Abstract
Background:
The present study was the first attempt for determination and measurement of pesticide residues in tomatoes used in salad production plants in Isfahan, central Iran.
Methods:
A multiresidue method based on modified QuEChERS (quick, easy, cheap, effective, rugged and safe) sample preparation, followed by Gas Chromatography Mass Spectrometry (GC–MS) was developed and validated for the determination of 15 pesticides (permethrin, primicarb, dichlorvos, diazinone, fenpropathrin, carbaryl, chlorpyrifos, malathion, chlortalonil, brompropilate, propargit, tetradifone, phosalone, iprodion and endosulfane) from different classes. The recovery yields ranged from 83.84 to 119.73%and the relative standard deviation (RSD) was below 20.54%. The limits of detection (LODs) were between 1.63to 10.5 mg/kg and the limits of quantifications (LOQs) were between 5.43to35 mg/kg. The method has been successfully applied to the analysis of 22 tomato samples obtained from salad production plants in Isfahan in Sep to Dec 2014.
Results:
An amount of 31.81% of samples showed contamination above maximum residue levels (MRLs) with pesticides. In addition, 13.6% of samples had contamination with diazinone and 18.18% of samples with chlorpyrifos.
Conclusion:
A full consideration is essential for tomatoes used in salad and food productions. It suggests a need for revision of the current trend of supervision for tomatoes and other vegetables presented in wholesale markets. In all, the study confirms that pesticide residue determination in food products, especially in raw fruits and vegetables, is a very demanding task in public health safety and trade.
Keywords: Pesticide residue, Tomato, QuEChERS, Gas chromatography, Mass spectrometry
Introduction
Tomatoes (Lycopersicum esculentum sin. Solanum lycopersicum, Lycopersicum lycopersicum) belong to the Solanaceae family and correspond to one of the most widely grown vegetables in the world (1). This fruit vegetable is typically produced in the spring–summer season, however, in many countries it is produced during all year in greenhouses (2). Thus, consumers can eat fresh tomato even during winter. Usually it is consumed in salad dishes without any cooking treatment. According to the Food and Agriculture Organization of the United Nations, Iran produced 6,000,000 tons tomato in 2012 (3).
Tomato is under the threat of various insect pests and diseases in the field, and pesticides are needed in different phases of cultivation to control pests and diseases that may cause yield reduction (4). To combat insect pests and diseases of this crop and to achieve higher production, many pesticides are used that may leave certain amounts of residues on the crops. Pesticides are extensively used to control various insect pests in cereals, vegetables and fruits (5). These residues, if present in excess, may act as a health hazard to the consumers and may cause chronic diseases.
Pesticides, which include insecticides, herbicides, fungicides, and others have been widely used in the cultivation and post-harvest storage of certain crops to control weeds, insect infestation and plant diseases and thus can improve yield as well as quality of the product (6). Despite their many merits and excessive use, pesticides are some of the most toxic substances contaminating the environment. Their excessive use can have negative environmental impacts on water quality, and terrestrial and aquatic biodiversity. Pesticide residues in foodstuffs can pose a risk to human health, varying from allergies to chronic diseases and cancer, depending on the intrinsic characteristics of their active substances and use patterns (7). Additionally, WHO has reported that roughly three million pesticide poisonings occur annually and result in 220,000 deaths worldwide (8).
Many methods have been developed in the last few years for the determination of pesticides. The most widely used methods are gas chromatography (GC) (9). GC is a separation technique widely used in the analysis of pesticide residues because of its high separation power and the variety of sensitive and selective detectors, such as electron capture detector (ECD), nitrogen-phosphorus detector (NPD), flame photometric detector (FPD) and mass spectrometry (MS). Gas chromatography coupled to mass detection is widely used in the analysis of pesticides that are highly volatile (10). Detection of pesticide residues by gas chromatography-mass spectrometry (GC–MS) provides good identification and quantification for many pesticides in food matrices.
QuEChERS analysis is based on acetonitrile extraction of different classes of pesticide residues that are widely used to control tomato pests (10–12). Advantages of this methodology are its flexibility, high degree of selectivity and sensitivity (13).
The aim of this study was evaluating the utility of QuEChERS method in combination with GC–MS for the identification and quantification of 15 multiclass pesticides in tomato. The validated method was also applied to the analysis of 22 tomato samples collected from salad production plants in Isfahan during the months of Sep to Dec 2014.
