Abstract
The present study determined the plasma amino acid status in children with cystic fibrosis (CF) and pancreatic insufficiency (PI) in the modern medical and nutritional care setting and investigated the effect of choline supplementation on amino acid status. A total of 110 children, ages 5 to 18 years, with CF and PI were randomized to receive choline-enriched structured lipid (LYM-X-SORB™, LXS) or placebo with similar calorie and fat content. Plasma amino acids were measured at baseline, 3, and 12 months. We hypothesized that choline supplementation would result in lower plasma homocysteine concentrations in children with CF. At baseline, dietary protein intake was high and the amino acid profile was within laboratory reference ranges in most subjects. Alanine and cysteine were elevated in 24% and 36% of subjects, respectively. Children with baseline alanine above reference range had improved weight, body mass index, and fat-free mass. Low homocysteine was found in 62% of children 11 years and older. After 3 and 12 months, there was no effect of choline supplementation on methionine or homocysteine status. Compared to placebo, choline supplementation resulted in increased glycine and decreased threonine, histidine, valine and total branch chained amino acids at 12 months. In conclusion, daily choline supplementation with LXS did not alter methionine-homocysteine metabolism but did result in alterations in other amino acids in children with CF and PI.
Keywords: Cystic Fibrosis, Amino Acids, Choline, Children, LYM-X-SORB™
1. Introduction
Cystic fibrosis (CF) is the most common fatal genetic disorder in the Caucasian population. Median age of survival increased significantly from 31.3 in 2002 to 41.1 years in 2012.[1] One factor contributing to clinical progress is a greater emphasis on optimizing nutritional status. Current nutritional recommendations include routine higher energy and fat intake.[2,3] However, decades of calorie and fat supplementation efforts have limited success, and have not resulted in normal growth and body composition, including muscle mass status in children and adolescents.[4] Fat-free mass (FFM) depletion was present in 25 to 30% of children with CF and has been associated with reduced lung function.[5] FFM depletion and related protein deficits occur frequently and are likely due to increased fecal nitrogen loss, protein breakdown, and decreased protein synthesis along with inadequate caloric intake to optimize growth.[6,7]
Amino acid metabolism is complex and involves various micronutrients such as choline, folic acid and vitamin B12. Choline depletion and elevated vitamin B12 concentrations have been described in children with CF.[8,9] Little is known about the effect of choline supplementation on amino acid status, particularly in the CF care setting where the daily intake from CF-specific vitamin products results in serum vitamin B12 concentrations that are relatively high.[10] In a study by Innis et al [11], high dose choline supplementation for two weeks in children with CF improved choline status, reduced plasma homocysteine and increased methionine concentrations. These effects may be explained by remethylation of homocysteine to methionine in two interrelated pathways that involve choline, folic acid and vitamin B12.[12] Hyperhomocysteinemia is a risk factor for cardiovascular disease, thrombosis and stroke in adults.[13] Because these conditions occur predominantly in older individuals and survival past middle-age in CF has been limited in the past, cardiovascular risk factors often have not been considered in disease management. CF survival has improved with medical advances and newborn screening. Yet, lifelong exposure to a higher fat diet, vitamin and mineral supplements and chronic malabsorption of fat, essential fatty acids and fat soluble vitamins may change this in the future. The impact of long-term supplementation of choline on homocysteine and other amino acid metabolism, and the potential effects on CF clinical outcomes have not been investigated. In this report, we hypothesized that long-term supplementation with choline would result in lower plasma homocysteine concentrations in children with CF and PI. The objectives were twofold: 1) to determine plasma amino acid status in stable children with CF and PI in the contemporary care setting; and 2) to evaluate the effect of a choline-rich easily absorbable fat supplement (LYM-X-SORB™ [LXS]) on plasma amino acid concentrations.
2. Methods and Materials
Here we report on data collected as part of a randomized, placebo controlled trial of a structured lipid (LXS), Biomolecular Products, Byfield, MA; Avanti Polar Lipids, Alabaster, AL) to evaluate changes in plasma and muscle choline status, dietary fat absorption and fatty acid status in children with CF and PI. The clinical trial registration is: Study of LYM-X-SORB™ to Improve Fatty Acid and Choline Status in Children with Cystic Fibrosis and Pancreatic Insufficiency, NCT00406536, https://clinicaltrials.govct2/show/NCT00406536. As part of a secondary aim of the study, this report describes the amino acid status of these children and explores alterations in amino acids and particularly the methionine-homocysteine pathway after LXS or placebo supplementation. LXS is a matrix of lysophosphatidylcholine (LPC), free fatty acids (FFA) and monoglycerides. It is well absorbed without pancreatic enzymes and has been shown to improve nutritional status in children with CF and PI.[14]
2.1 Study participants
Subjects ages 5.0 to 17.9 with CF and PI from ten US CF Centers were seen at The Children’s Hospital of Philadelphia (CHOP). The study was approved by the CHOP Institutional Review Board and at each CF Center. Verbal assent was obtained from subjects 6.0 to <18.0 years of age with written consent from all parents/guardians for subjects <18 years. Patients with forced expiratory volume in one second (FEV1) <40% predicted, residual pancreatic lipase activity (fecal elastase >15 μg/g stool), liver disease (serum gamma-glutamyl transferase [GGT] > 3 times reference range), or other chronic health conditions affecting gastrointestinal absorption or growth were excluded.
2.2 Study design and intervention
Study design details have been previously described.[15–17] Subjects were enrolled and randomized in a 1:1 ratio to receive daily oral LXS or placebo for 12 months. LXS is coated with wheat flour and sugar for mixing with food/beverages selected by the participant.[15] The placebo had similar calories, total fat, and macronutrient distribution (protein 6%, carbohydrate 58%, lipid 34% kcal), but less choline than LXS (39 vs 295 mg/packet). Each packet had 32g powder and subjects <12 years took two packets (304 kcal/d), and those ≥ 12 years old took three packets per day (456 kcal/d). Dietary intake was assessed using 3-day weighed food records, and macro- and micronutrient content analyzed using the Nutrition Data System for Research software (National Coordinating Center, University of Minnesota, Minneapolis, MN). [18] Protein, vitamins B6, folic acid and B12 intake were expressed as percent Recommended Dietary Intake (RDA) and choline as percent Adequate Intake (% AI).[19]
2.3 Nutritional Assessment and Clinical Status
Height and weight were measured using standard techniques [20], BMI derived as kg/m2, and age- and sex-specific Z-scores (HAZ, WAZ, BMIZ) were calculated.[21] Using mid-upper arm circumference and triceps skinfold thickness, upper arm muscle and fat areas were derived, and Z scores (UAMAZ, UAFAZ) were computed.[22] Total fat-free mass (FFM), fat mass (FM), and percent body fat were obtained by whole body dual energy X-ray absorptiometry (DXA, Delphi A, Hologic, Inc., Bedford, MA). Pubertal status was determined using Tanner stages with a validated self-assessment questionnaire.[23] Resting energy expenditure (REE) was measured by open circuit indirect calorimetry using a computerized metabolic cart (SensorMedics Spectra, Yorba Linda, CA) as previously described.[15] REE was calculated from the modified Weir equation.[24] REE (kcals/day) as a percent of predicted values (%REE) was derived from the Schofield equations that adjust for age, sex, weight, and height.[25] Pulmonary function was assessed and predicted percentage FEV1 calculated.[26,27]
2.4 Assessment of Amino Acids and Choline
Plasma samples were obtained in the morning after an overnight fast. Amino acids were measured (Clinical Laboratory, CHOP) by ion-exchange chromatography with post-column ninhydrin derivatization (Biochrom 30 amino acid analyzer, Holliston, MA). Essentially, 500 microliters of plasma was deproteinized by the addition of 50 microliters of 35% sulfosalicylic acid, centrifuged and 120 microliters of supernatant added to 100 microliters of lithium buffer (Biochrom US, Holliston, MA) containing 200 micromolar glucosaminic acid as internal standard. Forty microliters was injected directly into the amino acid analyzer using the standard long-run protocol recommended by Biochrom. This methodology was validated for clinical use according to Clinical Laboratory Improvement Amendments (CLIA) specifications.[28] The water soluble choline was measured in EDTA-plasma by an isotope dilution LC/MS/MS technique.[29,30] The internal standard compounds were dissolved and mixed in acetonitrile to contain 12.5 μM choline chloride-d9, 5 μM of N,N – dimethylglycine hydrochloride-d6 (SigmaAldreich, St. Louise, MO) and 44.4 μM N-carboxymethyl-trimethyl ammonium salt-d9 (CDN Isotopes, Point Clare, Quebec). Samples were extracted by combining 30 μL if of plasma and 10 μL of the internal standard mixture and 200 μL of acetonitrile and 0.1%/v formic acid. This mixture was vortex mixed for 30 seconds at 2500–3000 rpm, followed by room temperature sonication and vortex mixing for 30 seconds. The samples were centrifuged at ~17,000 g for 1 minute and the clear supernatant was transferred to silanized injection inserts and vials for LC/MS/MS analysis. Choline (μmol/L) was quantified from calibration curves generated from reference materials (SigmaAldrich-Fluka, St. Louis, MO) of choline chloride (50-1.6 μM) spiked with identical amount of internal standard compound as the plasma extracts. The calibration standards and plasma extracts were assayed as previously reported [8] using a Waters AcquityTM UPLC system/AB Sciex 5500 mass spectrometer at 0.5 mL/minute flow of 81:19 (v/v) acetonitrile: 15 mM ammonium formate with the addition of a 60:40 (v/v) acetonitrile: 15 mM ammonium formate mobile phase to elute the more polar GPC and CDPC compounds from an Agilent RX-Sil (2.1 × 50 mm, 1.8 μm particle size) column.
