Abstract
Background: In resource-limited settings in which child malnutrition is prevalent, humans live in close proximity to household livestock. However, the relation between household livestock and child nutrition represents a considerable knowledge gap.
Objective: We assessed whether household livestock ownership or livestock disease episodes were associated with growth in young children in western Kenya.
Methods: We incorporated monthly anthropometric measurements for children <5 y of age into an ongoing linked human and animal surveillance cohort in rural western Kenya. Using linear mixed models adjusted for age, sex, and household wealth, we tested whether baseline household livestock ownership was related to baseline child height for age or prospective growth rate. We also evaluated whether livestock disease episodes were associated with child growth rate over 11 mo of follow-up.
Results: We collected data on 925 children over the course of follow-up. Greater household livestock ownership at baseline was not related to baseline child height-for-age z score (adjusted β: 0.01 SD; 95% CI: −0.02, 0.04 SD) or child growth rate (adjusted β: 0.02 cm/y; 95% CI: −0.03, 0.07 cm/y). Livestock disease episodes were not significantly associated with child growth across the entire cohort (adjusted β: −0.007 cm/mo; 95% CI: −0.02, 0.006 cm/mo). However, children in households with livestock digestive disease between June and November gained less height than did children in households that did not report livestock disease (β: −0.063 cm/mo; 95% CI: −0.112, −0.016 cm/mo). Children <2 y of age in households with livestock digestive disease gained less weight than did those who did not report disease (β: −0.033 kg/mo; 95% CI: −0.063, −0.003 kg/mo).
Conclusion: In this cohort of young children in western Kenya, we did not find an association between ownership of livestock and child growth status. However, disease episodes in household livestock may be related to a lower child growth rate in some groups.
Keywords: child growth, stunting, nutrition, livestock, household environment
Introduction
Optimal early childhood growth is associated with long-term health and cognitive function (1). As such, child growth measures serve as important indicators for current and future population health and economic well-being (2). Child growth velocity varies by age and follows a pattern of rapid increases followed by stasis (3). Throughout infancy, children experience steep gains in length, the velocity of which slows at ∼1 y of age (4). Growth trajectory can be diminished by negative energy balance, either from decreased nutrient intake or increased metabolic demand from infections. Although catch-up growth can occur after these insults, it may be incomplete, leading to a permanent height deficit (5, 6). This height deficit, or stunting, is associated with a higher risk of acute morbidity, long-term chronic disease and education underperformance (1).
Household livestock production is a common income-generating activity in low-resource settings, and may benefit child nutrition through better access to health care, education, sanitation, and nutritionally rich foods (7). In addition, direct consumption of eggs, meat, and milk or milk products can improve children’s dietary diversity, which is an important contributor to linear growth (8, 9). Consumption of cow milk has been demonstrated to improve child growth in both developed and developing settings (10, 11). For these reasons, livestock interventions, such as livestock production intensification and livestock donation, often have the goal of improving child nutrition. However, child growth and health outcomes have not been evaluated commonly as metrics for these interventions. In a systematic review of the impact of livestock production improvement on nutrition-related outcomes, only one trial had evaluated child growth and no significant associations were reported (12). Furthermore, to our knowledge, only a few observational studies have tested the overall influence of household livestock ownership on child growth (13–15).
Although there are several conceptual benefits to livestock ownership, increasing numbers of household animals could include potential trade-offs. For example, dairy intensification may result in a decrease in exclusive breastfeeding behavior as a result of increased availability of cow milk (16). Livestock in resource-limited settings also often have a high incidence of disease, and many livestock pathogens have zoonotic potential (17–19). Environmental contamination with livestock fecal pathogens could increase the risk of clinical and subclinical infection in children, both of which can directly affect linear growth (20, 21). Disease in livestock may also lead to loss of household income through the loss of investment in these animals (22, 23).
As a result of the role of livestock as a source of wealth and food, and as a potential source of zoonotic pathogens, disentangling the relation between household livestock and child growth is inherently challenging. In this study, we evaluated the overall relations between household livestock ownership, episodes of livestock disease, and child growth trajectory in a linked human and animal health surveillance cohort in western Kenya.
Methods
Study design
This prospective cohort study was conducted within a human–animal health surveillance platform in western Kenya managed jointly between the Kenya Medical Research Institute, CDC Kenya, Washington State University, and the University of Washington. During the study, human health and livestock surveillance systems were ongoing in 10 villages (1800 households) within a 5-km radius near Lake Victoria (24). The primary economic activities in this area include fishing, smallholder mixed-crop farming, and livestock farming. Chickens are usually free range and are able to enter human homes. In some households, chickens are penned in a separate enclosure at night. Cattle, goats, and sheep graze during the day but are enclosed within the compound (a fenced group of familial housing) at night.
