Abstract
Background: Previous studies indicated that nonpurified and purified commercially available control murine diets have different metabolic effects with potential consequences on hepatic methionine metabolism and liver histology.
Methods: We compared the metabolic and histological effects of commercial nonpurified (13% calories from fat; 57% calories from carbohydrates with 38 grams/kg of sucrose) and purified control diets (12% calories from fat; 69% calories from carbohydrates with ∼500 grams/kg of sucrose) with or without choline supplementation administered to C3H mice with normal lipid and methionine metabolism. Diets were started 2 weeks before mating, continued through pregnancy and lactation, and continued in offspring until 24 weeks of age when we collected plasma and liver tissue to study methionine and lipid metabolism.
Results: Compared to mice fed nonpurified diets, the liver/body weight ratio was significantly higher in mice fed either purified diet, which was associated with hepatic steatosis and inflammation. Plasma alanine aminotransferase levels were higher in mice receiving the purified diets. The hepatic S-adenosylmethionine (SAM)/S-adenosylhomocysteine (SAH) ratio was higher in female mice fed purified compared to nonpurified diet (4.6 ± 2 vs. 2.8 ± 1.9; P < 0.05). Choline supplementation was associated with improvement of some parameters of lipid and methionine metabolism in mice fed purified diets.
Conclusions: Standard nonpurified and purified diets have significantly different effects on development of steatosis in control mice. These findings can help in development of animal models of fatty liver and in choosing appropriate laboratory control diets for control animals.
Introduction
Commercial murine purified and nonpurified diets are two common types of laboratory research diets, which have the potential to influence gene expression and alter hepatic metabolism. Previous studies in laboratory animals have demonstrated that purified and nonpurified diets variably impact cell physiology and metabolic processes, including lipid metabolism.1–6 A recent study showed that the composition of animal diets, including the use of natural versus purified components, markedly affected hepatic gene expression.1
The effects of purified and nonpurified diets on the physiology and pathophysiology of hepatic methionine metabolism in mice have not been studied. DNA methylation plays a crucial role in the control of gene expression and nuclear DNA architecture,7–9 including cell differentiation and embryonic development.10 The importance of epigenetic regulation through DNA methylation has been further noted for variably altering the onset and progression of many human diseases.11–14 Methionine metabolism is regulated by the principal methyl donor S-adenosylmethionine (SAM) to supply the methyl groups needed for all methylation reactions,7 including DNA methylation. SAM is generated from methionine, which is an essential amino acid acquired and maintained through dietary ingestion. Methionine can also be regenerated from the remethylation of homocysteine through methionine synthase (MS) or alternatively through betaine–homocysteine methyltransferase (BHMT). SAM allows for methylation reactions to occur, consequently forming S-adenosylhomocysteine (SAH), the principal inhibitor for the same methylation reactions.7,15,16 With SAM as the main methyl donor and SAH as the main inhibitor of methylation reactions, the balance between SAM and SAH is crucial in maintaining normal cell physiology.15,17,18 Disruption of hepatic methyl balance in mice and humans may change the levels of SAM and SAH, negatively affecting transmethylation reactions. Such phenomena have been shown to lead to the development of liver injury, including hepatic steatosis.7,15–17 Choline serves as a precursor to the methyl donor betaine, which is the substrate for the transmethylation reaction of homocysteine to methionine. Increasing choline increases the amount of methionine that can be converted to SAM, thus elevating the levels of SAM and consequently the SAM to SAH ratio.15
Choline deficiency has been associated with fatty liver in adult life.19
The methyl group content of maternal diets can affect DNA methylation with consequences on gene expression and eventual disease presentation in the offspring.12,20 The aim of the present analysis was to examine the effects of maternal purified and nonpurified diets on male and female progeny methionine and lipid metabolism.12,16 We compared the effects of two standard control diets (nonpurified and purified) and one supplemented purified diet characterized by different sucrose and choline content on wild-type mice with normal lipid and methionine metabolism. Diets were provided preconception through lactation and through 24 weeks of age in subsequent progeny.
Materials and Methods
Animal model and experimental protocols
We used the control C3HeB/FeJ (C3H) mouse model originally purchased from The Jackson Laboratory (www.jax.org) and maintained at our UC Davis in-house colony. C3H mice were maintained according to guidelines set by the American Association of Accreditation of Laboratory Animal Care. All feeding and animal care protocols were further reviewed and approved annually by the UC Davis IACUC (Institutional Animal Care and Use Committee, UC Davis, Davis, CA). The animal room was maintained at 20°C–23°C and 45%–65% relative humidity with a 14-hr light/10-hr dark light cycle.
Virgin female breeders were placed on their respective diets 2 weeks before mating and remained on their diets through mating, gestation, and lactation. Progeny were weaned at 3 weeks of age onto the same diet as their dam and remained on the diet until tissues were harvested. Progeny were anesthetized at 24 weeks of age with isoflurane, exsanguinated through retro-orbital bleed, and euthanized with isoflurane followed by cervical dislocation. Livers were dissected and weighed, flash-frozen in liquid nitrogen, and stored at −80°C until further analysis.
The study design does not include a nonpurified choline-supplemented diet since the present results derive from a secondary analysis of existing data.
