Abstract
Background: Body mass index (BMI, in kg/m2) is positively associated with plasma glucose in late pregnancy and with risk of adverse obstetric outcomes. Much of the existing research uses single-clinic measures of plasma glucose, which may not accurately reflect circulating glucose under free-living conditions. Furthermore, little is known about circulating glucose concentrations of African American women, who tend to have poorer diet quality and a greater risk of obstetric complications.
Objective: The objective of the study was to test the hypothesis that the positive association of BMI in early pregnancy with third-trimester circulating glucose concentrations measured under free-living conditions among African American women would be at least partially attributable to lower β-cell insulin secretion relative to insulin sensitivity [i.e., lower disposition index (DI)].
Methods: Using a prospective, observational design, 40 pregnant African American women (mean ± SD age: 23.1 ± 4.0 y; mean ± SD BMI: 28.4 ± 7.5) wore continuous glucose monitors and accelerometers for 3 d at 32–35 wk of gestation and concurrently maintained a food diary to report their self-selected meals. The DI was derived from a 75-g oral glucose tolerance test. Linear regression modeling was used to calculate the association of BMI with the 24-h glucose (GLUC24h) and 2-h (GLUC2hPP) postprandial glucose areas under the curve and with the percentage of time the glucose concentrations were >120 mg/dL.
Results: The positive associations between BMI and GLUC24h (standardized β = 0.36, P = 0.03) and the percentage of time glucose concentrations were >120 mg/dL (standardized β = 0.40, P = 0.02) were independent of total carbohydrate intake and physical activity and were attenuated when DI was added to the model. The positive association of BMI with GLUC2hPP was attenuated when DI was added to the model, and DI itself was independently associated with GLUC2hPP after self-selected breakfast and dinner (standardized β = −0.33 and −0.42, respectively; P = 0.01).
Conclusions: The association of BMI with high circulating glucose in free-living pregnant African American women is at least partially attributable to lower β-cell responsiveness.
Keywords: diet, carbohydrate, insulin sensitivity, β-cell function, glucose tolerance
Introduction
Overweight and obesity are prevalent among women of childbearing age. African American women are particularly burdened, with 80% being overweight or obese before conception, compared with 55% of non-Hispanic White women (1). Prepregnancy obesity is associated with a number of adverse outcomes during pregnancy, including greater risk of hypertension or gestational diabetes (2). Even in the absence of diabetes, women who are overweight or obese tend to have higher glucose concentrations during pregnancy (3). Over the long term, gestational diabetes and relatively high glucose concentrations during pregnancy are predictive of future metabolic health problems such as the metabolic syndrome, cardiovascular disease, and type 2 diabetes (4–7).
Several studies have used continuous glucose monitors (CGMs)10 to examine glucose profiles of free-living nondiabetic pregnant women. In a study of nondiabetic normal-weight compared with obese pregnant women, Yogev et al. (8) found no group difference in fasting and mean 24-h glucose concentrations, but postprandial glucose was higher in the obese group. Harmon and colleagues (9) also found higher postprandial and 24-h glucose concentrations in late pregnancy among women who were obese than among those who were lean despite a controlled diet. To our knowledge, no previous study has used CGMs to examine the circulating glucose profile of low-income African American women during pregnancy under free-living conditions. It would be of interest to do so, however, because low-income women have a poorer diet quality during pregnancy (10), and even among low-income groups, African American women have a greater risk of adverse pregnancy outcomes (11). Furthermore, previous studies have not examined whether physical activity during pregnancy is associated with glucose concentrations measured under free-living conditions or whether carbohydrate intake during individual self-selected meals is associated with postprandial glucose in late pregnancy.
The overall goal of this study was to explore associations among BMI (in kg/m2), insulin sensitivity, β-cell response, and mean 24-h and postprandial glucose concentrations, as well as the duration of time with glucose >120 mg/dL, under free-living conditions in late pregnancy among low-income African American women. We hypothesized that women with a higher BMI in early pregnancy would have higher 24-h glucose AUC (GLUC24h) and would spend more time with glucose >120 mg/dL in the third trimester of pregnancy, independent of their total carbohydrate intake and physical activity. We also hypothesized that these associations would be at least partially attributable to lower β-cell insulin secretion relative to insulin sensitivity. Furthermore, we also hypothesized that BMI and/or the β-cell response would be associated with 2-h postprandial glucose AUC (GLUC2hPP) after self-selected, free-living meals, independent of the carbohydrate content of the meal.
Methods
Participants.
African American women were recruited into this study during the third trimester of pregnancy from among those planning to deliver at a large urban university hospital. Mothers were eligible if they were aged ≥16 y at recruitment, had initiated prenatal care before 19 wk of gestation, and were experiencing a healthy singleton pregnancy. Women were excluded if they had type 1, 2, or gestational diabetes; had previously delivered a preterm (i.e., <37.0 wk) or low-birth-weight (i.e., <2500 g) infant; or had any medical condition during pregnancy believed to interfere with normal fetal growth.
Procedure.
