Abstract
Background
In the context of rising obesity in South Asia, it is unclear whether the “South Asian phenotype”—described as high glucose, low high density lipoprotein cholesterol (HDL) and high triglycerides at normal ranges of body mass index (BMI)—continues to be disproportionately exhibited by contemporary South Asians relative to other race/ethnic groups.
Aim
We assessed the distinctiveness of the South Asian cardiometabolic profile by comparing the prevalence of combined high glucose, high triglycerides, and low HDL (“combined dysglycemia and dyslipidemia”) in resident South Asians with four race/ethnic groups in the United States (US): Asians, Blacks, Hispanics, and Whites overall and by BMI category.
Methods
South Asian data were from the 2010–11 Center for cArdiometabolic Risk Reduction in South Asia Study, representative of Chennai and New Delhi, India and Karachi, Pakistan. United States data were from the 2011–2012 National Health and Nutrition Examination Survey, representative of the US population. Combined dysglycemia and dyslipidemia was defined as fasting blood glucose ≥ 126 mg/dL and triglyceride:HDL ratio>4. Logistic regression was used to estimate the relative odds (OR) and 95% CIs of combined dysglycemia and dyslipidemia associated with each race/ethnic group (ref=US Whites). Models were estimated among adults aged 20–79 years by sex and BMI category and accounted for age, education, and tobacco use.
Results
Data from 8,448 resident South Asians, 274 US Asians, 404 US Blacks, 308 US Hispanics, and 703 US Whites without previously known diabetes were analyzed. In the normal BMI range of 18.5 to 24.9 kg/m2, the prevalence of combined dysglycemia and dyslipidemia among men and women, respectively, was 33% and 11% in resident South Asians, 15% and 1% in US Asians, 5% and 2% in US Blacks, 11% and 2% in US Hispanics, and 8% and 2% in US Whites. Compared with US Whites, South Asians were more likely to present with combined dysglycemia and dyslipidemia at all categories of BMI for men and at BMI 18.5 to 29.9 for women in adjusted models. The most pronounced difference between South Asians and US Whites was observed at normal weight (adjusted odds ratio [aOR]= 4.98; 2.46–10.07 for men and aOR=9.09; 2.48–33.29 for women).
Conclusions
Between 8% to 15% of US men and 1% to 2% of US women of diverse race/ethnic backgrounds exhibited dysglycemia and dyslipidemia at levels of BMI considered “healthy,” consistent with the cardiometabolic profile described as the “South Asian Phenotype.” Urban South Asians, however, were 5 to 9 times more likely to exhibit dysglycemia and dyslipidemia in the “healthy” BMI range compared with any other US race/ethnic group.
INTRODUCTION
South Asians, or people who trace their ancestry to the Indian subcontinent, have been observed to exhibit higher indicators of cardiometabolic risk relative to White populations despite lower body mass index; this has been termed the “South Asian” or “Asian Indian phenotype” [1,2]. Among the consistently noted distinctive features of this phenotype are abdominal adiposity combined with glucose intolerance and specific dyslipidemias—such as low levels of high-density lipoprotein cholesterol (HDL), high levels of triglycerides and low-density lipoprotein (LDL), and high triglycerides relative to HDL—at levels of overall body mass considered normal in other populations[1–3]. Authors have also variously described additional features such as excess body fat per unit BMI, truncal subcutaneous fat, higher c-reactive protein, and lower adiponectin as part of the South Asian phenotypic package; others have equated the South Asian phenotype with metabolic syndrome [1–3]. The South Asian phenotype has been invoked to explain, in part, reports of elevated coronary heart disease, events, and cardiovascular mortality among migrant South Asians relative to local populations in diverse settings that date back several decades [4–8].
Although cardiometabolic risk associated with South Asian race/ethnicity may reflect any combination of common environmental exposures and stressors, genetic predisposition, shared behaviors, or similar social circumstances in migrant settings, long-term undernutrition at the population level is a leading hypothesis for the phenotype. Specifically, researchers posit that early life undernutrition (reflected in low birth weight and poor early life growth) predisposes South Asians to elevated metabolic risk later in life in this population [9–13]. Recently observed transitions from undernutrition to overnutrition, however, appear to be altering the low average weight status traditionally associated with South Asians. For example, the most recent national data indicated that overweight and obesity increased (and underweight decreased) from 1996 to 2006 among women in multiple South Asian countries [14], and the prevalence of overweight was higher than that of underweight among middle-aged urban adults in India [15]. Similarly, recent studies in India have also indicated that overweight exceeded underweight in urban adolescents in industrial settings [16]
In light of rising trend towards excess weight in urban populations in South Asian, we assessed the distinctiveness of contemporary urban South Asians with respect to their cardiometabolic phenotype. Specifically, we compared the prevalence of combined high fasting plasma glucose and high triglycerides relative to HDL among resident South Asians with prevalence of this combination in four race/ethnic groups residing in the United States (US), Asians, Blacks, Hispanics, and Whites, at varying levels of BMI. We focused on high glucose and high triglycerides relative to HDL because glucose intolerance and accompanying specific dyslipidemias are the hallmark of the South Asian phenotype [1,3]. We also sought to add to the literature by comparing South Asians with multiple race/ethnic groups because race/ethnic classifications are an imperfect yet pragmatic way to group people who may have common historical nutritional and social experiences relevant to their cardiometabolic phenotype [17–19].
METHODS
Participants and data sources
Data for this study were from two large population-based, representative samples: the 2010–2011 assessment of the Center for cArdiometabolic Risk Reduction in South Asia Study (CARRS) and 2011–2012 National Health and Nutrition Examination Survey (NHANES). CARRS is an ongoing cohort study designed to be representative of non-pregnant adults aged 20 years and older residing in Chennai and New Delhi, India and Karachi, Pakistan in 2010–2011. NHANES is a biennial cross-sectional study designed to be representative of the United States population. Details of both studies are published [20,21]. We restricted the current analysis to adults aged 20–79 years in demographic groups of interest without a prior diagnosis of diabetes (self-reported), with complete anthropometric (height, weight) and laboratory assessments (fasting plasma glucose, triglycerides, HDL), and BMI>18.5. A total of 8448 participants from CARRS and 1,789 participants from NHANES matched these criteria and were included in the study sample. Figure 1 shows a flow diagram of the inclusion/exclusion criteria that produced the analytic sample.
