the recombinant CBF-B subunit was expressed as a fusion with two different epitope tags, FLAG and HA, at its N-terminal end. The epitope tag fusion CBF-B polypeptide (flag-ha-CBF-B) was expressed stably in HeLa cells under the control of a tetracycline-inducible promoter. We purified the flag-ha-CBF-B polypeptide from HeLa cell extracts using two sequential immunoaffinity resins (Figure 1A). In the first step, anti-FLAG affinity resin was used to purify flag-ha-CBF-B from HeLa cell extracts. The polypeptide bound to the resin was eluted in buffer containing the FLAG peptide. To determine whether the flag-ha-CBF-B polypeptide was associated with the cellular CBF-A and CBF-C polypeptides, we examined the eluted material in a DNA-binding experiment using an electrophoretic mobility shift assay (EMSA). The eluted sample from the anti-FLAG resin formed a specific complex with DNA containing a CCAAT motif (data not shown). This indicated that the flag-ha-CBF-B polypeptide co-eluted with CBF-A and CBF-B as together they formed a CBF–DNA complex (21). To further purify the flag-ha-CBF-B complex, we fractionated the eluted material using an anti-HA affinity resin. The bound complex was eluted in a buffer containing the HA peptide, and again examined by EMSA. This shows that the eluted sample from the anti-HA resin also forms a specific CBF–DNA complex (data not shown), indicating that the flag-ha-CBF-B was eluted from the anti-HA resin as a complex with the CBF-A and CBF-C subunits. As a control, when extracts from the HeLa cells not expressing the flag-ha-CBF-B polypeptide were fractionated by the anti-FLAG resin and subsequently by the anti-HA resin, the eluted material did not form any specific complex with DNA containing the CCAAT motif.
Figure 1.
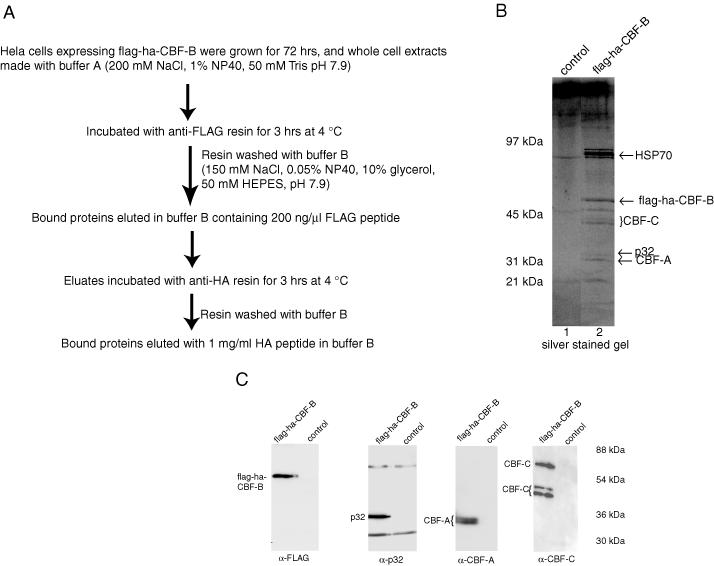
Purification and characterization of CBF-B complex. (A) Flow diagram for purification of the CBF-B complex from HeLa cells expressing flag-ha-CBF-B. (B) SDS–PAGE analysis of purified CBF-B complex eluted from anti-HA affinity resin. The lanes 1 and 2 were visualized by silver staining. The two different forms of CBF-A and three different forms of CBF-C subunits are probably not stained equally well with a silver stain. (C) Western blot of the purified CBF-B complex eluted from anti-HA resin with anti-FLAG, anti-p32, anti CBF-A and anti-CBF-C antibodies. Extracts from HeLa cells not expressing flag-ha-CBF-B were fractionated in the same way to serve as the control. In case of the blot with mouse monoclonal anti-p32 antibody, the IgG heavy and light chains, coming from the fractionation through anti-FLAG resin (containing mouse monoclonal FLAG antibody) are also detected.
The eluted sample from the anti-HA resin (for both the flag-ha-CBF-B and the control cells) was analyzed using SDS–PAGE followed by silver staining. The results showed that the eluted flag-ha-CBF-B sample contained several polypeptides, which were not present in the control sample (Figure 1B, lanes 1 and 2). Larger amounts of the eluted flag-ha-CBF-B sample were analyzed using SDS–PAGE followed by Colloidal Coomassie blue staining, each of the stained polypeptides was excised from the gel and then identified using mass spectrometry. This analysis showed that the major polypeptides in the flag-ha-CBF-B-eluted sample were CBF-B, CBF-A, CBF-C, HSP70, and p32, which was previously purified as a splicing factor ASF/SF2-associated protein (22). The mass spectrometric analysis, which led to the identification of p32, had nine tryptic polypeptides matching the sequence of p32 polypeptide spanning from amino acid 85 to 280 (Table 1).