Participants enrolled in the study at 32.0–34.6 wk of gestation. A CGM was placed on the abdomen on day 0, and women were provided with a glucometer for CGM calibration, an accelerometer to measure physical activity, and a food diary to record all of their intake during the free-living period (days 1 and 2). Women were instructed to go about their usual daily activities and to eat and drink as they normally would, with the exception of an overnight fast before returning to the clinic on day 3, when an oral-glucose-tolerance test (OGTT) was conducted. Maternal weight in early pregnancy was retrieved from medical records, and height (cm) and weight (kg) were measured on the day of CGM placement. Socioeconomic status was assessed with the Hollingshead survey (12), which combines education and occupation of the participant and her husband, if applicable, and family history of type 2 diabetes was determined by a survey designed specifically for this study. The Institutional Review Board for Human Use at the University of Alabama at Birmingham approved all study procedures. Informed consent was obtained from all participants, and consent was also obtained from a parent or guardian of women who were aged <19.0 y at enrollment (n = 5).
Dietary intake.
Participants were provided with a food diary and asked to complete it with as much detail as they could before the subsequent visit. Trained research assistants reviewed the food diary with each participant to clarify meal or snack content, portion sizes, and any other detail that was missing or inadequate. Data from the food diary were subsequently entered into ASA24 (13), and dietary outcomes of interest, including overall total (24-h) and per meal intake, were extracted, along with kcal from protein, carbohydrate, and fat. Overall diet quality was calculated by using the Diet Quality Index for Pregnancy (10). In brief, this index is based on how closely the diet adheres to recommended daily intake of grains, fruit, vegetables, fat, iron, calcium, folate, and the meal/snack pattern.
Physical activity.
Women wore Actigraph 3TX+ (ActiLife) accelerometers on an elastic band around the waist and above the right hip, during all waking hours, except when bathing or swimming. Data were uploaded and analyzed by using ActiLife 6.0 software (ActiLife). Epochs were 60 s in length. If an hour had 0 counts, it was considered nonwear time, and days with <8 h of wear time were discarded. Estimates of physical activity energy expenditure and time spent at each intensity of activity were derived by using the Freedson Combination approach as described in the ActiLife software. This approach uses the Freedson et al. (14) energy expenditure equations and cutoffs for sedentary, light, lifestyle, moderate, and vigorous activity when accelerometer counts are >1951/min, but because these equations are not valid for lower accelerometer counts, the Williams Work-Energy equation (15), developed by the manufacturer, is used for counts ≤1951/min.
Continuous glucose monitoring.
An indwelling glucose sensor and recorder (iPro; Medtronic MiniMed, Inc.) was placed on the abdomen of each woman on the self-reported nonsleeping side. The sensor measures glucose concentrations in the interstitial fluid, which, along with measures of blood glucose from a glucometer (Ultra OneTouch; Lifescan, Inc.), are used to estimate circulating glucose. Participants were instructed to perform a finger stick ≥3 times/d to measure their blood glucose, selecting premeal times when blood glucose was most likely to be stable. Participants performed at least one finger stick in the presence of the research nurse to be sure they could conduct the procedure correctly.
Data were uploaded by using the provided software (Solutions Software version 2.2; Medtronic MiniMed, Inc.), exported in tabulated form, and then visually inspected and manually cleaned following a previously published protocol (16). Data from day 0, days during which <2 calibrations were conducted, and days for which <90% of data remained after cleaning were discarded and not replaced. A random selection of 8 CGM files was inspected and cleaned by a second investigator. These files had excellent agreement regarding the number of epochs remaining after cleaning (r = 0.999) and the total sum of glucose concentrations in each cleaned file (r = 0.998). Given the overall consistency between the independent investigators, the final CGM files used in the analyses were those inspected and cleaned by the primary investigator. After cleaning, 34 participants had CGM data from 2 free-living days, and the remaining 6 participants had data from 1 free-living day. Descriptive statistics did not differ for women who had 1 compared with 2 free-living days, and so all files were included in the analyses.
Individual free-living meals were identified following a previously described protocol (16). In brief, the mealtimes reported on the food diaries were used to guide investigators to the approximate mealtimes in the CGM data file. The beginning of the meal was then considered a sustained glucose increase of ≥4 mg/dL at the approximate time a meal was reported on the food diary. It is likely that only meals containing carbohydrate were detected by the CGM, because those with fat and protein only were unlikely to evoke a change in glucose. Only discrete meals were included, operationally defined as those for which no other intake was reported or detected on the CGM output during the 2 h after meal initiation.
OGTT.
Women returned to the clinic on day 3 after a 12-h overnight fast. An intravenous catheter was placed to obtain blood samples during the 75-g OGTT. Blood draws were obtained at −10 and −5 min (averaged to provide fasting concentrations) and at 10, 20, 30, 60, 90, and 120 min relative to glucose consumption. Serum samples were separated and stored at −85°C until assayed for glucose, insulin, and C-peptide concentrations.
Serum assays.
Assays were conducted by the Core laboratory of the University of Alabama at Birmingham Nutrition Obesity Research Center and Diabetes Research Center. Glucose concentrations were measured with the SIRRUS analyzer (Stanbio Laboratory). This analysis had a mean intra-assay CV of 1.28% and an interassay CV of 2.56%. Insulin and C-peptide were assayed by using the TOSOH AIA-600 II automated analyzer (TOSOH Bioscience, Inc.). The intra- and interassay CVs were 4.42% and 1.49% for insulin and 1.67% and 2.59% for C-peptide, respectively.