Serum vitamin B12 was analyzed by quantitative chemoluminescent immunoassay (CHOP Clinical Laboratory). Plasma B6 (pyridoxine-5-phosphate) was determined by high performance liquid chromatography (ARUP Laboratory, Salt Lake City, UT). For serum B12 and B6, subjects were designated as having elevated concentrations if their B12 or B6 concentrations were above versus within the established age- and sex-specific clinical laboratory reference ranges [31–33]. Whole blood was collected for red blood cell folate concentrations by chemoluminescence (ARUP Laboratory) and reported correcting for the hematocrit. Assessment of glucose and the liver enzymes alanine transaminase (ALT), aspartate transaminase (AST) and gamma glutamyl transferase (GGT) was done using standard techniques (CHOP Clinical Laboratory).
2.5 Statistical Analyses
Descriptive statistics were presented as frequency counts and percentages for categorical variables and mean ± standard deviation (SD) or median (range) depending on skewness for continuous variables. Two-sample t-test or Mann-Whitney U test for continuous variables and chi-square tests or Fisher’s tests of independence for categorical variables were used to compare characteristics at baseline. Associations at each time point between plasma amino acid concentrations and growth, body composition, and pulmonary status were assessed using Pearson or Spearman rank correlation coefficients depending upon skewness. Simple linear regression was used to correct for potential or known confounding factors including age and sex. Changes in outcome measures over time (baseline, 3, 12 months), the main effect of randomization groups, and randomization group (R) by time (T) interactions on outcomes were investigated on an intent-to-treat basis using mixed-effects linear regression models accounting for correlations arising from repeated-measures. Mixed models allow using all data available from each subject under the assumption of missing at random. Whether changes in outcomes over time differed by randomization groups were evaluated by examining the interaction effects of group by time (R x T). If the residuals from the models were not normally distributed, log transformations were applied and reported. Sensitivity analyses were performed to examine the potential outliers and influential points. Preliminary analyses omitting these outliers did not influence results. Stata 13.1 (Stata Corporation, College Station, TX) was used, and statistical significance level was set at P < 0.05 for all tests. The amino acid analyses were part of the exploratory aim of the larger clinical trial. The sample size for the clinical trial was determined based on testing the primary outcomes related to choline and essential fatty acid status.[17]
3. Results
3.1 Nutritional Assessment
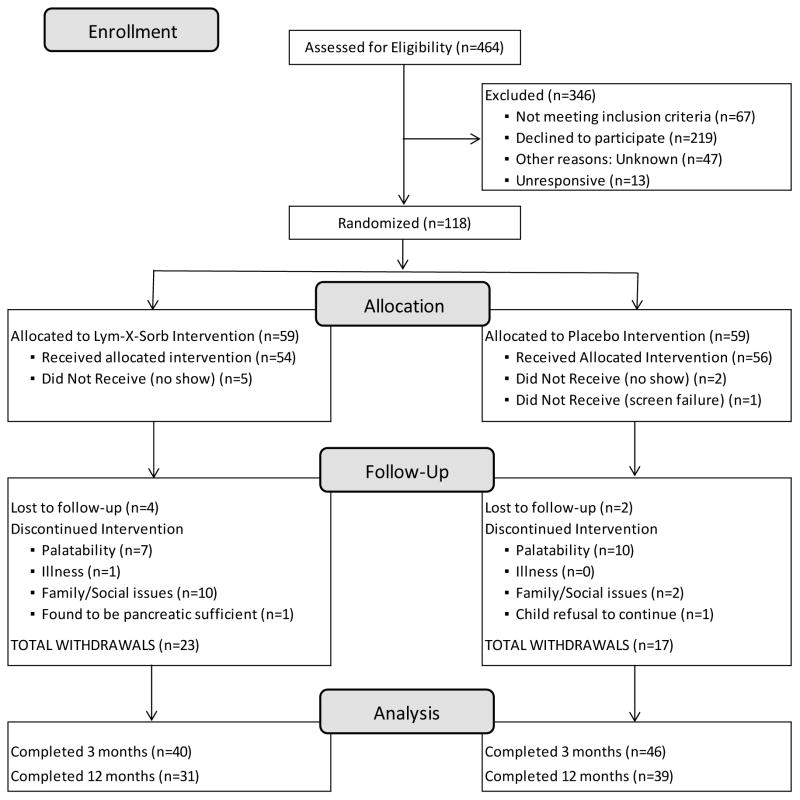
A total of 118 subjects were randomly assigned and 110 received an intervention (Figure 1): 54 subjects in the LXS group; 56 in placebo group. Eighty-six subjects completed 3 month follow-up, 40 in LXS and 46 in placebo, and 70 subjects completed 12 month follow-up, 31 in LXS and 39 in placebo group. Enrollment began in March 2007 and the final visit occurred in May 2011. Characteristics at baseline are presented in Table 1 by randomization group. Subjects had suboptimal growth status, mild to moderate lung disease, and mildly elevated resting energy expenditure. There were no differences between placebo and LXS groups at baseline. Table 2 presents changes over time in energy, macronutrients, choline, betaine, and selected B vitamin intakes. Both LXS and placebo supplements provided 300 to 450 kcal/day depending on age. Our group has previously reported some decrease in calories from food as a compensation for the increased intake from supplements.[15] Protein intake at each observation was high (% RDA > 250%) in both groups. Choline intake increased slightly in the placebo group, and by 2.7-fold in the LXS group by 3 and 12 months (P<0.001).
Figure 1.
Flow diagram for subjects randomized, drop-outs and completers in the placebo-controlled 12-month trial of daily LXS supplementation in children with cystic fibrosis and pancreatic insufficiency.
Table 1.