Table 1. Sequences of nine tryptic peptides, identified by the mass spectrometric analysis that matched the amino acid sequence of p32 protein in the database.
| Peptides matching with p32 | Peptide sequence |
|---|---|
| 1. | 81AFVDFLSDEIKEER94 |
| 2. | 105MSGGWELELNGTEAK119 |
| 3. | 105MSGGWELELNGTEAKLVR122 Oxidation (M) |
| 4. | 124VAGEKITVTFNINNSIPPTFDGEEEPSQGQK154 |
| 5. | 181ALVLDCHYPEDEVGQEDEAESDIFSIR207 Carbamidomethyl (C) |
| 6. | 208EVSFQSTGESEWK220 |
| 7. | 221DTNYTLNTDSLDWALYDHLMDFLADR246 |
| 8. | 247GVDNTFADELVELSTALEHQEYITFLEDLK276 |
| 9. | 247GVDNTFADELVELSTALEHQEYITFLEDLKSFVK280 |
To confirm the mass spectrometry results, we analyzed the flag-ha-CBF-B sample eluted from the anti-HA resin by western blotting using anti-p32 antibodies. The p32 polypeptide was present in the eluted flag-ha-B sample but not in the control sample (Figure 1C). Similarly the different subunits of CBF, flag-ha-CBF-B, CBF-A and CBF-C, are pulled down only in the flag-ha-CBF-B sample.
Specific interaction of cellular p32 with CBF subunits
To determine which CBF subunit interacted with the p32 protein, each recombinant CBF subunit was expressed as a fusion with the FLAG epitope in the HeLa cells. The expression of each CBF polypeptide was examined by western blotting using anti-FLAG antibody (Figure 2A). Each flag-CBF subunit was immunoprecipitated with anti-FLAG resin and then analyzed by western blot using anti-p32 antibody (Figure 2B). As expected, a considerable quantity of p32 was coprecipitated with flag-CBF-B. In contrast, a very small but detectable amount of p32 was coprecipitated with flag-CBF-A, and no p32 was present in the immunoprecipitate with flag-CBF-C. The presence of cellular CBF-B in the immunoprecipitates of flag-CBF-A and flag-CBF-C was also analyzed using anti-CBF-B antibodies. This showed that cellular CBF-B was coprecipitated with flag-CBF-A but not with flag-CBF-C. However, cellular CBF-A was coprecipitated with flag-CBF-B as well as with flag-CBF-C. This analysis indicated that the association of the p32 polypeptide with the CBF complex depended on the presence of the CBF-B subunit in the complex.
Figure 2.
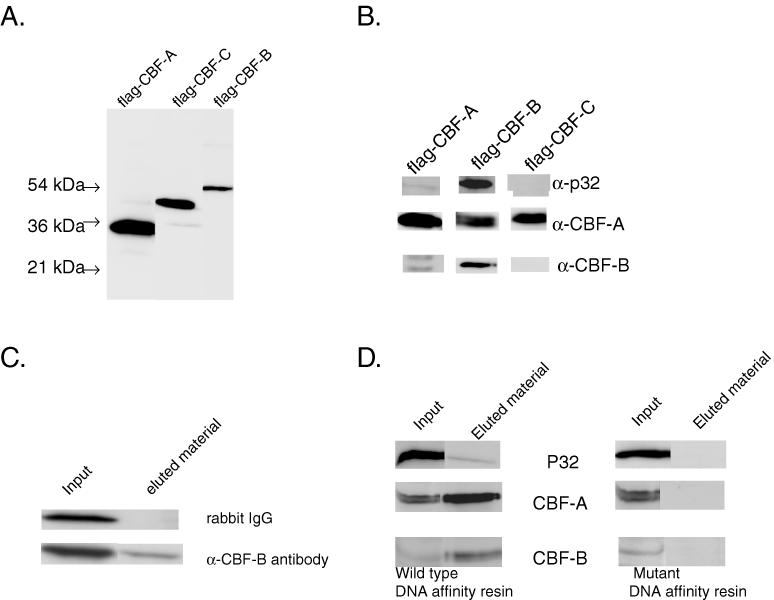
Interaction of cellular p32 with different CBF subunits. (A) Each CBF polypeptide expressed in the HeLa cells was detected by western blotting using anti-Flag antibody. Each CBF polypeptide, which is shown at the top of each lane, was expressed as a fusion with Flag epitope at N-terminal end. (B) The p32 polypeptide was detected in the immunoprecipitates containing different Flag-CBF polypeptides. Extract of HeLa cells expressing different flag-tagged CBF subunit was immunoprecipitated with anti-FLAG resin. The presence of p32 and the CBF subunits in the immunoprecipitate were detected by western blots using anti-p32, anti-CBF-B or anti CBF-A antibodies. (C) Detection of p32 polypeptide after immunoprecipitation of HeLa cell extracts with rabbit IgG as a control and with anti-CBF-B antibody. The presence of p32 polypeptide in the immunoprecipitate (eluted material) was determined by western blot with anti-p32 antibody. The crude Hela cell extracts were used to detect p32 shown as input. (D) Endogenous CBF from HeLa cell extracts was isolated with DNA affinity resin containing either wild-type or mutant CBF binding site. The resin-bound fractions (eluted material) were analyzed by western blot using anti-p32, anti-CBF-A or anti-CBF-B antibodies.