Diet composition of nonpurified, nonsupplemented purified, and choline-supplemented purified diets
Effects of three diets were examined: nonpurified (LabDiet 5001; Purina), nonsupplemented purified (AIN-76A, #110098; Dyets, Inc.), and choline-supplemented purified (modified AIN-76A, #110184; Dyets, Inc.). Table 1 describes the composition of each diet, the main difference occurring in the concentration of dietary choline: nonpurified (choline chloride 2250 ppm), nonsupplemented purified (choline chloride 1100 ppm), and choline-supplemented purified (choline chloride 5000 ppm). Sucrose was about 14 times higher in the purified diets than in the nonpurified diet. Although there were no differences in the caloric intake (%) of fat, the nonpurified diet contained a variety of fat products, including animal sources, whereas the fat source of the purified diets was solely corn oil.
Table 1.
Diet Composition
| Nonpurified | Nonsupplemented purified | Choline-supplemented purified | |
|---|---|---|---|
| Protein (grams/kg) | 304.7 | 203 | 203 |
| % Calories | 30 | 19 | 19 |
| Methionine (grams/kg) | 5.9 | 3 | 3 |
| Carbohydrates (grams/kg) | 580.8 | 660.9 | 657 |
| % Calories | 57 | 69 | 69 |
| Starch (grams/kg) | 210 | 150 | 150 |
| Sucrose (grams/kg) | 38.3 | 510.9 | 507 |
| Glucose (grams/kg) | 1.9 | — | — |
| Fructose (grams/kg) | 2.7 | — | — |
| Lactose (grams/kg) | 20.1 | — | — |
| Fat (grams/kg) | 60.9 | 50 | 50 |
| % Calories | 13 | 12 | 12 |
| Cholesterol (mg/kg) | 209 | — | — |
| Linoleic acid (%) | 1.05 | 60 | 60 |
| Linolenic acid (%) | 0.09 | 0.6 | 0.6 |
| Total saturated fatty acids (%) | 1.48 | 13 | 13 |
| Minerals (grams/kg) | 70 | 35* | 35a |
| Choline chloride (grams/kg) | 2.25 | 1.10 | 5.00 |
| Fiber (grams/kg) | 53 | 50 | 50 |
Minerals in purified diets derived from same components as nonpurified with exception of fluorine and cobalt.
Additional methods are described in the Supplementary Materials and Methods section (Supplementary Data are available online at www.liebertpub.com/met). Primer sequences used are listed in Table 2.
Table 2.
Quantitative Real-Time PCR Primer Sequences of Selected Genes
| Gene | Primer | Sequence 5′–3′ | |
|---|---|---|---|
| Acc1 | Acetyl-coenzyme A carboxylase alpha | F | CGAAACTCCCAGAACTGCTCTTAA |
| R | GAGTTGGGATACCTGCAGTTTGA | ||
| Dnmt1 | DNA methyltransferase 1 | F | CCAGCTGCCAAACGGAGA |
| R | CCTCGGGAGTCTCTGGAGCTA | ||
| Dnmt3a | DNA methyltransferase 3a | F | CACTGGAGTAGGCGCTGAGAC |
| R | CAGCAAAGGGCCTTCCATAG | ||
| Dnmt3b | DNA methyltransferase 3b | F | CCGTTCGACTTGGTGATTGG |
| R | GGGCAGGATTGACGTTAGAGAG | ||
| Fasn | Fatty acid synthase | F | AGCAGGCACACACAATGGAC |
| R | AGTGTTCGTTCCTCGGAGTGA | ||
| Gpat | Glycerol-3-phosphate acyltransferase, mitochondrial | F | GAACTGAACTGGAGAAGTGACGAG |
| R | AGCAGAGCTGTAGGCTTCTAGCA | ||
| Grp78 | Glucose-regulating protein 78 | F | GTGGAGATCATAGCCAACG |
| R | CACATACGACGGCGTGATGC | ||
| Pemt | Phosphatidylethanolamine N-methyltransferase | F | TACTTCCTGGGCCTTGCATTC |
| R | ACGCTGAAGGGAAATGTGGTC | ||
| Pparα | Peroxisome proliferator-activated receptor alpha | F | CGATGCTGTCCTTGATGA |
| R | GAAGTCAAACTTGGGTTCCATGAT | ||
| Sahh | S-adenosylhomocysteine hydrolase | F | ATCCTTGGCCGGCACTTT |
| R | TTCTTTAGCCAGTAGCGGTCCA | ||
| Scd1 | Stearoyl-coenzyme A desaturase 1 | F | TCATTCTCATGGTCCTGCTGC |
| R | CAGAGCGCTGGTCATGTAGTAGA | ||
| Srebf1 | Sterol regulatory element-binding transcription factor 1 | F | CTGGCTTGGTGATGCTATGTTG |
| R | GACCATCAAGGCCCCTCAA | ||
Results
Body weights, liver/body weight ratio, plasma alanine aminotransferase, and hepatic iron
Twenty-four-week-old mice fed nonsupplemented purified and choline-supplemented purified diets had higher body weights and liver/body weight ratios compared to mice fed nonpurified diet (Table 3). Within each sex, plasma alanine aminotransferase (ALT) levels were two to three times higher in mice fed nonsupplemented purified diets compared to mice fed nonpurified diets, and females fed choline-supplemented purified diet had decreased ALT levels by 50% compared to nonsupplemented purified females (P < 0.001). For the nonpurified diet groups, males presented with ALT levels 1.5-fold higher than females on the same diet. Mean hepatic iron concentrations in female mice fed the nonpurified diet were significantly 1.5 times higher compared to their male counterparts on the same diet. Male mice on purified diets had higher hepatic iron than males on nonpurified diets.