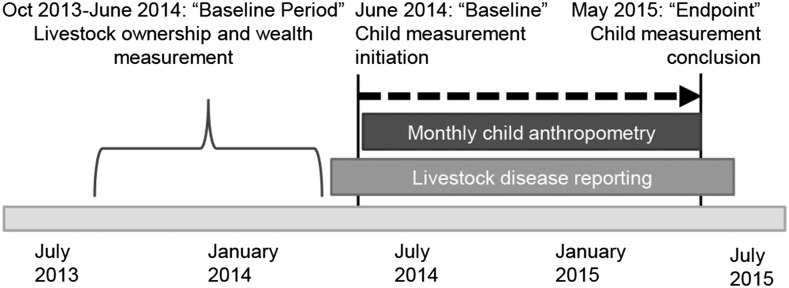
The anthropometric component of this study was approved by the Kenya Medical Research Institute Ethical Review Board as an amendment to an existing animal surveillance study (Scientific Steering Committee protocol no. 2250) and the human health surveillance study (Scientific Steering Committee protocol no. 1899) in March 2014. Any households enrolled in both human and animal surveillance systems with children who were <5 y of age in June 2014 were eligible for inclusion in this cohort. Caregivers provided informed consent for participation in the study. The follow-up timeline for the study is depicted in Figure 1.
FIGURE 1.
Follow-up timeline for measurements of household livestock and children <5 y of age in Asembo, Kenya.
Our sample size was confined by the population contained within the participating villages. We predicted that we would enroll ∼1000 children in 700 households and calculated the minimum detectable effect based on these numbers. To account for clustering within household, we calculated the anticipated design effect and cluster variability. Based on these values, we expected to have the capacity to detect an RR of stunting of ∼1.43, comparing those families that owned no animals to those that owned any animals. For the analysis of livestock disease and child growth, the primary exposure of interest was a time-varying covariate. We estimated that the incidence of livestock diarrheal disease would be 40 diagnoses/wk within the catchment area, yielding an 11% monthly cumulative incidence of the exposure at the household level.
Data collection
Four experienced community health interviewers were trained in questionnaire administration and anthropometric measurements with the use of the World Vision training package (25). From June 2014 to May 2015, the team collected the height and weight measurements of children <5 y of age every month during household visits. The team measured the length of children <2 y of age and the height of children ≥2 y of age with the use of a ShorrBoard (Weigh and Measure, LLC). Weight was measured with the use of a digital mother/child standing scale (Seca). Midupper arm circumference was assessed with the use of standardized tape for the purpose of screening and referral for acutely malnourished children [defined as an arm circumference of <11.5 cm for children >6 mo of age (26)]. Interobserver reliability assessments were conducted initially as a part of training. For ongoing quality control, managers conducted repeat visits with a subset of children each month. Children with abnormal height or weight values also were followed up after data cleaning.
Baseline livestock ownership and economic data were collected by a separate team through the administration of an in-depth quarterly economic questionnaire between October 2013 and June 2014, as described elsewhere (24). For these economic questionnaires, trained interviewers asked the household head a series of questions about assets, livestock (cows, sheep, goats, and chickens), income, and expenditures. Livestock disease information, collected between May 2014 and May 2015 by a team of veterinary technicians, was available throughout the child growth follow-up study (24). Community health interviewers requested information on whether or not there were any sick livestock in the household during biweekly interviews. Farmers could also report an animal disease directly through a toll-free phone line. If a household reported a livestock illness, veterinary technicians responded within 48 h to examine and diagnose the animals, collect diagnostic specimens, and provide veterinary treatment, if necessary. Treatment was provided in accordance with the best clinical diagnosis, as well as with laboratory results when available. Common treatments for infections included anthelminthics, antibiotics, and anti-inflammatory drugs. Veterinarians and animal health technicians also provided advice if the clinical diagnosis was considered to be of environmental or dietary origin. Livestock disease reports were subcategorized for cows, goats, and sheep into 9 syndromes: death, reproductive (abortion, stillbirths, or neonatal deaths), respiratory (cough, nasal discharges, or difficulty breathing), digestive (diarrhea, bloody diarrhea, or bloating), urogenital, mastitis, musculoskeletal, skin, and nervous system. Chicken reports were recorded based on episodic outbreak mortality in chicks, reflective of the most common losses in household poultry.
Data analyses
We conducted 3 main analyses. First, we evaluated the relations between livestock ownership and baseline anthropometric measurement status. Second, we assessed whether baseline livestock ownership was associated with subsequent annualized growth trajectory. Finally, we tested the relation between time-varying livestock disease episodes and monthly growth trajectory. All analyses were conducted in Stata/SE 13. Tests were considered to be statistically significant if the CIs did not include zero. No adjustments were made for multiple comparisons.