Interaction between endogenous CBF and p32 in HeLa cells
To determine whether endogenous CBF forms a complex with p32 present in the HeLa cells, the CBF complex was immunoprecipitated with anti-CBF-B antibody from HeLa cell extracts and then analyzed by western blot using anti-p32 antibody. The western blot analysis showed that p32 was immunoprecipitated with anti-CBF-B antibody but not with a control rabbit IgG (Figure 2C). This result indicated that p32 specifically associated with endogenous CBF-B present in HeLa cell extracts.
We also determined whether p32 associates with CBF-B when it is present in a CBF–DNA complex. The HeLa cell extracts were fractionated with a DNA affinity resin containing wild-type CBF binding site, and the resin bound proteins were analyzed by western blot using anti-CBF and anti-p32 antibodies. As a control, the HeLa cell extracts were fractionated with a DNA affinity resin containing mutant CBF site, and then the resin bound proteins were analyzed similarly by the western blot. As expected from our previous studies, CBF, present in HeLa cell extracts, could bind to the wild-type DNA affinity resin but not the mutant DNA affinity resin (Figure 2D). Interestingly, p32 was also bound to the wild-type resin but not to the mutant resin. This indicated that p32 was specifically associated with CBF, which is purified as a complex with DNA in the wild-type DNA affinity resin.
Interaction of recombinant p32 with CBF-B
The p32 protein is synthesized in cells as a pro-polypeptide of 282 amino acids, that is cleaved at the N-terminal end with the removal of the first 73 amino acids to form a mature p32 protein (23). To determine whether CBF-B interacts with the pro- or the mature p32 polypeptide, two forms of recombinant p32 proteins, one containing the full-length 282 amino acids and the other containing 75 to 282 amino acids, corresponding to the mature p32 form, were expressed in the HeLa cells. Each polypeptide was expressed as a fusion with Myc epitope tag, and was detected by western blotting using an anti-MYC antibody (Figure 3A). To examine the interaction between p32 and CBF-B, each form of p32 was first expressed in HeLa cells together with flag-CBF-B, and then immunoprecipitated with the anti-FLAG resin from the HeLa cell extracts. The precipitate was then analyzed by western blotting using the anti-MYC antibody. The immunoprecipitate was also analyzed with the anti-p32 antibody, which detects both the recombinant and the cellular p32 polypeptides. The results showed that the full-length p32 polypeptide, mycp32 (1–282), did not associate with flag-CBF-B (Figure 3B). In contrast, the truncated p32 polypeptide, mycp32, corresponding to the mature form of p32 protein, strongly associated with flag-CBF-B (Figure 3C). As a control, mycp32 was not precipitated with the anti-FLAG resin when flag-CBF-B was not coexpressed with mycp32. This result indicated that CBF-B specifically associated with the mature p32 protein.
Figure 3.
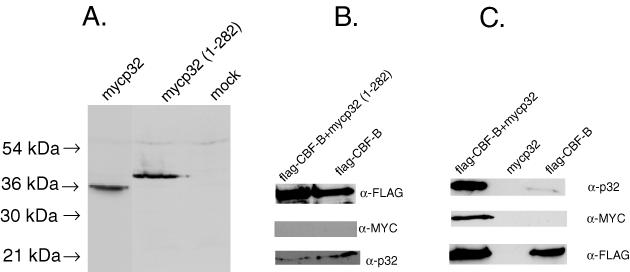
Interaction of recombinant p32 polypeptides with CBF-B in HeLa cells. (A) Two recombinant p32 polypeptides, one corresponding to the full- length p32, mycP32(1–282) and the other corresponding to the processed form of p32 (mycP32) were expressed in HeLa cells. Each polypeptide was expressed as a fusion with the myc epitope tag and was detected by western blot using anti-myc antibody. Cell extracts (mock) without expression of recombinant p32 were used as the control. (B and C) The flag-CBF-B polypeptide was expressed without or together with each recombinant p32 polypeptide in HeLa cells. The cell extracts were immunoprecipitated with an anti-FLAG resin, and then the immunoprecipitate was analyzed by western blot using specific antibodies.
Colocalization of CBF-B and p32 in HeLa cell nucleus
To determine the cellular compartment in which p32 and CBF-B interact with each other, the endogenous CBF-B and p32 polypeptides in HeLa cells were detected by immunostaining. The CBF-B was detected using anti-CBF-B antibody (rabbit polyclonal) and an anti-rabbit IgG coupled to Alexa Fluor 488, whereas the p32 polypeptide was detected using an anti-p32 antibody (mouse monoclonal) and an anti-mouse IgG coupled to Alexa Fluor 594. The nucleus was stained with DAPI in each case. Such staining showed that p32 (in red) was localized in both the cytosol and the nucleus (Figure 4). In contrast, CBF-B (in green) was extensively localized in the nucleus (Figure 4). The merge of red and green fluorescence data showed that a portion of CBF-B colocalizes with p32 in the nucleus indicated by the yellow spots.