Table 3.
Effects of Diets on Body and Liver Weight, ALT, Hepatic Iron, and Liver Histology
| Nonpurified | Nonsupplemented purified | Choline-supplemented purified | ||||
|---|---|---|---|---|---|---|
| Females (12) | Males (10) | Females (16) | Males (17) | Females (5) | Males (5) | |
| Body weight (grams) | 31.2 ± 1.0a | 36.9 ± 3.5*a | 40.2 ± 3.2b | 40.2 ± 2.1b | 38.6 ± 3.2b | 40.3 ± 2.4ab |
| Liver/body weight | 0.05 ± 0.0025a | 0.05 ± 0.0013*a | 0.07 ± 0.01b | 0.07 ± 0.01b | 0.06 ± 0.01a | 0.07 ± 0.01b |
| ALT (U/L) | 20.7 ± 4.4a | 31.2 ± 11.5*a | 66.8 ± 26.8b | 58.3 ± 24.6b | 29.3 ± 21.4a | 42.9 ± 14.5ab |
| Iron (μg/g liver) | 32.9 ± 3.2a | 17.5 ± 1.6*a | 25.6 ± 4.9b | 23.7 ± 3.03b | 33.4 ± 6.8a | 25.2 ± 6.9b |
| Steatosis score | 0a | 0a | 3.63 ± 0.8b | 3.35 ± 1.1b | 3.00 ± 1.0b | 3.20 ± 0.8b |
In parentheses is the number of mice in each dietary group. Data values are expressed as mean ± SD. Values with * are significantly different between sexes within dietary group. Values with different letters are significantly different between diets within the same sex (all P < 0.05).
ALT, alanine aminotransferase; SD, standard deviation.
Liver histology
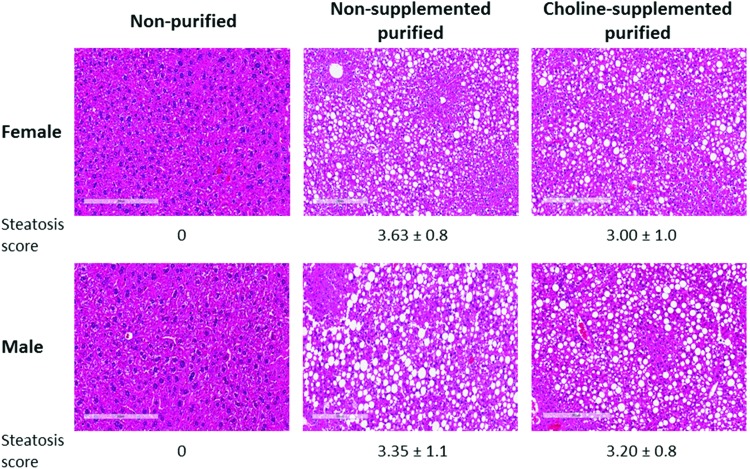
Liver histology scores demonstrated increased lipid deposition in mice fed both nonsupplemented and choline-supplemented purified diets compared to mice fed nonpurified diet where there was no hepatic steatosis. The steatosis score was mildly improved after choline supplementation in mice fed purified diets, but the difference was not significant (Fig. 1). There was a positive correlation between ALT and steatosis (r = 0.69, P < 0.0001).
FIG. 1.
Increased lipid deposition in livers from mice fed purified diets. Hepatic steatosis scores were measured in mice fed with nonpurified (female: n = 12; male: n = 10), nonsupplemented purified (female: n = 16; male: n = 17), and choline-supplemented purified diets (female: n = 5; male: n = 5). Steatosis scores were increased in mice fed with nonsupplemented (P < 0.05 for both female and male) or choline-supplemented purified diet (P < 0.05 for both female and male) compared to those seen in mice fed with nonpurified diet. However, there appeared to be no difference between mice fed nonsupplemented and choline-supplemented purified diets. Data shown are presented as mean ± standard deviation (SD). Representative images of liver histology from each group of mice have been shown. Color images available online at www.liebertpub.com/met
Plasma and liver cholesterol and triglycerides
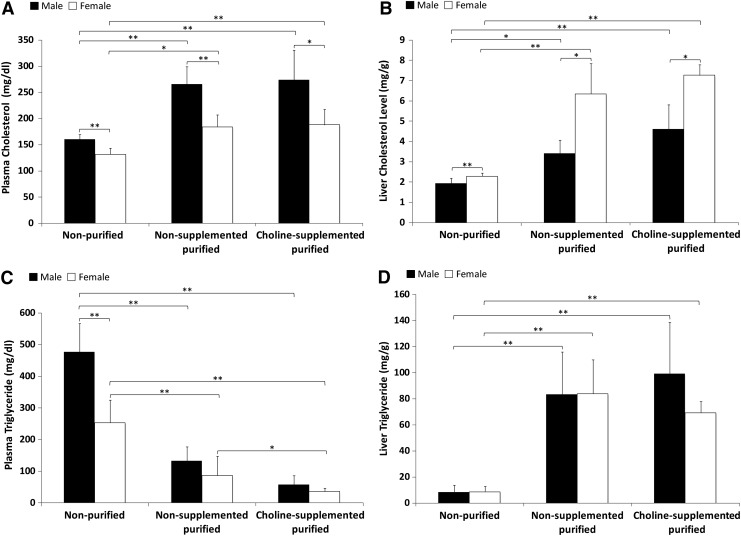
Overall, both sexes receiving nonpurified diet had significantly lower plasma and liver cholesterol levels compared to those on purified diets (Fig. 2A, B) (P < 0.0001). Liver and plasma cholesterol levels in mice fed the purified diets were not affected by choline supplementation. Males presented higher plasma cholesterol levels, whereas females presented higher liver cholesterol levels compared to their counterparts in every dietary group. Plasma triglyceride levels were higher in males and females fed the nonpurified diet, whereas liver triglycerides presented the opposite trend (Fig. 2C, D). Males on the nonpurified diet presented with plasma triglyceride values 61% higher than females on the same diet (P < 0.001). Choline supplementation was associated with reduction of plasma triglycerides in female mice only. There was a positive correlation between hepatic steatosis and plasma cholesterol (r = 0.27), liver cholesterol (r = 0.78), and liver triglyceride (r = 0.86). Plasma triglyceride was negatively correlated with steatosis when combining data from all groups (P < 0.001).
