Abstract
Aging has been related to diminished cognitive function, which could be a result of ineffective synaptic function. We have previously shown that synaptic plasma membrane (SPM) proteins supporting synaptic integrity and neurotransmission were down-regulated in docosahexaenoic acid (DHA)-deprived brains, suggesting an important role of DHA in synaptic function. In this study, we demonstrate aging-induced synaptic proteome changes and DHA-dependent mitigation of such changes using mass spectrometry (MS)-based protein quantitation combined with western blot or mRNA analysis. We found significant reduction of 15 SPM proteins in aging brains including fodrin-α, synaptopodin, PSD-95, SV2B, SNAP25, SNAP-α, NR2B, AMPA2, AP2, VGluT1, munc18-1, dynamin-1, VAMP2, rab3A, and EAAT1, most of which are involved in synaptic transmission. Notably, the first nine proteins were further reduced when brain DHA was depleted by diet, indicating that DHA plays an important role in sustaining these synaptic proteins down-regulated during aging. Reduction of two of these proteins was reversed by raising the brain DHA level by supplementing aged animals with an omega-3 fatty acid sufficient diet for 2 months. The recognition memory compromised in DHA-depleted animals was also improved. Our results suggest a potential role of DHA in alleviating aging-associated cognitive decline by offsetting the loss of neurotransmission-regulating synaptic proteins involved in synaptic function.
Keywords: Aging-related changes, Synaptic plasma membrane proteins, DHA, neurotransmission, MS-based protein quantitation, mass spectrometry, recognition memory
1. Introduction
Aging leads to decreased cognitive performance even in the absence of a diseased condition. Decrease in cognitive function reduces the quality of life of aging individuals as well as increases the costs of health care. It is known that during the normal aging process significant neuronal loss does not occur (Rapp and Gallagher, 1996; Rasmussen et al., 1996). Therefore, cognitive decline could be a result of dysregulation of synaptic function and neurotransmission. Previous studies have reported age-related changes in glucose metabolism and long term potentiation as well as levels of neurotransmitters (dopamine, norepinephrine, 5-HT, GABA, acetylcholine) and trophic factors like brain-derived neurotrophic factor, nerve growth factor, insulin-like growth factor-1 (Baxter et al., 1999; Foster, 2007; Gant et al., 2006; Gavilan et al., 2007; Hwang et al., 2006; Majdi et al., 2007; Mora et al., 2007; Rowe et al., 2007; Shi et al., 2007; Smith et al., 2000). Structural changes in the aging brain have also been demonstrated such as atrophy of various aspects of dendritic and synaptic morphology and vascular rarefaction (Dickstein et al, 2007; Shi et al 200; Sonntag et al 1997, 2000). Alterations in hippocampal expression of mRNAs with age have also been reported (Blalock et al., 2003; Kumar et al., 2007; Rowe et al., 2007). Previous proteomic studies identified modifications in multiple regulatory processes like metabolism, glutamate processing, protein synthesis (Poon et al, 2006), protein folding as well as response to oxidative stress (Gavilan et al., 2007, Poon et al., 2006). Recent age-related proteomic studies focused on the synaptic proteome have revealed changes in the expression of proteins involved in neurotransmitter vesicle exocytosis and recycling dynamics (VanGuilder et al., 2010). A large subset of synaptic proteins including NSF, SNAP25, syntaxin 1, VAMP2, synaptophysin, synapsin 1, dynamin 1, amphiphysin, clathrin, syndapin 1 and munc 18–1 which represent both effectors and regulators of neurotransmission were found to change during aging, indicating an age-based deterioration of hippocampal neurotransmission.
Docosahexaenoic acid (DHA), an n-3 highly unsaturated fatty acid enriched in the central nervous system, is important for normal brain development. Previous studies in our laboratory demonstrated that DHA promotes neurite outgrowth and synaptogenesis (Cao et al., 2009). We also reported significant differences in the synaptic proteome of DHA-adequate and DHA-deficient mouse brains (Sidhu et al., 2011). We found that some of the proteins important for synaptic integrity and synaptic neurotransmission were reduced in the DHA-deficient brains, providing a molecular basis for the significant impact of nutritional DHA on learning and memory function. Moreover, a recent study documented that twenty four week supplementation with 900 mg/day DHA improved learning and memory in healthy older adults with age-related cognitive decline (Yurko-Mauro et al., 2010). Our hypothesis in this study was that downregulation of synaptic proteins during aging may be dependent on the DHA-status in the diet. For this purpose, we compared the synaptic proteome of mice at different ages under DHA-adequate and deficient conditions. A quantitative nano-LC-ESI-MS/MS proteomics approach using 18O labeling or label-free analysis was used to identify proteome changes, which were further validated by western blot analysis with specific antibodies and/ or mRNA analysis using real-time quantitative PCR. Combining these approaches, we found that 15 SPM proteins were differentially expressed with age. Among them, six proteins were newly identified as age-regulated synaptic proteins. Intriguingly, we observed a positive effect of DHA on regulating nine proteins important for vesicle recycling and neurotransmission. These include fodrin-α, synaptopodin, PSD-95, synaptic vesicle glycoprotein 2B (SV2B), synaptosomal-associated protein 25 (SNAP25), alpha-soluble NSF attachment protein (SNAP-α), glutamate [NMDA] receptor subunit epsilon-2 precursor (NR2B), glutamate receptor ionotropic AMPA2 (AMPA2), and AP-2 complex subunit mu isoform b (AP2). Increasing brain DHA by supplementing the deficient group with an n-3 fatty acid adequate diet was able to restore levels of two out of six proteins that were significantly downregulated due to DHA deficiency in aged animals. The restoration of brain DHA also led to an improvement of recognition memory.
2. Methods
2.1 Experimental strategy
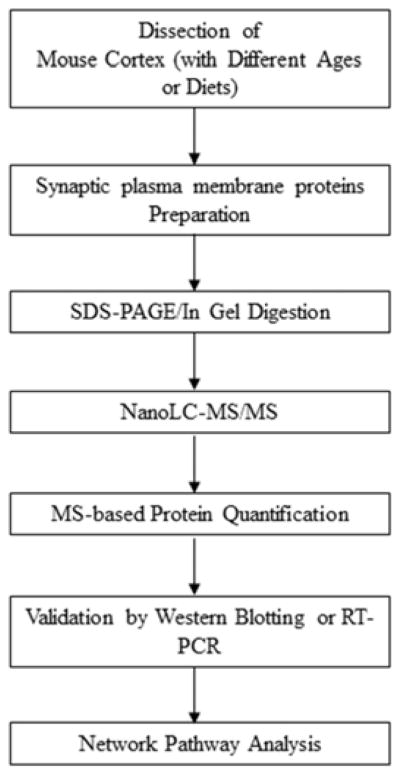
Brain cortices were collected from mice fed DHA-adequate or deficient diets at different ages. Synaptic plasma membranes (SPM) proteins were prepared by subcellular fractionation, and subjected to SDS-PAGE, in-gel tryptic digestion, mass spectrometric identification and quantitation. The changes in protein expression due to aging or DHA status revealed by MS were validated by western blotting or RT-PCR. The age- and DHA-related proteins were subjected to network pathway analysis (Scheme 1).
Scheme 1.
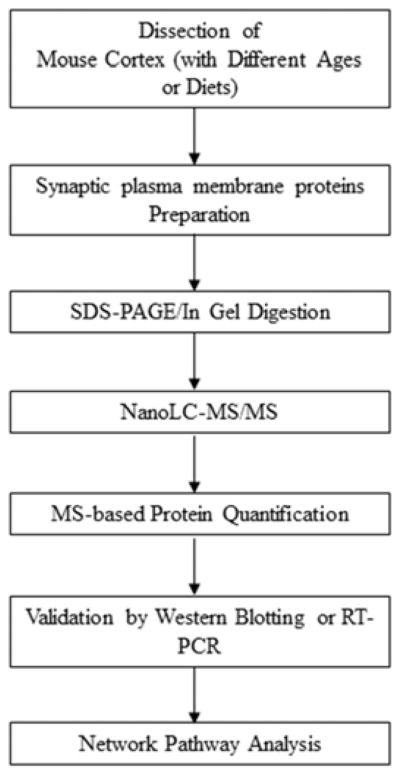
Experimental strategy for investigating the differential expression of synaptic plasma membrane (SPM) proteins affected by aging and DHA status.
2.2 Animals and diets
Pregnant C57/BL6 mice at 2 days of gestation were obtained from Charles River Laboratories (Frederick, MD, USA). The pregnant mice were allowed to feed on either an n-3 fatty acid adequate or deficient diet throughout the pregnancy and lactation period and their offspring were further continued on the same diet after weaning as described earlier (Cao et al., 2009). The n-3 fatty acid adequate and deficient semi-synthetic pelleted diets were based on the AIN-93G formula and differed only in fat composition (Dyets, Bethlehem, PA). The n-3 fatty acid adequate diet consisted of 7.45 %, 1.77 %, 0.48 % and 0.3 % (w/w) of hydrogenated coconut, safflower, flaxseed and DHASCO oil (Martek, Columbia, MD) while the deficient diet contained 8.1 % and 1.9 % (w/w) of hydrogenated coconut and safflower oil. The resulting n-3 fatty acid content was 2.5 % (w/w) linolenic acid (LNA) plus 0.9 % DHA (w/w) in the adequate diet, and only 0.09 % (w/w) of LNA in the deficient diet. The procedures employed in this study were approved by the National Institute on Alcohol Abuse and Alcoholism (LMS-HK31). Three different age groups were selected for the experiment: 3 weeks (young), 4 months (young-adults) and 15 months (aged). Animals were exposed to CO2 inhalation before sacrificing them by cervical dislocation. For DHA replenishment studies, 15 month old deficient mice were either continued on the same deficient diet or fed the adequate diet for additional 2 months. At 17 months of age, the novel object recognition test was performed and the brains were collected for protein analysis. For the adequate aged-control group, mice were fed the adequate diet for the entire 17 months.