Materials and Methods
Reagents and chemicals
All pesticide standards were supplied from Dr. Ehrenstorfer GmbH Co. (Germany). The selected pesticides were 15 pesticides pronounced permissible in cultivation of tomato by the “Iranian National Standards Organization” (14). The stock solution of these compounds were prepared in acetonitrile at a concentration of 1000 mg/L and stored at −18°C. Acetonitrile and toluene (all analytical reagent grades) were purchased from Romil (Italy). Anhydrous sodium sulphate and sodium chloride (extra pure), were obtained from Merck (Germany). PSA bonded silica (Primary secondary amin) used in sample clean-up step were purchased from Supelco (USA).
Tomato samples were purchased from 3 salad production plants in Isfahan in which 22 samples were collected between Sep to Dec 2014.
Apparatus
An Agilent Technologies 7890A Network GC System Chromatograph (Wilmington, USA) with a SQ detector equipped with an Agilent 7693 autosampler (Agilent technologies, USA) was used. A HP-5 capillary column (30 m × 0.25 mm I.D., 0.25 μm film thicknesses) was used for separation.
Instrumental conditions
An Agilent 5975C inert MSD with triple-Axis Detector was used. The MS was operated in the EI mode (70 eV). The GC–MS interface, ion source and quadruple temperatures were set at 266, 230 and 150 °C, respectively. For quantitative determination, the MS was operated in the time-scheduled selected ion monitoring (SIM) mode.
Helium (99.999%) was employed as carrier gas at the constant flow rate of 1.0 mL/min. The gas chromatograph was operated in the splitless mode and the split valve was kept closed for 0.75 min. The injector temperature was kept at 250 °C. The column temperature was raised from 75 °C (hold 3 min) to 120 °C at 25 °C/min, then to 300 °C at 5 °C/ min (hold 11 min). Total time for the GC analysis was 51.8 min. A split/splitless injector operated in the splitless mode was used. The carrier was hydrogen at 9 psi pressure. The flow of carrier gas was applied as 19 ml/min. The injection volume was 2 μl.
Standards
Individual certified pesticide standards (Table 1) were purchased from Dr. Ehrenstorfer (Augsburg, Germany). Individual pesticide stock standard solutions (1000 mg/L) were prepared in acetonitrile and stored at −18°C. From these individual stock solutions, a multi standard mixture, containing 10 mg/L of each pesticide was prepared in acetonitrile and stored at −18°C. Spiked calibration curves at 7 levels of 5, 10, 25, 50, 100, 250 and 500 ng/g triplicate were prepared by addition of 5μl, 10 μl, 25 μl, 50 μl, 100 μl, 250 μl and 500 μl of standard stock solution, respectively, to 10g of blank tomato samples. A stock solution of triphenylmethane (TPM) in acetonitrile at concentration of 0.5 mg/ml was used as internal standard and 10 μl of TPM solution was added to the spiked samples. The samples then were treated as described in the next section.
Table 1:
Ion parameters for the analysis of pesticides
| Pesticide | Pesticide group | MRL (mgkg−1) | Ions | tR (min) |
|---|---|---|---|---|
| Permethrin | Pyrethroid | 500 | 184.1, 183.1, 165 | 33.8, 34.12 |
| Primicarb | Carbamate | 500 | 238.1, 167.1, 166.1 | 19.6 |
| Dichlorvos | Organophosphate | 50 | 186.9, 185, 145 | 8.07 |
| Diazinone | Organophosphate | 50 | 304.1, 276, 248 | 18.7 |
| Fenpropathrin | Pyrethroid | 500 | 265, 209, 181 | 30.8 |
| Carbaryl | Carbamate | 50 | 144, 116, 115 | 12.7, 20.5 |
| Chlorpyrifos | Organophosphate | 50 | 315.9, 313.9, 257.9 | 22.3 |
| Malathion | Organophosphate | 200 | 173.1, 157.9, 143 | 21.9 |
| Chlortalonil | Chloronitrile | 5000 | 265.9, 267.9, 263.9 | 19.01 |
| Brompropilate | Chloronitrile | 500 | 340.9, 342.9, 338.9 | 30.39 |
| Propargit | Sulphite ester | 2000 | 350.1, 173.1, 201 | 29.3 |
| Tetradifone | Bridged diphenyl | 100 | 355.9, 357.9, 353.9 | 31.3 |
| Phosalone | Organophosphate | 50 | 366.9, 183.9, 183 | 31.67 |
| Iprodion | Dicarboximide | 5000 | 245.8, 243.9, 188.9 | 27.1 |
| Endosulfane | Organochlorine | 500 | 340.8, 338.8, 336.8 | 22.6 |
Sample preparation
A modified version of the QuEChERS method for sample preparation of vegetables was used (13).