Figure 1.
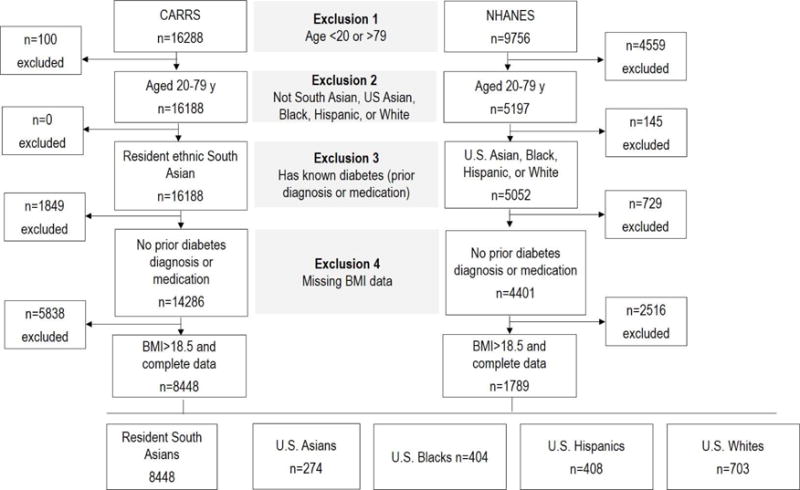
Participant exclusion criteria and sample size after each stage of restriction
Residential and race/ethnic classification
We categorized the sample into 5 mutually exclusive demographic groups based on residence and race/ethnicity. All participants from CARRS were classified as “resident South Asians.” In NHANES, race/ethnicity was self-reported and our sample was categorized into four groups: 1) US Asians; 2) US Blacks; 3) US Hispanics; and 4) US Whites. All NHANES participants beyond these categories were excluded from analysis. The US Asian group is composed of both East Asians and South Asians, and the publicly available NHANES data do not allow for distinguishing between these two groups.
Body mass index classification
We followed the international classification of body mass index (BMI). BMI<18.5 was excluded. BMI ranging from 18.5 to 24.9 kg/m2 was considered “normal weight;” BMI ranging from 25 to 30 was considered “overweight;” and BMI>30 was considered “obese.” These three categories, normal weight, overweight, and obese, were employed in the primary analysis.
Combined dysglycemia and dyslipidemia
We defined combined dysglycemia and dyslipidemia as the presence of fasting plasma glucose (FPG) ≥126 mg/dL and triglyceride to high-density lipoprotein cholesterol (HDL) ratio > 4. When this package of high glucose and dyslipidemias is present at normal BMI, it reflects the “South Asian” phenotype.
In the CARRS study, fasting blood samples were collected in the morning (minimum eight hours of fasting) at local camps when possible or in the home of participants. Samples were sent to an accredited laboratory in the respective city of data collection (Chennai, New Delhi, or Karachi) for analysis on the same day as they were collected. All three laboratories used by CARRS participated in a Randox International Quality Assessment Scheme (RIQAS) that standardized findings to a central laboratory at the Public Health Foundation of India/Centre for Chronic Disease Control in New Delhi. In the NHANES study, blood samples were collected at mobile examination centers; only participants who were examined in the morning were eligible for blood glucose and triglycerides assessment, and only those fasting for nine hours or more were assigned positive analytic weights. NHANES samples were analyzed at the University of Minnesota; vials were stored at −20 degrees C for shipment. Both CARRS and NHANES used standardized laboratory protocols for the assessment of plasma glucose (hexokinase/kinetic method), triglycerides (enzymatically with glycerol blanking in NHANES only), and HDL (the direct method).
Statistical analysis
Survey weights were used to age-standardize each group to the WHO standard population structure in descriptive analysis [22]. Analyses described the prevalence of combined dysglycemia dyslipidemia by ethnicity-residence group for each sex and BMI category separately. Within each sex-BMI category, differences in prevalence relative to resident South Asians were estimated using a binomial model with robust variance estimation [23]. Logistic regression was used to compute the odds (and 95% confidence intervals) of combined dysglycemia and dyslipidemia for each race/ethnic group relative to US Whites. US Whites, instead of resident South Asians, were used as the referent in the logistic models because US Whites were the largest US group for comparison and the magnitudes of odds ratio estimates were expected to mostly be in the positive direction. Adjusted logistic regression models accounted for age, college education, and tobacco use.
In addition, we conducted two sensitivity analyses. First, we specified an alternate definition of the cardiometabolic package, and analyzed differences in the “metabolic syndrome” defined per the 2009 American Heart Association Harmonization Panel [24]. This definition considers any 3 of 5 risk factors as the criterion for metabolic syndrome: high waist circumference (ethnic definitions, see [24]); triglycerides≥150 mg/dl; systolic blood pressure≥130 or diastolic blood pressure≥85 mmHg or hypertension medication; FPG≥100 mg/dl or medication; HDL <40 mg/dl. Participants with diabetes were not excluded from the metabolic syndrome analysis because diabetes medication is part of the definition of metabolic syndrome. Second, we specified alternate weight classification categories by using thresholds recommended for Asians (BMI 18.5–22.9 kg/m2 as normal, 23–24.9 kg/m2 as overweight, and 25 kg/m2 or above as obese).
Ethics approval
The CARRS study protocol was approved by ethics committees of the Public Health Foundation of India and AIIMS (Delhi), Madras Diabetes Research Foundation (Chennai), Aga Khan University (Karachi) and Emory University (Atlanta). The NHANES 2011–12 study protocol was approved by the National Center for Health Statistics Ethics Review Board (Protocol #2011-17).