2.3 Analysis of fatty acid in mouse brains
Lipids from brain homogenates were extracted and fatty acid contents were analyzed by gas chromatography after transmethylation with boron trifluoride-methanol as described earlier (Wen and Kim, 2004).
2.4 Preparation of synaptic plasma membranes
Synaptic plasma membranes (SPM) were prepared according to the protocol by Cottman and Matthews (1971) with a slight modification as described previously (Sidhu et al, 2011). The brain cortices obtained from 3 mice fed the DHA-adequate or deficient diet were homogenized in 0.32 M sucrose with a tissue/solution ratio of 20% (w/v). The homogenate was diluted to 10% (w/v) tissue/sucrose solution and centrifuged at 1,000 g at 4 °C for 5 min. The nuclear pellet was discarded and the supernatant was centrifuged at 11,000 g for 20 min to obtain the crude mitochondrial pellet. Plasma membranes were extracted from crude mitochondria by density gradient centrifugation using different concentrations of Ficoll (13%, 6% and 4%). Three fractions namely, myelin, synaptosomes and mitochondria were collected following centrifugation at 64,000 g for 45 min. Myelin was collected from the top layer of 4% Ficoll, synaptosomes were obtained at the interface between the 6% and 13% Ficoll layers while mitochondrial fraction was collected as a pellet at the bottom. Synaptosomes were washed twice with PBS buffer before obtaining SPM by osmotic shock. The synaptosomes were incubated with 5 mM Tris HCl buffer (pH 7.4) containing 1 mM EDTA at 4°C for one hour with gentle stirring and the suspension was centrifuged at 20,000 x g for 30 min to obtain the SPM pellet.
2.5 In-gel tryptic digestion
Eighty μg SPM proteins obtained from young (4 months) or aged (15 months) mice respectively, were incubated with lithium dodecyl sulfate (LDS) buffer (Invitrogen) at 70 °C for 5 min, and loaded side by side onto 10% Bis-Tris gels (Invitrogen). SDS-PAGE was carried out at a constant voltage of 100 V for 2 h using MOPS SDS running buffer. The separated proteins were visualized with Coomassie blue (SimplyBlue SafeStain, Invitrogen). The gel was cut in-pair (young/old) into 8–10 segments, destained, and subjected to reduction with DTT and alkylation with iodoacetamide. For in-gel digestion, the gel pieces were rehydrated with 12.5 ng/mL trypsin (Promega, Madison, WI) solution in 25 mM ammonium bicarbonate on ice for 30 min and incubated overnight at 37°C. Five standard peptides originated from non-mammalian sources (at 0.05 μg each) were added to each sample for the purpose of normalizing 18O/16O ratio. These peptides include PopD from pseudomonas aerigunosa, a peptide from plasmodium falciparum 3D7 (H[313–333]K), a peptide from plasmodium falciparum 3D7 (L[250–269]K), a fragment of pachytene arrest protein (1–50) from saccharomyces cerevisiae (New England Peptide Company, Gardner, MA) and a synthetic magnainin spacer peptide (American Peptide Company, Sunnyvale, CA). The resulting tryptic peptides were extracted with 5% formic acid/50% ACN, concentrated with a Speed Vac, and desalted using C18 ziptips before 18O labeling or injecting into the mass spectrometer.
2.6 18O labeling using immobilized trypsin
18O labeling was performed as described previously (Huang and Kim, 2012). Briefly, the desalted peptides were completely dried and reconstituted with 10 μL acetonitrile and 50 μL of 50 mM ammonium bicarbonate in either 16O water (for DHA-adequate sample) or 97% 18O enriched water (for DHA-deficient sample), followed by addition of 0.5 μl of 2 M calcium chloride and 2.5 μL immobilized trypsin. The mixture was incubated with constant rotation for 36 hours at 30°C. After centrifugation at 15,000 g for 5 min, the supernatant was collected and acidified to pH 3–4 by adding 1–2 μL of 10% TFA. The 16O-labeled DHA-adequate samples were mixed with 18O-labeled DHA-deficient samples from the corresponding gel fractions. The mixture was desalted by ziptip C18 column prior to the analysis by nano-LC/ESI-MS/MS.
2.7 Nano-LC/ESI-MS/MS analysis
Nano-LC/ESI-MS/MS was performed on an LTQ-Orbitrap XL mass spectrometer equipped with a nanospray source (Thermo Scientific) and an Eksigent nanoLC 1D system. The mobile phases consisted of 0.1% formic acid (solvent A) and 0.1% formic acid in 95% ACN (solvent B). Approximately 1 μg of labeled digests was loaded onto a C18 trap column (Zorbax 5 × 0.3 mm, Agilent) and separated by a 15 cm IntegraFrit column (ProteoPep™, New Objective, Woburn, MA) at a flow rate of 300 nL/min with a gradient from 5–40% solvent B in 150 min. The LC eluant was sprayed into the MS instrument with a glass emitter tip (PicoTip, New Objective) using a spray voltage of 2.0 kV in positive-ion mode. Full scan spectra from m/z 300 to 1700 at 60,000 resolution were acquired by the Orbitrap. Data-dependent MS/MS spectra of ten most intense ions were acquired by the LTQ-XL ion trap using CID with a normalized energy of 35. Dynamic exclusion for the ions for which MS/MS spectra had been already acquired used following parameters: exclusion time 180 s, repeat count 1, repeat duration 30 s, exclusion mass width 10 ppm, and exclusion size 500. Singly charged species were excluded from data-dependent MS/MS analysis.
2.8 Data analysis
The acquired data were searched against the NCBInr database with Mascot (v2.3, Matrix Sciex) for protein identification. Search parameters were set as follows: enzyme, trypsin; precursor ion mass tolerance, 10 ppm; fragment ion mass tolerance, 0.3 Da; maximum missed cleavages allowed 2; carbamidomethyl of cysteine residues for fixed modification; oxidation of methionine for variable modification. Only the proteins with at least 2 distinct peptides and a Mascot score more than 50 were considered for positive identification. Quantitation of the 18O/16O ratio was performed by Mascot Distiller (version 2.3) with the 18O-corrected method due to the use of 97% enriched 18O water. Peptide peaks were rejected for quantitation if peak correlation was below 0.7, standard error was greater than 0.1, or area fraction was below 0.5. Protein abundance ratio was presented by the average 18O/16O ratio of at least two peptides and normalized to the average 18O/16O value from five standard peptides. Label-free protein quantitation was performed using Progenesis LC-MS software (v2.0, Nonlinear Dynamics).
2.9 Western blot analysis
Proteins from whole homogenates or subcellular fractions were electrophoresed in 4–12% Bis-Tris gels, transferred to a PVDF membrane, and then blocked for 1 hr at room temperature with 5% milk in TBS containing 0.1% Tween 20 (TBS-T). Blots were incubated with primary antibody overnight at 4°C and washed three times with TBS-T. Blots were then incubated with peroxidase-conjugated secondary antibody for one hour at room temperature, washed three times with TBS-T, incubated with ECL detection reagent, and imaged with a Kodak Gel Logic 440 Imaging System and quantified by Kodak 1D Image Analysis software. The primary antibodies used in this study were anti-cadherin, anti-synaptopdin, anti-PSD-95, anti-fodrin-α, anti-rab3a, anti-SNAP25, anti-SNAP-α, anti-VAMP2, anti-VGlut1, anti-dynamin-1, anti-EAAT1, anti-SV2B, and anti-munc18 (obtained from Cell Signaling or Santa Cruz Biotechnology).
2.10 RNA extraction and Real time PCR
Twenty mg cortices were homogenized using a glass Teflon homogenizer and processed for total RNA isolation with an Aurun total RNA mini kit (Bio-Rad Laboratories, CA) in accordance with the manufacturer’s instructions. The concentration of the extracted RNA was measured by NanoDrop 1000 (Thermo Scientific, Waltham, MA). cDNA synthesis was performed using high capacity cDNA reverse transcription kit (Applied Biosystems, Foster city, CA) in accordance with the manufacturer’s instructions. Each RT reaction contained 1 μg of isolated RNA, 50 nM random RT primer, 1X RT buffer, 0.25 mM each of the dNTPs, 3.33 U/μL multiscribe reverse transcriptase and 0.25 U/μL RNAase inhibitor.