FIG. 2.
Plasma and liver cholesterol and triglycerides. Cholesterol levels in plasma (A) and liver (B) as well as triglyceride levels in plasma (C) and liver (D) were measured in mice fed nonpurified (female: n = 12; male: n = 10), nonsupplemented purified (female: n = 16; male: n = 17), and choline-supplemented purified diets (female: n = 5; male: n = 5) by enzymatic colorimetric assays. Data shown are presented as mean ± SD. *P < 0.05; **P < 0.01.
Hepatic SAM and SAH levels, SAH hydrolase and DNA methyltransferase transcript levels, and global DNA methylation
As shown in Table 4, SAM and SAH were both significantly higher in nonpurified diet male and female mice compared to nonsupplemented purified diet. SAH levels were lower in the nonsupplemented purified diet group than in the nonpurified or choline-supplemented group. Within the purified diet groups, choline supplementation restored SAM to the nonpurified diet group level and increased SAH in both sexes.
Table 4.
Hepatic Levels of SAM and SAH, Transcript Levels of SAH Hydrolase and DNA Methyltransferases
| Nonpurified | Nonsupplemented purified | Choline-supplemented purified | ||||
|---|---|---|---|---|---|---|
| Females (12) | Males (10) | Females (16) | Males (17) | Females (5) | Males (5) | |
| SAM (nmol/gram) | 79.5 ± 14.3a | 74.2 ± 13.7a | 55.9 ± 16.6b | 48.1 ± 14.1b | 95.9 ± 21a | 74.5 ± 18.6a |
| SAH (nmol/gram) | 35.6 ± 13.5a | 44.5 ± 16.1a | 18.1 ± 5.7b | 19.7 ± 8.9b | 23.0 ± 7.1ab | 27.6 ± 6.5ab |
| SAM/SAH | 2.8 ± 1.9 | 1.9 ± 0.96 | 3.4 ± 1.4 | 2.8 ± 1.1 | 4.6 ± 2 | 2.9 ± 1.5 |
| Sahh | 1.06 ± 0.13a | 0.94 ± 0.11*a | 0.36 ± 0.06b | 0.49 ± 0.09*b | 0.99 ± 0.11a | 0.90 ± 0.11a |
| Dnmt1 | 1.05 ± 0.10 | 0.96 ± 0.13 | 1.08 ± 0.12a | 0.83 ± 0.18b | 0.98 ± 0.13 | 0.93 ± 0.13 |
| Dnmt3a | 0.98 ± 0.18 | 1.06 ± 0.20 | 0.81 ± 0.14a | 0.78 ± 0.19 | 1.15 ± 0.18b | 0.98 ± 0.20 |
| Dnmt3b | 1.35 ± 0.65a | 0.92 ± 0.43a | 0.32 ± 0.07b | 0.27 ± 0.05b | 0.44 ± 0.07b | 0.57 ± 0.23a |
In parentheses is the number of mice in each dietary group. Data values are expressed as mean ± SD. Values with * are significantly different between sexes within dietary group. Values with different letters are significantly different between diets within the same sex (all P < 0.05).
SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine.
Hepatic transcript levels of SAH hydrolase (Sahh), the bidirectional enzyme responsible for converting SAH to homocysteine or vice versa, were two to three times downregulated in nonsupplemented purified diet compared to nonpurified diet (P < 0.001) (Table 4). Sahh transcript levels were negatively correlated with moderate (grade 3) steatosis (r = −0.56, P < 0.0001). Choline supplementation was associated with increased Sahh transcript levels, reaching nonpurified diet levels. Females on nonsupplemented purified diet presented with lower transcript levels of Sahh than their male counterparts (P < 0.05), whereas mice of each sex in the nonsupplemented purified diet group had lower Sahh transcript levels than in the nonpurified and choline-supplemented purified diet groups (P < 0.05). DNA methyltransferase 1 (Dnmt1) transcript levels were lower in male mice on nonsupplemented purified diet compared to females but no other differences were detected. Among females, Dnmt3a transcript levels were lower in the nonsupplemented purified diet group. Dnmt3b transcript levels were lower in both females and males fed a nonsupplemented purified diet but increased after choline supplementation especially in males (Table 4).