RESULTS
Table 1 describes participant characteristics by race/ethnic group and sex. Data from 8,448 resident South Asians, 274 US Asians, 404 US Blacks, 308 US Hispanics, and 703 US Whites without prior diabetes diagnosis were included in the analytic sample. The mean age of participants in each race/ethnic group, after age-standardization, ranged from 35 to 41 years among men and 37 to 42 years among women. The proportion of college graduates was smallest in resident South Asians and largest in US Asians. Tobacco use was highest in South Asians (40%) among men and in US Whites (18%) among women. Mean BMI was highest in US Hispanics (28.89±0.43 kg/m2) among men and in US Blacks (31.53±0.60 kg/m2) among women. Notably, South Asian men had BMI comparable to US Asian men (24.84 vs 24.86 kg/m2, respectively) and South Asian women had significantly higher BMI than US Asian women (26.84 vs 23.53 kg/m2, respectively); BMI among South and US Asians was lower than that of any other group.
Table 1.
Characteristics of CARRS and NHANES participants by sex and race/ethnicity
| Sex | Demographic Group | n | Age, y | College educated, % | Tobacco user, % | BMI, kg/m2 | HDL, mg/dL | Triglyerides, mg/dL | Triglyceride:HDL ratio | FPG, mg/dL |
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Resident South Asians | 370 5 |
38.69±0.31 | 21.5±1.7 | 39.6±1.6 | 24.84±0.15 | 38.2 (33.0–45.1) | 133.8 (96.8–195.3) | 3.4 (2.3–5.5) | 93.8 (87.4–102.2) |
| U.S. Asians | 143 | 37.36±1.58 | 78.0±3.4 | 21.3±4.1 | 24.86±0.55 | 46.9 (39.9–54.8) | 113.1 (77.6–176.6) | 2.5 (1.5–4.0) | 99.2 (93.9–104.5) | |
| U.S. Blacks | 175 | 37.36±1.19 | 49.4±5.8 | 26.0±4.3 | 28.55±0.61 | 47.9 (42.0–57.9) | 80.7 (58.0–118.7) | 1.7 (1.1–2.6) | 95.9 (89.3–102.8) | |
| U.S. Hispanics | 219 | 34.83±0.94 | 35.6±4.6 | 22.6±4.5 | 28.89±0.43 | 43.4 (38.6–50.1) | 116.3 (86.6–168.4) | 2.5 (1.8–4.2) | 99.2 (94.0–105.3) | |
| U.S. Whites | 354 | 40.93±0.79 | 67.1±4.6 | 26.7±3.5 | 28.37±0.31 | 45.8 (39.4–52.4) | 119.6 (79.4–162.3) | 2.6 (1.5–4.0) | 98.2 (92.0–104.7) | |
| Women | Resident South Asians | 474 3 |
37.59±0.32 | 13.1±1.4 | 5.0±0.5 | 26.84±0.13 | 43.0 (37.1–49.4) | 108.1 (79.6–146.6) | 2.5 (1.7–3.6) | 94.4 (88.2–102.2) |
| U.S. Asians | 131 | 38.90±1.03 | 79.4±3.3 | 6.0±2.4 | 23.53±0.39 | 57.8 (48.6–69.9) | 89.1 (66.2–119.9) | 1.4 (1.0–2.4) | 93.8 (88.4–100.5) | |
| U.S. Blacks | 229 | 38.09±0.75 | 67.4±4.2 | 19.5±2.7 | 31.53±0.60 | 56.1 (47.8–66.4) | 73.9 (54.1–100.6) | 1.3 (0.9–1.9) | 93.9 (88.0–101.3) | |
| U.S. Hispanics | 189 | 36.88±1.14 | 43.2±4.9 | 6.0±2.1 | 29.27±0.44 | 53.0 (45.6–61.9) | 98.4 (67.4–135.1) | 1.8 (1.2–2.8) | 93.4 (88.6–99.3) | |
| U.S. Whites | 349 | 42.38±0.96 | 80.2±3.4 | 17.8±2.3 | 28.25±0.51 | 55.9 (47.8–64.6) | 98.9 (74.2–135.2) | 1.8 (1.2–2.7) | 93.7 (88.0–101.3) |
Reported values are mean ±SE unless otherwise noted.
Median (interquartile range) is reported.
Figure 2 (and Appendix Table 1) shows the prevalence of combined dysglycemia and dyslipidemia, defined as FPG≥126 and triglyceride:HDL ratio>4, by demographic group. Combining all BMI categories, South Asians had the highest prevalence, 41% and 20% in men and women respectively. In the normal BMI range of 18.5 to 24.9 kg/m2, prevalence was 33% and 11% in resident South Asian, 15% and 1% in US Asian, 5% and 2% in US Blacks, 11% and 2% in US Hispanics, and 8% and 2% in US White men and women, respectively. Compared with South Asian men, the prevalence of combined dysglycemia and dyslipidemia was statistically significantly lower in US Black, Hispanic, and White men at all BMI levels; prevalence in US Asian men was significantly different in the normal (BMI 25.0 to 29.9 kg/m2) and overweight range (30.0 and above kg/m2). Compared with South Asian women, the prevalence of combined dysglycemia and dyslipidemia was statistically significantly lower among US Asian, Black, Hispanic, and White women at all BMI levels with the exception of Asian women in the overweight and obese range and Hispanic women in the obese range. Across weight categories, combined dysglycemia and dyslipidemia tended to be lowest in the normal weight group (ranges: 5% to 33% among men and 1% to 2% among women) and highest in the obese group (ranges: 15% to 53% among men and 10% to 30% among women). The prevalence of combined dysglycemia and dyslipidemia was lower among women compared with men in each BMI category for all race/ethnic groups.
Figure 2. Prevalence of combined dysglycemia and dyslipidemia by demographic group within each BMI category.