Descriptive statistics.
To describe the cohort, we calculated means and proportions of baseline child and household characteristics in total and across a binary variable indicating whether any livestock illness had been reported over the entire livestock disease reporting period (May 2014–May 2015). We described animal ownership over the baseline period by providing mean household counts and variability. Livestock disease reports are described as the total count and proportion of disease reports in each syndrome category. We also assessed nonlivestock predictors of growth trajectory (child age, season, child sex, number of household members, and household wealth) in univariable analyses.
Livestock ownership and child growth analysis.
In order to include as many households as possible, and to account for variability and wealth at the household level, we calculated baseline livestock and wealth scores over a 9-mo period (3 quarterly measurement points) before child growth measurements began (Figure 1). For baseline livestock counts, we created a single mean household count within each livestock species (chickens, cows, sheep, and goats). To generate a total livestock score, we combined livestock species into an unweighted sum per household. We calculated an asset-based wealth score with the use of the household’s ownership of farming implements, bikes, vehicles, radios, tractors, phones, motorbikes, televisions, computers, electronics, dwellings and buildings, and latrines in a principal component analysis based on cofactors from previous literature and cultural aspects relevant to our study population (27). The first component was used as a wealth score for each measurement point, which was then averaged across all baseline time points. Both livestock ownership and the wealth score were household-level variables.
We compared child height and weight measurements by age and sex to the WHO 2006 standards to create continuous measures for height-for-age z score (HAZ) and weight-for-height z score (WHZ) (28). Stunting and wasting were defined as less than −2 SDs below the WHO reference median. The child’s baseline HAZ, baseline WHZ, and prospective annualized growth rates were considered the outcomes for this analysis, which was conducted in a subset of the cohort for which an initial child height measurement was available within 3 mo of June 2014. Baseline HAZ and WHZ were used in their first available instance between June and August 2014. HAZ, WHZ, weight, and height measurements were excluded for biological implausibility a priori if the HAZ or WHZ was below −5 SD or above 5 SD. Annualized 12-mo growth rate [(last height measurement − first height measurement) × 12/total months contributed] was calculated for children with ≥2 measurements, ≥3 mo apart.
We used linear mixed regression to evaluate the relation between household livestock ownership and these child growth outcomes. Random effects were included for the household level, because many households included >1 child. The main predictor in this model was baseline livestock ownership, either as a total count or a count by species. After evaluating the unadjusted association, we controlled for the a priori covariates of child age, child sex, number of household members, and household wealth. Covariates were chosen as potentially important predictors of child height or weight gain, or as potential confounding factors in the relation between livestock and child growth. In order to encompass the entire effect between livestock ownership and child nutrition, we chose not to adjust for variables that might be considered within the overall pathways between livestock ownership, livestock disease, and child growth, such as child feeding practices or child disease status.
Livestock disease and child growth analysis.
For this analysis, livestock disease was defined as a report of any livestock syndrome within the household over time-varying 30-d increments. Households could report >1 disease/mo, so disease counts were treated as a continuous variable. Because of the large numbers of digestive syndromes reported, and because of interest in diarrheal disease specifically, we evaluated reports of animal digestive syndromes and diarrheal symptoms separately. Further disease stratifications by syndrome type or livestock species type were not conducted because of insufficient numbers of disease reports at the household level. Child growth was defined as the change in height or weight by month over the course of follow-up.
We again used linear mixed regression to evaluate whether time-varying livestock disease was related to monthly child growth rate. Random intercepts were included for both child and household levels to account for multiple measurements per child over time and for related children within households. We also included a random slope for the child level. The primary independent variable for this model was time, as measured by month of the child’s height and weight measurement. We tested whether livestock disease episodes significantly influenced the effect of time on child height or weight (i.e., growth) by evaluating an interaction between the exposure and month. In the primary analysis, we tested the effect by using a lag of 1 mo between livestock disease and the child’s growth measurements. In exploratory analyses, we also tested models with no lag and a lag of 3 mo, as well as change in HAZ and WHZ over time.
After evaluating the unadjusted associations in each of these models, we controlled for child baseline age, child sex, number of household members, household baseline wealth, and baseline household livestock ownership count. As above, covariates were chosen a priori as potentially important predictors of child height or weight gain, or as potential confounding factors in the relation between livestock syndromes and child growth. We provided overall results as well as results separately for children ≤2 of age and children >2 y because of the differences in growth slope at these ages. We also provided livestock disease model estimates stratified by 2 seasons, June–November and December–April, because of seasonal differences in growth.
Sensitivity analysis.