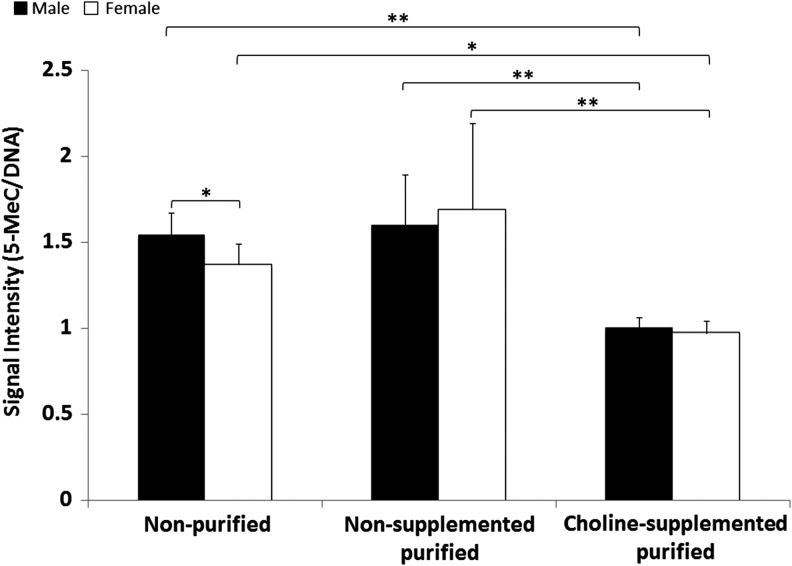
Dot blot analysis for hepatic global DNA methylation revealed overall decreased DNA methylation levels associated with choline-supplemented purified diet in both sexes (Fig. 3) (P < 0.001). Males fed nonpurified diet demonstrated 12% higher levels of global DNA methylation than the females in that same diet group.
FIG. 3.
Decreased global hepatic DNA methylation level in mice fed choline-supplemented purified diets. Global DNA methylation level was measured in mice fed nonpurified (female: n = 12; male: n = 10), nonsupplemented purified (female: n = 16; male: n = 17), and choline-supplemented purified diets (female: n = 5; male: n = 5) by dot-blot analyses. Data shown are presented as mean ± SD. *P < 0.05; **P < 0.01.
Hepatic transcript levels of selected genes related to lipogenesis, fatty acid synthesis, and fatty acid oxidation
Transcript levels of genes associated with increased fatty acid synthesis and lipogenesis, including Acc1 (acetyl-coenzyme A [CoA] carboxylases 1), Fasn (fatty acid synthase), Gpat (glycerol-3-phosphate acyltransferase, mitochondrial), and Scd1 (stearoyl-CoA desaturase 1), were significantly upregulated in the nonsupplemented purified diet group compared to nonpurified diet, whereas transcript levels of Pparα (peroxisome proliferator-activated receptor alpha), related to fatty acid oxidation, were downregulated (Table 5). Scd1 was greater in each purified diet than in the nonpurified diet. Of note, Pparα transcript levels were increased by choline supplementation reaching nonpurified diet levels in both sexes. Choline supplementation was associated with downregulation of Acc1 in female mice. Expression of Acc1 and Scd1 was highest among females on nonsupplemented purified diet compared to nonpurified (P < 0.001) and choline-supplemented purified diet (P < 0.05). There were positive correlations between the steatosis grade and transcript levels of Acc1 (r = 0.73), Fasn (r = 0.57), Gpat (r = 0.68), and Scd1 (r = 0.60) (all P < 0. 0001).
Table 5.
Hepatic Transcript Levels of Selected Genes Related to Lipid Metabolism
| Nonpurified | Nonsupplemented purified | Choline-supplemented purified | ||||
|---|---|---|---|---|---|---|
| Females (12) | Males (10) | Females (16) | Males (17) | Females (5) | Males (5) | |
| Acc1 | 1.33 ± 0.36a | 0.76 ± 0.17*a | 6.75 ± 1.97b | 3.8 ± 0.79*b | 4.43 ± 1.49c | 3.77 ± 0.65b |
| Fasn | 1.82 ± 1.25a | 0.84 ± 0.5*a | 4.18 ± 2.73b | 3.03 ± 1.45b | 3.63 ± 2.04ab | 3.37 ± 2.05b |
| Gpat | 1.06 ± 0.39a | 1.08 ± 0.39a | 3.34 ± 1.23b | 3.01 ± 0.68b | 3.16 ± 0.91b | 3.24 ± 0.54b |
| Pparα | 1.06 ± 0.34a | 1.02 ± 0.24a | 0.63 ± 0.25b | 0.75 ± 0.23b | 1.33 ± 0.29a | 1.00 ± 0.2a |
| Scd1 | 1.37 ± 0.69a | 0.87 ± 0.39a | 2.85 ± 0.44b | 2.67 ± 0.53b | 2.16 ± 0.95ab | 2.31 ± 0.21b |
| Pemt | 1.07 ± 0.23a | 0.96 ± 0.19*a | 0.66 ± 0.09b | 0.48 ± 0.11*b | 0.74 ± 0.14b | 0.45 ± 0.15b |
| Srebf1 | 1.12 ± 0.2a | 0.9 ± 0.18*a | 0.83 ± 0.16a | 0.88 ± 0.25a | 1.6 ± 0.4b | 1.24 ± 0.19b |
| Grp78 | 1.00 ± 0.37a | 1.09 ± 0.23a | 0.41 ± 0.04b | 0.41 ± 0.06b | 0.64 ± 0.08ab | 0.73 ± 0.17c |
In parentheses is the number of mice in each dietary group. Data values are expressed as mean ± SD. Values with * are significantly different between sexes within dietary group. Values with different letters are significantly different between diets within the same sex (all P < 0.05).
