Abstract
DNA double-strand breaks (DSBs) are repaired by either homologous recombination (HR) or non-homologous end joining (NHEJ) in mammalian cells. Repair with NHEJ or HR using single-strand annealing (SSA) often results in deletions and is generally referred to as non-conservative recombination. Error-free, conservative HR involves strand invasion and requires a homologous DNA template, and therefore it is generally believed that this type of repair occurs preferentially in the late S, G2 and M phases of the cell cycle, when the sister chromatid is available. There are several observations supporting this hypothesis, although it has not been tested directly. Here, we synchronize human SW480SN.3 cells in the G1/G0 (with serum starvation), S (with thymidine block) and M (with nocodazole) phases of the cell cycle and investigate the efficiency of conservative HR repair of an I-SceI-induced DSB. The frequency of HR repair of DSBs was 39 times higher in S-phase cells than in M-phase cells and 24-fold higher than in G1/G0 cells. This low level of conservative HR occurs even though a homologous template is present within the recombination substrate. We propose that this can be explained by an absence of recombination proteins outside the S phase or alternatively that there maybe factors that suppress HR in G1/G0 and M. Furthermore, we found that HR repair of DSBs involves short tract gene conversion in all the phases of the cell cycle. This indicates that the same pathway for conservative HR is employed in the repair of DSBs regardless of phase of the cell cycle and that only the frequency is affected.
INTRODUCTION
DNA double-strand breaks (DSBs) are highly cytotoxic lesions and may arise following exposure to ionizing radiation (IR), chemical exposure or following endogenous processes. There are two pathways for DSB repair in mammalian cells, non-homologous end joining (NHEJ) and homologous recombination (HR) (1). It has been suggested that these two pathways have overlapping roles (2). Cells with deficient NHEJ pathway have an increased level of HR and a functional NHEJ pathway suppresses HR induced by a DSB (3). Thus, it appears that HR can partly compensate for an NHEJ defect, although this effect is probably very slight since NHEJ-deficient cells are still highly sensitive to IR. The lack of complete compensation may be due to differences in the DNA lesions recognized by the two repair pathways (4). However, the stage of the cell cycle in which the damage occurs might also be of importance, as conservative HR requires a homologous DNA template. It has been suggested that the sister chromatid, present in the late S, G2 and M phases of the cell cycle, would favour conservative HR during these phases of the cell cycle (5). This is supported by the observation that the sister chromatid is used 100 times more often for HR repair of a DSB than the homologous chromosome (6,7). Also, HR-deficient cells are only slightly radiosensitive in the G1/G0 phase, but highly radiosensitive in the late S/G2 phases of the cell cycle, which indicates that conservative HR has a role in DSB repair in S/G2 (8). However, proteins involved in conservative HR, such as Rad51 are also found in foci at sites of DSBs in cells regardless of the phase of the cell cycle (9). Thus, there is still some ambiguity about the role of conservative HR in repair of DSBs in relation to the cell cycle.
MATERIALS AND METHODS
Cell lines
The SW480SN.3 cell line contains a stably integrated copy of the recombination reporter vector SCneo (10) and is cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal calf serum, penicillin (60 μg/ml), streptomycin (100 μg/ml) at 37°C under an atmosphere containing 5% CO2. The medium was supplemented with hygromycin (0.05 mM) in order to maintain the SCneo vector.
Recombination assay
In the SCneo recombination assay, a confluent Petri dish (100 mm diameter) of SW480SN.3 cells were expanded separately from 103 cells in order to avoid contamination of spontaneous G418R clones. From this plate, 1.5 × 106 SW480SN.3 cells were grown overnight before a synchronization with thymidine (2 mM), nocodazole (40 ng/ml) or serum starvation (1%) as indicated in Figure 2A. The arrested cells were transiently transfected for 5 h with the pEGFP-C2 (Clontech) or pCMV3xnlsI-SceI vectors using lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Following treatments, the cells were kept arrested for an additional 7 h with thymidine or nocodazole or overnight with serum starvation. Then, plates were rinsed in 2× phosphate-buffered saline (PBS), trypsinized and reseeded for cloning (2 plates with 500 cells/plate), and selected in G418 (1 mg/ml; 25 cells/mm2) or by FACS analysis for GFP expression.