Insulin sensitivity and secretion.
Glucose and insulin concentrations throughout the OGTT were used to derive whole-body insulin sensitivity by using the Matsuda index [whole-body insulin sensitivity index (WBISI) (17)]. Estimation of insulin secretion requires the measurement of C-peptide concentrations to isolate insulin secretion from insulin clearance (18–20). Mathematical modeling of C-peptide and glucose concentrations was used to derive an estimate of β-cell response to glucose across the entire test [glucose-stimulated β-cell insulin secretion (PhiTOT) (21)]. Given that insulin secretion should be interpreted relative to insulin sensitivity, we calculated the disposition index (DI), also known as the ability of β-cells to adequately respond to a decrease in WBISI with an increase in secretion, as the product of WBISI and PhiTOT. The DI will remain constant if a person is able to secrete sufficient insulin to compensate for reduced insulin sensitivity but will decrease if a person’s insulin secretion is inadequate (22).
Statistical analysis.
The primary outcomes for this study were 24-h interstitial fluid glucose-derived (IFGD) circulating GLUC24h, percentage of time spent with glucose >120 mg/dL, and GLUC2hPP, as collected by CGM. After CGM data were cleaned, summary statistics were derived following a previously published protocol (16). Fasting glucose was calculated as the mean of 6 consecutive glucose recordings beginning at 0600 on day 3, and GLUC24h and GLUC2hPP were calculated by using the trapezoid method. “Day” was defined as the period between 0630 and 2329 on days 1–2, and “night” was between 2330 and 0629 on days 0–1 and days 1–2. These days were selected to avoid the overnight fast that was required before day 3. The CV in IFGD circulating glucose concentrations across the free-living period was used as an index of variability in glucose. Premeal concentration was calculated as the mean of 3 IFGD circulating glucose concentrations immediately before the meal.
Due to non-normality of the data, insulin sensitivity and total insulin secretion were log-transformed before analysis. Simple Pearson correlations were used to examine associations among parameters describing the glucose profile measured under free-living conditions, as well as maternal BMI, diet, physical activity, and insulin sensitivity and secretion. Multiple linear regression modeling was then used to examine whether the associations of BMI with GLUC24h and the percentage of time glucose concentrations were >120 mg/dL were independent of carbohydrate content of the diet and time spent physically active and whether these associations were at least partially explained by DI. Linear regression modeling was also used to examine whether BMI was associated with GLUC2hPP after self-selected free-living meals, independent of the carbohydrate content of the meal, and whether this association was at least partially explained by DI. In models where DI was significantly associated with the dependent variable, a follow-up model was calculated with WBISI and PhiTOT replacing DI, in an attempt to explain the mechanism by which DI was associated with the dependent variable. A median split for DI was then used to group women into high and low DI groups, and the between-group differences in GLUC2hPP were analyzed with ANCOVA, adjusted for BMI and carbohydrate intake. All analyses were performed by using SPSS version 18 (SPSS, Inc.), and α was set at 0.05 for statistical significance.
Results
Forty-six pregnant African American women were enrolled in the study. Preliminary analyses showed that the 3 women with the highest BMIs (i.e., >49) in early pregnancy were multivariate outliers in terms of the association between BMI and GLUC24h glucose concentrations (as indicated by a Cook’s distance that was >4/n) (23). Given that weight status is known to be a strong correlate of circulating glucose concentrations during pregnancy, data from these women were removed from the subsequent analyses. In addition, no blood draws were obtained from 1 participant during the OGTT, and 1 participant was an outlier for WBISI and 1 for DI. Final analyses, therefore, were conducted for 40 women.
Most women in this cohort were single (95%) and had graduated high school (88%). Just over one-fourth (25%) reported being employed part-time or full-time at enrollment into the study. Four of the women (10%) reported that their mothers or a sibling had been diagnosed with type 2 diabetes, and none reported that their fathers were diabetic, although it should be noted that 4 did not know their fathers’ health status. Parity ranged from 0 to 4, and just under half were nulliparous (45%). Other characteristics of the mothers are shown in Table 1. Thirty-five percent of the sample met the 2009 Institute of Medicine recommendations for gestational weight gain, whereas 25% failed to meet and 40% exceeded recommendations. Mean reported energy intake ranged from 1250 to 5230 kcal/d, and the mean diet quality score was 45 of 80, which is slightly lower than that previously reported in a more diverse population (10). Women spent ~4 h/d in light or lifestyle-related activity, as defined by activity thresholds described previously (14). Fasting glucose ranged from 62 to 124 mg/dL, and the mean ± SD 24-h IFGD circulating glucose was 99.4 ± 11.7 mg/dL. Further dietary and IFGD circulating glucose are provided in the Supplemental Table 1. A total of 157 discrete meals (57 breakfasts among 37 women, 50 lunches among 34 women, and 50 dinners among 35 women) were identified in the records of women with complete data. The mean ± SD reported carbohydrate intake at breakfast, lunch, and dinner was 72.1 ± 34.2, 96.8 ± 66.1, and 87.2 ± 59.5 g, respectively, with 39.5 ± 22.8, 73.9 ± 148, and 37.5 ± 37.3 g of total sugar and 6.4 ± 5.2, 8.8 ± 9.4, and 5.7 ± 5.2 g of added sugar, respectively.