Transcript levels of Srebf1 (sterol regulatory element-binding transcription factor 1), associated with lipogenesis, were higher in mice fed choline-supplemented diet than nonsupplemented purified diet. Transcript levels of Grp78 (glucose-regulated protein 78), representing endoplasmic reticulum stress, were also increased with choline supplementation in males and females.
Pemt (phosphatidylethanolamine N-methyltransferase), which catalyzes the synthesis of phosphatidylcholine and whose reduced levels are linked to hepatic steatosis21 primarily due to reduced lipid export from the liver, was significantly upregulated in mice fed a nonpurified diet compared to the other diets and in females fed nonpurified diet compared to their male counterparts (P < 0.001). Pemt transcript levels were negatively correlated with steatosis grade (r = −0.76, P < 0.0001), plasma cholesterol (r = −0.86), liver cholesterol (r = −0.46), and liver triglycerides (r = −0.73) (all P < 0.0001).
Discussion
The present study provided several original findings. First, the composition of standard control purified diet (nonsupplemented) differs significantly from the nonpurified diet in one critical component, a 14-fold greater amount of sucrose, which is well-known to alter lipid metabolism.22,23 Second, mice fed purified diets presented with markedly greater hepatic steatosis compared to mice fed nonpurified diets, which was associated with an overall upregulation of gene transcripts relating to fatty acid synthesis and lipogenesis and an overall downregulation of gene transcripts relating to fatty acid oxidation. Third, choline supplementation of purified diets that was started in the prenatal phase offered significant improvement of parameters relating to methionine and lipid metabolism, suggesting that dietary choline exerts beneficial effects through its ability to restore methionine metabolism parameters to control levels. Fourth, relevant differences between males and females observed within each of the three diets suggested that, in addition to diet composition, sex plays an essential role in lipid deposition and development of hepatic steatosis, and males and females respond differently to methyl donor supplementation.
The primary carbohydrate in each purified diet was sucrose, which in high levels has been seen to alter lipid parameters as well as induce hepatic steatosis. The amount of sucrose found in both our purified diets (∼53% of total caloric value or ∼500 grams/kg diet) is comparable to these studies, whose high-sucrose diets induced elevated hepatic triglycerides (∼580 grams/kg sucrose),24 dyslipidemia and steatohepatitis (68% calories from sucrose),25,26 and hepatic steatosis among wild-type rats (30% sucrose calories from carbohydrate).27 A high-fat diet caused more steatosis, inflammation, and fibrosis only when associated with high sucrose (30%).28
Of note, purified diets also had a higher content of saturated fatty acids, which can potentially contribute to fatty liver.29 However, the total percentage of calories from fat was much lower compared to previously described high-fat diets30 and the concomitant presence of polyunsaturated fatty acids probably limits the contribution of dietary fat to hepatic steatosis in this model.
Purified diets caused macrovesicular steatosis consistent with previous dietary studies.22 Compared to nonpurified mice, plasma and liver cholesterol levels were significantly increased in purified diets, whereas plasma triglycerides were lower in purified diets. Liver triglycerides were still higher in both purified diets compared to nonpurified diets, and gene transcript levels indicating steatosis in the nonsupplemented purified diet were due to mechanisms of increased lipid synthesis (increased Acc1, Fasn, and Gpat), reduced oxidation (decreased Pparα), and reduced excretion from the liver (reduced Pemt). Downregulation of hepatic Pparα expression has been observed in patients with steatosis from nonalcoholic fatty liver disease.23,24 The absence of steatosis in the nonpurified diet group may have been partly due to the significantly greater level of Pemt, essential to maintain lipid export. Knockouts of Pemt and other choline-related gene deletions have been noted to lead to the development of fatty liver in several mouse models.31,32
Methionine metabolism parameters were significantly improved with choline supplementation by elevating Sahh transcript levels and restoring SAM to nonpurified control levels. Choline supplementation was associated with improvement of some parameters of lipid metabolism but the response was not consistent in all tested genes and in the two sexes. In particular, Acc1 and Pparα transcript levels improved in female mice after choline supplementation. Choline produced changes in plasma triglycerides only in females, but no significant changes in the steatosis score. Similarly, improvement of plasma ALT levels was more evident in females. This incomplete effect may be related to (1) the duration of treatment as longer treatment may have produced significant effects; (2) the opposite effect caused by changes in transcript levels of Pparα (when increased after choline supplementation, should improve steatosis) and Srebf1 (may worsen steatosis); or (3) the studied genes are regulated at different levels other than DNA or histone methylation.