Figure 2.
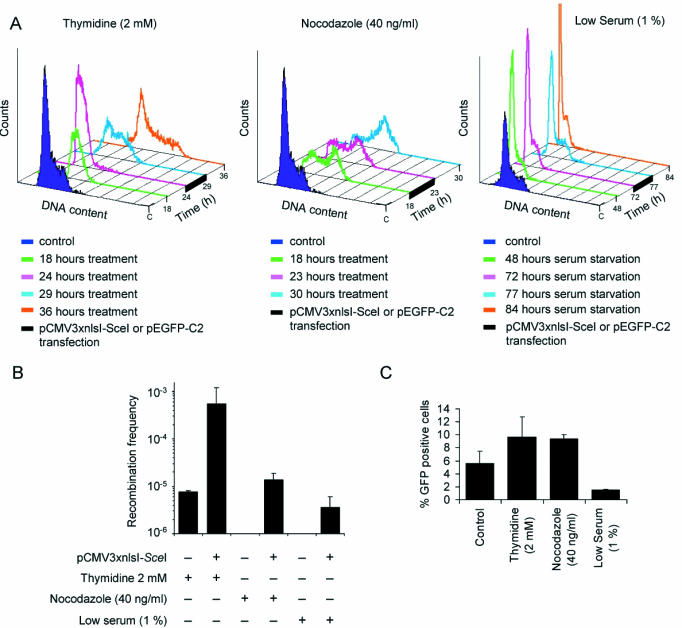
Conservative HR repairs DSBs primarily in cells in the S phase of the cell cycle. (A) Cell cycle profiles of SW480SN.3 cells arrested in the S, M or G1/G0 phase of the cell cycle with thymdine (2 mM), nocodazole (40 ng/ml) or serum starvation (1%), respectively. Cells arrested in the cell cycle were transfected with either pCMV3xnlsI-SceI or pEGFP-C2 vector for 5 h and at the same time kept in the given phase of the cell cycle. Cells were maintained in the arrested stage for an additional 7 h before continuation of recombination assay or GFP expression assay. (B) HR induced with or without a DSB at different phases of the cell cycle. (C) GFP expression from the CMV promoter at different phases of the cell cycle.
FACS analysis
Arrested cells were harvested, washed twice in PBS and fixed in 70% cold ethanol followed by treatment with 1 mg/ml RnaseA and staining with 100 μg/ml propidium iodide (PI). The proportion of the arrested cells in each phase of cell cycle was then determined by FACS Sort Vantage (Becton Dickinson).
Southern blotting
Genomic DNA was isolated from SW480SN.3 cells, phenol–chloroform treated and digested with SacI and/or NcoI. About 20 G418R clones were isolated DSB-induced recombinants from each phase of the cell cycle and genomic DNA extracted. From each clone, 10 μg of phenol–chloroform purified DNA was digested with SacI and HindIII restriction endonucleases and separated by gel electrophoresis. Southern blotting was carried out as previously described using the S2neo fragment as probe (11).
RESULTS
Conservative homologous recombination primarily repairs DSBs occurring in the S phase of the cell cycle
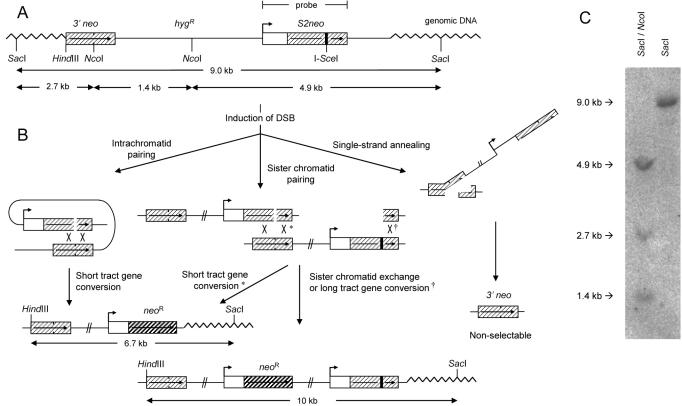
It is clear that both NHEJ and HR are involved in the repair of DSBs (2,12–14), and it has been suggested that conservative HR is involved in the repair of DSBs occurring in the late S and G2 phases of the cell cycle (5). We tested this hypothesis directly by utilizing a human cell line SW480SN.3 which carries a single integrated copy of the recombination reporter SCneo (Figure 1A) (10). The SCneo construct has two non-functional neoR genes, 3′neo and S2neo. A DSB can be specifically introduced in the S2neo repeat using a transient expression of the I-SceI restriction endonuclease. HR repair restores a functional neoR gene that can be selected with G418 (Figure 1B).