Figure 4.
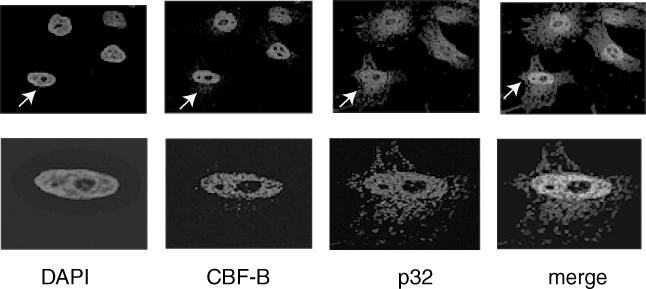
Colocalization of endogenous CBF and p32 proteins in HeLa cells. This was studied by immunostaining these proteins with their specific primary antibodies and fluorescent-labeled appropriate secondary antibodies. The nuclei were stained with DAPI. The red staining corresponds to p32 protein while the green corresponds to of CBF-B. The merge shows an overlay of red and green fluorescence data. The cell marked by arrow in the upper panel is shown in the lower panel for better details.
Role of p32 in CBF-mediated transcription
To determine the role of p32 in the DNA binding and transcription activity of CBF, we purified the p32 polypeptide expressed in bacteria as a fusion with a six-histidine tag (His-p32) (Figure 5A). The purified p32 was first tested for interaction with recombinant GST-CBF subunits by in vitro protein interaction assay using glutathione–agarose resin. Each GST-CBF polypeptide was first incubated with His-p32 and then precipitated with the glutathione–agarose resin. The precipitate was then analyzed by western blot using anti-p32 antibody. This showed that both full-length (GST-CBF-B) and a truncated CBF-B (GST-CBF-Bdbd, containing the DNA binding segment but lacking the transcription activation domain of CBF-B), but not GST or GST-CBF-A/CBF-C complex associated with His-p32 (Figure 5B). This indicated that the recombinant CBF-B polypeptides specifically and directly associated with the recombinant p32 protein. It also showed that the DNA binding segment of CBF-B is sufficient to form a CBF-B-p32 complex.
Figure 5.
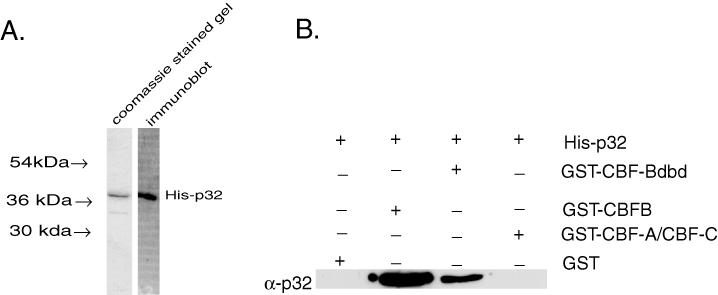
Interaction of the purified recombinant p32 polypeptide with the recombinant CBF subunits. (A) The left lane shows SDS–PAGE of purified fraction of recombinant p32 (His-p32), which was expressed in Escherichia coli. The gel was stained with Coomassie blueR250. The right lane shows western blot analysis of purified His-p32 using anti-p32 antibody. (B) Interaction of His-p32 with GST-CBF subunits. 75 ng of His-p32 and 150 ng of either GST-CBF-B or GST-CBF-AC or GST-CBF-Bdbd were used in a GST-pull down assay. The resin bound p32 polypeptide was detected by western blotting using anti-p32 antibody.
In a previous study, we found that recombinant CBF strongly activated transcription in vitro from an α2(1) collagen promoter containing four CBF binding sites (designated as FC1) (18). In this study we used the same in vitro transcription assay to determine the role of p32 in the CBF-mediated transcriptional activation of the α2(1) collagen promoter. When increasing amounts of His-p32 were added to the transcription reaction together with recombinant CBF polypeptides to HeLa cell nuclear extracts depleted of cellular CBF, it strongly inhibited CBF-dependent transcription of the α2(1) collagen promoter (Figure 6A). The addition of His-p32, however, did not change the transcription of the control promoter, which contains an α1(III) collagen promoter. Previous study showed that the α1(III) collagen promoter does not contain CBF binding site and is not activated by CBF in vitro (12). Similarly, the addition of His-p32 to the nuclear extracts with endogenous CBF protein also resulted in inhibition of transcription specifically from the α2(1) collagen promoter but not from the control α1(III) collagen promoter (Figure 6B). In this experiment, as another control, addition of same amount of GST protein did not change transcription activity of either the α2(1) or the α1(III) collagen promoter (data not shown). This result indicated that p32 specifically inhibited the CBF-mediated transcription activation in vitro.
Figure 6.