TABLE 1.
Characteristics of this sample of pregnant African American women, measured at gestational weeks 32.0–34.61
| Characteristic | Value |
| Age, y | 23.1 ± 4.0 (17.0–33.1) |
| BMI in early pregnancy, kg/m2 | 28.4 ± 7.5 (17.4–43.3) |
| Gestational age on day of test, wk | 33.9 ± 0.7 (32.0–34.6) |
| BMI on day of test, kg/m2 | 31.8 ± 6.5 (21.3–43.9) |
| Gestational weight gain, kg | 12.2 ± 7.1 (−2.3 to 27.3) |
| 1-h OGTT glucose,2 mg/dL | 110 ± 17 (83–150) |
| Energy intake, kcal/d | 2740 ± 829 (1250–5230) |
| Fat, % energy | 38.2 ± 5.8 (28.1–51.9) |
| Carbohydrate, % energy | 48.1 ± 8.1 (25.4–62.2) |
| Protein, % energy | 14.5 ± 3.2 (9.3–25.5) |
| Diet quality index score | 45.1 ± 11.2 (24.5–66.7) |
| Total physical activity, kcal/d | 356 ± 322 (56–1800) |
| Time spent active during accelerometer use, min/d | 281 ± 114 (87–623) |
| Fasting glucose, mg/dL | 86.5 ± 12.7 (61.7–124) |
| GLUC24h, g/L × 24 h | 1430 ± 169 (1140–1840) |
| Time of glucose <70 mg/dL, % | 5.6 ± 7.0 (0–26.6) |
| Time of glucose >100 mg/dL, % | 42.6 ± 15.8 (0.4–98.1) |
| Time of glucose >120 mg/dL, % | 14.6 ± 15.8 (0–60.1) |
| 24-h CV, % | 16.4 ± 5.4 (6.2–28.7) |
Data are means ± SDs (minimum–maximum); n = 40, unless noted. GDM, gestational diabetes mellitus; GLUC24h, 24-h glucose area under the curve; OGTT, oral-glucose-tolerance test.
From the 50-g glucola test to screen for GDM; data retrieved from medical records.
Simple Pearson correlations of the associations among parameters describing the IFGD circulating glucose profile measured under free-living conditions and BMI, diet, physical activity, and insulin sensitivity and secretion are shown in Supplemental Table 2. BMI was positively associated with GLUC24h (r = 0.39, P = 0.01) and with the percentage of time glucose concentrations were >120 mg/dL (r = 0.43, P < 0.01), and it was inversely associated with WBISI (r = −0.52, P < 0.01) and DI (r = -0.38, P = 0.02). Total carbohydrate intake was not associated with IFGD circulating glucose concentrations, but total fat consumption tended to be positively associated with the fasting glucose concentration (r = 0.30, P = 0.06). Parity was also positively associated with fasting glucose (r = 0.34, P = 0.04). Physical activity was positively associated with IFGD circulating glucose variability (r = 0.36, P = 0.02), but visual inspection of this association revealed that it was carried by data from 2 women who were outliers with high physical activity, and when the Spearman rank-order correlation was calculated, the association was lost (r = 0.26, P = 0.11).
The association of early pregnancy BMI with GLUC24h persisted after adjusting for maternal age, total carbohydrate intake, and time spent physically active (partial r = 0.35, P = 0.03; Table 2), although the overall model did not obtain statistical significance. When DI was added to the model (model 2), neither it nor BMI was associated with GLUC24h. The association of BMI with the percentage of time glucose concentrations were >120 mg/dL remained after adjusting for age, carbohydrate intake, and time spent physically active (partial r = 0.39, P = 0.02; Table 2), and when DI was added to the model (model 2), the association of BMI with the percentage of time glucose concentrations were >120 mg/dL weakened, and DI was independently associated with the percentage of time glucose concentrations were >120 mg/dL (partial r = −0.34, P = 0.04). In a follow-up model (adjusted R2 = 0.24, P = 0.02; not shown), WBISI and PhiTOT were entered into the model instead of DI, and only PhiTOT was a significant predictor of the percentage of time glucose concentrations were >120 mg/dL (WBISI: partial r = −0.27, P = 0.12; PhiTOT: partial r = −0.39, P = 0.02). With respect to fasting glucose, total fat consumption (partial r = 0.38, P = 0.02) and parity (partial r = 0.35, P = 0.04) remained significantly and positively associated with fasting glucose after adjusting for BMI and total energy intake (not shown).
TABLE 2.