Because this study was longitudinal and community-based, some anthropometric data points were missing throughout follow-up. As a sensitivity analysis, we imputed missing height values with the use of the Markov Chain Monte Carlo multivariate normal regression technique. We used 10 imputed datasets. Estimates of the imputed regressions were derived with the use of Rubin’s combining rules through Stata’s “mi” package (29).
Results
Cohort description.
During follow-up, we collected data on 925 children in 755 households. Eight hundred forty-one children had sufficiently complete data to be included in ≥1 adjusted analysis. Children were available for a mean of 8 of 11 growth measurement visits, and 77% of monthly height observations were available overall. Those children who were missing height measurement data points in this cohort were older and more likely to be female, and came from households with higher household livestock ownership. However, in the imputation analyses, no major differences in the size or direction of the associations reported below were noted (data not shown).
The baseline age of children ranged from 1 mo to 60 mo. Among those for whom baseline and endpoint values were available, 23.5% of children were stunted at baseline. In univariable models, child age, season, number of household members, household wealth, and child sex were related to child growth over time (Supplemental Table 1). Overall, children grew (mean ± SE) 0.68 ± 0.007 cm/mo and gained 0.15 ± 0.002 kg/mo. Children ≤2 y of age grew 0.80 ± 0.014 cm/mo and gained 0.16 ± 0.004 kg/mo; children >2 y of age grew 0.63 ± 0.005 cm/mo and gained 0.14 ± 0.003 kg/mo. Girls grew significantly faster than boys, by ∼0.05 cm/mo in height and 0.014 kg/mo in weight. The children’s height gain was greater between December 2014 and April 2015 than between June 2014 and November 2014 (β: 0.10 cm/mo for December–April compared to June–November, 95% CI: 0.06, 0.14 cm/mo).
At baseline, there were a few notable differences in characteristics between children whose families reported livestock disease and those whose families did not (Table 1). The mean WHZ and HAZ were higher in those families that reported any syndromes. Baseline wealth status quintile (excluding livestock) was higher, and baseline livestock ownership counts were much higher in those families who reported any syndromes throughout follow-up. Child age was fairly balanced between the 2 groups.
TABLE 1.
Baseline cohort characteristics of children <5 y of age and their households, Asembo, Kenya1
| Characteristic | Any livestock disease reported (n = 304) | No livestock disease reported (n = 554) | Overall (n = 858) |
| Child indicators | |||
| Age, mo | 33.0 ± 17.2 | 32.7 ± 17.4 | 32.8 ± 17.3 |
| Female | 163 (53.6) | 287 (51.8) | 450 (52.4) |
| HAZ | −1.1 ± 1.26 | −1.25 ± 1.1 | −1.2 ± 1.2 |
| WHZ | −0.02 ± 1.1 | −0.20 ± 1.1 | −0.12 ± 1.1 |
| Wasted | 9 (3.0) | 31 (5.7) | 40 (4.8) |
| Stunted | 65 (21.9) | 132 (24.4) | 197 (23.5) |
| Household indicators2 | |||
| Number of household members | 6.3 ± 1.9 | 5.6 ± 2.0 | 5.9 ± 2.0 |
| Household wealth quintile | 3.2 ± 1.4 | 2.9 ± 1.4 | 3.0 ± 1.4 |
| Livestock count | 4.8 ± 3.0 | 2.7 ± 2.3 | 3.4 ± 3.1 |
| Cattle, n | 3.8 ± 5.8 | 1.1 ± 2.2 | 2.1 ± 4.1 |
| Chickens, n | 10.4 ± 7.8 | 8.3 ± 7.6 | 9.1 ± 7.7 |
| Goats, n | 2.7 ± 3.6 | 0.8 ± 1.5 | 1.5 ± 2.6 |
| Sheep, n | 2.0 ± 4.1 | 0.4 ± 1.4 | 1.0 ± 2.8 |
Values are means ± SDs or n (%). HAZ, height-for-age z score; WHZ, weight-for-height z score.
Household mean over the past 9 mo before baseline.
Livestock ownership and child growth analysis.
Eight hundred thirty-eight children from 597 households had a baseline anthropometric measurement between June 2014 and August 2014 and were included in the analysis of livestock ownership and growth. The mean HAZ at baseline was −1.2 ± 1.2 SD and the mean WHZ was −0.12 ± 1.1 SD. Child age, child sex, number of household members, and household wealth score were associated with HAZ at baseline. Child age was the only covariate significantly related to WHZ; children between 1 and 2 y of age were the most likely to be wasted (Table 2). We did not find an association between livestock ownership, in combination or as separate species, and child baseline HAZ or WHZ (Table 2). Adjustment for child age, child sex, household members, and household wealth did not meaningfully change these associations. We also did not find an association between baseline livestock ownership and prospective annualized growth rate (Table 3). However, in exploratory analyses, baseline livestock ownership counts were significantly positively related to both baseline household wealth and livestock disease (data not shown).