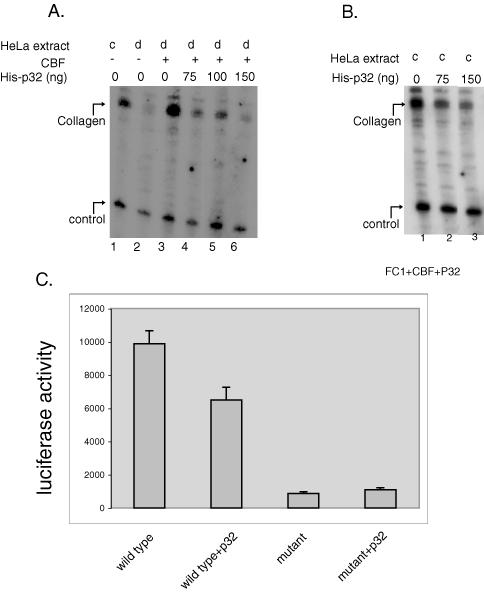
Role of p32 in CBF mediated transcription. (A) Increasing amounts of p32 protein were reconstituted in an in vitro transcription reaction together with the recombinant CBF protein (300 ng). In lanes 2 to 6, transcription reactions were performed with a CBF-depleted (d) HeLa nuclear extracts, whereas the reaction in lane 1 was carried out with undepleted or complete (c) HeLa nuclear extracts. (B) Increasing amounts p32 were reconstituted with the complete HeLa nuclear extracts in an in vitro transcription reaction. Two promoter templates, were used in the transcription reactions in vitro, one containing four CBF binding sites of mouse α2(1) collagen promoter (FC1) marked as collagen, the other, the control, a promoter of mouse type III collagen. (C) Transcription activity of a wild-type collagen promoter (FC1) and a mutant promoter containing mutations in the CBF binding sites (FC2) in HeLa cells untransfected/transfected with mycp32. The luciferase reporter activity was measured after 48 h of transfection.
The role of p32 in promoter activity in HeLa cells was also determined by transient transfection experiments. Two promoter–reporter (luciferase) constructs were used for this experiment, one containing the collagen promoter with four wild-type CBF binding sites as described earlier, and the other containing a mutant collagen promoter with single nucleotide mutations in each of the four CBF binding sites that inhibit CBF binding to the promoter (14). The results showed that expression of mycp32 caused a ∼30% decrease of the collagen promoter activity, but it did not change the activity of a collagen promoter with mutated CBF sites (Figure 6C). This result indicated that p32 also inhibited CBF mediated transcription in vivo in HeLa cells. The repression by mycp32 is less pronounced in these experiments probably due to the presence of endogenous p32 in HeLa cell nucleus, which already interacts with the cellular CBF complex present there.
DISCUSSION
In this study we identified p32 as a novel and specific corepressor of CBF-mediated transcription activation. The two successive steps of immunoaffinity fractionation method allowed us to purify recombinant CBF-B and its associated proteins from HeLa cell extracts with a high degree of purity. We found that CBF-B copurified with cellular CBF-A and CBF-C, indicating that CBF-B was purified as a heterotrimeric CBF–protein complex. Furthermore, with this purification method, CBF-B also copurified with several other cellular polypeptides. Here we identified and characterized the specific interaction of human protein p32 with CBF-B. The human p32 protein was previously purified from cell nuclear extracts as a pre-mRNA splicing protein complex, specifically associated with the ASF/SF2 splicing protein (22).
The cellular p32 protein is synthesized as a pro-polypeptide, which is cleaved to form a mature polypeptide after removal of N-terminal 73 amino acids. The pro-p32 protein is present in the cytosol with distinct localization in the mitochondria, and the mature p32 is found in cytosol as well as in nucleus (23). Our mass spectrometric analysis of p32 present in the purified CBF-B fraction identified nine tryptic peptides, which are located in the amino acid sequences of the mature p32 protein. This analysis however did not find any tryptic peptide that corresponded to pro-p32 protein. Furthermore, our analysis of the interaction with recombinant p32 polypeptide shows that CBF-B specifically interacts with the mature p32 protein. In this regard it is important to mention that analysis of HeLa cell extracts by western blot using the monoclonal anti-p32 antibody, which was used in our experiment, only detected the processed form of p32. Thus it is possible that the HeLa cells contain mostly the processed form of p32, or that the anti-p32 antibody only detects the processed p32 polypeptide. As mentioned earlier, the pro- or full-length p32 polypeptide was localized primarily in cytosol, possibly in mitochondria. Thus it is possible that the full-length p32 could not interact with CBF-B due to cellular localization, as CBF-B is primarily localized in nucleus.