Characteristics at baseline for placebo and LYM-X-SORB™ (LXS)
| All (110) | Placebo (56) | LXS (54) | |
|---|---|---|---|
| Age (y) | 10.4 ± 3.0 a | 10.2 ± 2.9 | 10.6 ± 3.1 |
| Sex, male | 63 (57%) b | 33 (59%) | 30 (56%) |
| Genotype, ΔF508 homozygous d | 62 (57%) | 30 (55%) | 32 (60%) |
| Pubertal status, pre–pubertal | 58 (53%) | 31 (55%) | 28 (52%) |
| Growth and Body Composition | |||
| Height for age Z score | −0.41 ± 0.92 | −0.46 ± 0.94 | −0.36 ± 0.90 |
| Weight for age Z score | −0.39 ± 0.78 | −0.36 ± 0.80 | −0.43 ± 0.77 |
| BMI for age Z score | −0.20 ± 0.77 | −0.10 ± 0.74 | −0.30 ± 0.81 |
| Whole Body DXA | |||
| FFM (kg) | 23.7 (13.3 – 65.3) c | 22.6 (13.3 – 61.4) | 24.1(14.1 – 65.3) |
| FM (kg) | 6.5 (3.0 – 18.9) | 6.5 (3.0 – 14.7) | 6.6 (3.2 – 18.9) |
| Percent Fat (%) | 21.4 ± 5.6 | 21.6 ± 5.5 | 21.2 ± 5.7 |
| UAMAZ e | −0.53 ± 0.82 | −0.49 ± 0.83 | −0.56 ± 0.81 |
| UAFAZ e | −0.29 ± 0.80 | −0.26 ± 0.84 | −0.32 ± 0.77 |
| Pulmonary Function and Energy Expenditure | |||
| FEV1 % predicted f | 95 ± 23 | 97 ± 24 | 93 ± 21 |
| REE, Kcal/day g | 1248 (862 – 2243) | 1211 (885 – 2201) | 1323 (862 – 2243) |
| REE % predicted g | 107 ± 9 | 107 ± 9 | 108 ± 9 |
FFM, fat free mass (kg); FM, fat mass (kg); UAMAZ, upper arm muscle area Z score; UAFAZ, upper arm fat area Z score; REE, resting energy expenditure (kcal/day); FEV1 % predicted, forced expiratory volume at 1 second, percent predicted value; REE % predicted, resting energy expenditure percent predicted value using Schofield equations. No differences between LXS and placebo randomization groups at baseline, by unpaired student’s t test or Mann-Whitney U test for continuous variables, and chi-squared or Fisher’s exact test for categorical variables.
Continuous normally distributed variables given as means ± SD
Categorical variables are given as numbers and percentages
Continuous variables with significant skewness are given as medians (range)
Sample sizes were 108, 55 and 53 for All, Placebo and LYX groups, respectively
Sample sizes were 109, 55, and 54 for All, Placebo and 54 LYX groups, respectively
Sample sizes were 107, 56 and 51 for All, Placebo and 51 LYX groups, respectively
Sample sizes were 103, 54 and 49 for All, Placebo and 49 LYX groups, respectively
Table 2.
Total daily energy, macronutrients, choline, betaine and B vitamin intake in placebo and LYM–X–SORB™ (LXS) groups at baseline, 3, and 12 months.
| Nutrient | n | Baseline | n | 3 months | n | 12 months | P0–3 | P0–3–12 |
|---|---|---|---|---|---|---|---|---|
| Energy (kcal) | ||||||||
| Placebo | 54 | 2438 ± 798 a | 37 | 2609 ± 563 | 35 | 2618 ± 667 | 0.76 | 0.95 |
| LXS | 44 | 2423 ± 618 | 31 | 2567 ± 580 | 23 | 2704 ±643 | ||
| Protein (gm/kg) | ||||||||
| Placebo | 54 | 2.7 ± 1.0 | 37 | 2.6 ± 1.0* | 35 | 2.5 ± 1.0** | 0.92 | 0.86 |
| LXS | 44 | 2.6 ± 0.8 | 31 | 2.4 ± 0.7 | 23 | 2.5 ± 0.8 | ||
| Protein as % RDA | ||||||||
| Placebo | 54 | 282 ± 108 | 37 | 273 ± 108* | 35 | 265 ± 109** | 0.97 | 0.83 |
| LXS | 44 | 274 ± 79 | 31 | 260 ± 76 | 23 | 265 ± 89 | ||
| Protein as % kcal | ||||||||
| Placebo | 54 | 13.6 ± 2.9 | 37 | 12.9 ± 2.6* | 35 | 12.9 ± 3.1 | 0.77 | 0.78 |
| LXS | 44 | 13.3 ± 2.6 | 31 | 12.5 ± 2.2* | 23 | 13.0 ± 2.3 | ||
| Fat as % kcal | ||||||||
| Placebo | 54 | 35.9 ± 6.1 | 37 | 34.9 ± 5.8 | 35 | 36.3 ± 4.5 | 0.79 | 0.36 |
| LXS | 44 | 36.1 ± 4.6 | 31 | 34.4 ± 6.9 | 23 | 34.6 ± 5.1 | ||
| Carbohydrate as % kcal | ||||||||
| Placebo | 54 | 51.8 ± 7.2 | 37 | 52.6 ± 6.2 | 35 | 51.1 ± 5.3 | 0.59 | 0.44 |
| LXS | 44 | 52.7 ± 6.0 | 31 | 53.5 ± 8.2* | 23 | 53.0 ± 6.4 | ||
| Choline (mg/day) | ||||||||
| Placebo | 54 | 308 (144 – 736) b | 37 | 358 (193 – 773)*** | 35 | 361 (183 – 765)*** | <0.001 | <0.001 |
| LXS | 44 | 291 (129 – 735) | 31 | 831 (444 – 1691)*** | 23 | 812 (244 – 1727)*** | ||
| Choline as % AI | ||||||||
| Placebo | 54 | 88 (37 – 288) | 37 | 92 (52 – 309)** | 35 | 108 (49 – 292)** | <0.001 | <0.001 |
| LXS | 44 | 87 (34 – 221) | 31 | 248 (134 – 524)*** | 23 | 235 (61 – 350)*** | ||
| Betaine (mg/day) | ||||||||
| Placebo | 54 | 135 (25 – 359) | 37 | 124 (73 – 339) | 35 | 112 (31 – 734) | 0.006 | 0.021 |
| LXS | 44 | 135 (47 – 344) | 31 | 106 (9 – 300)*** | 23 | 121 (15 – 392)* | ||
| Total B6 (μg/day) | ||||||||
| Placebo | 54 | 4.6 (1.9 – 32.4) | 37 | 5.5 (1.4 – 40.7) | 35 | 4.5 (1.1 – 8.9) | 0.29 | 0.38 |
| LXS | 44 | 4.7 (2.4 – 78.2) | 31 | 5.0 (1.9 – 12.0) | 23 | 4.6 (2.6 – 11.5) | ||
| Total B6 as % RDA | ||||||||
| Placebo | 54 | 554 (291 – 2496) | 37 | 572 (228 – 4073) | 35 | 529 (148 – 1221)* | 0.37 | 0.64 |
| LXS | 44 | 542 (243 – 7827) | 31 | 547 (188 – 1526) | 23 | 497 (265 – 945) | ||
| Total folic acid (ug/day) | ||||||||
| Placebo | 54 | 800 (340 – 1872) | 37 | 744 (250 – 1954) | 35 | 631 (230 – 1307)** | 0.96 | 0.78 |
| LXS | 44 | 747 (335 – 1684) | 31 | 738 (262 – 1546) | 23 | 681 (338 – 1471) | ||
| Total folic acid as % RDA | ||||||||
| Placebo | 54 | 280 (113 – 840) | 37 | 251 (99 – 800)* | 35 | 227 (106 – 497)*** | 0.73 | 0.94 |
| LXS | 44 | 286 (128 – 562) | 31 | 263 (97 – 674) | 23 | 247 (118 – 423)** | ||
| Total B12 (μg/day) | ||||||||
| Placebo | 54 | 18.7 (5.3 – 109.8) | 37 | 19.3 (6.1 – 111.6) | 35 | 15.2 (3.0 – 36.4)*** | 0.62 | 0.37 |
| LXS | 44 | 17.4 (5.6 – 158.9) | 31 | 18.6 (4.7 – 40.3) | 23 | 17.4 (9.1 – 36.8) | ||
| Total B12 as % RDA | ||||||||
| Placebo | 54 | 1163 (442 – 4575) | 37 | 1093 (354 – 4649) | 35 | 893 (250 – 2271)*** | 0.73 | 0.63 |
| LXS | 44 | 1168 (310 – 8826) | 31 | 1035 (319 – 2519) | 23 | 966 (508 – 2043) | ||
AI: adequate intake. RDA: recommended dietary intake
P0–3 is testing for partial randomization group x time interaction between baseline and 3 month from a mixed effects linear regression model that included baseline, 3, and 12 months data (i. randomization group x time interaction was used).
P0–3–12 is testing for randomization group x time interaction between baseline and 3 and 12 month.
(P≤0.05)
(P≤0.01)
(P≤0.001) difference from baseline within randomization groups.