The mRNA expression was measured by qPCR using an ABI Prism 7900 HT and gene-specific flourogenic TaqMan probes (Applied Biosystems, Foster City, CA). Each 10 μL polymerization chain reaction contained 1 μL relevant cDNA, 5μL of TaqMan Gene expression master mix, 0.5 μL each specific primer and the housekeeping gene, glyceraldehydes-3-phosphate dehydrogenase (GAPDH) which served as an internal control. The thermal cycling program was composed of 2 minutes at 50 °C, 10 minutes at 95 °C followed by 40 cycles of 15 seconds at 95 °C and 1 minute at 60 °C. For qPCR analysis, each sample was run in triplicates. The quantification was made by selecting the amplification cycle when the PCR product of interest was first detected, also called threshold cycle (Ct). The reactions were repeated in triplicates for each of the three biological replicates and the average Ct value was used in all analysis. Relative quantification was performed using the comparative threshold (CT) method after determining the Ct values for reference (GAPDH) and target genes in each sample sets according to the 2−DDCt method (Pfaffl, 2001) as described by the manufacturer (Applied Biosystems; User Bulletin 2). Changes in mRNA expression level of different age/diet groups were calculated after normalization to the value obtained for the 15 month old adequate diet group. The following formulas were used in the analysis.
2.11 Novel object recognition test
The novel object recognition test based on the one-trial model (Ennaceur and Delacour, 1988) was performed as described previously (Desai et. al., 2014). All behavioral procedures were performed between 11:00 and 18:00 h. The habituation phase consisted of allowing the mice to individually explore an open, empty, black plexiglass box (40 cm2) freely for 30 min. On the day following the day of habituation, each mouse was allowed to explore a pair of identical objects placed side by side in the box for a period of 10 min. After an intertrial interval of 3 h, the mouse was placed back in the box, which now consisted of one object used in the familiarization trial or sample phase and a novel object of comparable dimensions. The time spent in exploring the two objects was then recorded for a period of 6 min, and memory was expressed as percent of time spent in novel object exploration. The box and the objects were cleaned with quatricide and allowed to dry completely in-between tests to avoid any olfactory ques. The mouse was considered to explore an object if its nose was 2 cm or less from the object and the mouse was looking at the object. Sitting on the object was ignored.
2.12 Network Pathway Analysis
Using MetaCore, ontology enrichment analysis of process networks which scores and ranks the most relevant cellular processes for a dataset was performed on the differentially regulated proteins identified by nano-LC/ESI-MS/MS. The interaction network for the proteins was built using canonical pathway modeling and standard Dijkstra’s shortest paths algorithm (the maximum number of steps to connect seed nodes set at 2). For clarity, the network generated was simplified by excluding some unseeded proteins.
2.13 Statistical analysis
Unless specified, data were analyzed by one-way ANOVA and significant differences between different age-groups and diets were determined by Student’s t-test.
3. Results
3.1 Age-related changes in the synaptic proteome
To assess the changes in the synaptic proteome due to aging, we prepared the SPM fractions from the cortices of the DHA adequate young (4 months) and old (15 months) mice and assayed the differential expression of synaptic proteins using nano-LC-ESI-MS/MS combined with SDS-PAGE and in-gel digestion (Scheme 1). Half of the tryptic digests were analyzed by 18O-labeling and the other half by label-free technique using the Progenesis software.
The label-free protein quantitation indicated a total of 15 proteins that were down-regulated significantly in their levels at 15 months compared to 4 months (Table 1, supplemental Table 1). The decrease of the protein level in nine of these proteins was confirmed by 18O-labeling technique (supplemental Table 2), which normally quantifies fewer proteins due to the loss of samples during labeling procedures but provides more accurate results compared to the label-free approach. These 15 proteins include fodrin-α, synaptopodin, PSD-95, synaptic vesicle SV2B, SNAP25, SNAP-α, NR2B, AMPA2, AP2, vesicular glutamate transporter 1 (VGluT1), munc18-, dynamin-1, vesicle associated membrane protein 2 (VAMP2), ras-related protein rab3A (Rab3A) and excitatory amino acid transporter 1 (EAAT1). Among these proteins, EAAT1, VGluT1, fodrin-α, AP2, synaptopodin and SNAP-α were identified for the first time as the age-related synaptic proteins, while the rest of them have been reported previously to down-regulate during the normal aging process (Clayton and Browning, 2001; Dyall et. al., 2007; Hof et. al., 2002; Kadish et. al., 2009; VanGuilder et. al., 2010, 2011).
Table 1.
Synaptic proteins downregulated in aged mouse brains revealed by quantitative mass spectrometry.
| Proteins | Gene symbol | Accession number | Molecular mass (kDa) |
|---|---|---|---|
| Fodrin-α | Spna2 | 223462890 | 250 |
| Synaptopodin | Synpo | 158303337 | 104 |
| Discs, large homolog 4 (PSD-95) | dlg4 | 148680577 | 95 |
| Synaptic vesicle glycoprotein 2B (SV2B) | Sv2b | 27261824 | 78 |
| Synaptosomal-associated protein 25 (SNAP25) | Snap25 | 6755588 | 25 |
| Alpha-soluble NSF attachment protein (SNAP-α) | Napa | 13385392 | 33 |
| Glutamate [NMDA] receptor subunit epsilon-2 precursor (NR2B) | Grin2b | 117168299 | 168 |
| Glutamate receptor, ionotropic, AMPA2 (AMPA2) | Gria2 | 148683502 | 104 |
| AP-2 complex subunit mu isoform b (AP2) | Ap2m1 | 68799814 | 109 |
| Vesicular glutamate transporter 1 (VGluT1) | Vglut1 | 123793848 | 52 |
| Munc18-1 | Stxbp1 | 17225417 | 68 |
| Dynamin-1 | Dnm1 | 123230374 | 98 |
| Vesicle associated membrane protein 2 (VAMP2) | Vamp2 | 2253399 | 13 |
| Ras-related protein rab3A (Rab3A) | Rab3a | 6679593 | 25 |
| Excitatory amino acid transporter 1 (EAAT1) | Eaat1 | 24233554 | 60 |
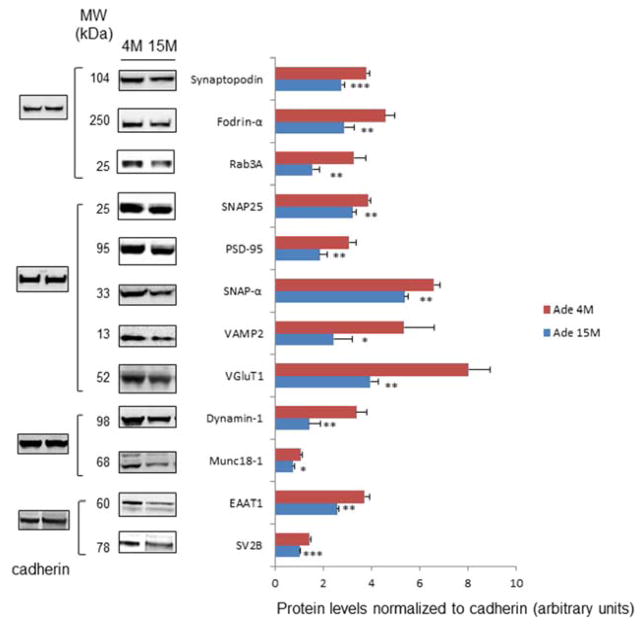
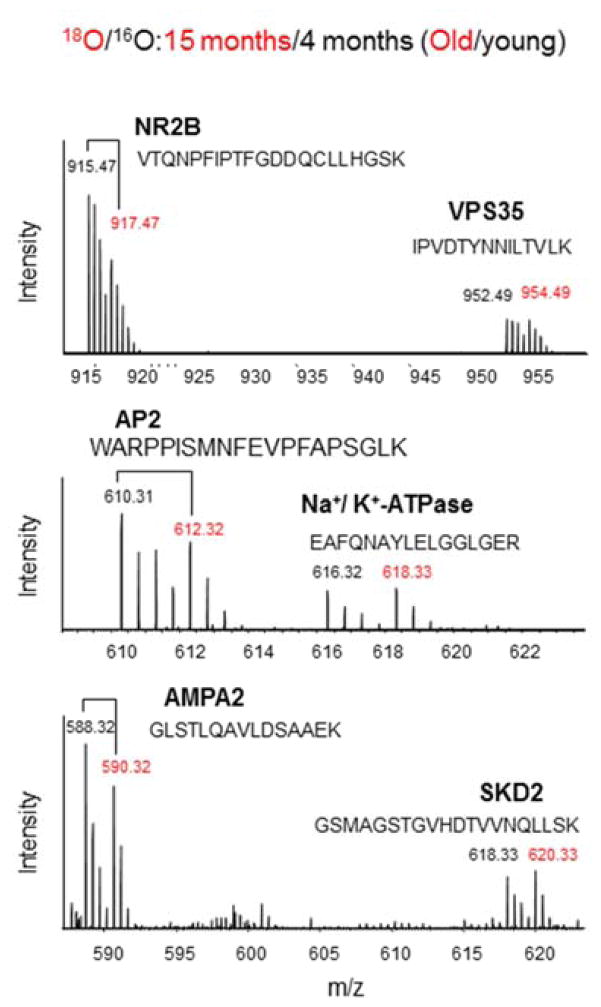
The changes in protein levels identified by the MS-based approach were validated by western blot analysis using specific antibodies. Out of 15 proteins identified as down-regulated, 12 proteins were analyzed by western blotting. Densitometric analysis indicated that they all showed significant down-regulation at 15 months in comparison to 4 months (Fig. 1). Since NR2B and AMPA2 are already known to be down-regulated during aging (Clayton and Browning, 2001; Dyall et. al., 2007; Hof et. al., 2002), western blot analysis for these two proteins was not performed. The antibody for AP2 was not available. For these three proteins (NR2B, AMPA2 and AP2), individual peptides were quantified by 18O/16O labeling and representative spectra for these proteins are shown in Fig. 2. Each spectrum contained 18O/16O labeled peptides belonging to the indicated proteins. The reduction is clearly shown for the peptide peaks representing the aged (18O-labeled) compared to the young mouse brain sample (16O-labeled), while no change is apparent for control proteins such as VPS35, Na+/K+-ATPase and SKD2. Of note, all these 15 proteins down-regulated during the aging process are known to be involved in neurotransmission and synaptic vesicle trafficking or docking.