Linear regression models predicting GLUC24h and percentage of time glucose concentrations were >120 mg/dL, at 32.0–34.6 wk of gestation among pregnant African American women (n = 40)1
| Adjusted R2 | Unstandardized β | Standardized β | P value | |
| GLUC24h, g/L × 24h | ||||
| Model 1 | 0.12 | 0.07 | ||
| BMI, kg/m2 | 804 | 0.36 | 0.03 | |
| Model 2 | 0.14 | 0.07 | ||
| BMI, kg/m2 | 604 | 0.27 | 0.13 | |
| DI | −25.1 | −0.21 | 0.20 | |
| Time of glucose >120 mg/dL, % | ||||
| Model 1 | 0.14 | 0.05 | ||
| BMI, kg/m2 | 0.84 | 0.40 | 0.02 | |
| Model 2 | 0.22 | 0.02 | ||
| BMI, kg/m2 | 0.54 | 0.26 | 0.12 | |
| DI | −0.04 | −0.33 | 0.04 | |
Models adjusted for age, mean daily carbohydrate intake, and mean time spent physically active each day. DI, disposition index; GLUC24h, 24-h glucose area under the curve.
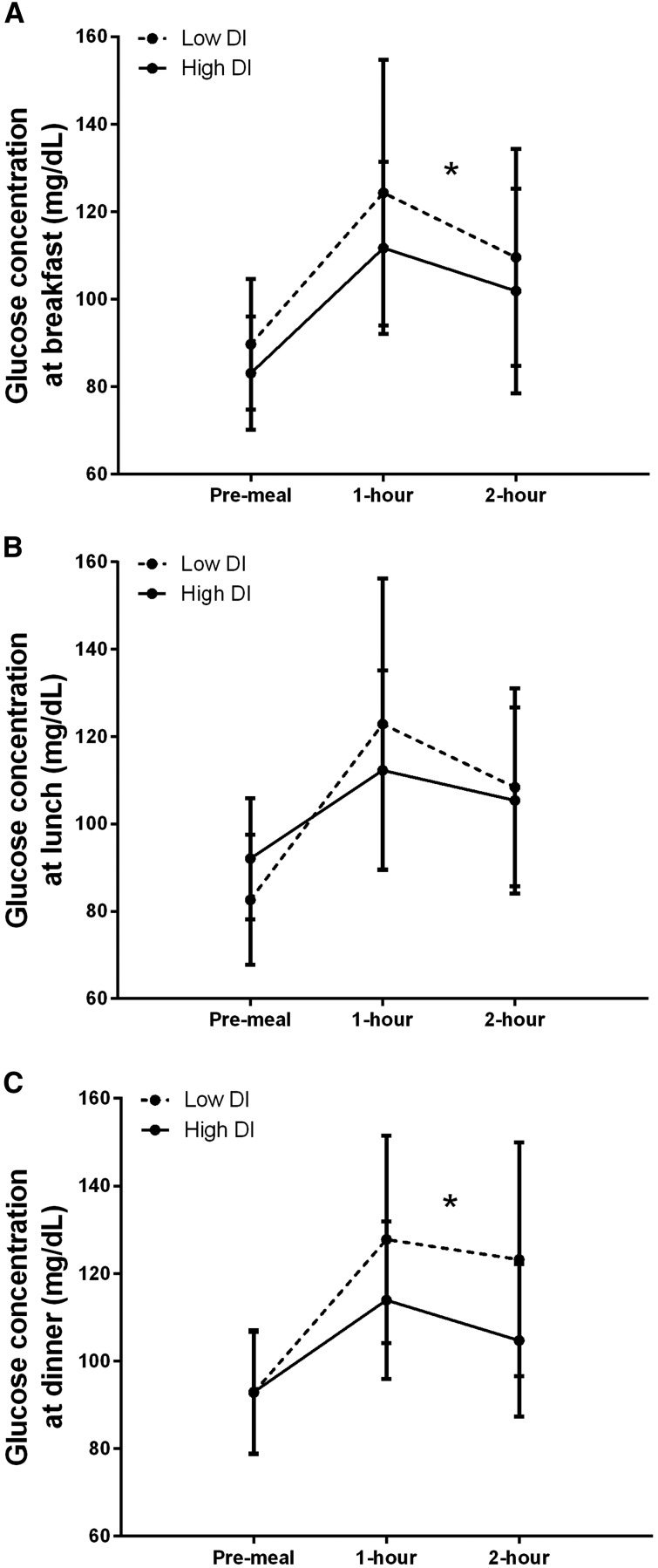
In models predicting GLUC2hPP, BMI was positively associated with glucose after breakfast (partial r = 0.37, P = 0.01) and lunch (partial r = 0.31, P = 0.03), independent of carbohydrate intake at those meals (Table 3). When DI was added to the models, the association of BMI with GLUC2hPP weakened, and DI was inversely and independently associated with GLUC2hPP at breakfast (partial r = −0.34, P = 0.01) and dinner (partial r = −0.39, P = 0.01). It should also be noted that for the breakfast model that included DI, carbohydrate consumed during the meal was also positively associated with GLUC2hPP (partial r = 0.31, P = 0.02; not shown). This association was not found for other meals. In follow-up models, WBISI and PhiTOT were entered into the breakfast and dinner models instead of DI (not shown), and each was found to be independently associated with GLUC2hPP (WBISI: breakfast partial r = −0.32, P = 0.02; dinner partial r = −0.36, P = 0.01; PhiTOT: breakfast partial r = −0.40, P < 0.01; dinner partial r = −0.37, P = 0.01; not shown). The addition of total steps or total accelerometer counts measured during the 2 h after breakfast did not change the outcome of the breakfast model, and these activity variables were not themselves independently associated with GLUC2hPP (not shown). Postprandial glucose concentrations are shown in Figure 1, dichotomized by individuals with high compared with low DI (median = 302). The results of the between-groups analysis were consistent with that shown by the continuous analysis in that women in the low DI group had higher GLUC2hPP after breakfast (F = 4.44, P = 0.04) and dinner (F = 5.46, P = 0.02), after adjusting for BMI and carbohydrate intake at the respective meal.