TABLE 2.
Linear mixed-model results of household livestock ownership, total count and by species, and anthropometric measurement status in children <5 y of age at baseline (n = 838)1
| Baseline child HAZ |
Baseline child WHZ |
|||
| Model | Unadjusted β (95% CI) | Adjusted β (95% CI)2 | Unadjusted β (95% CI) | Adjusted β (95% CI)2 |
| Total livestock ownership | ||||
| Baseline livestock count3 | 0.01 (−0.01, 0.04) | 0.01 (−0.02, 0.04) | −0.002 (−0.03, 0.02) | −0.002 (−0.03, 0.03) |
| Child age (mo), linear | −0.10 (−0.12, −0.08) | 0.03 (0.009, 0.05) | ||
| Child age (mo), quadratic | 0.001 (0.001, 0.002) | −0.0004 (−0.0007, −0.0001) | ||
| Child sex (female = 1) | 0.24 (0.09, 0.40) | 0.16 (−0.001, 0.32) | ||
| Number of household members | −0.08 (−0.13, −0.04) | −0.03 (−0.07, 0.02) | ||
| Household wealth score | 0.11 (0.03, 0.19) | 0.06 (−0.02, 0.14) | ||
| Separate livestock species3 | ||||
| Poultry | 0.003 (−0.008, 0.14) | −0.0005 (−0.01, 0.01) | −0.0003 (−0.01, 0.01) | −0.0003 (−0.01, 0.01) |
| Cows | 0.03 (−0.05, 0.11) | 0.04 (−0.04, 0.12) | 0.001 (−0.07, 0.08) | −0.02 (−0.10, 0.06) |
| Goats | 0.010 (−0.03, 0.05) | 0.004 (−0.03, 0.04) | −0.02 (−0.06, 0.01) | −0.02 (−0.06, 0.02) |
| Sheep | −0.008 (−0.04, 0.03) | 0.02 (−0.02, 0.05) | 0.02 (−0.02, 0.05) | 0.03 (−0.01, 0.06) |
| Child age (mo), linear | −0.10 (−0.12, −0.07) | 0.03 (0.009, 0.05) | ||
| Child age (mo), quadratic | 0.001 (0.001, 0.002) | −0.0004 (−0.0007, −0.0001) | ||
| Child sex (female = 1) | 0.24 (0.09, 0.40) | 0.17 (0.01, 0.33) | ||
| Number of household members | −0.10 (-0.12, −0.08) | −0.03 (−0.07, 0.02) | ||
| Household wealth score | 0.11 (0.03, 0.19) | 0.07 (−0.01, 0.15) | ||
HAZ, height-for-age z score; WHZ, weight-for-height z score.
Adjusted for household wealth, child sex, child age, and number of household members.
Mean livestock numbers owned over the previous 9 mo.
TABLE 3.
Linear mixed-model results of household livestock ownership, total count and by species, and annualized growth rate in children <5 y of age (n = 841)
| Annualized growth rate, cm/y |
||
| Model | Unadjusted β (95% CI) | Adjusted β (95% CI)1 |
| Overall livestock ownership | ||
| Baseline livestock count2 | 0.01 (−0.04, 0.07) | 0.02 (−0.03, 0.07) |
| Child age (mo), linear | −0.13 (−0.17, −0.09) | |
| Child age (mo), quadratic | 0.001 (0.0004, 0.002) | |
| Child sex (female = 1) | 0.79 (0.49, 1.10) | |
| Number of household members | −0.08 (−0.17, −0.004) | |
| Household wealth score | 0.15 (0.01, 0.29) | |
| Separate livestock species2 | ||
| Poultry | 0.01 (−0.01, 0.03) | 0.01 (−0.008, 0.03) |
| Cattle | 0.05 (−0.10, 0.21) | 0.06 (−0.08, 0.20) |
| Goats | 0.01 (−0.06, 0.09) | 0.006 (−0.06, 0.07) |
| Sheep | −0.05 (−0.12, 0.02) | −0.04 (−0.11, 0.02) |
| Child age (mo), linear | −0.13 (−0.17, −0.09) | |
| Child age (mo), quadratic | 0.001 (0.0004, 0.002) | |
| Child sex (female = 1) | 0.77 (0.47, 1.08) | |
| Number of household members | −0.08 (−0.16, −0.001) | |
| Household wealth score | 0.14 (−0.003, 0.28) | |
Adjusted for household wealth, child sex, child age, and number of household members.
Mean livestock numbers owned over the previous 9 mo.
Livestock disease and child growth analysis.