Fig. 1.
Western blot analysis of SPM proteins obtained from young (4 months) and old (15 months) mice raised with DHA-adequate diet. Quantitation was based on the band intensity normalized to cadherin using three biological replicates. The data are expressed as mean ± standard deviation (n=3–4). Statistical analysis was performed using Student’s t-tests. *, p< 0.05; **, p< 0.01. 4M; 4 month-old, 15M; 15 month-old, Ade, DHA-adequate.
Fig. 2.
Representative mass spectra from 16O-labeled sample (4 month-old mice) mixed with 18O labeled sample (15 month-old mice). The brain samples were obtained from DHA-adequate mice. The 18O/16O peak intensity ratio was used for relative quantitation of the peptides.
3.2 Effect of DHA on age-related changes in the synaptic proteome
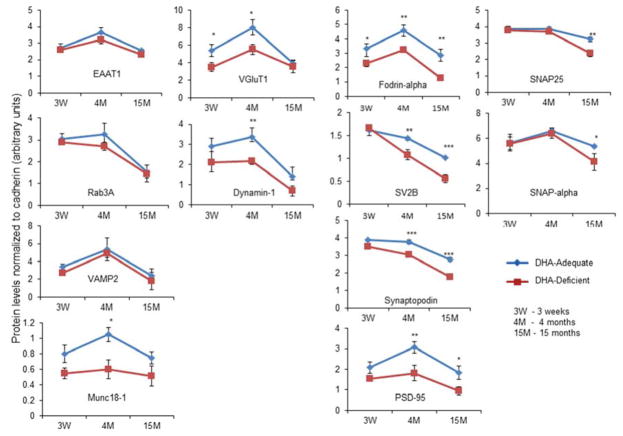
We have previously shown that DHA deficiency can result in a loss of a number of important synaptic proteins involved in neurotransmission and synaptic plasticity (Sidhu et al., 2011). In this study, we tested if depleting DHA would exacerbate the age-related loss of the synaptic proteins. To this end, mice were treated with DHA-deficient or adequate diet as described in the experimental section. Analysis of fatty acid contents indicated at least 35% reduction in brain DHA level in mice fed the deficient diet compared to those fed the adequate diet (supplemental Table 3). SPM proteins from DHA-adequate and deficient mice were analyzed by SDS-PAGE followed by mass spectrometric approaches. Intriguingly, nine of the synaptic proteins that were down-regulated during aging were found to be further decreased as a result of DHA deficiency in aged (15 months old) mice (supplemental Table 4). These proteins include fordin-α, synaptopodin, PSD-95, SV2B, SNAP25, SNAP-α, NR2B, AMPA2, and AP2. The MS-based results were evaluated by western blot analysis based on the availability of antibodies. For the western blot analysis, we included another age-group, 3 week-old mice, to better understand the synaptic proteome changes during the course of development and aging (Fig. 3, supplemental Fig. 1). There was significant down-regulation in the levels of VGluT1 at 3 week- and 4 month-old brains, and fodrin-α at all ages tested in DHA-deficient brains. In addition, under the DHA-depleted condition, the down-regulation of brain SV2B, synaptopodin and PSD-95 was observed in mouse brains at 4 and 15 months, while reduced levels in SNAP25 and SNAP-α were found at 15 months only. The western blot data also indicated that proteins including fodrin-α, SV2B, synaptopodin, PSD-95, SNAP25 and SNAP-α were down-regulated at 15 months compared to 4 months, regardless of the DHA status (Fig. 3, supplemental Fig. 1). The DHA effect was also observed at 4 months in many of the SPM proteins such as dynamin-1 and munc18–1 as reported in our previous study (Sidhu et. al., 2011). Overall, the western blot data was in good agreement with our MS data.
Fig. 3.
Effects of the DHA status on SPM proteins during aging. The effect of DHA on SPM proteins down-regulated during aging (4 months to 15 months) was evaluated at 3 weeks, 4 months and 15 months of age. The western blot data from three biological replicates expressed as mean ± standard deviation (n=3–4) is presented. Statistical analysis was performed for the differences between DHA-adequate and deficient samples using Student’s t-tests. *, p< 0.05; **, p< 0.001; ***, p<0.0001.
3.3 Effect of DHA on age-related changes in the synaptic protein gene expression
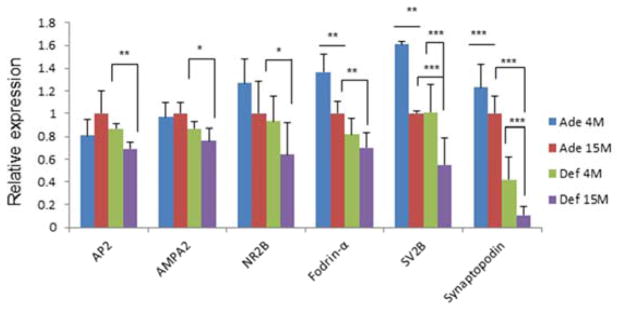
For the SPM proteins that were found to change under the influence of DHA-depletion, we performed qRT-PCR to evaluate changes in gene expression (Fig. 4). The positive effect of DHA at 15 months of age was also observed at the gene level for NR2B, AP2 and AMPA2 for which western blot analyses were not performed. Similarly, mRNA levels of fodrin-α, SV2B and synaptopodin were also higher in DHA-adequate samples in comparison to the deficient brains at 15 months. Although gene and protein expression levels do not necessarily correlate, these results appeared to be consistent with the protein data based on western blot and MS analyses as summarized in Table 2. From 4 to 15 months of age, gene expression of fodrin-α, SV2B and synaptopodin was significantly down-regulated. Despite the decrease at the protein level detected by mass spectrometry, AP2, AMPA2, and NR2B at the gene level were not significantly affected by aging in DHA-adequate brains. With the exception of AP2, DHA deficiency resulted in a general trend of decrease in the levels of all genes tested also at 4 months (Fig. 4).
Fig. 4.
Effects of aging and DHA status on gene expression in mouse brains. The Y-axis represents the mRNA expression relative to that of the 15 month adequate group. The data were obtained from three biological replicates using real time qPCR and expressed as mean ± standard deviation (n=3). Statistical analysis was performed using Student’s t-tests. *, p< 0.05; **, p< 0.001; ***, p<0.0001.
Table 2.
Summary of aging- and DHA-related changes in synaptic protein expression.
| Proteins | Aging-related changes (15 vs. 4 months) | DHA-related changes at 15 months | |||
|---|---|---|---|---|---|
|
| |||||
| MS | WB | MS | WB | RT-PCR | |
| Fodrin-α* | Down | Down | + | + | + |
| Synaptopodin* | Down | Down | + | + | + |
| Discs, large homolog 4 (PSD-95) | Down | Down | + | + | NT |
| Synaptic vesicle glycoprotein 2B (SV2B) | Down | Down | + | + | + |
| Synaptosomal-associated protein 25 (SNAP25) | Down | Down | + | + | NT |
| Alpha-soluble NSF attachment protein (SNAP- α) * | Down | Down | + | + | NT |
| Glutamate [NMDA] receptor subunit epsilon-2 precursor (NR2B) | Down | NT | + | NT | + |
| Glutamate receptor, ionotropic (AMPA2) | Down | NT | + | NT | + |
| AP-2 complex subunit mu isoform b (AP2)* | Down | NT | + | NT | + |
| Vesicular glutamate transporter 1 (VGluT1)* | Down | Down | − | − | NT |
| Munc18-1 | Down | Down | − | − | NT |
| Dynamin-1 | Down | Down | − | − | NT |
| Vesicle associated membrane protein 2 (VAMP2) | Down | Down | − | − | NT |
| Ras-related protein rab3A (Rab3A) | Down | Down | − | − | NT |
| Excitatory amino acid transporter 1 (EAAT1)* | Down | Down | − | − | NT |
MS, mass spectrometry; WB, western blot analysis; Down, down-regulated; NT, not tested
, newly identified as synaptic proteins down-regulated with aging; +, further down-regulation upon DHA depletion; −, no change upon DHA depletion.