Continuous normally distributed variables given as means ± SD
Continuous variables with significant skewness are given as medians (range). Log transformation was applied for skewed variables in the mixed models.
3.2 Amino Acid Status at Baseline
Baseline concentrations of most plasma amino acids were within the laboratory reference range for healthy children (Table 3). The exceptions were alanine, cysteine, histidine, and lysine with 12 to 36% of subjects above reference ranges. Homocysteine was below the reference range in more than half of children ≥ 11 years. As presented in Table 3, the reference range for homocysteine increases with age in healthy children. In contrast, subjects with CF and PI showed no increase in plasma homocysteine as expected with age.
Table 3.
Plasma amino acids at baseline in subjects with CF compared to reference ranges (RR).
| Amino acid | Reference range (RR: nmol/mL) | n | Plasma concentration (nmol/mL) | Proportion of subjects (%) | ||
|---|---|---|---|---|---|---|
| within RR | < RR | > RR | ||||
| Alanine | 88 – 440 | 110 | 384 ± 109 a | 84 (76%) b | 0 | 26 (24%) |
| Arginine | 15 – 120 | 110 | 76 ± 25 | 107 (97%) | 0 | 3 (3%) |
| Asparagine | 19 – 106 | 110 | 55 ± 12 | 110 (100%) | 0 | 0 |
| Aspartic Acid | 0 – 31 | 110 | 6.2 ± 2.5 | 110 (100%) | 0 | 0 |
| Cysteine | 2 – 47 | 110 | 44 ± 10 | 71 (65%) | 0 | 39 (36%) |
| Glutamic Acid | 24 – 163 | 110 | 54 ± 24 | 105 (96%) | 4 (4%) | 1 (1%) |
| Glutamine | 285 – 832 | 110 | 551 ± 89 | 109 (99%) | 0 | 1 (1%) |
| Glycine | 103 – 424 | 110 | 321 ± 78 | 102 (93%) | 0 | 8 (7%) |
| Histidine | 38 – 132 | 110 | 101 (60 – 176) c | 98 (89%) | 0 | 12 (11%) |
| Isoleucine | 17 – 106 | 110 | 63 ± 13 | 109 (99%) | 0 | 1 (1%) |
| Leucine | 42 – 188 | 110 | 118 ± 21 | 109 (99%) | 0 | 1 (1%) |
| Lysine | 71 – 217 | 109 | 177 ± 30 | 99 (90%) | 0 | 11 (10%) |
| Methionine | 8 – 49 | 110 | 26 ± 9 | 107 (97%) | 0 | 3 (3%) |
| Phenylalanine | 23 – 95 | 110 | 56 ± 10 | 110 (100%) | 0 | 0 |
| Proline | 83 – 346 | 110 | 166 (82 – 396) | 107 (97%) | 1 (1%) | 2 (2%) |
| Serine | 50 – 215 | 110 | 113 ± 19 | 110 (100%) | 0 | 0 |
| Threonine | 86 – 321 | 110 | 142 ± 38 | 108 (98%) | 2 (2%) | 0 |
| Tyrosine | 22 – 102 | 110 | 63 (32 – 134) | 108 (98%) | 0 | 2 (2%) |
| Valine | 39 – 321 | 110 | 221 (152 – 353) | 109 (99%) | 0 | 1 (1%) |
| Ornithine | 13 – 151 | 110 | 82 ± 23 | 110 (100%) | 0 | 0 |
| α-aminobutyric | 0 – 43 | 108 | 21 ± 8 | 105 (97%) | 0 | 3 (3%) |
| Taurine | 55 – 204 | 109 | 78 ± 22 | 103 (94%) | 6 (6%) | 0 |
| Homocysteine d | ||||||
| 5 – 10.9 y | 0.8 – 6.5 | 68 | 4.9 ± 1.2 | 63 (93%) | 0 | 5 (7%) |
| 11–16.9 y | 5.7 – 11.7 | 39 | 5.5 ± 1.5 | 16 (41%) | 23 (59%) | 0 |
| ≥17 y | 10.5 – 16.5 | 3 | 6.0 ± 0.6 | 0 | 3 (100%) | 0 |
| BCAA | - | 110 | 403 ± 67 | - | - | - |
| AAA | - | 110 | 121 ± 21 | - | - | - |
| BCAA:AAA ratio | - | 110 | 3.41 ± 0.62 | - | - | - |
BCAA, branch- chained amino acids (Valine, Leucine, Isoleucine). AAA, aromatic amino acids (Tyrosine, Phenylalanine).
Continuous normally distributed variables given as means ± SD
Categorical variables are given as numbers (percentages)
Continuous variables with significant skewness are given as medians (range)
The reference ranges for homocysteine are given by age group.
Further analysis of the elevated alanine was conducted and after adjusting for age and sex, subjects with alanine above the reference range had better nutritional status than those within the range, with higher WAZ (0.00 ± 0.67 vs. −0.51 ± 0.77; P= 0.001), BMIZ (0.18 ± 0.63 vs. −0.31 ± 0.8; P 0.001), and FFM (30.9 ± 12.7 vs. 24.8 ± 8.6 kg; P= 0.002). Furthermore, alanine concentrations were positively correlated with FFM (r= 0.36; p<0.001). Although all values were within the reference range, serum glucose positively predicted plasma alanine (β coef= 2.9, SE= 1.0; P= 0.009) using a linear regression model adjusted for age and sex. Four subjects had CF-related diabetes mellitus and were on insulin treatment. Alanine was higher than the reference range in only one of them.
There were no significant differences in growth, body composition, BMI or pulmonary function between those with plasma cysteine or homocysteine above vs. within the reference range. However, those with cysteine above the reference range had higher serum vitamin B6 (above the reference range) than those within the range (35% and 9% respectively, P = 0.01). In contrast, subjects with homocysteine below the range were more likely to have high serum vitamin B12 (above the reference range) than subjects within the range (77% and 46% respectively, p= 0.006). These amino acid and vitamin B associations seen at baseline were also present at 3 and 12 months.
3.3 12-Month Amino Acids Evaluation
Plasma amino acids are presented by randomization group at baseline, 3 and 12 months in Table 4. Compared to placebo, plasma glycine increased over 3 and 12 months in the LXS group (P0–3–12 =0.046). Threonine increased in placebo and slightly decreased in LXS. Total branched chain amino acids (BCAA: leucine, valine, isoleucine) decreased with LXS and did not change with placebo over 12 months (P0–3–12≤0.01). The BCAA decrease in the LXS group was due to a significant decrease in valine and smaller decline in leucine. Total aromatic amino acids (AAA) also declined significantly in the LXS group only (P <0.001).
Table 4.
Plasma amino acids in placebo and LYM–X–SORB™ (LXS) groups at baseline, 3, and 12 months.