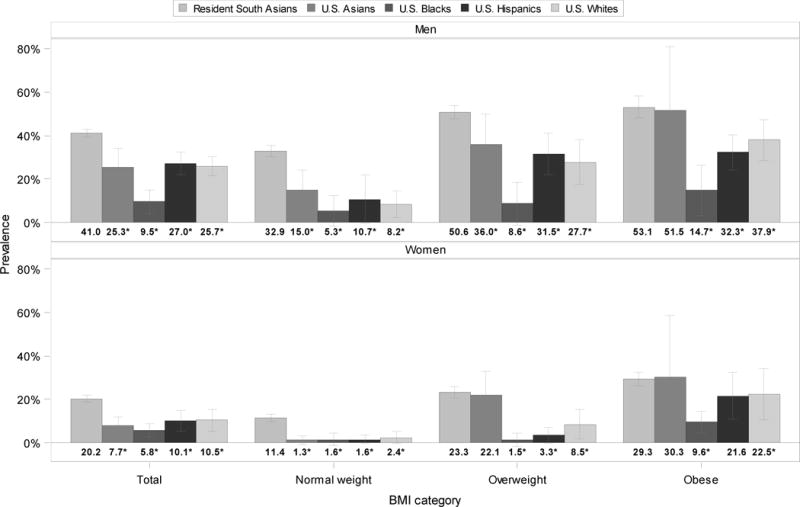
*p<.05 for difference in combined dysglycemia and dyslipidemia prevalence between resident South Asians (referent) and other race/ethnic groups.
Figure 3 (and Appendix Table 2) shows the relative odds of combined dysglycemia and dyslipidemia associated with race/ethnic group (ref=US Whites) overall and by BMI levels. Compared with US Whites, adjusted relative odds (aOR; 95% confidence intervals) of combined dysglycemia and dyslipidemia were aOR=1.94 (1.48–2.54) and aOR=2.55 (1.53–4.27) for South Asian men and women, respectively. At each BMI level, with the exception of obese women, South Asian ethnicity was associated with the highest adjusted relative odds of combined dysglycemia and dyslipidemia. Comparing South Asians with US Whites, odds ratios tended to be largest in the normal BMI range and smallest in the obese range. Normal weight South Asian men were nearly five times as likely to have combined dysglycemia and dyslipidemia as normal weight US White men (aOR=4.98; 2.46–10.07). South Asian women had greater odds of combined dysglycemia and dyslipidemia compared with US women in the normal (aOR=9.09; 2.48–33.29) and overweight (aOR=3.14; 1.34–7.36), but not obese, BMI range. With the exception of US Black men (OR=0.30; 0.16–0.60), no other US group statistically differed from US Whites with respect to combined dysglycemia and dyslipidemia in the overall sample. In analyses stratified by BMI, relative to US Whites, Black men had lesser odds of combined dysglycemia and dyslipidemia in the overweight and obese range, Black women had lesser odds in the obese range, and Hispanic women had lesser odds in the overweight range.
Figure 3. Relative odds of combined dysglycemia and dyslipidemia associated with race/ethnic groups by BMI category.
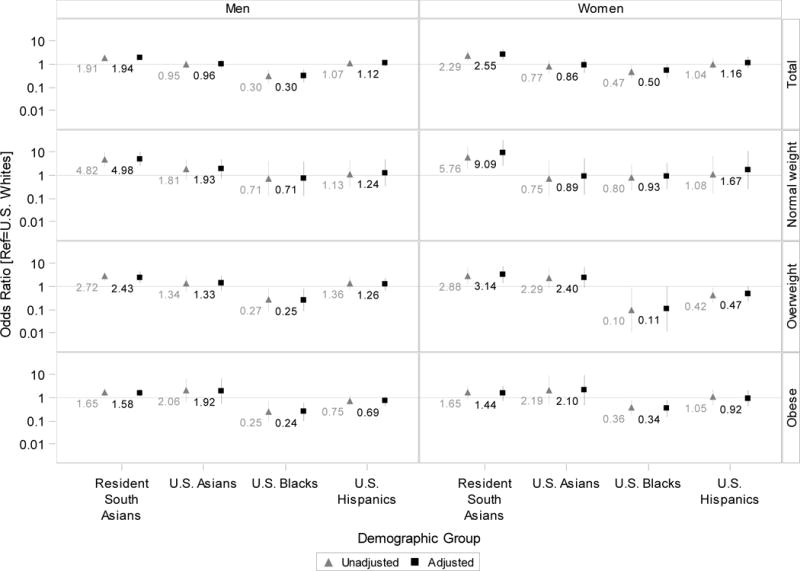
Odds ratios are plotted on a logarthmic axis (base 10) to better display positive and inverse asociations on the same chart. The horizontal line marks the null value (odds ratio=1).
Appendix Tables 1 and 2 show the sensitivity analysis examining metabolic syndrome by BMI levels and race/ethnicity. The prevalence of metabolic syndrome was between 1.5 to 5 times higher than that of combined dysglycemia and dyslipidemia in US Black, Hispanic and White men and in women of all race/ethnic groups (Appendix Table 1). The adjusted relative odds of metabolic syndrome was higher among South Asians compared with US Whites at all levels of BMI; associations were attenuated compared with those observed for combined dysglycemia and dyslipidemia. Appendix Table 3 shows the sensitivity analysis examining combined dysglycemia and dyslipidemia within BMI categories suggested for Asians. South Asians had the highest prevalence of combined dysglycemia and dyslipidemia in all categories of BMI defined using Asian-specific thresholds.
DISCUSSION
Between 11% to 15% of US men and 1% to 2% of US women of varying race/ethnic backgrounds exhibited combined dysglycemia and dyslipidemia at levels of BMI considered healthy (i.e., “normal” BMI range of 18.5–24.9), consistent with the cardiometabolic profile described as the “South Asian Phenotype.” Urban South Asians, however, were 5 to 9 times more likely to exhibit this combination at normal weight compared with any other US race/ethnic group. These findings, based on recent population-based data of multiple race/ethnic groups, are consistent with a large body of literature reporting that South Asians tend to experience worse cardiometabolic health despite lower body mass index levels compared with other ethnic groups [25–27].
Although combined dysglycemia and dyslipidemia was more common among normal weight South Asian individuals than normal weight individuals from other groups, 5% to 15% of US men with normal weight had this metabolic package. A separate literature has described a phenotype described as “metabolically obese but normal weight” [28–32]. Higher percent body fat and lower fat-free mass has been noted among metabolically obese but normal weight individuals [28], and in keeping with this, higher metabolic abnormalities have been observed among normal weight people with higher body fat content [33]. The metabolic obese but normal weight phenotype has been examined among migrant and resident South Asians, using definitions of metabolic syndrome that included hypertension [34,35]. Whether there is a causal convergence between the so-called “metabolically obese but normal weight” and “South Asian” phenotypes [34] would be an interesting topic of future research and possibly important to identify individuals with adverse cardiometabolic profiles who are otherwise considered low risk based on anthropometric screening.