3.4 Effect of aging and brain DHA status on cognitive function
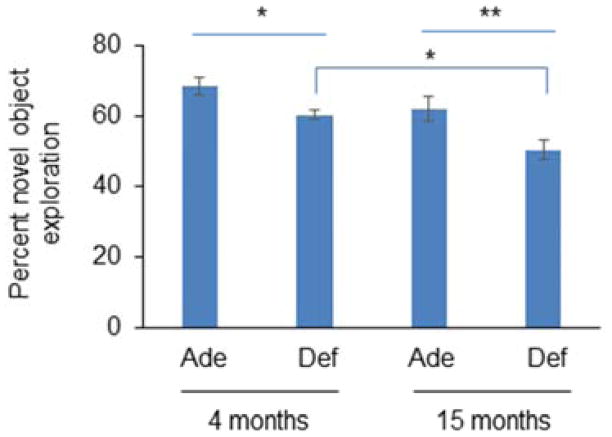
To examine possible impact of the loss of SPM proteins on neurological function, we evaluated cognitive function, particularly recognition memory, by novel object recognition test. Depletion of brain DHA significantly decreased recognition memory in both young (68.5 ± 2.6 vs. 60.5 ± 1.3 %; p<0.05) and aged animals (62.0 ± 3.3 vs. 50.5 ± 2.8 %; p<0.01) tested at 4 and 15 months, respectively (Fig. 5). Despite a decreasing trend, the difference in recognition memory between 4 and 15 months did not reach the statistical significance in DHA-adequate animals. However, aging significantly decreased cognitive function in DHA-deficient mice (60.5 ± 1.3 vs. 50.5 ± 2.8 % ; p<0.05), which is consistent with the aggravated loss of synaptic proteins in these animals. These data suggested a positive correlation between the observed synaptic proteome loss and cognitive decline.
Fig. 5.
Effect of aging and brain DHA status on cognitive function. DHA-deficiency in brain compromises cognitive function evaluated by novel object recognition test in young (4 months) and aged mice (15 months). Aging significantly affected recognition memory only in DHA-depleted animals. Data are presented as mean ± SEM (n=6–9). *, p<0.05 ; **, p<0.01.
3.5 Effect of raising brain DHA content on aging-related changes in the synaptic proteome
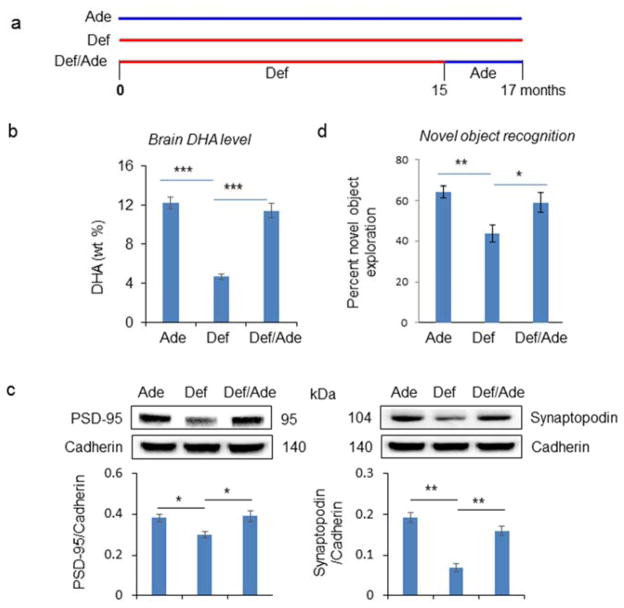
We tested whether increasing brain DHA in aging brains could reverse synaptic protein loss and improve cognitive function. We used DHA-depleted aged mice as a model since the synaptic protein loss was exaggerated in these animals. DHA-deficient mice at 15 month of age were supplemented with the n-3 fatty acid adequate diet for the subsequent 2 months (diet reversal group). In parallel, DHA-adequate and deficient animals were continued on their corresponding diets until 17 months of age (Fig. 6a). At 17 months, western blot analysis was performed to evaluate the six synaptic proteins that were shown to be significantly down-regulated at 15 months and by DHA deficiency as shown in Fig. 3. Similar to the data obtained at 15 months (Supplemental Table 3), the brain DHA content in the deficient mice was significantly lower than that of the adequate mice at 17 months (12.2 ± 0.6 vs. 4.7 ± 0.3 %; p<0.01). Two months of the diet reversal markedly increased DHA to nearly the level of the adequate mice (11.5 ± 0.7 %) (Fig. 6b). Western blot analysis revealed significant increases of two proteins, namely synaptopodin and PSD-95, in the reversal group in comparison to the deficient animals (Fig. 6c). We also performed the novel object recognition test to evaluate cognitive function for DHA-adequate, DHA-deficient and diet reversal groups at 17 months. As observed with 15-month old animals (Figure 5), the recognition memory was significantly lower in DHA-deficient animals compared to the adequate group at 17 months (64.2 ± 2.9 vs. 43.7.5 ± 4.1 %; p<0.01). After 2 months of diet reversal, the cognitive function was improved (58.9 ± 5.1 %) nearly up to the level observed for the DHA-adequate group (Fig. 6d). These data indicated that the synaptic protein loss as well as cognitive decline in aging animals exacerbated under a DHA-depleted condition can be reversed by raising the brain DHA content.
Fig. 6.
Effect of raising brain DHA content on synaptic protein level and cognitive function in aged mice. a, Timeline of the diet. Mice were fed with DHA-adequate (Ade) or DHA-deficient diet (Def) diet for 17 months, or fed with the deficient diet for 15 months followed by the adequate diet for 2 months to increase the brain DHA level. b–c, Brain DHA (b) and synaptic protein levels (c) at 17 months of age after feeding different diets. d, Effect of brain DHA status on cognitive function evaluated by novel objective recognition test. The 17-month DHA-deficient mice showed significantly shorter novel object exploration as compared to the adequate group, indicating impaired recognition memory. This difference in the novel object recognition performance disappeared when 15 month old DHA-deficient mice were placed on the adequate diet for 2 months. Student’s paired t-test was used to assess statistical differences. Data are presented as mean ± standard deviation (in b,c; n=3–4) or SEM (in d; n=6–8). ***, p < 0.001; **, p < 0.01; *, p < 0.05.
3.6 Network pathway analysis
The 15 proteins altered in expression by aging and brain DHA level were subjected to the MetaCore enrichment analysis to determine their functional involvement in the context of cellular processes (Bessarabova et al., 2012). Based on the score and ranking calculated by this functional analysis, the most relevant cellular processes were found to include transport (synaptic vesicle exocytosis), development (neurogenesis/synaptogenesis), neurophysiological process (GABAergic neurotransmission), and cell adhesion (synaptic contact) (Table 3). Of note, synaptopodin and PSD-95 are involved in the synaptogenesis and synaptic contacts (Table 3, Fig. S2 and 3). To assess the connectivity within the synaptic proteins, the interaction network analysis was performed using the shortest path algorithm (Bessarabova et al., 2012). As shown in Fig. 7, many of these proteins are found to interact with each other. Interestingly, all these 15 synaptic proteins are linked to cAMP responsive element binding protein (CREB) (Fig. 7).
Table 3.
Enrichment analysis of process networks from 15 synaptic proteins altered in expression by aging and brain DHA level
| Top 10 Networks | p-value | FDR | Ratio | Network Objects |
|---|---|---|---|---|
| Transport/Synaptic vesicle exocytosis | 1.042E-10 | 2.918E-09 | 9/175 | SV2B, VAMP2, SLC17A7/VGluT1, Dynamin- 1, GLAST1/EAAT1, Rab-3A, SNAP-25, Rab-3, Munc18 |
| Development/Neurogenesis/ Synaptogenesis | 4.913E-09 | 6.879E-08 | 8/180 | SNAP-α, GluR2/AMPA2, VAMP2, Synaptopodin, SNAP-25, PSD-95, NR2B, Munc18 |
| Neurophysiological process/GABAergic neurotransmission | 2.299E-08 | 2.146E-07 | 6/138 | AP complex 2, VAMP2, Dynamin-1, SNAP-25, NR2B, Munc18 |
| Cell adhesion/Synaptic contact | 3.864E-06 | 2.705E-05 | 6/184 | Synaptopodin, SLC17A7/3 VGluT1, SNAP-25, PSD-95, Fodrin-α, NR2B |
| Neurophysiological process/Long-term potentiation | 1.051E-03 | 5.884E-03 | 3/82 | GluR2/AMPA2, NR2B, NR2 |
| Signal transduction/Nitric oxide signaling | 1.290E-03 | 6.019E-03 | 3/88 | PSD-95, NR2B, NR2 |
| Neurophysiological process/Transmission of nerve impulse | 1.513E-02 | 6.053E-02 | 3/212 | GluR2/AMPA2, PSD-95, NR2B |
| Cytoskeleton/Cytoplasmic microtubules | 3.323E-02 | 1.163E-01 | 2/115 | PSD-95, Fodrin-3α |
| Development/Neuromuscular junction | 5.189E-02 | 1.615E-01 | 2/147 | VAMP2, SNAP-25 |
| Cell adhesion/Cell junctions | 6.169E-02 | 1.715E-01 | 2/162 | PSD-95, Fodrin-α |
FDR: false discovery rate
Fig. 7.
Protein interaction network analysis indicating the involvement of CREB signaling. The analysis was conducted on 15 SPM proteins altered by aging and brain DHA levels. The network was built with the shortest paths algorithm using MetaCore software.
4. Discussion
Aging is a known risk factor for cognitive decline. It has been reported that a large number of synaptic proteins involved in neurotransmission are altered during aging (VanGuilder et al., 2010). We have previously demonstrated that DHA promotes hippocampal neurite outgrowth, synaptogenesis and synaptic activity (Calderon and Kim, 2004; Cao et. al., 2009). Our study also indicated that DHA depletion leads to down-regulation of SPM proteins involved in synaptic neurotransmission (Sidhu et. al, 2011) and impairs long-term potentiation in hippocampal slices (Cao et. al., 2009). Based upon these studies, we hypothesized that DHA can offset synaptic proteome changes during aging, which may alleviate aging-related cognitive decline.