Figure 1.
The SCneo substrate for conservative HR in SW480SN.3 cells. (A) Schematic illustration of the SCneo recombination substrate that contains two non-functional copies of the neoR gene in the SW480SN.3 cell line. (B) Following an I-SceI-induced DSB, a functional neoR gene can be gained by HR through intrachromatid pairing or sister chromatid pairing. Intrachromatid pairing is possible throughout the cell cycle and sister chromatid pairing is only possible during late S or G2/M phases of the cell cycle. A short tract gene conversion HR event gives a 6.7 kb product, while SCE or long tract gene conversion restores a neoR gene within a 10 kb product (14). Non-conservative SSA does not produce a functional neoR gene. (C) Southern blot of DNA isolated from the SW480SN.3 cell line containing one copy of the intact SCneo substrate, using S2neo as probe.
HR-mediated DSB repair may occur through either conservative strand invasion of a homologous DNA molecule or non-conservative single-strand annealing (SSA) between two repeated sequences within the recombination reporter substrate (Figure 1B). SSA may be independent of the cell cycle since it does not requires the presence of a sister chromatid. The advantage using the SCneo construct for these experiments is that SSA does not produce a functional neoR gene (Figure 1B). Therefore, the SCneo reporter reveals only DSB repair events using conservative HR, i.e. strand invasion.
We first verified the correct integration of the SCneo vector in the SW480SN.3 cell line using Southern blotting. Genomic DNA isolated from SW480SN.3 was digested with NcoI and/or SacI (Figure 1C). The NcoI and SacI digestion produced 3 bands with a size of 4.9, 2.7 and 1.4 kb, which reveals that the internal structure of the SCneo construct is preserved also when integrated into the SW480SN.3 cell line. Digestion with SacI alone produced only 1 band that was 9 kb (Figure 1C). The presence of a single band following cleavage with SacI shows that SCneo is integrated as a single copy, since SacI does not cleave the SCneo vector. It has been previously reported that transient transfection with the pCMV3xnlsI-SceI induces HR about 100-fold in SW480SN.3 cells showing that this recombination reporter works (10).
We arrested SW480SN.3 cells in the S, M and G1/G0 phases of the cell cycle, using thymidine (2 mM), nocodazole (40 ng/ml) or serum starvation (1%), respectively (Figure 2A). Thymidine is converted to dTTP that inhibits reduction of UDP and CDP by ribonucleotide reductase resulting in lower [dCTP] levels, which slow replication and arrest cells in the S phase of the cell cycle (15). Nocodazole binds to various sites on β-tubulin and suppresses microtubule dynamics or induces microtubule depolymerization, which results in accumulation of cells in the early M phase of the cell cycle (16). Serum starvation depletes the medium of growth factors, which arrests cells in G1/G0.
The arrested cells were transiently transfected with the pCMV3xnlsI-SceI vector for 5 h (Figure 2A). Following the transfection, we kept the cells arrested for 7 h more to ensure expression of the I-SceI restriction endonuclease and DSB formation while cells were still in the same phase of the cell cycle. The frequency of neoR recombinants was determined at each phase of the cell cycle by plating cells in G418 selection media. The S-phase arrested cells produced neoR recombinants at a frequency of 5.4 × 10−4, while M-phase or G1/G0-phase arrested cells produced 39 or 148 times fewer neoR recombinants, respectively (Figure 2B). The reduced HR levels in M or G1/G0 cells are not a reflection of increased toxicity following noncodazole or serum starvation, since reduced cloning efficiency were compensated for when calculating HR frequencies. Thus, there is clearly a stimulatory effect to produce neoR recombinants when cells are present in the S phase of the cell cycle. Since neoR recombinants can be produced only by HR involving strand invasion, our result indicates that conservative HR preferentially repairs DSBs present in the S phase of the cell cycle.