Freezed tomatoes were blended in a warring blender to become homogenized. 10 g of the homogenized tomato sample was weighted in a 50 ml centrifuge tube. Then 10 μl TPM (5 mg/ml) was added as the internal standard and 10 ml acetonitrile was added afterwards. In this stage, the centrifuge tube was shaken for 1 min on the vortex at the full speed.
Then 1 g of sodium chloride was added. Tubes were shaken for another1 minute and then were centrifuged at 4500 rpm for 5 min at −5 °C. 7 ml of the upper phase was transferred to a 10 mL centrifuge tube containing 2 g anhydrous magnesium sulfate and 0.35 g PSA and was shaken for 60 sec. Then tubes were centrifuged for 5 min at 4500 rpm at −5 °C.
4 ml aliquot was transferred to dark vials and its solvent was evaporated under nitrogen. 1ml toluene was added to vials and then was shaken for 3 sec. Extracts were transferred into auto sampler vials. The samples were placed onto a tray for automated GC/MS analysis (15).
Method validation: Linearity of the calibration curves
All pesticides showed linearity in the SIM mode. Linear spiked calibration curves for all of the pesticides under study were obtained with correlation factors >0.99 (Table 2).
Table 2:
Linearity and correlation factors of spiked calibration curves
| Pesticide | Equation of regression | Correlation coefficient (r2) |
|---|---|---|
| Permethrin | y = 0.0002x − 0.0028 | 0.9903 |
| Primicarb | y = 0.0004x − 0.005 | 0.9961 |
| Dichlorvos | y = 7E − 05x − 0.0006 | 0.9911 |
| Diazinone | y = 0.003x − 0.0033 | 0.9966 |
| Fenpropathrin | y = 0.0001x − 0.0026 | 0.998 |
| Carbaryl | y = 0.0013x − 0.0085 | 0.9977 |
| Chlorpyrifos | y = 0.0002x − 0.0028 | 0.9974 |
| Malathion | y = 0.0006x − 0.013 | 0.9905 |
| Chlortalonil | y = 0.0002x − 0.0043 | 0.9968 |
| Brompropilate | y = 0.0019x − 0.0391 | 0.9915 |
| Propargit | y = 3E − 05x − 0.0005 | 0.9921 |
| Tetradifone | y = 0.0002x − 0.0021 | 0.9921 |
| Phosalone | y = 7E − 05x − 0.0018 | 0.9925 |
| Iprodion | y = 5E − 05x − 0.0007 | 0.9957 |
| Endosulfane | y = 9E − 05x − 0.0004 | 0.997 |
Limits of detection and limits of quantification
The quantification limits (LOQs) and detection limits (LODs) were calculated based on the standard deviations of the intercept and calibration curve parameters (16). Good LODs were achieved under the optimized experimental conditions, ranging between 1.63–10.5 mg/kg. Good LOQs were also obtained for tomato samples, ranging between 5.43–35mg/kg (Table 3).
Table 3:
Limits of detection and limits of quantification
| Pesticide | LOD (mg/kg) | LOQ (mg/kg) |
|---|---|---|
| Permethrin | 3.6 | 12 |
| Primicarb | 7.44 | 24.79 |
| Dichlorvos | 9.72 | 32.4 |
| Diazinone | 8.63 | 28.763 |
| Fenpropathrin | 4.5 | 14.99 |
| Carbaryl | 2.9 | 9.65 |
| Chlorpyrifos | 6.37 | 21.23 |
| Malathion | 8.04 | 26.81 |
| Chlortalonil | 12 | 40 |
| Brompropilate | 1.713 | 5.71 |
| Propargit | 10.5 | 35 |
| Tetradifone | 5.13 | 17.1 |
| Phosalone | 6.87 | 22.9 |
| Iprodion | 3.69 | 12.3 |
| Endosulfane | 1.63 | 5.43 |
Recovery
The recovery and repeatability validation experiments were conductedin tomato matrix at three spiking levels for each pesticide. Results are presented in Table 4. The recovery of pesticides at 3 concentration levels triplicates was in the range of 83.84–119.73%. In terms of repeatability, the majority of the pesticides gave arelative standard deviation (RSD) <20.54%.