4.1 DHA ameliorates aging-related loss of brain SPM proteins important for proper cognitive function
By comparing the SPM proteins from young (4 months) and aged (15 months) mouse brains, we identified dysregulation of 15 synaptic proteins. Among those, 6 proteins including fodrin-α, synaptopodin, SNAP-α, AP2, VGluT1, and EAAT1 were not reported to change in the earlier aging studies. More importantly, we found that DHA-depletion exacerbates the aging-related decline of some of these synaptic proteins, indicating a role of DHA in mitigating aging-induced decline of synaptic proteins (Table 2). Significant synaptic protein loss and cognitive deficit observed in DHA-deficient aging mice was reversed when the brain DHA level was normalized, supporting a role of DHA in ameliorating aging-induced cognitive decline that parallels synaptic proteome restoration.
Previous proteomics studies have reported age-related reduction of many synaptic proteins such as, PSD-95, munc18, synapsin 1, synapsin 2, SNAP25, VAMP2, dynamin 1, syntaxin 1, hippocalcin and 14-3-3 isoforms in the hippocampus, most of which are involved in neurotransmission and synaptic plasticity (VanGuilder et. al, 2010, 2011). SV2B and Rab3A have also been demonstrated to decrease in expression during aging in Fischer 344 rats of ages 3, 12 and 23 months (Kadish et. al., 2009). Previous studies have also reported down-regulation of glutamate receptors, AMPA2 and NR2B with aging (Clayton and Browning, 2001; Dyall et. al., 2007; Hof et. al., 2002). Our MS-based strategy of 18O labeling or label-free mass spectrometric analysis allowed the detection of most of these proteins down-regulated during aging along with 6 additional SPM proteins as described above. Age-related protein up-regulation has been reported mainly for enzymes that mediate energy production and oxidative stress such as, transketolase and mitochondrial creatine kinase (Yang et. al., 2008). For SPM proteins, however, we observed only age-related down-regulation and no increases in protein expression were detected.
DHA produced a significant effect on the levels of some SPM proteins that were down-regulated during aging. These include fodrin-α, SV2B, synaptopodin, PSD-95, SNAP25 and SNAP-α (Fig. 3). Gene expression levels of AP2, AMPA2 and NR2B receptors at 15 months were also found to decrease significantly under DHA-deficient conditions (Fig. 4, Table 2). Most of the proteins down-regulated due to DHA deficiency play an important role in synaptic plasticity. PSD-95 is known to recruit and cluster glutamate receptors (NMDA and AMPA), ion channels as well as signal transduction molecules for proper synaptic activity (Beique et. al, 2006; Kim et. al., 1995, Vickers et. al., 2006). This protein is also required for maturation of the pre-synaptic terminals and dendritic spines and for stabilizing established synapses (El-Husseini et. al., 2000, Okabe et. al., 2001). Synaptopodin, an actin-binding protein found in a subset of dendritic spines containing the spine apparatus organelle (Deller et al., 2000), has been shown to be important for long-term potentiation in vitro (Deller et al., 2003) and in vivo (Jedlicka et al., 2009) as well as in spatial learning. SNAP25, a presynaptic plasma membrane protein, is a part of SNARE (soluble N-ethylmaleimide sensitive factor attachment protein receptor) core complex formed together with VAMP2 and syntaxin1a (Chen et al., 2002). This protein functions in the regulation of neurotransmitter release by associating with the proteins involved in vesicle docking and fusion (Blasi et al., 1993; Hodel, 1998). Fodrin-α is involved in stabilizing membrane structure, maintaining cell shape and linking the cytoskeleton to plasma membranes or intracellular vesicles (Bennett, 1990), Bourguignon et al., 1985). SV2B is an isoform of SV2 protein that comprises 12 transmembrane regions (Heese et al., 2001; Janz et al., 1999). SV2 proteins have been reported to participate in the regulation of calcium-mediated synaptic transmission and also play a role in vesicle trafficking by binding to other cell surface proteins (Lazzell et al., 2004; Schivell et al., 1996). SNAP-α is required for vesicle fusion with the target membrane (Beckers et al., 1989; Block et al., 1988, Diaz et al., 1989, Rodriguez et al., 1994). An ATP-controlled highly specific interaction has been reported between SNAP-α and N-ethylmaleimide-sensitive fusion protein (NSF) (Wilson et al., 1992). ATP hydrolysis leads to rearrangement of the SNARE complex and to membrane fusion and release of the soluble components of the reaction from the membrane (Hayashi et al., 1995; Sollner et al., 1993). Since aging results in significant loss of these important synaptic proteins and DHA deficiency leads to their further decrease, it can be concluded that DHA helps to prevent down-regulation and maintain the level of these SPM proteins in the brain during aging.
The aging-induced reduction of fodrin-α, SV2B and synaptopodin proteins was also observed at the gene level (Fig. 4). However, gene expression of AP2, AMPA2 and NR2B in DHA-adequate brains, which declined during aging from 4 to 15 months at the protein level (Fig. 2, Supplemental Table 1), was not correspondingly altered. This discrepancy could be due to complicated biological processes, such as transcriptional splicing, post-transcriptional splicing, translational modifications, translational regulation, and protein complex formation, which can influence mRNA and protein correlation. It is also possible that stability of some proteins were affected by aging despite the absence of altered gene expression. While some studies have found a good correlation between mRNA and protein expression levels, others have also reported discrepancies as observed for AP2, AMPA2 and NR2B in our study (Guo et. al., 2008; Gygi et. al., 1999). In terms of the DHA effects, we observed significant decreases in mRNA levels in gria2, grin2b, spna2, sv2b and synpo genes at 15 months under DHA deficient conditions, which is consistent with protein levels obtained by mass spectrometry and/or western blot analysis (Figs. 3,4, Table 1). Unlike the case at 15 months, the reduction of gria2 and grin2b did not reach statistical significance despite the decreasing tendency under DHA deficient conditions at 4 months of age. It is possible that the down-regulation of AMPA2 and NR2B previously observed (Sidhu et al., 2012) may not occur at mRNA level at this age. It can be also speculated that protein degradation at 4 months of age is slower compared to 15 months age.
Our data clearly indicated aging-induced reduction of synaptic proteome exacerbated in DHA-deficient brains (Fig. 3), while such synaptic protein loss was ameliorated after increasing the brain DHA level by switching to an n-3 fatty acid supplemented diet (Fig. 6), demonstrating a role of DHA in sustaining synaptic proteome. Although only two out of the six down-regulated synaptic proteins, synaptopodin and PSD-95, showed statistically significant increases after diet reversal, (Fig. 6c), the other four SPM proteins (fodrin-α, SV2B, SNAP25 and SNAP-α) also showed an increasing trend (data not shown). It is possible that a longer n-3 fatty acid supplementation is required for a significant recovery of these four SPM proteins.
DHA-depletion and aging caused impairment of recognition memory (Fig. 5) along with synaptic protein reduction (Fig. 3), suggesting a positive correlation between synaptic protein expression and cognitive function. Raising the brain DHA content by diet reversal restored synaptic protein loss as well as the recognition memory impaired by the DHA-deficiency in 17 month old mice (Fig. 6d), which is consistent with previous studies indicating that DHA administration improves cognitive function in aged animals (Gamoh et al, 2001; Jiang et al, 2009). The ability of DHA to maintain important synaptic proteins which are otherwise decreased by aging may be an important mechanism connecting DHA and cognitive improvement in aged animals.
4.2 Proposed mechanisms for the effect of DHA in maintaining SPM protein levels
There have been conflicting reports on the effects of normal aging on brain DHA levels in both rodent and humans. While aging-related decline in DHA levels was observed in various rodent brain regions (Lopez et al., 1995; Little et al., 2007; Barcelo-Coblijn et al., 2003; Favrelier et al., 2000), others found lack of such effects (Yetmler et al., 2012). Some human studies indicated that aging has no appreciable influence on DHA contents in cerebral cortex (Carver et al., 2001), frontal cortex, hippocampus and pons (Soderberg M, 1991). In another study, healthy aging was shown to be associated with a progressive decline in polyunsaturated fatty acid (PUFA) composition, including DHA and arachidonic acid (AA, 20:4n-6) in the orbitofrontal cortex gray matter (McNamara et al., 2008). In contrast, a recent report indicated that normal adult aging increases DHA-containing phospholipids in the dorsolateral prefrontal cortex from subjects aged from 20 to 100 years (Norris et al., 2015). The present study showed no significant changes in the brain DHA content during normal aging. The 2-way ANOVA also indicated no significant interaction between aging and DHA for the six synaptic proteins that were down-regulated at 15 months and influenced by DHA, with the exception of SNAP25 (Supplemental Table 5). These findings suggest that aging and DHA may not have identical mechanisms to alter synaptic proteome, although shared targets can be postulated in the downstream signaling.