However, the lower frequency of recombinants in the M and G1/G0 phases of the cell cycle could be the result of a lower level of induced DSBs in these cells following transfection with the pCMV3xnlsI-SceI vector. To test this possibility, we transfected cells in the different phases of the cell cycle with the pEGFP-C2 vector, expressing the green fluorescent protein (GFP) from the same CMV promoter as the I-SceI restriction endonuclease. We found that ∼10% of S- or M-phase arrested cells express GFP, while only 1.6% of G1/G0-arrested cells expressed GFP (Figure 2C). About 6% of unarrested cells expressed GFP. Thus, it is likely that 6 times fewer DSBs were induced by I-SceI in G1/G0-arrested cells. When taking this into account, 148 times reduction of conservative HR in G1/G0 cells might be an overestimate and the actual reduction of HR in G1/G0 as compared to S-phase arrested cells might be 24-fold, which is still a significant reduction in HR frequency (Table 1).
Table 1. HR frequencies and transfection efficiency in SW480SN.3 cells following arrest of cells in different phases of the cell cycle.
| HR frequency in SCneo − pCMV3xnlsI-SceI | HR frequency in SCneo + pCMV3xnlsI-SceI | DSB-induced HR (fold induction) | Transfection efficiency % GFP expressing cells | Relative level of DSB-induced HRa | Relative level of DSB-induced HR (GFP compensated)a | |
|---|---|---|---|---|---|---|
| Thymidine | 7.4 ± 0.5 × 10−6 | 5.4 ± 3.6 × 10−4 | 73 | 9.6 ± 3.1 | 1/1 | 1/1 |
| Nocodazole | <2.3 × 10−6 | 1.4 ± 0.5 × 10−5 | >6 | 9.4 ± 0.6 | 1/39 | 1/39 |
| Serum starvation | <0.2 × 10−6 | 3.6 ± 2.5 × 10−6 | >18 | 1.6 ± 0.1 | 1/148 | 1/24 |
aRelative thymidine-treated cells.
The background HR frequencies varied following the different treatments (Figure 2B). The 36 h thymidine treatment produced neoR recombinants at a frequency of 7.9 × 10−6 (Table 1), which is 3.4 times higher than in untreated control cells (10). We found no neoR recombinants in the control plates in either the M or G1/G0 arrested cells. This suggests that spontaneous HR using strand invasion is uncommon in these phases of the cell cycle. However, we found that an I-SceI-induced DSB triggered conservative HR to increase in both the M and G1/G0 arrested cells at least 6- to 18-fold (Table 1). A DSB induced HR to increase 73-fold in S-phase arrested cells (Table 1). Thus, we have no reason to believe that the DSB-induced HR differs between S-phase and M or G1/G0 arrested cells. In conclusion, our data suggest that only the overall HR frequency is reduced in M or G1/G0 arrested cells.
Gene conversion repairs DSBs throughout the cell cycle
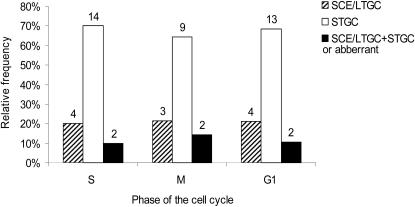
There are several different conservative HR pathways used for DSB repair in mammalian cells that may result in SCE or gene conversion (17). It is possible that the same HR pathway is operating inside and outside the S phase, although at different frequencies. Alternatively, a different HR pathway may be responsible for the HR outside the S phase. To test this, we isolated about 20 DSB-induced G418R clones from each stage of the cell cycle. We determined if the neoR gene was produced following HR involving either short tract gene conversion (STGC), sister chromatid exchange (SCE) or long tract gene conversion (LTGC) by isolating genomic DNA from each clone and analysing these with Southern blotting. We found that the spectrum of recombinants produced following HR repair of S-phase-induced DSBs includes a majority of STGC events. This is in agreement with what is found in non-arrested cells (14). The spectra of recombinants produced following HR repair involved in DSB-repair in M or G1/G0 arrested cells also include a majority of STGC events (Figure 3).