The immunofluorescence-staining analysis demonstrates that CBF-B localizes primarily in the nucleus whereas p32 is present throughout cytosol and nucleus. This study showed that a portion of CBF-B is colocalized with p32 in the nucleus. The colocalization of cellular CBF-B with p32 is consistent with our observation that endogenous p32 was immunoprecipitated with cellular CBF-B by anti-CBF-B antibody from HeLa cell extracts. Our study of in vitro protein–protein interactions using purified recombinant polypeptides showed that CBF-B but not CBF-A/CBF-C hetrodimer interacts directly with p32. We also found that a 96 amino acid segment of CBF-B containing the C-terminal DNA-binding region is sufficient to form a CBF-B/P32 complex. When HeLa cell extracts were fractionated by a DNA affinity resin containing CBF binding site, p32 was bound to the resin and was coeluted with CBF subunits. In contrast, neither p32 nor CBF subunits could bind to a DNA affinity resin containing mutant CBF binding site. This study indicated that p32 associates with CBF-B in CBF–DNA complex. Since p32 did not associate with the mutant DNA affinity resin, it is clear that p32 does not bind to DNA by itself. Altogether these studies suggest that nuclear p32 can be recruited to promoter DNA through CBF–DNA complex in vivo.
The data from our in vitro transcription experiments showed that p32 specifically represses recombinant CBF-mediated transcription activation as well as activation by cellular CBF. In contrast, the transcription from the control promoter, not activated by CBF, remained unaffected by p32. These results indicated that p32 acts as a specific corepressor of CBF-mediated transcription in vitro. This conclusion is also supported by the data obtained from transient transfection experiments in which expression of mycp32 inhibited reporter gene expression specifically from a collagen promoter containing multiple CBF binding sites. The corresponding mutant promoter construct with mutated CBF sites was used as a control and expression of mycp32 did not alter its activity. The modest effect of mycp32 in this assay was probably due to already existing endogenous p32 in HeLa cells, which interacts with the available CBF-B in the nucleus.
A previous analysis of crystal structure of the mature human p32 protein revealed that p32 is a novel class of protein, and that it has three monomeric p32 polypeptides that together form a doughnut-shaped quaternary structure with an unusual surface charge distribution containing a high negative charge on one surface (24). Although CBF-B contains many basic amino acids, particularly in the DNA-binding domain, we believe that interaction of p32 with CBF-B is not an acid–base amino acid interaction. First, the interaction of p32 with CBF-B remains very stable at 300 mM salt. Second and more importantly, p32 associates with the CBF–DNA complex. Moreover, p32 did not inhibit or stimulate binding of CBF to DNA in a DNA binding reaction using electrophoretic mobility shift assay (data not shown). It is important to note that most of the basic amino acids of CBF-B are involved in DNA binding. This involvement strongly indicates that p32 interacts with regions of CBF-B other than its DNA-binding domain and its domains for interaction with CBF-A/CBF-C (25). Since p32 forms a trimeric structure, association of p32 with CBF–DNA complex in a minimum 1:1 stoichiometry recruits three p32 polypeptides. Thus it is possible that the association of p32 with the CBF–DNA complex could sterically hinder function of the two activation domains of CBF and inhibit the CBF-mediated transcription activation. However, we cannot rule out the possibility that recruitment of p32 in a promoter DNA could also result in an inhibition of interaction with basal transcription factors. Nevertheless, our results clearly demonstrated that p32 is a specific corepressor for the CBF-mediated transcription in vitro.
Although p32 was originally isolated as an ASF/SF2-associated protein, many studies have shown that p32 interacts with various cellular and viral proteins, such as lamin B receptor, HIV-1 Tat and Rev, EBNA-1 of the Epstein–Barr virus, ORF P of the herpes simplex virus, and core protein V of adenovirus (17,23,26–29). The p32 protein also interacts with hyaluronic acid (30). Studies of interaction between p32 and ASF/SF2 showed that p32 inhibits RNA binding of ASF/SF2 protein, and thus it acts as an inhibitor of mRNA splicing (31). However, the way p32 interacts with various other cellular and viral proteins remains unclear. Localization studies of p32 after viral infection showed that in many circumstances p32 migrates from the mitochondria to the nucleus together with the associated viral proteins. This indicated that P32 is a shuttling protein between the mitochondria and the nucleus (17).
The p32 protein is an evolutionarily conserved protein that is present in yeast to human. The normal cellular function of p32 in yeast Saccharomyces cerevisae was studied by deletion of p32 homologous gene. This deletion resulted in a slower growth of yeast specifically in a glycerol medium though not in glucose medium, suggesting that p32 might play a role in mitochondrial oxidative phosphorylation. Human p32 was found to complement this defect (32). Interestingly, the disruption of HAP2, HAP3 and HAP5 genes, which are homologues of the CBF subunits in yeast, also resulted in a specific inhibition of mitochondrial oxidative phosphorylation in yeast (33,34). The HAP complex in yeast controls the expression of several nuclear genes that play a role in mitochondrial oxidative phosphorylation. Although it is not known whether HAP2, a homologue of CBF-B, interacts with yeast p32 or not, these previous studies indicate that both p32 and CBF proteins are evolved in lower eukaryotes to control mitochondrial function. Hence we speculate that the interactions between CBF-B and p32 play a role in signaling between the mitochondria and the nucleus.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Henry Adams for helping with the immunofluorescence studies and Andrea Cervin for technical assistance in Mass Spectrometry. We thank Françoise Coustry for helping with the in vitro transcription reactions and Gayle Nesom for editing the manuscript. This work was supported by National Institutes of Health Grant AR46264 (to S.N.M), and the DNA sequencing facility at The University of Texas M.D. Anderson Cancer center was supported by the National cancer Institute Grant CA16672.