The CREB-mediated gene transcription may be a common target mechanism for DHA- and aging-related synaptic proteome changes and behavioral consequences. We have recently demonstrated that cAMP/PKA/CREB signaling is activated by DHA and its metabolite N-docosahexaenoylethanolamine in cultured neural stem cells (Rashid et al., 2013; Rashid and Kim 2016). We have also shown that SPM proteins down-regulated due to DHA deficiency are downstream of CREB (Sidhu et al., 2011). According to the interaction network analysis in the present study, most of the synaptic proteins altered by aging are also upregulated by CREB through direct or indirect interaction (Fig. 7), suggesting CREB-activated gene transcription as a key shared factor in the aging- and DHA-mediated regulation of the synaptic proteome. Significant loss of the synaptic proteins important for neurotransmission may lead to cognitive decline during aging (VanGuilder et. al, 2010, 2011). CREB is known to play an important role in learning and memory (Dubynina and Dolotov, 2009). A significantly impaired LTP has been reported in hippocampal slices of CREB mutant mice (Bourtchuladze et al., 1994). Similar impairment of LTP was also observed in hippocampi of DHA-depleted mice where CREB downstream synaptic proteins such as synapsin I and NR2B were reduced (Cao et al., 2009). These findings suggest that CREB signaling for the expression and maintenance of synaptic proteins is an important mechanism for the DHA-enhanced learning and memory function in aging brains.
In addition to the CREB signaling, the Akt-mediated neuronal survival pathway may also play an important role in synaptic proteome changes by DHA. DHA was shown to promote neuronal survival through increasing phosphatidylserine in cell membranes, which in turn facilitates membrane translocation and activation of Akt (Akbar et al., 2005; Huang et al. 2011). Conversely, DHA-depletion was shown to increase neuronal susceptibility to cell death (Akbar et al., 2005), reducing neuron numbers. In a recent study, DHA ingestion increased newborn neurons at 18 month old rats, although statistical significance was not reached (Tokuda et al., 2014), also indicating the influence of DHA on neuron numbers. The number of surviving neurons decreased by DHA-deficiency may be a contributing factor for the exacerbated loss of synaptic proteins in DHA deficient aging brains.
In conclusion, our results indicate that the loss of synaptic proteins associated with synaptic functions can be exacerbated by DHA-deficiency, leading to cognitive impairment. By maintaining the key SPM proteins, DHA may reduce the risk of age-related decline in cognitive functions.
Supplementary Material
Highlights.
Aging-induced synaptic proteome changes and DHA-dependent mitigation of such changes.
Synaptic proteome changes correlate with the recognition memory.
Mass spectrometry-based quantitation combined with western blot or mRNA analysis.
CREB signaling implicated in aging- and DHA-dependent synaptic proteome changes.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Alcohol Abuse and Alcoholism, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: A positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barceló-Coblijn G, Högyes E, Kitajka K, Puskás LG, Zvara A, Hackler L, Jr, Nyakas C, Penke Z, Farkas T. Modification of docosahexaenoic acid of age-induced alterations in gene expression and molecular composition of rat brain phospholipids. Proc Nat Acad Sci USA. 2003;100:11321–11326. doi: 10.1073/pnas.1734008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter MG, Frick KM, Price DL, Brekler SJ, Markowska AL, Gorman LK. Presynaptic markers of cholinergic function in the rat brain: relationship with age and cognitive status. Neuroscience. 1999;89:771–779. doi: 10.1016/s0306-4522(98)00374-1. [DOI] [PubMed] [Google Scholar]
- 4.Beckers CJM, Block MR, Glick BS, Rothman JE, Balch WE. Vesicular transport between the endoplasmic reticulum and Golgi stack requires the NEM-sensitive fusion protein. Nature. 1989;339:397–398. doi: 10.1038/339397a0. [DOI] [PubMed] [Google Scholar]
- 5.Beique JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci USA. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennet V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev. 1990;70:1029–1065. doi: 10.1152/physrev.1990.70.4.1029. [DOI] [PubMed] [Google Scholar]
- 7.Bessarabova M, Ishkin A, JeBailey L, Nikolskaya T, Nikolsky Y. Knowledge-based analysis of proteomics data. BMC Bioinformatics. 2012;13(Suppl 16):S13. doi: 10.1186/1471-2105-13-S16-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Sudhof TC, Niemann H, Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365:160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- 10.Block MR, Glick BS, Wilcox CA, Wieland FT, Rothman JE. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc Nod Acad Sci USA. 1988;85:7852–7856. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourguignon LY, Suchard SJ, Nagpal ML, Glenney JR. A T-lymphoma transmembrane glycoprotein (gp180) is linked to the cytoskeletal protein, fodrin. J Cell Biol. 1985;101:477–487. doi: 10.1083/jcb.101.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 13.Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- 14.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Sudhof TC, Rizo J. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- 17.Clayton DA, Browning MD. Deficits in the expression of the NR2B subunit in the hippocampus of aged Fisher 344 rats. Neurobiol Aging. 2001;22:165–168. doi: 10.1016/s0197-4580(00)00196-2. [DOI] [PubMed] [Google Scholar]
- 18.Cotman CW, Matthews DA. Synaptic plasma membranes from rat brain synaptosomes: isolation and partial characterization. Biochem Biophys Acta. 1971;249:380–394. doi: 10.1016/0005-2736(71)90117-9. [DOI] [PubMed] [Google Scholar]
- 19.Deller T, Korte M, Chabanis S, Drakew A, Schwegler H, Stefani GG, Zuniga A, Schwarz K, Bonhoeffer T, Zeller R, Frotscher M, Mundel P. Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. Proc Natl Acad Sci USA. 2003;100:10494–10499. doi: 10.1073/pnas.1832384100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deller T, Merten T, Roth SU, Mundel P, Frotscher M. Actin-associated protein synaptopodin in the rat hippocampal formation: localization in the spine neck and close association with the spine apparatus of principal neurons. J Comp Neurol. 2000;418:164–181. doi: 10.1002/(sici)1096-9861(20000306)418:2<164::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One. 2014;9(1):e86472. doi: 10.1371/journal.pone.0086472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz R, Mayorga LS, Weidman PJ, Rothman JE, Stahl PD. Vesicle fusion following receptor-mediated endocytosis requires a protein active in Golgi transport. Nature. 1989;339:398–400. doi: 10.1038/339398a0. [DOI] [PubMed] [Google Scholar]
- 23.Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007;6:275–284. doi: 10.1111/j.1474-9726.2007.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubynina EV, Dolotov OV. The CREB transcription factor and processes of memory formation. Neurochemical Journal. 2009;3:155–63. [Google Scholar]
- 25.Dyall SC, Michael GJ, Whelpton R, Scott AG, Michael-Titus AT. Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain. Neurobiol Aging. 2007;28:424–439. doi: 10.1016/j.neurobiolaging.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 26.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 27.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural Brain Research. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 28.Favrelière S, Stadelmann-Ingrand S, Huguet F, De Javel D, Piriou A, Tallineau C, Durand G. Age-related changes in ethanolamine glycerophosphatide fatty acid levels in rat frontal cortex and hippocampus. Neurobiol Aging. 2000;21:653–660. doi: 10.1016/s0197-4580(00)00170-6. [DOI] [PubMed] [Google Scholar]
- 29.Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 30.Gamoh S, Hashimoto M, Hossain S, Masumura S. Chronic administration of docosahexaenoic acid improves the performance of radial arm maze task in aged rats. Clin Exp Pharmacol Physiol. 2001;28:266–270. doi: 10.1046/j.1440-1681.2001.03437.x. [DOI] [PubMed] [Google Scholar]
- 31.Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J Neurosci. 2006;26:3482–3490. doi: 10.1523/JNEUROSCI.4171-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gavilan MP, Revilla E, Pintado C, Castano A, Vizuete ML, Moreno-Gonzalez I, Baglietto-Vargas D, Sanchez-Varo R, Vitorica J, Gutierrez A, Ruano D. Molecular and cellular characterization of the age-related neuroinflammatory processes occurring in normal rat hippocampus: potential relation with the loss of somatostatin GABAergic neurons. J Neurochem. 2007;103:984–996. doi: 10.1111/j.1471-4159.2007.04787.x. [DOI] [PubMed] [Google Scholar]
- 33.Guo Y, Xiao P, Lei S, Deng F, Xiao GG, Liu Y, Chen X, Li L, Wu S, Chen Y, Jiang H, Tan L, Xie J, Zhu X, Liang S, Deng H. How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim Biophys Sin. 2008;40:426–436. doi: 10.1111/j.1745-7270.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 34.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi T, Yamasaki S, Nauenburg S, Binz T, Niemann H. Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. EMBO J. 1995;14:2317–2325. doi: 10.1002/j.1460-2075.1995.tb07226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heese K, Nagai Y, Sawada T. Identification of a new synaptic vesicle protein 2B mRNA transcript which is up-regulated in neurons by amyloid beta peptide fragment (1–42) Biochem Biophys Res Commun. 2001;289:924–928. doi: 10.1006/bbrc.2001.5932. [DOI] [PubMed] [Google Scholar]
- 37.Hodel A. Molecules in Focus: SNAP-25. Int J Biochem Cell Boil. 1998;30:1069–1073. doi: 10.1016/s1357-2725(98)00079-x. [DOI] [PubMed] [Google Scholar]