Figure 3.
Similar spectra of recombinants in HR repair of DSBs at different phases of the cell cycle. Spectrum of DSB-induced recombinant clones that arose in the S, M or G1/G0 phase of the cell cycle. Numbers indicate the number of clones within each group.
DISCUSSION
Here, we report 39- and 24-fold reduced levels of DSB-induced HR using strand invasion in cells arrested in the M or G1/G0 phases of the cell cycle, respectively (Table 1). These values might not describe the absolute difference of conservative HR within the cell cycle. First, one confounding factor is that the cells are not completely synchronized to one phase (Figure 2A). A small fraction of S-phase cells still present in the nocodazole-treated or serum starved cells might be responsible for the HR that is scored. If this is so, the reduction of HR in the M and G1/G0 phases of the cell cycle may be an underestimate of the real values. Thus, the conservative HR repair of a DSB in the M and G1/G0 phases of the cell cycle is lower than in S-phase cells.
Here, we used the I-SceI restriction endonuclease to induce a DSB in the recombination reporter. We found that the transfection and/or expression differ between the phases of the cell cycle by expressing the GFP protein from the same promoter (Figure 2C). We compensated the HR frequencies with the difference in transfection and expression of the GFP protein (Table 1). However, it cannot be ruled out that the I-SceI enzyme has different activity when expressed in the different phases of the cell cycle. Also, two copies of the restriction site are present in cells following replication. Such differences may affect the results obtained, but it is unlikely that they would account for the 24- to 39-fold difference found in conservative HR.
Our observation that conservative HR is most frequently used in the S phase of the cell cycle is in line with that cells deficient in proteins involved in conservative HR show enhanced sensitivity to ionizing radiation only when present in the S phase of the cell cycle (8). Also, the HR protein RAD51 is more abundant and form foci preferentially in S phase cells (12,18,19). This is likely to reflect the important role for conservative HR in repair of replication errors (20,21).
The SCneo construct contains both the 3′neo and S2neo copies of the neo gene. Thus, a functional neoR copy could be produced using intrachromatid HR by strand invasion (Figure 1B). This HR pathway may be used in all phases of the cell cycle, since it does not depend on the presence of a second copy of SCneo on a sister chromatid. Bearing this in mind, the suppressed level of conservative HR in the M and G1/G0 phases of the cell cycle is surprising, since a template for HR is present. Our results suggest that the cell does not use the intrachromatid HR mechanism to restore the neoR gene. This suggests that there are factors that suppress HR outside the S phase rather than that the absence of a homologous template suppresses conservative HR. Alternatively, factors required for conservative HR might not be present outside the S phase of the cell cycle. It is well established that the level of HR proteins, e.g. RAD51 and RAD52, is highest in the S phase of the cell cycle (18,22).
We found no spontaneous recombinants in the absence of an I-SceI-induced DSB in M or G1/G0 arrested cells. However, S-phase arrested cells with thymidine show high background HR frequency (7.4 × 10−6), which is 3.4 times higher than in untreated cells (10). This is in agreement with that thymidine alone induces HR in the SCneo substrate (21,23). The reason for the low background levels of HR in M or G1/G0 arrested cells could be that conservative HR is not frequently used in these phases of the cell cycle. However, a DSB trigger conservative HR in both M and G1/G0 arrested cells indicates that HR using strand invasion is indeed triggered by a DSB in these cells. Since we found no spontaneous neoR clones the relative induction of HR following a DSB is unknown. However, the induction is more than 6- or 18-fold in the M or G1/G0 arrested cells, respectively (Table 1). The neoR recombinants produced by I-SceI in nocodazole-treated or serum-starved cells could be explained by that S-phase cells evading the cell cycle arrest (Figure 2A). Alternatively, cells in the M or G1/G0 phases may use intrachromatid HR to repair the DSB. In any case, it is clear that a DSB triggers HR by strand invasion also in nocodazole-treated or serum-starved cells. This argues for that only the level of conservative HR is affected by the phase of the cell cycle.