Between May 2014 and May 2015, 1181 cases of any animal disease or death were reported in 304 households. Digestive disorders were most commonly reported, followed by death, skin disorders, and respiratory disorders (Supplemental Table 2). Over any given month of follow-up, ∼92% of households reported no livestock disease, 6% reported 1 livestock disease, and 2% reported >1 livestock disease.
Nine hundred twenty-one children were included in the livestock disease and child growth analysis, and 800 had complete data for the adjusted analysis (Table 4). The models provided in Table 4 represent the association between child growth and livestock disease exposure in the previous month. We did not find any associations between livestock disease and child growth measured concurrently (no lag), or when using a lag of 3 mo.
TABLE 4.
Overall and stratified linear mixed-model results of time-varying livestock disease, livestock digestive disease, and livestock diarrheal disease with mean monthly growth in children <5 y of age (n = 800)
| Adjusted β (95% CI)1 |
|||||
| Exposure and outcome | Overall (n = 800) | <2 y of age (n = 267) | 2–5 y of age (n = 533) | June–November exposure (n = 792) | December–April exposure (n = 713) |
| Any livestock disease | |||||
| Height gain, cm/mo | −0.007 (−0.02, 0.006) | 0.0002 (−0.036, 0.037) | −0.011 (−0.024, 0.002) | −0.019 (−0.040, 0.002) | −0.047 (−0.101, 0.006) |
| Weight gain, kg/mo | −0.006 (−0.02, 0.004) | −0.016 (−0.035, 0.003) | −0.002 (−0.015, 0.011) | −0.009 (−0.024, 0.007) | −0.012 (−0.068, 0.044) |
| Livestock digestive disease2 | |||||
| Height gain, cm/mo | −0.016 (−0.039, 0.007) | 0.009 (−0.048, 0.064) | −0.023 (−0.046, 0.000) | −0.063 (−0.112, −0.016) | −0.048 (−0.123, 0.033) |
| Weight gain, kg/mo | −0.007 (−0.025, 0.010) | −0.033 (−0.063, −0.003) | 0.003 (−0.019, 0.025) | −0.003 (−0.037, 0.030) | −0.004 (−0.080, 0.071) |
| Livestock diarrheal disease2 | |||||
| Height gain, cm/mo | −0.011 (−0.042, 0.020) | −0.009 (−0.079, 0.061) | −0.014 (−0.047, 0.018) | −0.056 (−0.129, 0.016) | −0.076 (−0.171, 0.020) |
| Weight gain, kg/mo | 0.009 (−0.016, 0.033) | −0.0002 (−0.038, 0.038) | 0.016 (−0.014, 0.048) | 0.009 (−0.043, 0.062) | −0.033 (−0.131, 0.064) |
Adjusted for household wealth, child sex, child age, number of household members, and baseline livestock ownership.
Livestock digestive disease is a category within any livestock disease; livestock diarrheal disease is a category within livestock digestive disease.
In the overall models, almost all of the livestock disease exposures were inversely related to child growth, even though these associations were not statistically significant (Table 4). In the models that were separated by child age, children <2 y of age (n = 267) in households that experienced a single livestock digestive disorder report in the previous month grew significantly less in weight than did those reporting no disease (β: −0.033 kg/mo; 95% CI: −0.063, −0.003 kg/mo) (Table 4). In the models stratified by season, children in households with livestock digestive disease between June and November gained significantly less in height than did children in households that did not report livestock disease (β: −0.063 cm/mo; 95% CI: −0.112, −0.016 cm/mo) (Table 4). Livestock disease was not consistently significantly associated with child HAZ or WHZ over time, although the direction of effect was similar to above (data not shown).
Discussion
This study describes household livestock ownership and health in relation to the growth of children in rural western Kenya over a follow-up of 11 mo. We did not find a relation between owning higher numbers of household livestock and child baseline anthropometric measures or subsequent child linear growth outcomes. However, there was an indication that livestock disease was associated with some measures of diminished growth.
There are several conceptual benefits of livestock ownership for children <5 in rural families. However, in this cohort, livestock ownership was not related to baseline HAZ, baseline WHZ, or prospective growth rate. Other studies have shown that higher numbers of owned livestock are associated with better child anthropometric measures (8, 14, 30). It is possible that the families in this cohort interacted with their livestock differently from other populations, and thus are not receiving the potential nutritional benefits. We did identify that higher numbers of livestock were related to higher wealth scores, but owning more animals might not directly translate to increased consumption of nutritionally rich foods or health care access.
In this cohort, we found a strong seasonal association with child linear growth. Children had a faster rate of linear growth between December and January. Consequently, the HAZ of children decreased between July and November and then increased between December and January. During the study time period, the peak rains in the Kisumu area were in August, followed by a sharp decrease in rain between December and March (31). The trend in child growth may be related to the seasonality of diarrheal disease, malaria, and other febrile diseases (32–34); access to food; or other factors.