This study was motivated in part by the hypothesis that the nutrition transition from undernutrition to overweight may be reducing differences between South Asians and other populations with respect to cardiometabolic risk profile. Our results suggest that, to the contrary, South Asians continue to exhibit specific glucose-associated dyslipidemias at levels disproportionate to other groups. One possible explanation for the findings is continued suboptimal early life nutrition. The prevalence of low birth weight has been slow to decline in India—going from 25% in 1992–93 to 21% in 2005–06 [36]. In fact, persistent low birth weight coupled with overweight/obesity later in life would be expected to further increase the prevalence of the thin-fat body type, which has been implicated in the South Asian phenotype [37]. Future studies tracking the weight status of South Asian populations across the lifecourse may be informative to assess whether the high distribution of this phenotype in South Asians is indeed driven by poor early life nutrition.
We recognize that the race/ethnic groupings examined did not capture the vast heterogeneity within each race/ethnic group. Over 60 ethnic groups are represented among Asian Americans alone [38], and we were unable to distinguish between NHANES participants who originated from South Asia and those who originated from other regions of Asia (e.g., Chinese Americans). Because South and East Asians differ with respect to ethnic ancestry, it is important to acknowledge that observed differences between South Asians in CARRS and all Asians in NHANES may be due to a combination of hereditary, environmental, and/or cultural/behavioral factors that are observed to cluster within race/ethnic groupings.
Among the limitations of this study is the lack of a universal definition of the distinctive metabolic risk profile attributed to South Asians. For example, abdominal adiposity is often described as part of the South Asian phenotype, and we did not include abdominal adiposity in our primary analysis. We, however, applied a second definition in our sensitivity analysis. Using metabolic syndrome as the outcome, we still observed that South Asians were more likely to have worse metabolic health than US Whites at normal weight. This is reassuring that the ethnic differences reported here are robust to alternate specifications of metabolic risk, including a definition that incorporated abdominal adiposity and hypertension. Further investigation may benefit from examining measures of cardiometabolic risk (FPG, triglycerides, HDL, hypertension) separately to better understand differences by race/ethnic groups. Another major limitation was the possible lack of power to detect meaningful differences because laboratory data required for our outcome were available for only a small sample of NHANES participants.
We sought to investigate the distinctiveness of the South Asian cardiometabolic phenotype by describing cardiometabolic risk by BMI classification for five groups defined by contrasting race/ethnic and residential characteristics: Resident South Asians, US Asians, US Hispanics, US Blacks, and US Whites. In doing so, the study had several strengths. We combined two recent, large population-based datasets with fairly comprehensive anthropometric and metabolic assessments to shed light on cardiometabolic health in resident South Asians and US populations of diverse race/ethnic background. Participants were assessed close in calendar time, diminishing concerns regarding differences due to secular changes in cardiometabolic health. We included multiple race/ethnic comparison groups residing in the US. Future investigation may fruitfully pool prospective data from multiple sources in an effort to disentangle the modifiable causes of observed differences between resident South Asians and US groups to better guide intervention efforts.
Highlights.
Dysglycemia and dyslipidemia at normal weight characterize the South Asian phenotype
8%–15% of US men and 1%–2% of US women possess this phenotype
Resident South Asians exhibited this phenotype 5–9 times more often than US adults
Acknowledgments
FUNDING
The CARRS Study was funded in part by the National Heart, Lung, and Blood Institute of the National Institutes of Health, Department of Health and Human Services (contract no. HHSN268200900026C) and the United Health Group (Minneapolis, MN, USA). Additional support was provided by the Fogarty International Centre and the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health (grant number 1 D43 HD065249).
Abbreviations
- BMI
body mass index
- TG
triglycerides
- HDL
high density lipoprotein cholesterol
- FPG
fasting plasma glucose
Appendix Table 1.
Prevalence (95%CI) of combined dysglycemia and dyslipidemia by demographic group within BMI category
| Sex | Demographic group | Total BMI 18.5–30+ | Normal weight BMI 18.5–25 | Overweight BMI 25–30 | Obese BMI 30+ |
|---|---|---|---|---|---|
| Combined dysglycemia and dyslipidemia (FPG≥126 & TG:HDL>4) | |||||
| Men | Resident South Asians | 41.0 (39.3,42.7) | 32.9 (30.2,35.6) | 50.6 (47.6,53.7) | 53.1 (48.0,58.1) |
| US Asians | 25.3 (16.3,34.3) | 15.0 (6.0,24.0) | 36.0 (22.1,49.8) | 51.5 (22.1,80.9) | |