| Amino acids (nmol/mL) | n | Baseline | n | 3 months | n | 12 months | P0–3 | P0–3–12 |
|---|---|---|---|---|---|---|---|---|
| Alanine | ||||||||
| Placebo | 56 | 384 ± 99 a | 46 | 405 ± 120 | 39 | 401 ± 106 | 0.50 | 0.43 |
| LXS | 54 | 383 ± 119 | 40 | 423 ± 127* | 31 | 392 ± 104 | ||
| α–aminobutyric | ||||||||
| Placebo | 56 | 22 ± 9 | 45 | 20 ± 7 | 38 | 22 ± 8 | 0.70 | 0.82 |
| LXS | 52 | 20 ± 7 | 40 | 17 ± 8 | 31 | 18 ± 7 | ||
| Arginine | ||||||||
| Placebo | 56 | 81 ± 26 | 46 | 81 ± 26 | 39 | 77 ± 24 | 0.15 | 0.13 |
| LXS | 54 | 70 ± 23 ¥ | 40 | 77 ± 21* | 31 | 64 ± 20* | ||
| Asparagine | ||||||||
| Placebo | 56 | 54 ± 10 | 46 | 58 ± 28 | 39 | 58 ± 9 | 0.35 | 0.51 |
| LXS | 54 | 55 ± 13 | 40 | 55 ± 13 | 31 | 56 ± 10 | ||
| Aspartic Acid | ||||||||
| Placebo | 56 | 6.1 ± 2.6 | 45 | 5.7 ± 2.4 | 39 | 5.0 ± 2.7* | 0.41 | 0.65 |
| LXS | 54 | 6.4 ± 2.3 | 38 | 5.7 ± 2.7 | 31 | 5.6 ± 4.1 | ||
| Cysteine | ||||||||
| Placebo | 56 | 45 ± 10 | 46 | 46 ± 9 | 39 | 46 ± 11 | 0.33 | 0.31 |
| LXS | 54 | 43 ± 10 | 40 | 46 ± 8 | 31 | 44 ± 9 | ||
| Glutamic Acid | ||||||||
| Placebo | 56 | 54 ± 25 | 46 | 55 ± 26 | 39 | 55 ± 22 | 0.27 | 0.51 |
| LXS | 54 | 55 ± 24 | 40 | 50 ± 17 | 31 | 55 ± 28 | ||
| Glutamine | ||||||||
| Placebo | 56 | 568 ± 87 | 46 | 571 ± 107 | 39 | 593 ± 100 | 0.12 | 0.27 |
| LXS | 54 | 533 ± 88 ¥ | 40 | 564 ± 120 | 31 | 555 ± 105 | ||
| Glycine | ||||||||
| Placebo | 56 | 321 ± 81 | 46 | 309 ± 87 | 39 | 320 ± 60 | 0.017 | 0.046 |
| LXS | 54 | 320 ± 76 | 40 | 340 ± 88 | 31 | 336 ± 73 | ||
| Histidine | ||||||||
| Placebo | 56 | 102 (60 – 176) b | 46 | 91 (60 – 148)* | 39 | 93 (59 – 179) | 0.052 | 0.005 |
| LXS | 54 | 99 (71 – 150) | 40 | 107 (65 – 159) | 31 | 88 (58 – 192)* | ||
| Homocysteine | ||||||||
| Placebo | 56 | 4.8 ± 1.3 | 46 | 4.9 ± 1.4 | 39 | 5.3 ± 1.5** | 0.17 | 0.31 |
| LXS | 54 | 5.4 ± 1.3 ¥ | 40 | 5.3 ± 1.4 | 31 | 5.9 ± 1.3 | ||
| Isoleucine | ||||||||
| Placebo | 56 | 64 ± 10 | 46 | 64 ± 14 | 39 | 66 ± 10 | 0.43 | 0.23 |
| LXS | 54 | 62 ± 14 | 40 | 59 ± 10 | 31 | 59 ± 11 | ||
| Leucine | ||||||||
| Placebo | 56 | 119 ± 17 | 46 | 119 ± 24 | 39 | 126 ± 20 | 0.62 | 0.059 |
| LXS | 54 | 117 ± 24 | 40 | 113 ± 19 | 31 | 109 ± 24 | ||
| Lysine | ||||||||
| Placebo | 55 | 179 ± 27 | 46 | 185 ± 38 | 39 | 179 ± 25 | 0.23 | 0.13 |
| LXS | 54 | 174 ± 33 | 39 | 171 ± 37 | 31 | 160 ± 30* | ||
| Methionine | ||||||||
| Placebo | 56 | 27 ± 9 | 46 | 26 ± 9 | 39 | 27 ± 9 | 0.29 | 0.069 |
| LXS | 54 | 25 ± 8 | 40 | 27 ± 10 | 31 | 23 ± 6 | ||
| Ornithine | ||||||||
| Placebo | 56 | 81 ± 19 | 45 | 82 ± 24 | 39 | 81 ± 18 | 0.064 | 0.17 |
| LXS | 54 | 82 ± 26 | 39 | 73 ± 22* | 31 | 78 ± 27 | ||
| Phenylalanine | ||||||||
| Placebo | 56 | 57 ± 9 | 45 | 59 ± 13 | 39 | 56 ± 10 | 0.09 | 0.14 |
| LXS | 54 | 55 ± 11 | 40 | 52 ± 7 | 31 | 50 ± 9* | ||
| Proline | ||||||||
| Placebo | 56 | 165 (82 – 396) | 45 | 165 (9 – 380) | 39 | 172 (115 – 325) | 0.10 | 0.17 |
| LXS | 54 | 168 (87 – 294) | 40 | 187 (120 – 378)** | 31 | 178 (93 – 347) | ||
| Serine | ||||||||
| Placebo | 56 | 114 ± 17 | 46 | 109 ± 19 | 39 | 111 ± 21 | 0.32 | 0.26 |
| LXS | 54 | 111 ± 22 | 40 | 110 ± 23 | 31 | 104 ± 25 | ||
| Taurine | ||||||||
| Placebo | 56 | 74 ± 22 | 45 | 73 ± 22 | 38 | 72 ± 19 | 0.053 | 0.13 |
| LXS | 53 | 82 ± 21 ¥ | 40 | 72 ± 17* | 31 | 81 ± 33 | ||
| Threonine | ||||||||
| Placebo | 56 | 144 ± 35 | 46 | 155 ± 51 | 39 | 165 ± 40** | 0.14 | 0.029 |
| LXS | 54 | 140 ± 41 | 40 | 133 ± 48 | 31 | 129 ± 38 | ||
| Tyrosine | ||||||||
| Placebo | 56 | 67 (32 – 134) | 45 | 67 (41 – 132) | 39 | 59 (33 – 121) | 0.71 | 0.18 |
| LXS | 54 | 60 (39 – 95) | 40 | 64 (39 – 122) | 31 | 53 (25 – 92) | ||
| Valine | ||||||||
| Placebo | 56 | 221 (169 – 307) | 46 | 224 (154 – 340) | 39 | 229 (181 – 337) | 0.88 | 0.043 |
| LXS | 54 | 219 (152 – 353) | 40 | 212 (151 – 279) | 31 | 200 (130 – 318)* | ||
| BCAA | ||||||||
| Placebo | 56 | 408 (318 – 570) | 46 | 387 (278–628) | 39 | 414 (314–594) | 0.81 | 0.047 |
| LXS | 54 | 386 (278 – 606) | 40 | 388 (286–533) | 31 | 377 (245–547) | ||
| AAA | ||||||||
| Placebo | 56 | 126 (77–194) | 44 | 129 (83–232) | 39 | 119 (80–179) | 0.63 | 0.21 |
| LXS | 54 | 113 (80–172) | 40 | 117 (74–190) | 31 | 103 (65–152)*** | ||
| BCAA: AAA ratio | ||||||||
| Placebo | 56 | 3.38 ± 0.59 | 44 | 3.26 ± 0.62 | 39 | 3.69 ± 0.99 | 0.99 | 0.68 |
| LXS | 54 | 3.44 ± 0.64 | 40 | 3.35 ± 0.55 | 31 | 3.67 ± 0.62 | ||
BCAA, branch- chained amino acids (Valine + Leucine + Isoleucine); AAA, aromatic amino acids (Tyrosine + Phenylalanine).
P0–3 is testing for partial randomization group x time interaction between baseline and 3 month from a mixed effects linear regression model that included baseline, 3, and 12 months data (i. randomization group x time interaction was used).
P0–3–12 is testing for randomization group x time interaction between baseline and 3 and 12 month.
(P≤0.05)
(P≤0.01)
(P≤0.001) difference from baseline within randomization groups.
(P≤0.05) differences between LYX and Placebo randomization groups at baseline.
Continuous normally distributed variables given as means ± SD
Continuous variables with significant skewness are given as medians (range). Log transformation was applied for skewed variables in the mixed models.
3.4 Other Outcomes
Table 5 presents plasma choline, serum vitamin B status and liver enzymes for placebo and LXS groups at baseline, 3 and 12 months. The changes in amino acid status were accompanied by a significant increase in plasma choline in the LXS group and decrease in the placebo group (P0–3–12 =0.037). There were no changes in B vitamin status or liver enzymes over time in either group. Serum B12 concentrations were above reference ranges in > 50% of subjects as previously reported.[10] Growth status improved and REE declined in both LXS and placebo groups as previously reported.[15] FEV1 declined over the 12 months as the subjects aged, with no differences between randomization groups (data not presented).[17]
Table 5.