We found that conservative HR involves a majority of STGC events regardless of the phase of the cell cycle (Figure 3). This result adds to the argument that the same HR pathway operates in repair of DSBs outside the S phase but less efficiently. This would be the case if the same proteins execute DSB repair with HR, using the 3′neo repeat present on the same chromatid as template and the reason for a lower frequency would be because the level of HR proteins differs between the phases of the cell cycle, as the RAD51 and RAD52 proteins do (18,22). Since the level of RAD51 alone regulates HR (24,25) it is possible that not all HR proteins need to differ during the cell cycle.
In this study, we do not attempt to discuss the relative contribution of NHEJ in combination with the cell cycle. Also, we do not know the contribution of non-conservative HR using SSA. It has previously been reported that half or more than half of DSBs are repaired by NHEJ (2,14). Also, non-conservative HR using SSA has been reported to be important for repair of a DSB between repeated sequences (13). Thus, it is likely that most DSBs induced in this study are still repaired with NHEJ or with SSA and the relative contribution of these pathways in relation with the cell cycle is not dealt with in this study.
In conclusion, we report that conservative HR repair is predominant in the S phase of the cell cycle. The abundance of HR proteins is likely to determine if HR using strand invasion will take place, rather than the presence of a homologous template for repair.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Mark Meuth, Atul Mohindra and members of the Helleday laboratory for help and valuable discussions. The Biological & Biotechnological Sciences Research Council, the Swedish Cancer Society, the Swedish Research Council and Yorkshire Cancer Research supported this work financially. N.S.G. has a scholarship from the Kerman University of Medical Science, Iran.
REFERENCES
- 1.van Gent D.C., Hoeijmakers,J.H. and Kanaar,R. (2001) Chromosomal stability and the DNA double-stranded break connection. Nature Rev. Genet., 2, 196–206. [DOI] [PubMed] [Google Scholar]
- 2.Sargent R.G., Brenneman,M.A. and Wilson,J.H. (1997) Repair of site-specific double-strand breaks in a mammalian chromosome by homologous and illegitimate recombination. Mol. Cell. Biol., 17, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen C., Kurimasa,A., Brenneman,M.A., Chen,D.J. and Nickoloff,J.A. (2002) DNA-dependent protein kinase suppresses double-strand break-induced and spontaneous homologous recombination. Proc. Natl Acad. Sci. USA, 99, 3758–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ristic D., Modesti,M., Kanaar,R. and Wyman,C. (2003) Rad52 and Ku bind to different DNA structures produced early in double-strand break repair. Nucleic Acids Res., 31, 5229–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendrickson E.A. (1997) Cell-cycle regulation of mammalian DNA double-strand-break repair. Am. J. Hum. Genet., 61, 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson R.D. and Jasin,M. (2001) Double-strand-break-induced homologous recombination in mammalian cells. Biochem. Soc. Trans., 29, 196–201. [DOI] [PubMed] [Google Scholar]
- 7.Richardson C., Moynahan,M.E. and Jasin,M. (1998) Double-strand break repair by interchromosomal recombination: suppression of chromosomal translocations. Genes Dev., 12, 3831–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothkamm K., Kruger,I., Thompson,L.H. and Lobrich,M. (2003) Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol. Cell. Biol., 23, 5706–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aten J.A., Stap,J., Krawczyk,P.M., van Oven,C.H., Hoebe,R.A., Essers,J. and Kanaar,R. (2004) Dynamics of DNA double-strand breaks revealed by clustering of damaged chromosome domains. Science, 303, 92–95. [DOI] [PubMed] [Google Scholar]