These data suggest that livestock disease, specifically livestock digestive disorders, may be associated with poor subsequent child growth in the following month. There are several potential reasons for this signal. First, it is possible that sick household livestock serve as an indicator for poor overall household health. A recent study from the same cohort showed that livestock disease reports were significantly associated with human disease reports within the same household (24), which is supported by previous studies of animal husbandry (35). A second possibility is that sick livestock serve as source of zoonotic pathogens in the household. Given that childhood diarrhea has widely known impacts on child growth (36), there is the potential that these infections could result in infection-mediated linear growth failure (21). Finally, there are dietary and economic costs incurred as a result of livestock disease. Livestock illness could result in decreased production and direct consumption of animal-sourced foods, and might further represent a loss in household wealth. These losses could result in poorer child growth after a livestock disease.
This study has several key strengths, as well as limitations. First, the use of systematically collected data on childhood growth, animal ownership and animal illness is unique. In addition, the multidirectional interactions driving these associations were considered carefully in the analysis. We used time-varying linear mixed models of livestock syndrome report and child growth outcomes, which allowed us to evaluate a temporally appropriate relation between livestock disease and child growth. However, despite a reasonably high overall incidence, livestock disease reports were low in number at the household level. For most of the prospective livestock disease and growth analyses, we were unable to divide by species and syndrome type because of small numbers, and the effect estimates might be more statistically precise with more incidence data. In addition, in the course of the program, veterinary care was provided to sick animals requiring treatment, which may have altered the association between untreated disease and child growth. Although the animal disease surveillance system includes both active and passive reporting, there remains a potential for underreporting, which further could have led to a nondifferential exposure misclassification bias in the estimates. Moreover, we anticipate that the potential for residual confounding between livestock disease, household wealth, and child growth could have attenuated an inverse association between livestock disease and child growth. Missing data were another limitation of the analysis, although the multiple imputations models showed a size and direction of effect for livestock ownership and disease similar to the complete case analysis. Finally, we were unable to include some major predictors of child growth, such as parental height and child birth weight. Although we believe that we have included the most important confounding factors, the precision of the estimates could have been improved by including these strong predictors of the outcome.
Domestic livestock are ubiquitous in rural households throughout many resource-limited settings, and provide a means of economic development for families. However, the household relations between livestock and humans are complex, and this study encourages further examination into these relations. Optimizing the benefits of livestock for child health requires integrated programs that include veterinary care, water and sanitation interventions, child feeding education, and childhood disease prevention and control.
Acknowledgments
We thank Godfrey Bigogo, George Aol, and Penina Munyua for their help with data collection and work establishing the data collection systems. EM, SMT, TFM, MKN, PMR, AR-R, MLN, SM, GHP, and JLW designed the research; EM, SMT, and EO conducted the research; EM, SMT, and JLW drafted the manuscript; and EM analyzed the data, performed the statistical analysis, and had primary responsibility for the final content. All authors read and approved the final manuscript.
References
- 1.Dewey KG, Begum K. Long-term consequences of stunting in early life. Matern Child Nutr 2011;7 Suppl 3:5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382:427–51. [DOI] [PubMed] [Google Scholar]
- 3.Lampl M, Johnson ML, Frongillo EA Jr. Mixed distribution analysis identifies saltation and stasis growth. Ann Hum Biol 2001;28:403–11. [DOI] [PubMed] [Google Scholar]
- 4.Cameron N. The biology of growth. Nestle Nutr Workshop Ser Pediatr Program 2008;61:1–19. [DOI] [PubMed] [Google Scholar]
- 5.Richard SA, Black R, Gilman RH, Guerrant RL, Lanata CF, Molbak K, Rasmussen ZA, Sack RB, Valentiner-Branth P, Checkley W, et al. Catch-up growth occurs after diarrhea in early childhood. J Nutr 2014;144:965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gat-Yablonski G, Phillip M. Nutritionally-induced catch-up growth. Nutrients 2015;7:517–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pica-Ciamarra U, Dhawan M. Small-scale poultry farming and poverty reduction in South Asia. Rome, Italy: Food and Agriculture Organization, 2010. [Google Scholar]
- 8.Krebs NF, Mazariegos M, Tshefu A, Bose C, Sami N, Chomba E, Carlo W, Goco N, Kindem M, Wright LL, et al. Meat consumption is associated with less stunting among toddlers in four diverse low-income settings. Food Nutr Bull 2011;32:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones AD, Ickes SB, Smith LE, Mbuya MN, Chasekwa B, Heidkamp RA, Menon P, Zongrone AA, Stoltzfus RJ. World Health Organization infant and young child feeding indicators and their associations with child anthropometry: a synthesis of recent findings. Matern Child Nutr 2014;10:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiley AS. Cow milk consumption, insulin-like growth factor-I, and human biology: a life history approach. Am J Hum Biol 2012;24:130–8. [DOI] [PubMed] [Google Scholar]
- 11.Iannotti L, Lesorogol C. Animal milk sustains micronutrient nutrition and child anthropometry among pastoralists in Samburu, Kenya. Am J Phys Anthropol 2014;155:66–76. [DOI] [PubMed] [Google Scholar]
- 12.Leroy JL. Can interventions to promote animal production ameliorate undernutrition? J Nutr 2007;137:2311–6. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson C, Mwangi L, Staal S, Thornton P. Dairy cow ownership and child nutritional status in Kenya. AAEA Annual Meeting; Montreal, Canada 2003.