Plasma choline, serum B vitamins and liver enzymes in placebo and LYM–X–SORB™ (LXS) groups at baseline, 3, and 12 months.
| Variable | n | Baseline | n | 3 months | n | 12 months | P0–3 | P0–3–12 |
|---|---|---|---|---|---|---|---|---|
| Plasma Choline | ||||||||
| Choline (μmol/L) | ||||||||
| Placebo | 56 | 6.9 (4.2 – 12.8) a | 46 | 7.1 (5.0 – 11.5) | 39 | 6.6 (3.6 – 11.4)** | 0.011 | 0.037 |
| LXS | 52 | 7.6 (4.6 – 12.2) | 40 | 9.3 (3.9 – 15.6)** | 31 | 7.9 (4.1 – 12.8) | ||
| Serum B Vitamins | ||||||||
| B6 (ng/mL) | ||||||||
| Placebo | 56 | 17.4 (5.2 – 91.7) | 46 | 16.1 (4.7 – 93.8) | 38 | 18.7 (5.8 – 74.4) | 0.89 | 0.39 |
| LXS | 50 | 16.9 (5.0 – 55.5) | 38 | 17.2 (3.2 – 77.8) | 31 | 18.1 (4.2 – 72.9) | ||
| RBC folate (ng/mL) | ||||||||
| Placebo | 53 | 507 ± 157 b | 46 | 515 ± 167 | 39 | 479 ± 152 | 0.84 | 0.85 |
| LXS | 47 | 534 ± 181 | 37 | 546 ± 145 | 31 | 482 ± 182 | ||
| B12 (pg/mL) | ||||||||
| Placebo | 55 | 1142 (387 – 2861) | 46 | 1259 (387 – 2861) | 39 | 1296 (387 – 2861)* | 0.22 | 0.26 |
| LXS | 51 | 1040 (371 – 3455) | 39 | 1073 (457 – 3455) | 31 | 1099 (457 – 2257) | ||
| Liver Enzymes | ||||||||
| ALT (U/L) | ||||||||
| Placebo | 56 | 41 ± 25 | 46 | 42 ± 29 | 39 | 46 ± 60 | 0.59 | 0.86 |
| LXS | 54 | 37 ± 17 | 40 | 35 ± 15 | 31 | 44 ± 69 | ||
| AST (U/L) | ||||||||
| Placebo | 56 | 44 ± 26 | 46 | 49 ± 51 | 39 | 47 ± 45 | 0.47 | 0.57 |
| LXS | 54 | 41 ± 18 | 40 | 40 ± 17 | 31 | 57 ± 116 | ||
| GGT (U/L) | ||||||||
| Placebo | 56 | 29 ± 23 | 46 | 33 ± 38 | 39 | 31 ± 25 | 0.39 | 0.68 |
| LXS | 54 | 24 ± 15 | 40 | 24 ± 17 | 31 | 28 ± 28 | ||
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase;
Log transformation was applied for skewed variables in the mixed models.
P0–3 is testing for partial randomization group x time interaction between baseline and 3 month from a mixed effects linear regression model that included baseline, 3, and 12 months data (i. randomization group x time interaction was used).
P0–3–12 is testing for randomization group x time interaction between baseline and 3 and 12 month.
(P≤0.05)
(P≤0.01) difference from baseline within randomization groups.
Continuous variables with significant skewness are given as medians (range)
Continuous normally distributed variables given as means ± SD
4. Discussion
4.1 Amino Acid Status in Children with CF and PI
In the present study, most plasma amino acids were within the laboratory reference range in this sample of clinically stable children with CF and PI in the modern care and nutritional status setting. High alanine and cysteine concentrations were relatively common. Low homocysteine was prevalent in peri- and post-pubertal children. In 1981, Hubbard and Schulman described normal plasma amino acids in subjects with CF age 16–30 years with the exception of high glycine.[34] However, high glycine has been described in one third of healthy adults but was rarely seen in children.[35] The children in the current study were younger than those in the Hubbard et al. study and the clinical care, nutritional status and outcomes have changed over the two decades.[34] The higher glycine concentration found in the participants from the Hubbard and Schulman study may be unrelated to CF.
The elevated plasma alanine in 24% of subjects was an unexpected finding in this cohort of children with CF. Alanine plays a key role in gluconeogenesis. It carries ammonia and pyruvate carbon skeletons between muscle tissue and liver protein metabolism.[36] Pyruvate is used to produce glucose and returned to muscle. Thus, the positive relationship between high plasma alanine and better FFM status at baseline in our study may be partially explained. Alteration in the alanine-glucose cycle that leads to increased serum and presumably tissue alanine aminotransferase (ALT) has been linked to diabetes mellitus in non-CF adults.[37–39] The specific cystic fibrosis-related diabetes is found in 19% of adolescents and 40 to 50% of adults with CF. The elevated alanine may be of relevance to this CF comorbidity which has increased in prevalence with longer survival.[40] Enhanced gluconeogenesis and reduced suppression of gluconeogenesis by insulin leading to impaired glucose tolerance is not uncommon in CF.[41,42] The impact of early and sustained elevated alanine and glucose on the risk of CFRD is unknown and merits further investigation.
Elevated cysteine in 36% of sujects was also an unexpected finding. Cysteine is a conditionally essential amino acid and can be synthesized by adding sulfur from methionine through the formation of homocysteine.[43] Homocysteine is generally metabolized via either remethylation, which converts homocysteine back to methionine, or trans-sulfuration, which catabolizes homocysteine to cysteine.[43] Vitamin B12 and folate are essential for remethylation and vitamin B6 is required for trans-sulfuration. Our study identified an inverse relationship with concentrations of homocysteine and both B6 and B12. Children with high plasma cysteine were more likely to have high serum B6. Waskiewicz et al. [44] documented lower homocysteine in healthy adults receiving B6, B12 and folate supplementation. Daily oral supplementation with as little as 0.5 mg B12 alone reduced homocysteine by 7% in adults.[45] High serum B12 has been reported in the majority of children with CF and PI and was likely related to the high vitamin B12 content of CF-specific vitamin and mineral products.[10] There is no clinical indication for high B12 doses in CF. These CF products are commonly used to provide high fat soluble vitamin doses to compensate for the chronic fat malabsorption in CF. The B12 supplement intake was 6 to 10 times the RDA and strongly predicted the serum B12 concentrations. Children in the higher serum B12 group were older and had poorer growth status as indicated by BMIZ and lower lung function.[10] The current findings of high cysteine and low homocysteine may be due to enhanced remethylation and trans-sulfuration related to the unusually high B12 and B6 intake common in current CF clinical care.
Plasma homocysteine concentrations typically increase with age in healthy children. In this CF cohort, homocysteine was low in 62% of children 11years and older. In the presence of high B12 and B6, this expected increase in homocysteine did not occur in the children in this study. While high plasma homocysteine is a risk for coronary heart disease in otherwise healthy adults, individuals with low homocysteine may also be at risk for other conditions due to limited capacity to respond to oxidative stress, including the production of glutathione, a free-radical scavenger, and also taurine and sulfate. Low homocysteine was indicated in the malnutrition-inflammation complex that predicted lower survival in patients on hemodialysis.[46] The long-term effect of sustained low homocysteine concentrations in children with CF during the adolescent growth phase is unknown. These current results suggest that there may be a need to reduce vitamin B12 intake in these children with high B12 to support a more normal homocysteine status. Further investigation of the long-term health impact of chronically low homocysteine in children with CF is warranted.
4.2 Effect of Choline on Amino Acids
Choline supplementation over 12 months did not alter methionine-homocysteine metabolism as indicated by plasma concentrations, thus rejecting the hypothesis that long term choline supplementation lowers plasma homocysteine concentrations in children with CF and PI. Innis and colleagues have shown that supplementations with much higher dose of choline for 14 days in children with CF resulted in a significant decrease in homocysteine.[11] The average dose of choline in LXS supplementation (591 mg/day) was considerably lower than that used by Innis et al (1850 mg/day).[11] The homocysteine concentration also was lower at baseline in our sample (5.4 ± 1.5 μmol/L) compared to Innis et al (7.94 ± 0.45 μmol/L). Furthermore, serum vitamin B12 (1040 ng/ml) was higher in our subjects compared to Innis et al (594 ± 35 ng/mL).[11,47] The remethylation of homocysteine to methionine using choline-betaine pathway acts as an alternate pathway to remethylation by the B12 and folate-dependent pathway.[12] Increased activity of choline-betaine for remethylation of homocysteine has been well described in animals and humans with B12 or folate deficiency.[48,49] The effect of higher choline intake on the choline-remethylation process in the presence of high serum B12 has not been described previously. The magnitude of the effect of choline supplementation may be decreased based on the competitive interrelation between the two remethylation pathways.