TABLE 3.
Linear regression models showing associations of BMI and DI with GLUC2hPP at discrete breakfast, lunch, and dinner meals, for pregnant African American women at 32.0–34.6 wk of gestation1
| Adjusted R2 | Unstandardized β | Standardized β | P value | |
| Breakfast GLUC2hPP, g/L × 2 h | ||||
| Model 1 | 0.16 | <0.01 | ||
| BMI, kg/m2 | 115 | 0.36 | 0.01 | |
| Model 2 | 0.24 | <0.01 | ||
| BMI, kg/m2 | 74.4 | 0.23 | 0.07 | |
| DI | −5.31 | −0.33 | 0.01 | |
| Lunch GLUC2hPP, g/L × 2 h | ||||
| Model 1 | 0.11 | 0.03 | ||
| BMI, kg/m2 | 98.8 | 0.31 | 0.03 | |
| Model 2 | 0.10 | 0.05 | ||
| BMI, kg/m2 | 86.0 | 0.27 | 0.07 | |
| DI | −1.90 | −0.11 | 0.47 | |
| Dinner GLUC2hPP, g/L × 2 h | ||||
| Model 1 | <0.01 | 0.35 | ||
| BMI, kg/m2 | 56.3 | 0.20 | 0.17 | |
| Model 2 | 0.14 | 0.02 | ||
| BMI, kg/m2 | 9.68 | 0.03 | 0.82 | |
| DI | −5.76 | −0.42 | 0.01 |
Models adjusted for carbohydrate intake at breakfast, lunch, or dinner, as appropriate. Data used in the analyses were from 57 breakfast meals among 37 women, 50 lunch meals among 34 women, and 50 dinner meals among 35 women. DI, disposition index; GLUC2hPP, 2-h postprandial glucose area under the curve.
FIGURE 1.
Pre- and postprandial glucose concentrations are shown for the discrete meals reported by this cohort of pregnant African American women, dichotomized by high compared with low DI. Values are means ± SDs for the meals. Figures reflect data from 57 breakfasts reported by 37 women (A), 50 lunches reported by 34 women (B), and 50 dinners reported by 35 women (C). Linear regression modeling was used to compare GLUC2hPP after each meal, after adjusting for BMI and carbohydrate content of the meal. *The low DI group had greater GLUC2hPP than did the high DI group at breakfast (F = 4.44, P = 0.04) and dinner (F = 5.46, P = 0.02). DI, disposition index; GLUC2hPP, 2-h postpartum area under the curve.
Discussion
The overall objective of this study was to examine associations among early pregnancy BMI, lifestyle factors, β-cell function, and IFGD circulating glucose concentrations measured under free-living conditions during the third trimester of pregnancy. These analyses were conducted in a cohort of low-income African American women, which is a demographic group with a relatively greater risk of adverse obstetric outcomes. Results of this study extend previous findings by showing that the effect of BMI on GLUC24h, GLUC2hPP, and percentage of time glucose concentrations were >120 mg/dL was at least partially attributable to a reduced DI, and DI was inversely associated with GLUC2hPP after breakfast and dinner. These findings suggest that β-cell function influences mean IFGD circulating glucose measured under free-living conditions in African American women in the third trimester of pregnancy, even in the absence of diabetes.
Summary statistics characterizing the glucose profile in this cohort of African American women, when measured under free-living conditions, were very similar to those previously published in a cohort of predominantly Caucasian women (9). Also consistent with the previous literature was the pattern for a positive association between maternal BMI in early pregnancy and GLUC24h and percentage of time glucose concentrations were >120 mg/dL (8, 9). In this study, we added the finding, which, to our knowledge, is novel, that BMI was positively associated with GLUC2hPP after the self-selected breakfast and lunch meals, independent of the carbohydrate content of those meals. In general, the associations of BMI with IFGD circulating glucose concentrations diminished when the DI was added to the models, suggesting that the effect of BMI on circulating glucose may be at least partially attributable to a lower β-cell response among women with a higher BMI. These findings are consistent with previous research among normoglycemic women showing that there is less of an increase in insulin response to glucose among obese than among normal-weight women with advancing gestational age (24). Although the mechanisms underlying the association between adiposity and β-cell response have not been elucidated, it is possible that persistently higher circulating glucose and/or free FA concentrations in obese women may have suppressed insulin secretion (25, 26) or that higher leptin concentrations suppressed insulin secretion (27). More research is needed to explain the association of adiposity and β-cell response, particularly during pregnancy.