- 10.Mohindra A., Hays,L.E., Phillips,E.N., Preston,B.D., Helleday,T. and Meuth,M. (2002) Defects in homologous recombination repair in mismatch-repair-deficient tumour cell lines. Hum. Mol. Genet., 11, 2189–2200. [DOI] [PubMed] [Google Scholar]
- 11.Schultz N., Lopez,E., Saleh-Gohari,N. and Helleday,T. (2003) Poly(ADP-ribose) polymerase (PARP-1) has a controlling role in homologous recombination. Nucleic Acids Res., 31, 4959–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tashiro S., Walter,J., Shinohara,A., Kamada,N. and Cremer,T. (2000) Rad51 accumulation at sites of DNA damage and in postreplicative chromatin. J. Cell Biol., 150, 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang F., Han,M., Romanienko,P.J. and Jasin,M. (1998) Homology-directed repair is a major double-strand break repair pathway in mammalian cells. Proc. Natl Acad. Sci. USA, 95, 5172–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson R.D. and Jasin,M. (2000) Sister chromatid gene conversion is a prominent double-strand break repair pathway in mammalian cells. EMBO J., 19, 3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjursell G. and Reichard,P. (1973) Effects of thymidine on deoxyribonucleoside triphosphate pools and deoxyribonucleic acid synthesis in Chinese hamster ovary cells. J. Biol. Chem., 248, 3904–3909. [PubMed] [Google Scholar]
- 16.Zieve G.W., Turnbull,D., Mullins,J.M. and McIntosh,J.R. (1980) Production of large numbers of mitotic mammalian cells by use of the reversible microtubule inhibitor nocodazole. Nocodazole accumulated mitotic cells. Exp. Cell Res., 126, 397–405. [DOI] [PubMed] [Google Scholar]
- 17.Helleday T. (2003) Pathways for mitotic homologous recombination in mammalian cells. Mutat. Res., 532, 103–115. [DOI] [PubMed] [Google Scholar]
- 18.Flygare J., Benson,F. and Hellgren,D. (1996) Expression of the human RAD51 gene during the cell cycle in primary human peripheral blood lymphocytes. Biochim. Biophys. Acta, 1312, 231–236. [DOI] [PubMed] [Google Scholar]
- 19.Raderschall E., Golub,E.I. and Haaf,T. (1999) Nuclear foci of mammalian recombination proteins are located at single-stranded DNA regions formed after DNA damage. Proc. Natl Acad. Sci. USA, 96, 1921–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonoda E., Sasaki,M.S., Buerstedde,J.M., Bezzubova,O., Shinohara,A., Ogawa,H., Takata,M., Yamaguchi-Iwai,Y. and Takeda,S. (1998) Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J., 17, 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundin C., Erixon,K., Arnaudeau,C., Schultz,N., Jenssen,D., Meuth,M. and Helleday,T. (2002) Different roles for nonhomologous end joining and homologous recombination following replication arrest in mammalian cells. Mol. Cell. Biol., 22, 5869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen F., Nastasi,A., Shen,Z., Brenneman,M., Crissman,H. and Chen,D.J. (1997) Cell cycle-dependent protein expression of mammalian homologs of yeast DNA double-strand break repair genes Rad51 and Rad52. Mutat. Res., 384, 205–11. [DOI] [PubMed] [Google Scholar]
- 23.Mohindra A., Bolderson,E., Stone,J., Wells,M., Helleday,T. and Meuth,M. (2004) A tumour-derived mutant allele of XRCC2 preferentially suppresses homologous recombination at DNA replication forks. Hum. Mol. Genet., 13, 203–12. [DOI] [PubMed] [Google Scholar]
- 24.Vispe S., Cazaux,C., Lesca,C. and Defais,M. (1998) Overexpression of Rad51 protein stimulates homologous recombination and increases resistance of mammalian cells to ionizing radiation. Nucleic Acids Res., 26, 2859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnaudeau C., Helleday,T. and Jenssen,D. (1999) The RAD51 protein supports homologous recombination by an exchange mechanism in mammalian cells. J. Mol. Biol., 289, 1231–8. [DOI] [PubMed] [Google Scholar]