| US Blacks | 9.5 (3.9,15.1) | 5.3 (−1.7,12.4) | 8.6 (−1.1,18.3) | 14.7 (3.3,26.2) | |
| US Hispanics | 27.0 (21.8,32.2) | 10.7 (−0.5,22.0) | 31.5 (21.9,41.0) | 32.3 (24.2,40.3) | |
| US Whites | 25.7 (21.3,30.2) | 8.2 (2.0,14.3) | 27.7 (17.4,38.1) | 37.9 (28.5,47.3) | |
| Women | Resident South Asians | 20.2 (18.8,21.7) | 11.4 (9.7,13.0) | 23.3 (20.8,25.8) | 29.3 (26.1,32.6) |
| US Asians | 7.7 (3.7,11.8) | 1.3 (−0.7,3.3) | 22.1 (11.5,32.7) | 30.3 (2.1,58.5) | |
| US Blacks | 5.8 (2.7,8.9) | 1.6 (−1.5,4.6) | 1.5 (−1.5,4.5) | 9.6 (4.6,14.6) | |
| US Hispanics | 10.1 (5.4,14.8) | 1.6 (−0.5,3.6) | 3.3 (−0.2,6.9) | 21.6 (11.0,32.2) | |
| US Whites | 10.5 (5.5,15.5) | 2.4 (−0.3,5.1) | 8.5 (1.9,15.2) | 22.5 (10.7,34.3) | |
| Metabolic syndrome* | |||||
|
|
|||||
| Men | Resident South Asians | 47.4 (45.5,49.3) | 31.8 (29.6,34.0) | 66.4 (63.5,69.3) | 80.0 (76.6,83.4) |
| US Asians | 41.5 (29.0,54.0) | 16.2 (4.8,27.7) | 61.7 (48.0,75.4) | 69.4 (43.0,95.9) | |
| US Blacks | 28.1 (19.9,36.4) | 10.4 (0.8,20.0) | 11.6 (6.0,17.2) | 53.3 (38.0,68.6) | |
| US Hispanics | 47.9 (40.7,55.2) | 5.0 (−6.1,16.1) | 44.3 (35.4,53.3) | 61.8 (55.6,68.0) | |
| US Whites | 48.7 (43.3,54.1) | 11.7 (3.0,20.4) | 45.1 (36.6,53.5) | 67.2 (57.9,76.5) | |
| Women | Resident South Asians | 42.0 (39.9,44.1) | 23.7 (20.7,26.7) | 44.6 (41.3,47.9) | 60.6 (57.7,63.4) |
| US Asians | 21.0 (10.3,31.7) | 6.4 (2.8,9.9) | 39.4 (18.1,60.7) | 42.9 (13.3,72.4) | |
| US Blacks | 29.0 (24.2,33.8) | 15.0 (7.5,22.6) | 17.5 (9.5,25.6) | 38.4 (30.6,46.2) | |
| US Hispanics | 25.2 (20.1,30.4) | 1.3 (−1.4,4.1) | 11.8 (7.0,16.6) | 43.9 (34.2,53.7) | |
| US Whites | 29.8 (23.6,35.9) | 12.4 (3.8,20.9) | 22.0 (14.8,29.2) | 49.4 (39.2,59.5) | |
Harmonized definition released by American Heart Association 2009.
Appendix Table 2.
Associations (odds ratios [95% CI]) between race/ethnic group and combined dysglycemia and dyslipidemia within BMI category
| Total; BMI 18.5–30+ | BMI 18.5–25 kg/m2 | BMI 25–30 kg/m2 | BMI > 30 kg/m2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | ||
| Relative Odds of Combined Dysglycemia and Dyslipidemia* | |||||||||
|
|
|||||||||
| Men | Resident South Asians | 1.91 (1.45–2.51) | 1.94 (1.48–2.54) | 4.71 (2.41–9.20) | 4.98 (2.46–10.07) | 2.57 (1.48–4.49) | 2.43 (1.39–4.24) | 1.65 (1.05–2.58) | 1.58 (1.05–2.36) |
| US Asians | 0.95 (0.60–1.50) | 0.96 (0.62–1.51) | 1.89 (0.75–4.76) | 1.93 (0.74–5.04) | 1.39 (0.71–2.71) | 1.33 (0.62–2.85) | 2.16 (0.66–7.08) | 1.92 (0.53–6.92) | |
| US Blacks | 0.30 (0.15–0.59) | 0.30 (0.16–0.60) | 0.66 (0.13–3.46) | 0.71 (0.13–3.88) | 0.24 (0.08–0.71) | 0.25 (0.08–0.76) | 0.27 (0.12–0.60) | 0.24 (0.10–0.59) | |
| US Hispanics | 1.07 (0.80–1.43) | 1.12 (0.83–1.50) | 1.09 (0.30–4.04) | 1.24 (0.32–4.72) | 1.31 (0.67–2.57) | 1.26 (0.66–2.42) | 0.74 (0.47–1.17) | 0.69 (0.43–1.11) | |
| US Whites | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| Women | Resident South Asians | 2.29 (1.40–3.76) | 2.55 (1.53–4.27) | 5.37 (2.01–14.29) | 9.09 (2.48–33.29) | 2.78 (1.25–6.20) | 3.14 (1.34–7.36) | 1.70 (0.82–3.50) | 1.44 (0.68–3.04) |
| US Asians | 0.77 (0.38–1.55) | 0.86 (0.43–1.72) | 0.95 (0.16–5.66) | 0.89 (0.15–5.41) | 2.58 (1.05–6.35) | 2.40 (0.88–6.58) | 1.65 (0.43–6.32) | 2.10 (0.46–9.64) | |
| US Blacks | 0.47 (0.23–0.93) | 0.50 (0.25–1.02) | 0.66 (0.18–2.50) | 0.93 (0.25–3.38) | 0.21 (0.05–1.01) | 0.11 (0.01–1.00) | 0.38 (0.17–0.84) | 0.34 (0.15–0.77) | |
| US Hispanics | 1.04 (0.57–1.88) | 1.16 (0.63–2.13) | 0.96 (0.16–5.75) | 1.67 (0.24–11.44) | 0.37 (0.17–0.78) | 0.47 (0.23–0.97) | 1.00 (0.47–2.15) | 0.92 (0.42–2.04) | |
| US Whites | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| Sensitivity Analysis: Relative Odds of “Metabolic Syndrome”** | |||||||||
|
|
|||||||||
| Men | Resident South Asians | 0.98 (0.79–1.21) | 1.27 (1.00–1.60) | 2.74 (1.40–5.36) | 3.73 (1.90–7.36) | 2.17 (1.54–3.05) | 2.64 (1.86–3.74) | 1.64 (1.06–2.54) | 1.76 (1.08–2.87) |
| US Asians | 0.71 (0.43–1.17) | 0.78 (0.46–1.31) | 1.31 (0.59–2.92) | 1.62 (0.74–3.53) | 1.63 (0.89–2.98) | 1.65 (0.89–3.06) | 1.09 (0.30–4.01) | 1.70 (0.52–5.58) | |
| US Blacks | 0.46 (0.29–0.71) | 0.52 (0.32–0.84) | 0.74 (0.31–1.76) | 0.87 (0.40–1.89) | 0.23 (0.13–0.41) | 0.23 (0.12–0.45) | 0.58 (0.32–1.05) | 0.65 (0.36–1.17) | |
| US Hispanics | 0.94 (0.69–1.28) | 1.32 (0.94–1.84) | 0.24 (0.02–2.55) | 0.37 (0.04–3.49) | 0.97 (0.61–1.55) | 1.21 (0.73–2.01) | 0.79 (0.52–1.20) | 1.11 (0.68–1.79) | |
| US Whites | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| Women | Resident South Asians | 1.59 (1.16–2.19) | 2.40 (1.67–3.44) | 2.05 (1.03–4.08) | 4.13 (1.65–10.32) | 2.17 (1.36–3.45) | 3.11 (1.88–5.16) | 1.49 (0.96–2.32) | 2.28 (1.37–3.78) |
| US Asians | 0.63 (0.34–1.19) | 0.72 (0.39–1.32) | 0.68 (0.33–1.38) | 0.85 (0.35–2.04) | 1.76 (0.77–4.00) | 1.88 (0.80–4.42) | 0.87 (0.27–2.83) | 1.18 (0.37–3.72) | |
| US Blacks | 0.99 (0.68–1.44) | 1.26 (0.80–1.99) | 1.72 (0.81–3.68) | 2.03 (0.76–5.44) | 0.75 (0.37–1.52) | 0.89 (0.42–1.87) | 0.67 (0.40–1.12) | 0.84 (0.49–1.43) | |
| US Hispanics | 0.77 (0.55–1.08) | 1.04 (0.75–1.46) | 0.15 (0.02–1.21) | 0.27 (0.03–2.21) | 0.47 (0.27–0.81) | 0.59 (0.35–0.99) | 0.78 (0.52–1.18) | 0.99 (0.65–1.50) | |
| US Whites | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
FPG>126 mg/dL and triglyceride:HDL ratio > 4.