LXS supplementation resulted in lower plasma BCAA, especially valine and to a lesser extent leucine. These findings were unlikely related to the dietary protein intake which was high and sustained in both groups throughout the study. The reduction in plasma valine and trend toward lower leucine indicated that LXS may increase BCAA catabolism or increase uptake by muscle. BCAA catabolism is unique, as it is controlled by a common flux generating step that occurs largely in skeletal muscle.[50] No relationship between the common BCAA metabolic pathway and plasma choline has been previously reported. This study demonstrated higher glycine and lower threonine in children receiving the choline-rich LXS. This finding can be explained, at least in part by the fact that glycine is synthesized from choline and threonine in humans.[51] The choline supplementation may result in greater conversion of threonine to glycine in these subjects.
Potential limitations of the study were that these findings for amino acids status were part of a secondary and exploratory analyses of the original clinical trial and the lack of a healthy control group for comparison of response to choline supplementation. A major strength of the study is the collection of high quality longitudinal amino acid data that provides updated information on amino acid status for children with CF and PI receiving clinical care in the current environment. There has been no comprehensive update of amino acid status within the past 25 years.
In conclusion, clinically stable children with CF and PI had an amino acid profile that was within the reference range with the exception of high plasma alanine and cysteine, and low homocysteine. High alanine was likely due to increased endogenous gluconeogenesis. The altered cysteine and homocysteine concentrations may be related to excessive vitamin B6 and B12 intake. In this CF cohort, choline supplementation at the daily doses used here in a setting of high serum B12 did not alter methionine-homocysteine metabolism. Supplementation resulted in lower total BCAA suggesting reduced skeletal muscle catabolism or improved anabolism in children with CF and PI. Further studies are needed to confirm these findings and consider the clinical implications of altered plasma amino acid concentrations and high B12 during childhood growth for risk of complications such as CF-related diabetes and cardiovascular disease, as patients with CF now will commonly live decades into adult life.
Acknowledgments
This study was supported by NIDDK (R44DK060302), and the Nutrition Center at the Children’s Hospital of Philadelphia. The project described was also supported by the National Center for Research Resources, Grant UL1RR024134, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding sponsors were not involved in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication. All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors, and declare no conflict of interest. We thank the subjects, parents, other care providers, and all the CF Centers that participated in the study: Children’s National Medical Center, Washington, DC; Children’s Hospital of Philadelphia, Philadelphia, PA; Monmouth Medical Center, Long Branch, NJ; The Pediatric Lung Center, Fairfax, VA; Cystic Fibrosis Center of University of Virginia, Charlottesville, VA; Children’s Hospital of the King’s Daughters, Eastern Virginia Medical School, Norfolk, VA; Yale University School of Medicine, New Haven, CT; Cohen Children’s Medical Center, New Hyde Park, NY; St Joseph’s Children’s Hospital, Paterson, NJ and the Pediatric Specialty Center at Lehigh Valley Hospital, Bethlehem, PA. We thank Dr. Dr. Walter Shaw and the Avanti Polar Lipid, Inc. team for production of the LXS and placebo products and choline analyses. We thank Megan Johnson, Thananya Wooden, Elizabeth Matarrese and Nimanee Mosley Harris for their valuable contributions to the study.
Abbreviations
- AAA
Aromatic amino acids
- AI
Adequate Intake
- ALT
Serum alanine aminotransferase
- AST
Serum aspartate aminotransferase
- BCAA
Branch chain amino acids
- BMIZ
BMI-for-age Z score
- CF
Cystic Fibrosis
- CHOP
The Children’s Hospital of Philadelphia
- DRI
Dietary Reference Intake
- DXA
Dual energy x-ray absorptiometry
- FEV1
Forced expiratory volume at one second, % predicted
- FFA
Free fatty acids
- FFM
Fat-free mass kg
- FM
Fat mass, kg
- GGT
Serum gamma-glutamyl transferase
- HAZ
Height-for-age Z score
- LPC
Lysophosphatidylcholine
- LXS
LYM-X-SORB™
- PI
Pancreatic Insufficiency
- RDA
Recommended dietary allowance
- REE
Resting energy expenditure
- WAZ
Weight-for-age Z score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Belal Alshaikh, Email: belal.alshaikh@albertahealthservices.ca.
Joan I. Schall, Email: schall@email.chop.edu.
Asim Maqbool, Email: maqbool@email.chop.edu.
Maria Mascarenhas, Email: mascarenhas@email.chop.edu.
Michael J. Bennett, Email: bennettmi@email.chop.edu.
Virginia A. Stallings, Email: stallingsv@email.chop.edu.
Reference List
- 1.Cystic Fibrosis Foundation Patient Registry: 2012 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation; 2013. [Google Scholar]
- 2.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002;35(3):246–59. doi: 10.1097/00005176-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Smyth RL, Walters S. Oral calorie supplements for cystic fibrosis. Cochrane Database Syst Rev. 2012;10:CD000406. doi: 10.1002/14651858.CD000406.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Kalnins D, Corey M, Ellis L, Pencharz PB, Tullis E, Durie PR. Failure of conventional strategies to improve nutritional status in malnourished adolescents and adults with cystic fibrosis. J Pediatr. 2005;147(3):399–401. doi: 10.1016/j.jpeds.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 5.Engelen MP, Schroder R, Van der Hoorn K, Deutz NE, Com G. Use of body mass index percentile to identify fat-free mass depletion in children with cystic fibrosis. Clin Nutr. 2012;31(6):927–33. doi: 10.1016/j.clnu.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Milla PJ, Fenton TR. Small intestinal motility patterns in the perinatal period. J Pediatr Gastroenterol Nutr. 1983;2(Suppl 1):S141–S144. doi: 10.1097/00005176-198300201-00020. [DOI] [PubMed] [Google Scholar]
- 7.Geukers VG, Oudshoorn JH, Taminiau JA, van der Ent CK, Schilte P, Ruiter AF, et al. Short-term protein intake and stimulation of protein synthesis in stunted children with cystic fibrosis. Am J Clin Nutr. 2005;81(3):605–10. doi: 10.1093/ajcn/81.3.605. [DOI] [PubMed] [Google Scholar]
- 8.Innis SM, Hasman D. Evidence of choline depletion and reduced betaine and dimethylglycine with increased homocysteine in plasma of children with cystic fibrosis. J Nutr. 2006;136(8):2226–31. doi: 10.1093/jn/136.8.2226. [DOI] [PubMed] [Google Scholar]
- 9.Lindemans J, Abels J, Neijens HJ, Kerrebijn KF. Elevated serum vitamin B12 in cystic fibrosis. Acta Paediatr Scand. 1984;73(6):768–71. doi: 10.1111/j.1651-2227.1984.tb17773.x. [DOI] [PubMed] [Google Scholar]
- 10.Maqbool A, Schall JI, Mascarenhas MR, Dougherty KA, Stallings VA. Vitamin B12 Status in Children with Cystic Fibrosis and Pancreatic Insufficiency. J Pediatr Gastroenterol Nutr. 2014;58:733–8. doi: 10.1097/MPG.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Innis SM, Davidson AG, Melynk S, James SJ. Choline-related supplements improve abnormal plasma methionine-homocysteine metabolites and glutathione status in children with cystic fibrosis. Am J Clin Nutr. 2007;85(3):702–8. doi: 10.1093/ajcn/85.3.702. [DOI] [PubMed] [Google Scholar]