REFERENCES
- 1.Maity S.N. and de Crombrugghe,B. (1998) Role of the CCAAT-binding protein CBF/NF-Y in transcription. Trends Biochem Sci., 5, 174–178. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani R. (1999) The molecular biology of the CCAAT-binding factor NF-Y. Gene, 239, 15–27. [DOI] [PubMed] [Google Scholar]
- 3.Hu Q., Bhattacharya,C. and Maity,S,N. (2002) CCAAT binding factor (CBF) binding mediates cell cycle activation of topoisomerase IIalpha. Conventional CBF activation domains are not required. J. Biol. Chem., 277, 37191–37200. [DOI] [PubMed] [Google Scholar]
- 4.Katula K.S., Wright,K,L., Paul,H., Surman,D,R., Nuckolls,F,J., Smith,J,W., Ting,J,P., Yates,J. and Cogswell,J,P. (1997) Cyclin-dependent kinase activation and S-phase induction of the cyclin B1 gene are linked through the CCAAT elements. Cell Growth Differ., 8, 811–820. [PubMed] [Google Scholar]
- 5.Wasner M., Haugwitz,U., Reinhard,W., Tschop,K., Spiesbach,K., Lorenz,J., Mossner,J. and Engeland,K. (2003) Three CCAAT-boxes and a single cell cycle genes homology region (CHR) are the major regulating sites for transcription from the human cyclin B2 promoter. Gene, 312, 225–237. [DOI] [PubMed] [Google Scholar]
- 6.Zwicker J., Gross,C., Lucibello,F,C., Truss,M., Ehlert,F., Engeland,K. and Muller,R. (1995) Cell cycle regulation of cdc25C transcription is mediated by the periodic repression of the glutamine-rich activators NF-Y and Sp1. Nucleic Acids Res., 23, 3822–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caretti G., Salsi,V., Vecchi,C., Imbriano,C. and Mantovani,R. (2003) Dynamic recruitment of NF-Y and histone acetyltransferases on cell-cycle promoters. J. Biol. Chem., 278, 30435–30440. [DOI] [PubMed] [Google Scholar]
- 8.Roy B. and Lee,A,S. (1995) Transduction of calcium stress through interaction of the human transcription factor CBF with the proximal CCAAT regulatory element of the grp78/BiP promoter. Mol. Cell. Biol., 4, 2263–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kokame K., Kato,H. and Miyata,T. (2001) Identification of ERSE-II, a new cis-acting element responsible for the ATF6-dependent mammalian unfolded protein response. J. Biol. Chem., 276, 9199–9205. [DOI] [PubMed] [Google Scholar]
- 10.Tasanen K., Oikarinen,J., Kivirikko,K.I. and Pihlajaniemi,T. (1992) Promoter of the gene for the multifunctional protein disulfide isomerase polypeptide. Functional significance of the six CCAAT boxes and other promoter elements. J. Biol. Chem., 267, 11513–11519. [PubMed] [Google Scholar]
- 11.Yoshida H., Okada,T., Haze,K., Yanagi,H., Yura,T., Negishi,M. and Mori,K. (2000) ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol., 20, 6755–6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maity S.N., Golumbek,P,T., Karsenty,G. and de Crombrugghe,B. (1988) Selective activation of transcription by a novel CCAAT binding factor. Science, 241, 582–585. [DOI] [PubMed] [Google Scholar]
- 13.Ting J.P. and Trowsdale,J. (2002) Genetic control of MHC class II expression. Cell, 109(Suppl), S21–S33. [DOI] [PubMed] [Google Scholar]
- 14.Coustry F., Maity,S,N., Sinha,S. and de Crombrugghe,B. (1996) The transcriptional activity of the CCAAT-binding factor CBF is mediated by two distinct activation domains, one in the CBF-B subunit and the other in the CBF-C subunit. J. Biol. Chem., 271, 14485–14491. [DOI] [PubMed] [Google Scholar]
- 15.Hu Q. and Maity,S,N. (2000) Stable expression of a dominant negative mutant of CCAAT binding factor/NF-Y in mouse fibroblast cells resulting in retardation of cell growth and inhibition of transcription of various cellular genes. J. Biol. Chem., 275, 4435–4444. [DOI] [PubMed] [Google Scholar]
- 16.Van Leeuwen H.C. and O'Hare,P. (2001) Retargeting of the mitochondrial protein p32/gC1Qr to a cytoplasmic compartment and the cell surface. J. Cell. Sci., 114, 2115–2123. [DOI] [PubMed] [Google Scholar]
- 17.Matthews D.A. and Russell,W,C. (1998) Adenovirus core protein V interacts with p32—a protein which is associated with both the mitochondria and the nucleus. J. Gen. Virol., 79, 1677–1685. [DOI] [PubMed] [Google Scholar]
- 18.Coustry F., Maity,S,N. and de Crombrugghe,B. (1995) Studies on transcription activation by the multimeric CCAAT-binding factor CBF. J. Biol. Chem., 270, 468–475. [DOI] [PubMed] [Google Scholar]