Although not an explicit hypothesis of this study, the models showed that carbohydrate intake at breakfast was also associated with GLUC2hPP, independent of BMI and DI. To our knowledge, only one previous study of pregnant women has reported a positive association between carbohydrate consumption during meals and postprandial glucose, but only women with gestational diabetes were enrolled in that study, and whether the association was independent of BMI and DI was not explored (28). Our findings are also consistent with a study of abdominally obese nonpregnant individuals (29). It is possible that this association was found only at breakfast because women may have been more accurate in recording breakfast foods or because there was less of an influence of previous intake or activity on the glucose response to breakfast. It is unlikely that this finding was attributable to consumption of poorer quality carbohydrate at breakfast than at other meals because total and added sugar consumption was not higher at breakfast. It will be of interest in future research to reliably measure carbohydrate intake to assess the magnitude of its effect on circulating glucose across meals.
Overall fat intake was positively associated with fasting glucose concentrations independent of BMI and overall energy intake. Dietary fat has previously been associated with increased risk of impaired glucose tolerance and gestational diabetes (30, 31), and we have previously shown that a diet with lower fat compared with lower carbohydrate was associated with lower fasting glucose in nonpregnant individuals (32). Fasting glucose is thought to reflect hepatic glucose production, which increases late in pregnancy (33). It is possible that a high-fat diet-induced increase in circulating FAs contributes to hepatic insulin resistance and thereby impairs the suppression of glucose production, raising circulating glucose (34, 35). Together, these findings suggest that an intervention to reduce dietary fat or improve fat quality might be beneficial, particularly for women with impaired fasting glucose.
Strengths of this study include the objective assessment of IFGD circulating glucose concentrations and physical activity of low-income African American women measured under free-living conditions, although we were limited to a 72-h period for valid CGM sensor use. The short duration of accelerometer use and/or the relatively sedentary habits of women in this cohort limited the opportunity to detect an effect of activity on circulating glucose concentrations. The use of food diaries to record self-selected meals under free-living conditions is often considered problematic because of poor compliance and potentially biased underreporting (36); however, the results of this study suggest that food diaries were useful to characterize the dietary patterns of this cohort, although it is not possible to know if the absolute amount of energy consumed was accurate.
If confirmed in a larger cohort, results of this study suggest that lower β-cell responsiveness to glucose at least partially explains the association of BMI with higher IFGD circulating glucose among pregnant, low-income African American women. It would be of interest in future research to continue longer follow-up assessments, particularly among nonpregnant individuals, to examine whether dietary patterns and physical activity are predictive of future changes in β-cell response and glucose tolerance. Furthermore, the association of dietary fat with higher fasting glucose and of carbohydrate intake during meals with postprandial glucose concentrations suggests that there may be dietary targets for intervention that could improve specific features of the glucose profile and thereby potentially reduce the risk of impaired fasting glucose or glucose tolerance. Future research with this cohort will continue to explore associations of parameters describing maternal IFGD circulating glucose measured under free-living conditions during pregnancy with offspring outcomes.
Acknowledgments
We thank Britney Blackstock, Rachel Copper, and Judy Sheppard for administrative support, nursing, and data collection; and Maryellen Williams, Cindy Zeng, and Heather Hunter for conducting the laboratory analyses. PCC-L, MSM, JRB, and BAG designed the study; PCC-L, DNS, CRS, and LAF conducted the study; PCC-L and WMG analyzed the data; PCC-L, DNS, CRS, and LAF wrote the paper; and PCC-L had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CGM, continuous glucose monitoring; DI, disposition index; GLUC2hPP, 2-h postprandial glucose area under the curve; GLUC24h, 24-h glucose area under the curve; IFGD, interstitial fluid glucose derived; OGTT, oral-glucose-tolerance test; PhiTOT, glucose-stimulated β-cell insulin secretion; WBISI, whole-body insulin sensitivity index.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006;113:1126–33. [DOI] [PubMed] [Google Scholar]
- 3.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol 2007;50:938–48. [DOI] [PubMed] [Google Scholar]
- 4.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care 2008;31:2026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Beta-cell function declines within the first year postpartum in women with recent glucose intolerance in pregnancy. Diabetes Care 2010;33:1798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Retnakaran R, Qi Y, Connelly PW, Sermer M, Zinman B, Hanley AJ. Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J Clin Endocrinol Metab 2010;95:670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karoli R, Siddiqi Z, Fatima J, Shukla V, Mishra PP, Khan FA. Assessment of noninvasive risk markers of subclinical atherosclerosis in premenopausal women with previous history of gestational diabetes mellitus. Heart Views. 2015 2015;16:13–8. [DOI] [PMC free article] [PubMed]