Table 4:
Average recoveries (%) and range of relative standard deviations (%) at 3 spiking levels (n = 3)
| Pesticide | Average recovery (%) (n=3) | RSD (%) | ||||
|---|---|---|---|---|---|---|
| 15 | 60 | 150 | 15 | 60 | 150 | |
| Permethrin | 117.39 | 103.52 | 100.2 | 6.05 | 9.86 | 20.54 |
| Primicarb | 113.35 | 84.77 | 93.8 | 10.33 | 3.77 | 8.50 |
| Dichlorvos | 118.86 | 95.82 | 105.21 | 12.10 | 3.56 | 7.65 |
| Diazinone | 110.89 | 94.17 | 92.82 | 3.87 | 2.31 | 7.98 |
| Fenpropathrin | 117.63 | 95.85 | 88.69 | 4.81 | 4.56 | 8.63 |
| Carbaryl | 112.83 | 89.72 | 99.62 | 12.17 | 4.64 | 2.92 |
| Chlorpyrifos | 119.73 | 95.20 | 93.38 | 5.40 | 2.80 | 7.85 |
| Malathion | 111.89 | 87.13 | 87.63 | 3.77 | 5.48 | 18.18 |
| Chlortalonil | 116.26 | 83.84 | 89.39 | 5.80 | 6.32 | 15.82 |
| Brompropilate | 117.63 | 90.38 | 88.79 | 3.98 | 9.85 | 18.82 |
| Propargit | 111.45 | 93.49 | 85.80 | 3.38 | 4.63 | 15.51 |
| Tetradifone | 115.92 | 93.81 | 99.60 | 4.52 | 7.39 | 8.43 |
| Phosalone | 117.25 | 91.25 | 85.77 | 4.25 | 10.96 | 27.07 |
| Iprodion | 113.51 | 87.89 | 88.78 | 9.12 | 3.72 | 17.82 |
| Endosulfane | 118 | 97.65 | 97.79 | 5.90 | 5.9 | 7.83 |
Results
Pesticide residues in real samples
Twenty-two samples were milled and analyzed according to the described method. It was obtained that all of the 22 tomato samples were free of primicarb, dichlorvos, carbaryl, malathion, brompropilate, propargit, tetradifone, phosalone, iprodion and endosulfane (below LOD).
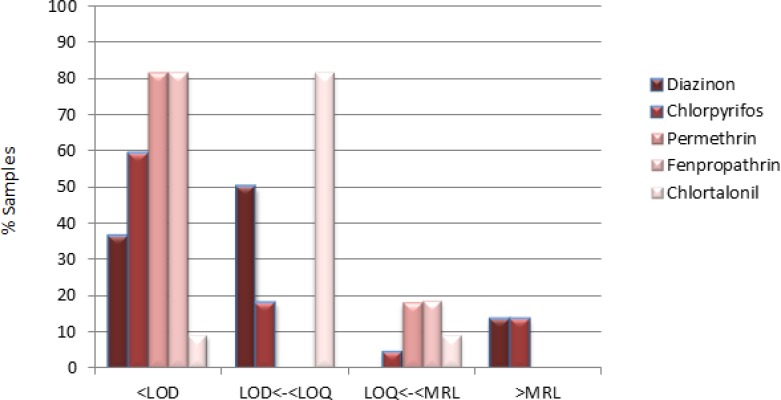
Seven (31.81%) of the 22 samples showed contamination above maximum residue levels (MRLs) with diazinone and chlorpyrifos. None of the samples had contamination with more than one pesticide. 3 of the samples (13.6%) were contaminated with diazinone and 4 samples (18.18%) with chlorpyrifos. Fig. 1 shows the detected residue levels in samples. Concentration of pesticides in the contaminated samples is shown in Table 5. There were contaminated samples to permethrin, fenpropathrin and chlortalonil but the concentration of pesticide was below MRL in these samples. Fig. 1 also shows distribution of contamination in these samples.