Harmonized definition released by American Heart Association 2009.
Appendix Table 3.
Sensitivity Analysis: Prevalence of combined dysglycemia and dyslipidemia by Asian BMI categories
| Sex | Demographic group | BMI 18.5–23* | BMI 23–25 | BMI>25 |
|---|---|---|---|---|
| Men | Resident South Asians | 39.1 (35.1,43.2) |
48.8 (44.5,53.0) |
53.0 (50.1,55.9) |
| US Asians | 33.7 (6.6,60.8) |
44.2 (17.7,70.6) |
44.3 (34.6,54.0) |
|
| US Blacks | 10.2 (−6.9,27.3) |
12.2 (−12.7,37.0) |
14.5 (7.1,21.8) |
|
| US Hispanics | 0.0 (0.0,0.0) |
49.2 (23.8,74.6) |
36.5 (30.0,42.9) |
|
| US Whites | 19.1 (2.0,36.2) |
23.4 (−5.8,52.7) |
34.8 (27.9,41.7) |
|
| Women | Resident South Asians | 16.9 (13.1,20.7) |
22.6 (17.8,27.4) |
27.5 (25.5,29.6) |
| US Asians | 5.4 (−2.4,13.1) |
1.6 (−2.0,5.2) |
25.7 (16.9,34.5) |
|
| US Blacks | 0.0 (0.0,0.0) |
5.6 (−4.3,15.6) |
7.3 (3.6,11.1) |
|
| US Hispanics | 0.0 (0.0,0.0) |
5.1 (−1.3,11.6) |
12.5 (6.1,18.9) |
|
| US Whites | 3.4 (−3.4,10.3) |
5.1 (−0.5,10.6) |
15.4 (8.3,22.5) |
There were 30 or fewer individuals in each of the US groups at this BMI level.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Enas EA, Mohan V, Deepa M, Farooq S, Pazhoor S, Chennikkara H. The Metabolic Syndrome and Dyslipidemia Among Asian Indians: A Population With High Rates of Diabetes and Premature Coronary Artery Disease. J Cardiometab Syndr. 2007;2:267–75. doi: 10.1111/j.1559-4564.2007.07392.x. [DOI] [PubMed] [Google Scholar]
- 2.Unnikrishnan R, Anjana RM, Mohan V. Diabetes in South Asians: Is the Phenotype Different? Diabetes. 2014;63:53–5. doi: 10.2337/db13-1592. [DOI] [PubMed] [Google Scholar]
- 3.Prasad DS, Kabir Z, Dash AK, Das BC. Abdominal obesity, an independent cardiovascular risk factor in Indian subcontinent: A clinico epidemiological evidence summary. J Cardiovasc Dis Res. 2011;2:199–205. doi: 10.4103/0975-3583.89803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKeigue PM, Ferrie JE, Pierpoint T, Marmot MG. Association of early-onset coronary heart disease in South Asian men with glucose intolerance and hyperinsulinemia. Circulation. 1993;87:152–61. doi: 10.1161/01.cir.87.1.152. [DOI] [PubMed] [Google Scholar]
- 5.McKeigue PM, Miller GJ, Marmot MG. Coronary heart disease in South Asians overseas: A review. J Clin Epidemiol. 1989;42:597–609. doi: 10.1016/0895-4356(89)90002-4. [DOI] [PubMed] [Google Scholar]
- 6.McKeigue PM, Marmot MG. Mortality from coronary heart disease in Asian communities in London. BMJ. 1988;297:903. doi: 10.1136/bmj.297.6653.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker ARP. The epidemiology of ischaemic heart disease in the different ethnic populations in Johannesburg. Afr Med J. 1980;57:748–742. [PubMed] [Google Scholar]
- 8.Wild S, Mckeigue P. Cross sectional analysis of mortality by country of birth in England and Wales, 1970–92. BMJ. 1997;314:705. doi: 10.1136/bmj.314.7082.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fall CHD, Sachdev HS, Osmond C, Lakshmy R, Biswas SD, Prabhakaran D, et al. Adult Metabolic Syndrome and Impaired Glucose Tolerance Are Associated With Different Patterns of BMI Gain During Infancy Data from the New Delhi birth cohort. Diabetes Care. 2008;31:2349–56. doi: 10.2337/dc08-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 11.Sachdev HS, Fall CH, Osmond C, Lakshmy R, Biswas SKD, Leary SD, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–66. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 12.Yajnik CS. Obesity epidemic in India: intrauterine origins? Proc Nutr Soc. 2004;63:387–96. doi: 10.1079/PNS2004365. [DOI] [PubMed] [Google Scholar]
- 13.Yajnik CS. The lifecycle effects of nutrition and body size on adult adiposity, diabetes and cardiovascular disease. Obes Rev. 2002;3:217–24. doi: 10.1046/j.1467-789X.2002.00072.x. [DOI] [PubMed] [Google Scholar]
- 14.Balarajan Y, Villamor E. Nationally Representative Surveys Show Recent Increases in the Prevalence of Overweight and Obesity among Women of Reproductive Age in Bangladesh, Nepal, and India. J Nutr. 2009;139:2139–44. doi: 10.3945/jn.109.112029. [DOI] [PubMed] [Google Scholar]
- 15.Patel SA, Narayan KMV, Cunningham SA. Unhealthy weight among children and adults in India: urbanicity and the crossover in underweight and overweight. Ann Epidemiol. 2015 doi: 10.1016/j.annepidem.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Jeemon P, Prabhakaran D, Mohan V, Thankappan KR, Joshi PP, Ahmed F, et al. Double burden of underweight and overweight among children (10–19years of age) of employees working in Indian industrial units. Natl Med J India. 2009;22:172–6. [PubMed] [Google Scholar]