- 38.Hof PR, Duan H, Page TL, Einstein M, Wicinski B, He Y, Erwin JM, Morrison JH. Age-related changes in GluR2 and NMDAR1 glutamate receptor subunit protein immunoreactivity in corticocortically projecting neurons in macaque and patas monkeys. Brain Res. 2002;928:175–186. doi: 10.1016/s0006-8993(01)03345-5. [DOI] [PubMed] [Google Scholar]
- 39.Huang BX, Kim HY. Effect of ethanol on conformational changes of Akt studied by chemical cross-linking, mass spectrometry and 18O labeling. ACS Chem Biol. 2012;7:387–394. doi: 10.1021/cb2003237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang BX, Akbar M, Kevala K, Kim HY. Phosphatidylserine is a critical modulator for Akr activation. J Cell Biol. 2011;192:979–992. doi: 10.1083/jcb.201005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang IK, Yoo KY, Jung BK, Cho JH, Kim DH, Kang TC, Kwon YG, Kim YS, Won MH. Correlations between neuronal loss, decrease of memory, and decrease expression of brain-derived neurotrophic factor in the gerbil hippocampus during normal aging. Exp Neurol. 2006;201:75–83. doi: 10.1016/j.expneurol.2006.02.129. [DOI] [PubMed] [Google Scholar]
- 42.Janz R, Goda Y, Geppert M, Missler M, Sudhof TC. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron. 1999;24:1003–1016. doi: 10.1016/s0896-6273(00)81046-6. [DOI] [PubMed] [Google Scholar]
- 43.Jedlicka P, Schwarzacher SW, Winkels R, Kienzler F, Frotscher M, Bramham CR, Schultz C, Bas OC, Deller T. Impairment of in vivo theta-burst long-term potentiation and network excitability in the dentate gyrus of synaptopodin-deficient mice lacking the spine apparatus and the cisternal organelle. Hippocampus. 2009;19:130–140. doi: 10.1002/hipo.20489. [DOI] [PubMed] [Google Scholar]
- 44.Jiang LH, Shi Y, Wang LS, Yang ZR. The influence of orally administered docosahexaenoic acid on cognitive ability in aged mice. J Nutr Biochem. 2009;20(9):735–41. doi: 10.1016/j.jnutbio.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Landfield PW. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J Neurosci. 2009;29:1805–1816. doi: 10.1523/JNEUROSCI.4599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K- channels by direct interaction with the PSD-95/SAP90 family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 47.Kumar A, Thinschmidt JS, Foster TC, King MA. Aging effects on the limits and stability of long-term synaptic potentiation and depression in rat hippocampal area CA1. J Neurophysiol. 2007;98:594–601. doi: 10.1152/jn.00249.2007. [DOI] [PubMed] [Google Scholar]
- 48.Lazzell DR, Belizaire R, Thakur P, Sherry DM, Janz R. SV2B regulates synaptotagmin 1 by direct interaction. J Biol Chem. 2004;279:52124–52131. doi: 10.1074/jbc.M407502200. [DOI] [PubMed] [Google Scholar]
- 49.Little SJ, Lynch MA, Manku M, Nicolaou A. Docosahexaenoic acid-induced changes in phospholipids in cortex of young and aged rats: a lipidomic analysis. Prostaglandins Leukot Essent Fatty Acids. 2007;77:155–162. doi: 10.1016/j.plefa.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Majdi M, Ribeiro-da-Silva A, Cuello AC. Cognitive impairment and transmitter-specific pre- and postsynaptic changes in the rat cerebral cortex during ageing. Eur J Neurosci. 2007;26:3583–3596. doi: 10.1111/j.1460-9568.2007.05966.x. [DOI] [PubMed] [Google Scholar]
- 51.McNamara RK, Liu Y, Jandacek R, Rider T, Tso P. The aging human orbitofrontal cortex: Decreasing polyunsaturated fatty acid composition associated increases in lipogenic gene expression and stearoyl CoA desaturase activity. Prostagl Leukot Essent Fatty Acids. 2008;78:293–304. doi: 10.1016/j.plefa.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norris SE, Friedrich MG, Mitchell TW, Truscott RJ, Else PL. Human prefrontal cortex phospholipids containing docosahexaenoic acid increase during normal adult aging, whereas those containing arachidonic acid decrease. Neurobiol Aging. 2015;36:1659–1669. doi: 10.1016/j.neurobiolaging.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Mora F, Segovia G, Del AA. Glutamate-dopamine-GABA interactions in the aging basal ganglia. Brain Res Rev. 2007;58:340–353. doi: 10.1016/j.brainresrev.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Okabe S, Miwa A, Okado H. Spine formation and correlated assembly of presynaptic and postsynaptic molecules. J Neurosci. 2001;21:6105–6114. doi: 10.1523/JNEUROSCI.21-16-06105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poon HF, Shepherd HM, Reed TT, Calabrese V, Stella AM, Pennisi G, et al. Proteomics analysis provides insight into caloric restriction mediated oxidation and expression of brain proteins associated with age-related impaired cellular processes: mitochondrial dysfunction, glutamate dysregulation and impaired protein synthesis. Neurobiol Aging. 2006;27:1020–1034. doi: 10.1016/j.neurobiolaging.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 57.Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci USA. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rashid MA, Katakura M, Kharebava G, Kevala K, Kim HY. N-Docosahexaenoylethanolamine is a potent neurogenic factor for neural stem cell differentiation. J Neurochem. 2013;125:869–884. doi: 10.1111/jnc.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rashid MA, Kim HY. N-Docosahexaenoylethanolamine ameliorates ethanol-induced impairment of neural stem cell neurogenic differentiation. Neuroparmacol. 2016;102:174–185. doi: 10.1016/j.neuropharm.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rasmussen T, Schliemann T, Sorensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol Aging. 1996;17:143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez L, Stirling CJ, Woodman PG. Multiple N-ethylmaleimidesensitive components are required for endosomal vesicle fusion. Mol Biol Cell. 1994;5:773–783. doi: 10.1091/mbc.5.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM, Rose GM, Landfield PW. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci. 2007;27:3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schivell AE, Batchelor RH, Bajjalieh SM. Isoform-specific, calcium-regulated interaction of the synaptic vesicle proteins SV2 and synaptotagmin. J Biol Chem. 1996;271:27770–27775. doi: 10.1074/jbc.271.44.27770. [DOI] [PubMed] [Google Scholar]
- 64.Shi L, Adams MM, Linville MC, Newton IG, Forbes ME, Long AB, Riddle DR, Brunso-Bechtold JK. Caloric restriction eliminates the aging-related decline in NMDA and AMPA receptor subunits in the rat hippocampus and induces homeostasis. Exp Neurol. 2007;206:70–79. doi: 10.1016/j.expneurol.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi L, Linville MC, Tucker EW, Sonntag WE, Brunso-Bechtold JK. Differential effects of aging and insulin-like growth factor-1 on synapses in CA1 of rat hippocampus. Cereb Cortex. 2005;15:571–577. doi: 10.1093/cercor/bhh158. [DOI] [PubMed] [Google Scholar]
- 66.Sidhu VK, Huang BX, Kim HY. Effects of docosahexaenoic acid on mouse brain synaptic plasma membrane proteome analyzed by mass spectrometry and 16O/18O labeling. J Proteome Res. 2011;10:5472–5480. doi: 10.1021/pr2007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Söderberg M, Edlund C, Kristensson K, Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids. 1991;26:421–425. doi: 10.1007/BF02536067. [DOI] [PubMed] [Google Scholar]
- 69.Söllner T, Bennett MK, Whiteheart S, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 70.Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 71.Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197(Pt 4):575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tokuda H, Kontani M, Kawashima H, Kiso Y, Shibata H, Osumi N. Differential effect of arachidonic acid and docosahexaenoic acid on age-related decreases in hippocampal neurogenesis. Neurosci Res. 2014;88:58–66. doi: 10.1016/j.neures.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 73.VanGuilder HD, Yan H, Farley JA, Sonntag WE, Freeman WM. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J Neurochem. 2010;113:1577–1588. doi: 10.1111/j.1471-4159.2010.06719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.VanGuilder HD, Farley JA, Yan H, Van Kirk CA, Mitschelen M, Sonntag WE, Freeman WM. Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol Dis. 2011;43(1):201–212. doi: 10.1016/j.nbd.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vickers CA, Stephens B, Bowen J, Arbuthnott GW, Grant SG, Ingham CA. Neurone specific regulation of dendritic spines in vivo by post synaptic density 95 protein (PSD-95) Brain Res. 2006;1090:89–98. doi: 10.1016/j.brainres.2006.03.075. [DOI] [PubMed] [Google Scholar]
- 76.Wen Z, Kim H-Y. Alternations in hippocammpal phospholipid profile by prenatal exposure to ethanol. J Neurochem. 2004;89:1368–1377. doi: 10.1111/j.1471-4159.2004.02433.x. [DOI] [PubMed] [Google Scholar]
- 77.Wilson DW, Whiteheart SW, Wiedmann M, Brunner M, Rothman JE. A multisubunit particle implicated in membrane fusion. J Cell Biol. 1992;117:531–538. doi: 10.1083/jcb.117.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang S, Liu T, Li S, Zhang X, Ding Q, Que H, Yan X, Wei K, Liu S. Comparative proteomic analysis of brains of naturally aging mice. Neuroscience. 2008;154:1107–1120. doi: 10.1016/j.neuroscience.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 79.Yetimler B, Ulusoy G, Çelik T, Jakubowska-Doğru E. Differential effect of age on the brain fatty acid levels and their correlation with animal cognitive status in mice. Pharmacol Biochem Behav. 2012;103:53–59. doi: 10.1016/j.pbb.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 80.Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, Salem N, Jr, Stedman M. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010;6:456–464. doi: 10.1016/j.jalz.2010.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.