Fig. 1:
Results obtained for the 15 pesticides detected in 22 tomato samples
Table 5:
Concentration of pesticides in contaminated samples
| Sample | Pesticide | Concentration of contamination (μg/g) | MRL (μg/g) |
|---|---|---|---|
| 1 | Diazinone | 107.67 | 50 |
| 2 | Diazinone | 258.39 | 50 |
| 3 | Diazinone | 579.81 | 50 |
| 4 | Chlorpyrifos | 144.92 | 100 |
| 5 | Chlorpyrifos | 212.56 | 100 |
| 6 | Chlorpyrifos | 234.69 | 100 |
| 7 | Chlorpyrifos | 254.84 | 100 |
Discussion
The developed QuEChERS method proved an effective method for determination of pesticide residues as was shown by the results of validation analysis with an R2> 0.99.
As mentioned before, tomato is usually consumed raw as a main salad ingredient. The importance of the research comes with the fact that non-permissible levels of pesticides in tomato samples can result in various threats to human health varying from allergies to chronic diseases and cancer.
There has been another attempt to measure the level of toxicity by pesticides in the domestic tomato. Hadian and Azizi analyzed 30 samples of vegetable products including tomato and detected pesticide levels less than the allowable limits (17). Khanyeki et al. (18) detected pesticeds levels more than MRL in most of tomato and cucumber samples they analyzed. Mohammadi and Imani (19) reported that 24% and 80% of the tomato samples possessed chlorpyrifos levels above consumption and national codex permissible levels and 56% of the samples were infected by deltametrin based on the consumption codex.
Table 5 and Fig. 1 of this study on the pesticide residues in 22 tomato samples collected from Isfahan salad production plants show the prevailing condition regarding the toxicity over the permissible levels. According to Fig. 1, 15% of the samples show contamination with two pesticides over MRL levels. While it is steel smaller than the contamination levels for example observed in India (35%) and China (30%), it is much more than the corresponding values detected in Brazil (14.2%), United States (0.9%), and Portugal (0%) mentioned in the following studies.
The level of toxicity obtained in (20) was considerable where several insecticides were found in 26 out of 75 (35%) tomato samples commercialized in India.
In a recent study by Zhao et al. (21) 186 pesticides were monitored in tomato and tomato products, including 10 tomato, 5 tomato juice, and 5 ketchup samples, from China. From the analytical results, triadimefon was not detected in any of the samples, while chlorpyrifos (1.6–8.1 lg/kg), procymidone (17–51 lg/kg), flucythrinate (5.6–8.7 lg/kg), and metalaxyl (2.3–11.2 lg/kg) were detected in six (30%) tomato samples.
In Portugal, twenty tomato samples were analyzed for detection of 30 pesticide residues. Six kinds of pesticides including azoxystrobin, trifloxystrobin, k-cyhalothrin, fenhexamid, tolyfluanid and cyprodinil were detected in 23% of samples, but all values were below the EU MRLs (22).
In Brazil, according to the data presented in 2010 (23) 2.1% of samples contained pesticides above the MRL. In addition, Brazil still faces challenges concerning the use of unauthorized pesticides in the tomato horticulture (14.2% of total samples) (24).
In Europe, the European Food Safety Authority (25) is responsible for risk analysis of pesticides and for the Annual Report on Pesticide Residues, which compiles and analyzes information provided by monitoring programs conducted in Member States. European Union (EU) legislation lists 450 pesticides with MRLs for tomato (26). The Codex Alimentarius described 71 pesticides with MRLs (27). In 2008, 21% of tomato samples analyzed showed residues of multiple pesticides such as acephate, dimethoate, and oxamyl, (methamidophos and bromuconazole were not analyzed) and 34 tomato samples imported from Morocco exceeded the EU MRL.
In the United States, the Food and Drug Administration (FDA)releases an annual report that compiles the results of monitoring programs for pesticide residues (28). Again, in 2008, 25.5% of samples were contaminated and 0.9% exceeded the US MRL. Among imported tomatoes, 31.7% were contaminated with pesticides 2.4% above the MRL.
According to a recently released international report on tomato cultivation (29), the production area of tomato in Iran has increased by 1.55 times from 2000 to 2011. In the same period, tomato production volume has increased by 2.1 times reaching about 7,000,000 tones and showing better production practices in Iran, possibly with more use of pesticides. Out of a total 184,000 ha cultivation area of tomato, only 30 ha has been specified to the organic tomato in 2011 in Iran. In 2011, Iran exported about 265,000 tons of tomato, or about 3.7% of the total tomato exports worldwide, to countries including Azerbaijan, Belarus, France, Kazakhstan, Moldova, Russian Federation, Turkey, and Ukraine.