- 8.Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol 2004;191:949–53. [DOI] [PubMed] [Google Scholar]
- 9.Harmon KA, Gerard L, Jensen DR, Kealey EH, Hernandez TL, Reece MS, Barbour LA, Bessesen DH. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: metabolic determinants of fetal growth. Diabetes Care 2011;34:2198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodnar LM, Siega-Riz AM. A Diet Quality Index for Pregnancy detects variation in diet and differences by sociodemographic factors. Public Health Nutr 2002;5:801–9. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Cardarelli K, Shim R, Ye J, Booker KL, Rust G. Racial disparities in economic and clinical outcomes of pregnancy among Medicaid recipients. Matern Child Health J 2013;17:1518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollingshead AB. Four-factor index of social status. New Haven (CT): Yale University Press; 1975. [Google Scholar]
- 13.National Cancer Institute. Automated self-administered 24-hour recall (ASA24). Bethesda (MD): National Cancer Institute; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777–81. [DOI] [PubMed] [Google Scholar]
- 15.Williams R. Kcal estimates from activity counts using the Potential Energy Method. Pensacola (FL): CSA, Inc.; 1998. [Google Scholar]
- 16.Hernandez TL, Barbour LA. A standard approach to continuous glucose monitor data in pregnancy for the study of fetal growth and infant outcomes. Diabetes Technol Ther 2013;15:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuda M, DeFronzo R. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–70. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe RM, Steil GM, Bergman RN. Critical evaluation of the combined model approach for estimation of prehepatic insulin secretion. Am J Physiol 1998;274:E172–83. [DOI] [PubMed] [Google Scholar]
- 19.Toffolo G, De Grandi F, Cobelli C. Estimation of beta-cell sensitivity from intravenous glucose tolerance test C-peptide data: knowledge of the kinetics avoids errors in modeling the secretion. Diabetes 1995;44:845–54. [DOI] [PubMed] [Google Scholar]
- 20.Polonsky KS, Rubenstein A. C-peptide as a measure of the secretion and hepatic extraction of insulin: pitfalls and limitations. Diabetes 1984;33:486–94. [DOI] [PubMed] [Google Scholar]
- 21.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001;50:150–8. [DOI] [PubMed] [Google Scholar]
- 22.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes 2002;51(Suppl 1):S212–20. [DOI] [PubMed] [Google Scholar]
- 23.Bollen KA, Jackman RW. Regression diagnostics: an expository treatment of outliers and influential cases. In: Fox J, Long JS, editors. Modern methods of data analysis. Newbury Park (CA): Sage; 1990. p. 257–91. [Google Scholar]
- 24.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol 1999;180:903–16. [DOI] [PubMed] [Google Scholar]
- 25.Purrello F, Rabuazzo AM, Anello M, Patanè G. Effects of prolonged glucose stimulation on pancreatic beta cells: from increased sensitivity to desensitization. Acta Diabetol 1996;33:253–6. [DOI] [PubMed] [Google Scholar]
- 26.Zhou YP, Grill V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab 1995;80:1584–90. [DOI] [PubMed] [Google Scholar]
- 27.Kulkarni RN, Wang ZL, Wang RM, Hurley JD, Smith DM, Ghatei MA, Withers DJ, Gardiner JV, Bailey CJ, Bloom SR. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest 1997;100:2729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson CM, Jovanovic-Peterson L. Percentage of carbohydrate and glycemic response to breakfast, lunch, and dinner in women with gestational diabetes. Diabetes 1991;40(Suppl 2):172–4. [DOI] [PubMed] [Google Scholar]
- 29.Kochan AM, Wolever TM, Chetty VT, Anand SS, Gerstein HC, Sharma AM. Glycemic index predicts individual glucose responses after self-selected breakfasts in free-living, abdominally obese adults. J Nutr 2012;142:27–32. [DOI] [PubMed] [Google Scholar]
- 30.Saldana TM, Siega-Riz AM, Adair LS. Effect of macronutrient intake on the development of glucose intolerance during pregnancy. Am J Clin Nutr 2004;79:479–86. [DOI] [PubMed] [Google Scholar]
- 31.Park S, Kim MY, Baik SH, Woo JT, Kwon YJ, Daily JW, Park YM, Yang JH, Kim SH. Gestational diabetes is associated with high energy and saturated fat intakes and with low plasma visfatin and adiponectin levels independent of prepregnancy BMI. Eur J Clin Nutr 2013;67:196–201. [DOI] [PubMed] [Google Scholar]
- 32.Gower BA, Goree LL, Chandler-Laney PC, Ellis AC, Casazza K, Granger WM. A higher-carbohydrate, lower-fat diet reduces fasting glucose concentration and improves β-cell function in individuals with impaired fasting glucose. Metabolism 2012;61:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okereke NC, Huston-Presley L, Amini SB, Kalhan S, Catalano PM. Longitudinal changes in energy expenditure and body composition in obese women with normal and impaired glucose tolerance. Am J Physiol Endocrinol Metab 2004;287:E472–9. [DOI] [PubMed] [Google Scholar]
- 34.Boden G, Jadali F. Effects of lipid on basal carbohydrate metabolism in normal men. Diabetes 1991;40:686–92. [DOI] [PubMed] [Google Scholar]
- 35.Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Invest 1983;72:1737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh R, Martin B, Hickey Y, Teegarden D, Campbell W, Craig B, Schoeller D, Kerr D, Weaver C. Comparison of self-reported, measured, metabolizable energy intake with total energy expenditure in overweight teens. Am J Clin Nutr 2009;89:1744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]