A previous study by Zhu et al.24 demonstrated choline reversal of methylation status of various genes, including Pparα, whose increased transcript levels (relevant to fatty acid oxidation) in our present study were negatively correlated with the reduction in DNA methylation. Furthermore, the improvement in ALT levels, signaling a decrease in liver injury, was correlated with the degree of steatosis. The reduced global DNA methylation following methyl-group supplementation is an apparent discrepancy as previously described.33 Cordero et al.34 assessed global DNA methylation by [3H]dCTP extension assay for rats fed standard control and high-fat sucrose diet before and after supplementing with a methyl-donor cocktail. Rats assigned to control diet were found to have decreased liver global DNA methylation after methyl-donor supplementation. The authors attributed it to potential compensatory mechanisms that affect interconnecting transmethylation metabolic pathways.35
Finally, we found that sex was an important factor in determining the dietary influence on hepatic methionine metabolism and steatosis development. Female mice on the nonsupplemented purified diet developed more steatosis than male counterparts, which was consistent with highest levels of ALT, increased gene transcript level of Acc1 and increased liver triglyceride. Females also demonstrated the most improvement after choline supplementation in the expression of selected gene transcripts and markers of hepatic methionine metabolism with increases in Sahh, Dnmt1, and Pparα, which ultimately lessened the development of steatosis. A previous study also demonstrated strikingly different effects of maternal high-fat diet on the transcriptome and on genes related to epigenetic mechanisms of gene expression regulation.36
While the study design was not originally intended for a comparative diet study, this does not invalidate the findings. Additional groups would be needed to determine if these effects are solely due to maternal diet influence in utero, diet influence during growth and/or adulthood, or a combination of both. The limitations of our study include the lack of a group of mice fed a nonpurified diet supplemented with choline as this would ultimately clarify the effect of choline in the absence of high sucrose concentration. We did not conduct pair-fed experiments and results could be further extended by studies of mitochondrial complex activities.
In conclusion, our study provides novel insight into the potentially detrimental effects on lipid and methionine metabolism caused by the difference in composition of standard nonpurified and purified diets with further differences relevant to sex. Almost all findings can be related to elevated sucrose in the purified diets even though choline did have predicted effects on methionine metabolism. Although we did not directly focus on how sucrose connects to these specific physiological changes, we believe that the consequential effects of purified diet may have been due to the higher composition of sucrose compared to the nonpurified diet. These findings will aid in the selection of appropriate control diets for control animals as well as the development and evaluation of potential fatty liver animal models utilizing nonpurified and purified diets, and will address the importance of methyl-group provision in modifying the risk of developing fatty liver.
Supplementary Material
Acknowledgments
V.M. designed and conducted the research, wrote the article, and had the primary responsibility for the final content; R.S. and N.M.S. conducted the research and wrote the article; K.K.K., R.J.S., K.O., A. Y., J.C.R., and K.J.C. provided essential analysis and contributed to writing the article; K.K. performed statistical analysis and contributed to writing the article; C.H.H. designed the research and provided major contribution to article preparation. All authors had read and approved the final article. This work was supported by funding from NIH grants K08DK084111, R03DK099427 (to V.M.), R01AG039094, R01AG045541, and the Richard A. and Nora Eccles Harrison Endowed Chair in Diabetes Research (to J.C.R.), Veterans Health Administration Biomedical Laboratory Research and Development National Merit Review grant award BX001155 (to K.K.K.). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Renaud HJ, Vui JY, Lu H, et al. Effect of diet on expression of genes involved in lipid metabolism, oxidative stress, and inflammation in mouse liver-insights into mechanisms of hepatic steatosis. PLoS One 2014;9:e88584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoekstra M, Stitzinger M, van Wanrooij EJ, et al. Microarray analysis indicates an important role for FABP5 and putative novel FABPs on a Western-type diet. J Lipid Res 2006;47:2198–2207 [DOI] [PubMed] [Google Scholar]
- 3.Kozul CD, Nomikos AP, Hampton TH, et al. Laboratory diet profoundly alters gene expression and confounds genomic analysis in mouse liver and lung. Chem Biol Interact 2008;173:129–140 [DOI] [PubMed] [Google Scholar]
- 4.Goto H, Takemura N, Ogasawara T, et al. Effects of fructo-oligosaccharide on DSS-induced colitis differ in mice fed nonpurified and purified diets. J Nutr 2010;140:2121–2127 [DOI] [PubMed] [Google Scholar]
- 5.Sahasakul Y, Takemura N, Sonoyama K. Gastric emptying is involved in Lactobacillus colonisation in mouse stomach. Br J Nutr 2014;112:408–415 [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi N, Sonoyama K, Kikuchi H, et al. Gastric colonization of Candida albicans differs in mice fed commercial and purified diets. J Nutr 2005;135:109–115 [DOI] [PubMed] [Google Scholar]