The above data and the level of toxicity observed in the samples analyzed in this study, shows that how important are detection of pesticides in the domestically produced tomato and strict exercise of the worldwide limitations. Moreover, development of methods other than GC to detect non amenable GC pesticides and implementation of pesticide detection in larger volumes of tomato samples are dire needs of the relevant market to protect human health more effectively. As a limitation, at this point it must be ascertained that though the presented procedure is quick and efficient in the laboratory, it does not mean that it has a direct effect on the level of safety regarding what is consumed by the general public. While the researcher is responsible for an accurate measurement and a fair conclusion, when he/she is working in the laboratory on the samples, the same infected product is being circulated and consumed in the society. Fortunately, it does not downgrade such efforts since they can act as a precaution for those who are responsible for prohibitive actions against overuse of pesticides in cultivation of agricultural products.
Conclusion
A rapid and sensitive analytical method for the simultaneous determination of multiple pesticides in tomato was validated. The modified QuEChERS-based sample preparation and subsequent quantification by GC–MS method showed satisfactory specificity, linearity (R2>0.99) and LOD/LOQ for selected 15 pesticides in tomato, with high precision (RSD<20.54%, in all cases). A total of 22 tomato samples were monitored using this validated method where it was revealed that seven samples (31.81%) were infected with non-permissible levels of diazinone and chlorpyrifos.
Ethical considerations
Ethical issues (including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
We sincerely acknowledge Dr Maryam Amirahmadi from the Food and Drug Laboratory Research Center (Tehran, Iran) for making a number of helpful suggestions.
Sincere thanks are forwarded to the Research Council of Isfahan University of Medical Sciences for supporting this research as the Project Number 292229. The authors declare that there is no conflict of interest.
References
- 1. Engindeniz S. (2006). Economic analysis of pesticide use on processing tomato growing: A case study for Turkey. Crop Protection, 25: 534–541. [Google Scholar]
- 2. Bidari A, Ganjali MR, Norouzi P, Hosseini MRM, Assadi Y. (2011). Sample preparation method for the analysis of some organophosphorus pesticides residues in tomato by ultrasound-assisted solvent extraction followed by dispersive liquid–liquid microextraction. Food Chemistry, 126: 1840–1844. [DOI] [PubMed] [Google Scholar]
- 3. United Nations (2012). Food and agriculture organization. FAOSTAT. Available from: http://faostat.fao.org/default.aspx.
- 4. Gambacorta G, Faccia M, Lambacchia C, Di Luccia A, La Notte E. (2005). Pesticide residues in tomato grown in open field. Food Control, 16: 629–632. [Google Scholar]
- 5. Worthing CR, Hance R. (1991). Compounds in use or being. The Pesticide Manual: A World Compendium. 9th ed. The British Crop Protection Council; , UK: . pp. 121 , 126 , , 166 , and 243 . [Google Scholar]
- 6. Walorczyk S, Drozdzynski D, Kowalska J, Remlein-Starosta D, Ziolkowski A, Przewozniak M. (2013). Pesticide residues determination in Polish organic crops in 2007–2010 applying gas chromatography–tandem quadrupole mass spectrometry. Food Chemistry ,139 : 482– 487 . [DOI] [PubMed] [Google Scholar]
- 7. Fenik J, Tankiewicz M, Biziuk M. (2011). Properties and determination of pesticides in fruits and vegetables. Trends Anal Chem, 30: 814–826. [Google Scholar]
- 8. World Health Organization (WHO) (1992). Our planet, our health. Report of the WHO Commission on Health and Environment, Geneva, Switzerland.