- 12.Ueland PM. Choline and betaine in health and disease. J Inherit Metab Dis. 2011;34(1):3–15. doi: 10.1007/s10545-010-9088-4. [DOI] [PubMed] [Google Scholar]
- 13.Kolling J, Scherer EB, da Cunha AA, da Cunha MJ, Wyse AT. Homocysteine induces oxidative-nitrative stress in heart of rats: prevention by folic acid. Cardiovasc Toxicol. 2011;11(1):67–73. doi: 10.1007/s12012-010-9094-7. [DOI] [PubMed] [Google Scholar]
- 14.Lepage G, Yesair DW, Ronco N, Champagne J, Bureau N, Chemtob S, et al. Effect of an organized lipid matrix on lipid absorption and clinical outcomes in patients with cystic fibrosis. Journal of Pediatrics. 2002;141(2):178–85. doi: 10.1067/mpd.2002.124305. [DOI] [PubMed] [Google Scholar]
- 15.Groleau V, Schall JI, Dougherty KA, Latham NE, Maqbool A, Mascarenhas MR, Stallings VA. Effect of a dietary intervention on growth and energy expenditure in children with cystic fibrosis. J Cyst Fibros. 2014;13(5):768–71. doi: 10.1016/j.jcf.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertolaso C, Groleau V, Schall JI, Maqbool A, Mascarenhas M, Latham NE, et al. Fat-soluble vitamins in cystic fibrosis and pancreatic insufficiency: efficacy of a nutrition intervention. J Pediatr Gastroenterol Nutr. 2014;58(4):443–8. doi: 10.1097/MPG.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schall JI, Mascarenhas MR, Maqbool A, Dougherty KA, Elci O, Wang D-J, et al. Choline supplementation with a structured lipid in children with cystic fibrosis: a randomized placebo-controlled trial. J Pediatr Gastroenterol Nutr. 2015 Oct 9; doi: 10.1097/MPG.0000000000001004. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989;30(1):47–57. doi: 10.1016/0169-2607(89)90122-3. [DOI] [PubMed] [Google Scholar]
- 19.Otten JJ, Hellwig JP, Meyers LD. Dietary DRI Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, D.C: The National Academies Press; 2006. [Google Scholar]
- 20.Lohman T, Roche AR, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 22.Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. 1981;34(11):2540–5. doi: 10.1093/ajcn/34.11.2540. [DOI] [PubMed] [Google Scholar]
- 23.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–80. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 24.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutrition. 1990;6(3):213–21. [PubMed] [Google Scholar]
- 25.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 26.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15(2):75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 27.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 28.Lepage N, McDonald N, Dallaire L, Lambert M. Age-specific distribution of plasma amino acid concentrations in a healthy pediatric population. Clin Chem. 1997;43(12):2397–402. [PubMed] [Google Scholar]
- 29.Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem. 2003;49(2):286–94. doi: 10.1373/49.2.286. [DOI] [PubMed] [Google Scholar]
- 30.Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem. 2002;74(18):4734–40. doi: 10.1021/ac025624x. [DOI] [PubMed] [Google Scholar]
- 31.Hicks JM, Cook J, Godwin ID, Soldin SJ. Vitamin B12 and folate. Pediatric reference ranges. Arch Pathol Lab Med. 1993;117(7):704–6. [PubMed] [Google Scholar]
- 32.Soldin OP, Sharma H, Husted L, Soldin SJ. Pediatric reference intervals for aldosterone, 17alpha-hydroxyprogesterone, dehydroepiandrosterone, testosterone and 25-hydroxy vitamin D3 using tandem mass spectrometry. Clin Biochem. 2009;42(9):823–7. doi: 10.1016/j.clinbiochem.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu AHB. Tietz Clinical Guide to Laboratory Tests. 4. St. Louis, Missouri: W. B. Saunders Company, Elsevier Press; 2006. [Google Scholar]
- 34.Hubbard VS, Towne D, Schulman JD. Plasma, leukocyte and fibroblast amino acid levels in cystic fibrosis (CF) Pediatr Res. 1981;15:632. [Google Scholar]
- 35.Armstrong MD, Stave U. A study of plasma free amino acid levels. VI. High plasma glycine levels of some women. Metabolism. 1973;22(6):835–9. doi: 10.1016/0026-0495(73)90055-3. [DOI] [PubMed] [Google Scholar]
- 36.Nelson DL, Cox MM. Amino Acids Oxidation and the Production of Urea. 5. New York: Sara Tenney; 2008. [Google Scholar]
- 37.Sattar N, Scherbakova O, Ford I, O’Reilly DS, Stanley A, Forrest E, et al. Elevated alanine aminotransferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the west of Scotland coronary prevention study. Diabetes. 2004;53(11):2855–60. doi: 10.2337/diabetes.53.11.2855. [DOI] [PubMed] [Google Scholar]
- 38.Ohlson LO, Larsson B, Bjorntorp P, Eriksson H, Svardsudd K, Welin L, et al. Risk factors for type 2 (non-insulin-dependent) diabetes mellitus. Thirteen and one-half years of follow-up of the participants in a study of Swedish men born in 1913. Diabetologia. 1988;31(11):798–805. doi: 10.1007/BF00277480. [DOI] [PubMed] [Google Scholar]
- 39.Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51(6):1889–95. doi: 10.2337/diabetes.51.6.1889. [DOI] [PubMed] [Google Scholar]
- 40.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32(9):1626–31. doi: 10.2337/dc09-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardin DS, Ahn C, Rice J, Rice M, Rosenblatt R. Elevated gluconeogenesis and lack of suppression by insulin contribute to cystic fibrosis-related diabetes. J Investig Med. 2008;56(3):567–73. doi: 10.2310/JIM.0b013e3181671788. [DOI] [PubMed] [Google Scholar]
- 42.Austin A, Kalhan SC, Orenstein D, Nixon P, Arslanian S. Roles of insulin resistance and beta-cell dysfunction in the pathogenesis of glucose intolerance in cystic fibrosis. J Clin Endocrinol Metab. 1994;79(1):80–5. doi: 10.1210/jcem.79.1.8027259. [DOI] [PubMed] [Google Scholar]
- 43.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–77. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 44.Waskiewicz A, Sygnowska E, Broda G. Dietary intake of vitamins B6, B12 and folate in relation to homocysteine serum concentration in the adult Polish population - WOBASZ Project. Kardiol Pol. 2010;68(3):275–82. [PubMed] [Google Scholar]
- 45.Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trial. JAMA. 2002;288(8):973–9. doi: 10.1001/jama.288.8.973. [DOI] [PubMed] [Google Scholar]
- 46.Kalantar-Zadeh K. Recent advances in understanding the malnutrition-inflammation-cachexia syndrome in chronic kidney disease patients: What is next? Semin Dial. 2005;18(5):365–9. doi: 10.1111/j.1525-139X.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 47.Innis SM, Davidson AG, Chen A, Dyer R, Melnyk S, James SJ. Increased plasma homocysteine and S-adenosylhomocysteine and decreased methionine is associated with altered phosphatidylcholine and phosphatidylethanolamine in cystic fibrosis. J Pediatr. 2003;143(3):351–6. doi: 10.1067/S0022-3476(03)00326-3. [DOI] [PubMed] [Google Scholar]
- 48.Holm PI, Bleie O, Ueland PM, Lien EA, Refsum H, Nordrehaug JE, et al. Betaine as a determinant of postmethionine load total plasma homocysteine before and after B-vitamin supplementation. Arterioscler Thromb Vasc Biol. 2004;24(2):301–7. doi: 10.1161/01.ATV.0000114569.54976.31. [DOI] [PubMed] [Google Scholar]
- 49.Jacob RA, Jenden DJ, Allman-Farinelli MA, Swendseid ME. Folate nutriture alters choline status of women and men fed low choline diets. J Nutr. 1999;129(3):712–7. doi: 10.1093/jn/129.3.712. [DOI] [PubMed] [Google Scholar]
- 50.Brosnan JT, Brosnan ME. Branched-chain amino acids: enzyme and substrate regulation. J Nutr. 2006;136(1 Suppl):207S–11S. doi: 10.1093/jn/136.1.207S. [DOI] [PubMed] [Google Scholar]
- 51.Remesy C, Fafournoux P, Demigne C. Control of hepatic utilization of serine, glycine and threonine in fed and starved rats. J Nutr. 1983;113(1):28–39. doi: 10.1093/jn/113.1.28. [DOI] [PubMed] [Google Scholar]