- 7.Mato JM, Martínez-Chantar ML, Lu SC. Methionine metabolism and liver disease. Annu Rev Nutr 2008;28:273–293 [DOI] [PubMed] [Google Scholar]
- 8.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet 2007;8:286–298 [DOI] [PubMed] [Google Scholar]
- 9.Strahl BD, Allis CD. The language of covalent histone modifications. Nature 2000;403:41–45 [DOI] [PubMed] [Google Scholar]
- 10.Phillips T. The role of methylation in gene expression. Nat Educ 2008;1:116 [Google Scholar]
- 11.Kashireddy PR, Rao MS. Sex differences in choline-deficient diet-induced steatohepatitis in mice. Exp Biol Med 2004;229:158–162 [DOI] [PubMed] [Google Scholar]
- 12.Medici V, Shibata NM, Kharbanda KK, et al. Maternal choline modifies fetal liver copper, gene expression, DNA methylation, and neonatal growth in the tx-j mouse model of Wilson disease. Epigenetics 2013;9:286–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker DJ, Eriksson JG, Forsén T, et al. Fetal origins of adult disease: Strength of effects and biological basis. Int J Epidemiol 2002;31:1235–1239 [DOI] [PubMed] [Google Scholar]
- 14.Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr 2007;27:363–388 [DOI] [PubMed] [Google Scholar]
- 15.Le A, Shibata NM, French SW, et al. Characterization of timed changes in hepatic copper concentrations, methionine metabolism, gene expression, and global DNA methylation in the Jackson toxic milk mouse model of Wilson disease. Int J Mol Sci 2014;15:8004–8023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medici V, Shibata NM, Kharbanda KK, et al. Wilson's disease: Changes in methionine metabolism and inflammation affect global DNA methylation in early liver disease. Hepatology 2013;57:555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu SC, Mato JM. S-adenosylmethionine in liver health, injury, and cancer. Physiol Rev 2012;92:1515–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen NC, Yang F, Capecci LM, et al. Regulation of homocysteine metabolism and methylation in human and mouse tissues. FASEB J 2010;24:2804–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leclercq IA, Farrell GC, Field J, et al. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest 2000;105:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davison JM, Mellott TJ, Kovacheva VP, et al. Gestational choline supply regulates methylation of histone H3, expression of histone methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA methylation of their genes in rat fetal liver and brain. J Biol Chem 2009;284:1982–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu X, Song J, Mar MH, et al. Phosphatidylethanolamine N-methyltransferase (PEMT) knockout mice have hepatic steatosis and abnormal hepatic choline metabolite concentrations despite ingesting a recommended dietary intake of choline. Biochem J 2003;370(Pt 3):987–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.del Bas JM, Caimari A, Ceresi E, et al. Differential effects of habitual chow-based and semi-purified diets on lipid metabolism in lactating rats and their offspring. Br J Nutr 2015;113:758–769 [DOI] [PubMed] [Google Scholar]
- 23.Kohjima M, Enjoji M, Higuchi N, et al. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med 2007;20:351–358 [PubMed] [Google Scholar]
- 24.Zhu J, Wu Y, Tang Q, et al. The effects of choline on hepatic lipid metabolism, mitochondrial function and antioxidative status in human hepatic C3A cells exposed to excessive energy substrates. Nutrients 2014;6:2552–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Alessandro ME, Oliva ME, Fortino MA, et al. Maternal sucrose-rich diet and fetal programming: Changes in hepatic lipogenic and oxidative enzymes and glucose homeostasis in adult offspring. Food Funct 2014;3:446–453 [DOI] [PubMed] [Google Scholar]
- 26.Huang W, Dedousis N, O'Doherty RM. Hepatic steatosis and plasma dyslipidemia induced by a high-sucrose diet are corrected by an acute leptin infusion. J Appl Physiol 2007;102:2260–2265 [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Wang C, Chen H. Green, oolong and black tea extracts modulate lipid metabolism in hyperlipidemia rats fed high-sucrose diet. J Nutr Biochem 2001;12:14–20 [DOI] [PubMed] [Google Scholar]
- 28.Ishimoto T, Lanaspa MA, Rivard CJ, et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology 2013;58:1632–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res 2013;52:165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Wit N, Derrien M, Bosch-Vermeulen H, et al. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol 2012;303:G589–G599 [DOI] [PubMed] [Google Scholar]
- 31.Zeisel SH. Choline: Critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 2006;26:229–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waite KA, Cabilio NR, Vance DE. Choline deficiency-induced liver damage is reversible in Pemt(−/−) mice. J Nutr 2002;132:68–71 [DOI] [PubMed] [Google Scholar]
- 33.Bermingham EN, Bassett SA, Young W, et al. Post-weaning selenium and folate supplementation affects gene and protein expression and global DNA methylation in mice fed high-fat diet. BMC Med Genomics 2013;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cordero P, Campion J, Milagro FI, et al. Transcriptomic and epigenetic changes in early liver steatosis associated to obesity: Effect of dietary methyl donor supplementation. Mol Genet Metab 2013;110:388–395 [DOI] [PubMed] [Google Scholar]
- 35.Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine and choline. J Nutr 2002;132(8 Suppl):2333S–2335S [DOI] [PubMed] [Google Scholar]
- 36.Gabory A, Ferry L, Fajardy I, et al. Maternal diets trigger sex-specific divergent trajectories of gene expression and epigenetic systems in mouse placenta. PLos One 2012;7:e47986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.