- 9. Cortes JM, Sanchez R, Diaz-Plaza EM, Villen J, Vazquez A. (2006). Large volume GC injection for the analysis of organophosphorus pesticides in vegetables using the through oven transfer adsorption desorption (TOTAD) interface. J Agri Food Chem, 54: 1997–2002. [DOI] [PubMed] [Google Scholar]
- 10. Likas DT, Tsiropoulos NG, Miliadis GE. (2007). Rapid gas chromatographic method for the determination of famoxadone, trifloxystrobin and fenhexamid residues in tomato, grape and wine samples. J Chromato A, 1150: 208–214. [DOI] [PubMed] [Google Scholar]
- 11. Angioni A, Porcu L, Dedola F. (2011). Determination of famoxadone, fenamidone, fenhexamid and iprodione residues in greenhouse tomatoes. Pest Management Science. Available from: http://dx.doi.org/10.1002/ps.2287. [DOI] [PubMed]
- 12. Lehotay SJ, Son AK, Kwon H, Koesukwiwat U, Fu W, Mastovska K. (2010). Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J Chromato A, 1217: 2548–2560. [DOI] [PubMed] [Google Scholar]
- 13. Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ. (2003). Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solidphase extraction” for the determination of pesticide residues in products. J. AOAC Int, 86: 412–431. [PubMed] [Google Scholar]
- 14. Iranian National Standards Organization (2011). National Standard No. 1389. Tehran, Iran. [Google Scholar]
- 15. Amirahmadi M, Yazdanpanah H, Shoeibi SH, PiraliHamedani M, OstadGholami M, Mohseninia MF, Kobarfard F. (2013). Simultaneous Determination of 17 Pesticide Residues in Rice by GC/MS using a Direct Sample Introduction Procedure and Spiked Calibration Curves. Iran J Pharm Res, 2: 295–302. [PMC free article] [PubMed] [Google Scholar]
- 16. Miller JN, Miller JC. (2005). Statistics and chemometrics for analytical chemistry , 5th ed. , Pearson Education Limited; , England: . [Google Scholar]
- 17. Hadian Z, Azizi MH. (2006). Pesticide residues in vegetables marketed in the main wholesale fruit and vegetable market in Tehran as determined by gas chromatography/mass spectrometry. Iran J Nutr Sci Food Technol, 1 ( 2): 13–20. [Google Scholar]
- 18. JahedKhanyeki GR, Fanaei AM, Sadeghi M, Mardani G. (2011). Study of residue OxiDimetonMetil pesticide in greenhouse cucumber and tomato in Chahar Mahal Bakhtiari province. Shahrekord Med Sci J, 12 ( 4): 9–17. [Google Scholar]
- 19. Mohammadi S, Imani S. (2012). Deltametrin and chlorpyrifos residue determination on greenhouse tomato in Karaj by solid phase extraction. Plant Protec J, 4: 57–66. [Google Scholar]
- 20. Kumari BR, Rao GVR, Sahrawat KL, Rajasekhar P. (2012). Occurrence of insecticide residues in selected crops and natural resources. Bullet Environ Contamination Toxicol, 89: 187–192. [DOI] [PubMed] [Google Scholar]
- 21. Zhao P, Huang B, Li Y, Han Y, Zou N, Gu K. (2014). Rapid multiplug filtration cleanup with multiple-walled carbon nanotubes and gas chromatography–triple–quadrupole mass spectrometry detection for 186 pesticide residues in tomato and tomato products. J Agri Food Chem, 62: 3710–3725. [DOI] [PubMed] [Google Scholar]
- 22. Melo A, Cunha SC, Mansilha C, Aguiar A, Pinho O, Ferreira I. (2012). Monitoring pesticide residues in greenhouse tomato by combining acetonitrile-based extraction with dispersive liquid–liquid microextraction followed by gas-chromatography–mass spectrometry. Food Chem, 135: 1071–1077. [DOI] [PubMed] [Google Scholar]
- 23. Brasil Ministerio da Agricultura, Pecuaria e Abastecimento (2003). AGROFIT: Sistema de Agrotoxicos Fitossanitarios. Available from: http://extranet.agricultura.gov.br/agrofit_ cons/principal_agrofit_cons.
- 24. Brasil Agencia Nacional de Vigilancia Sanitaria (2011). Programa de Anglise de Residuos de Agrotoxicosem Alimentos (PARA). Available from: http://portal.anvisa.gov.br/wps/portal/anvisa/anvisa/home/agrotoxicotoxicologia.
- 25. EU Pesticides Database (2012). Available from: http://ec.europa.eu/sanco_pesticides/public/index.cfm.event=substance.selection&ch=1.
- 26. European Food Safety Authority, EFSA (2013). Available from: http://www.efsa.europa.eu/en/efsajournal/pub/1646.htm.
- 27. Codex Alimentarius (2012). Pesticide Residues in Food and Feed. Available from: http://www.codexalimentarius.net/pestres/data/pesticides/index.html.
- 28. U.S. Food and Drug Administration, FDA (2010). Residue Monitoring Reports. Available from: http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/Pesticides/ResidueMonitoringReports/default.htm.
- 29. EPPO (2015). EPPO Technical Document No. 1068, EPPO Study on Pest Risks Associated with the Import of Tomato Fruit. EPPO, Paris: . [Google Scholar]