- 19.Coustry F., Hu,Q., de Crombrugghe,B. and Maity,S,N. (2001) CBF/NF-Y functions both in nucleosomal disruption and transcription activation of the chromatin-assembled topoisomerase IIalpha promoter. Transcription activation by CBF/NF-Y in chromatin is dependent on the promoter structure. J. Biol. Chem., 276, 40621–40630. [DOI] [PubMed] [Google Scholar]
- 20.Majumdar M., Meenakshi,J., Goswami,S,K. and Datta,K. (2002) Hyaluronan binding protein 1 (HABP1)/C1QBP/p32 is an endogenous substrate for MAP kinase and is translocated to the nucleus upon mitogenic stimulation. Biochem. Biophys. Res. Commun., 291, 829–837. [DOI] [PubMed] [Google Scholar]
- 21.Maity S.N., Sinha,S., Ruteshouser,E,C. and de Crombrugghe,B. (1992) Three different polypeptides are necessary for DNA binding of the mammalian heteromeric CCAAT binding factor. J. Biol. Chem., 267, 16574–16580. [PubMed] [Google Scholar]
- 22.Krainer A.R., Mayeda,A., Kozak,D. and Binns,G. (1991) Functional expression of cloned human splicing factor SF2: homology to RNA-binding proteins, U1 70K, and Drosophila splicing regulators. Cell, 66, 383–394. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y., Finan,J.E., Middeldorp,J.M. and Hayward,S.D. (1997) P32/TAP, a cellular protein that interacts with EBNA-1 of Epstein–Barr virus. Virology, 236, 18–29. [DOI] [PubMed] [Google Scholar]
- 24.Jiang J., Zhang,Y., Krainer,A,R. and Xu,R,M. (1999) Crystal structure of human p32, a doughnut-shaped acidic mitochondrial matrix protein. Proc. Natl Acad. Sci. USA, 96, 3572–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maity S.N. and de Crombrugghe,B. (1992) Biochemical analysis of the B subunit of the heteromeric CCAAT-binding factor. A DNA-binding domain and a subunit interaction domain are specified by two separate segments. J. Biol. Chem., 267, 8286–8292. [PubMed] [Google Scholar]
- 26.Simos G. and Georgatos,S,D. (1994) The lamin B receptor-associated protein p34 shares sequence homology and antigenic determinants with the splicing factor 2-associated protein p32. FEBS Lett., 346, 225–228. [DOI] [PubMed] [Google Scholar]
- 27.Yu L., Loewenstein,P.M., Zhang,Z. and Green,M. (1995) In vitro interaction between human immunodeficiency virus type 1 Rev protein and splicing factor ASF/SF2-associated protein, p32. J. Virol., 69, 3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tange T.O., Jensen,T,H. and Kjems,J. (1996) In vitro interaction between human immunodeficiency virus type 1 Rev protein and splicing factor ASF/SF2-associated protein, p32. J. Biol. Chem., 271, 10066–10072. [DOI] [PubMed] [Google Scholar]
- 29.Bruni R. and Roizman,B. (1996) Open reading frame P–a herpes simplex virus gene repressed during productive infection encodes a protein that binds a splicing factor and reduces synthesis of viral proteins made from spliced mRNA. Proc. Natl Acad. Sci. USA, 93, 10423–10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S., Batchu,R,B. and Datta,K. (1991) Purification, partial characterization of rat kidney hyaluronic acid binding protein and its localization on the cell surface. Eur. J. Cell. Biol., 56, 58–67. [PubMed] [Google Scholar]
- 31.Petersen-Mahrt S.K., Estmer,C., Ohrmalm,C., Matthews,D,A., Russell,W,C. and Akusjarvi,G. (1999) The splicing factor-associated protein, p32, regulates RNA splicing by inhibiting ASF/SF2 RNA binding and phosphorylation. EMBO J., 18, 1014–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muta T., Kang,D., Kitajima,S., Fujiwara,T. and Hamasaki,N. (1997) p32 protein, a splicing factor 2-associated protein, is localized in mitochondrial matrix and is functionally important in maintaining oxidative phosphorylation. J. Biol. Chem., 272, 24363–24370. [DOI] [PubMed] [Google Scholar]
- 33.Pinkham J.L. and Guarente,L. (1985) Cloning and molecular analysis of the HAP2 locus: a global regulator of respiratory genes in Saccharomyces cerevisiae. Mol. Cell. Biol., 5, 3410–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNabb D.S., Xing,Y. and Guarente,L. (1995) Cloning of yeast HAP5: a novel subunit of a heterotrimeric complex required for CCAAT binding. Genes Dev., 9, 47–58. [DOI] [PubMed] [Google Scholar]