- 17.Williams DR, Lavizzo-Mourey R, Warren RC. The concept of race and health status in America. Public Health Rep. 1994;109:26. [PMC free article] [PubMed] [Google Scholar]
- 18.Krieger N, Chen JT, Waterman PD, Kosheleva A, Beckfield J. History, haldanes and health inequities: exploring phenotypic changes in body size by generation and income level in the US-born White and Black non-Hispanic populations 1959–1962 to 2005–2008. Int J Epidemiol. 2013;42:281–95. doi: 10.1093/ije/dys206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krieger N, Kiang MV, Kosheleva A, Waterman PD, Chen JT, Beckfield J. Age at Menarche: 50-Year Socioeconomic Trends Among US-Born Black and White Women. Am J Public Health. 2014;105:388–97. doi: 10.2105/AJPH.2014.301936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Questionnaire, Examination Protocol, and Laboratory Protocol. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 21.Nair M, Ali MK, Ajay VS, Shivashankar R, Mohan V, Pradeepa R, et al. CARRS Surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health. 2012;12:701. doi: 10.1186/1471-2458-12-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad O, Boschi-Pinto C, Lopez A, Murray C, Lozano R, Inoue M. Age Standardisation of Rates: a New WHO Standard. World Health Organization; 2001. [Google Scholar]
- 23.Spiegelman D, Hertzmark E. Easy SAS Calculations for Risk or Prevalence Ratios and Differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 24.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 25.Bodicoat DH, Gray LJ, Henson J, Webb D, Guru A, Misra A, et al. Body Mass Index and Waist Circumference Cut-Points in Multi-Ethnic Populations from the UK and India: The ADDITION-Leicester, Jaipur Heart Watch and New Delhi Cross-Sectional Studies. PLoS ONE. 2014;9:e90813. doi: 10.1371/journal.pone.0090813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gujral UP, Narayan KMV, Pradeepa RG, Deepa M, Ali MK, Anjana RM, et al. Comparing Type 2 Diabetes, Prediabetes, and Their Associated Risk Factors in Asian Indians in India and in the U.S.: The CARRS and MASALA Studies. Diabetes Care. 2015;38:1312–8. doi: 10.2337/dc15-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misra A, Shrivastava U. Obesity and Dyslipidemia in South Asians. Nutrients. 2013;5:2708–33. doi: 10.3390/nu5072708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conus F, Allison DB, Rabasa-Lhoret R, St-Onge M, St-Pierre DH, Tremblay-Lebeau A, et al. Metabolic and Behavioral Characteristics of Metabolically Obese but Normal-Weight Women. J Clin Endocrinol Metab. 2004;89:5013–20. doi: 10.1210/jc.2004-0265. [DOI] [PubMed] [Google Scholar]
- 29.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and Body Composition Factors in Subgroups of Obesity: What Do We Know? J Clin Endocrinol Metab. 2004;89:2569–75. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 30.Lee K. Metabolically obese but normal weight (MONW) and metabolically healthy but obese (MHO) phenotypes in Koreans: characteristics and health behaviors. Asia Pac J Clin Nutr. 2009;18:280. [PubMed] [Google Scholar]
- 31.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 32.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the us population (nhanes 1999–2004. Arch Intern Med. 2008;168:1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 33.Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2009:ehp487. doi: 10.1093/eurheartj/ehp487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flowers E, Molina C, Mathur A, Reaven GM. Adiposity and Cardiovascular Risk Clustering in South Asians. Metab Syndr Relat Disord. 2013;11:434–40. doi: 10.1089/met.2013.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geetha L, Deepa M, Anjana RM, Mohan V. Prevalence and clinical profile of metabolic obesity and phenotypic obesity in Asian Indians. J Diabetes Sci Technol. 2011;5:439–46. doi: 10.1177/193229681100500235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhat BV, Adhisivam B. Trends and Outcome of Low Birth Weight (LBW) Infants in India. Indian J Pediatr. 2013;80:60–2. doi: 10.1007/s12098-012-0922-6. [DOI] [PubMed] [Google Scholar]
- 37.Kurpad AV, Varadharajan KS, Aeberli I. The thin-fat phenotype and global metabolic disease risk. Curr Opin Clin Nutr Metab Care. 2011;14:542–7. doi: 10.1097/MCO.0b013e32834b6e5e. [DOI] [PubMed] [Google Scholar]
- 38.Narayan KMV, Aviles-Santa L, Oza-Frank R, Pandey M, Curb JD, McNeely M, et al. Report of a National Heart, Lung, and Blood Institute Workshop: Heterogeneity in Cardiometabolic Risk in Asian Americans in the U.S.: Opportunities for Research. J Am Coll Cardiol. 2010;55:966–73. doi: 10.1016/j.jacc.2009.07.075. [DOI] [PubMed] [Google Scholar]