- 14.Jin M, Ianotti L. Livestock production, animal source food intake, and young child growth: the role of gender for ensuring nutrition impacts. Soc Sci Med 2014;105:16–21. [DOI] [PubMed] [Google Scholar]
- 15.Azzari C, Cross E, Heile B, Zezza A. Does livestock ownership affect animal source foods consumption and child nutritional status? Washington (DC): World Bank, 2014. [Google Scholar]
- 16.Wyatt AJ, Yount KM, Null C, Ramakrishnan U, Webb Girard A. Dairy intensification, mothers and children: an exploration of infant and young child feeding practices among rural dairy farmers in Kenya. Matern Child Nutr 2015;11:88–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aganga AO, Belino ED, Adegboye DS, Ilemobade AA. A serological survey of toxoplasmosis in food animals (cattle, sheep, goats and swine) in two northern states of Nigeria. Int J Zoonoses 1981;8:57–62. [PubMed] [Google Scholar]
- 18.Kagira JM, Kanyari PW. Occurrence of risk factors for zoonoses in Kisumu City, Kenya: a questionnaire survey. East Afr J Public Health 2010;7:1–4. [DOI] [PubMed] [Google Scholar]
- 19.Marquis GS, Ventura G, Gilman RH, Porras E, Miranda E, Carbajal L, Pentafiel M. Fecal contamination of shanty town toddlers in households with non-corralled poultry, Lima, Peru. Am J Public Health 1990;80:146–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keusch GT, Rosenberg IH, Denno DM, Duggan C, Guerrant RL, Lavery JV, Tarr PI, Ward HD, Black RE, Nataro JP, et al. Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low- and middle-income countries. Food Nutr Bull 2013;34:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, Morris SS, Molbak K, Valentiner-Branth P, Lanata CF, Black RE, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol 2008;37:816–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peyre M, Chevalier V, Abdo-Salem S, Velthuis A, Antoine-Moussiaux N, Thiry E, Roger F. A systematic scoping study of the socio-economic impact of Rift Valley Fever: research gaps and needs. Zoonoses Public Health 2015. [DOI] [PubMed] [Google Scholar]
- 23.Sinkala Y, Simuunza M, Pfeiffer DU, Munang'andu HM, Mulumba M, Kasanga CJ, Muma JB, Mweene AS. Challenges and economic implications in the control of foot and mouth disease in sub-Saharan Africa: lessons from the Zambian experience. Vet Med Int 2014;2014:373921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thumbi SM, Njenga MK, Marsh TL, Noh S, Otiang E, Munyua P, Ochieng L, Ogola E, Yoder J, Audi A, et al. Linking human health and livestock health: a “one-health” platform for integrated analysis of human health, livestock health, and economic welfare in livestock dependent communities. PLoS One 2015;10:e0120761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Vision Nutrition Centre of Expertise. Measuring and promoting child growth manual. Ontario, Canada: 2012. [Google Scholar]
- 26.World Health Organization. UNICEF. WHO child growth standards and the identification of severe acute malnutrition in infants and children. Geneva, Switzerland: WHO Press, 2009. [PubMed] [Google Scholar]
- 27.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan 2006;21:459–68. [DOI] [PubMed] [Google Scholar]
- 28.Child Growth Standards Table WHO [Internet]. Boys. Geneva, Switzerland: World Health Organization; 2006 [cited 2014 Jul 7]. Available from: http://www.who.int/childgrowth/standards/tab_lhfa_boys_p_0_2.txt. [Google Scholar]
- 29.Little RJA, Rubin DB. Statistical analysis with missing data. New York: John Wiley and Sons; 1987. [Google Scholar]
- 30.Bwibo NO, Neumann CG. The need for animal source foods by Kenyan children. J Nutr 2003;133:3936S–40S. [DOI] [PubMed] [Google Scholar]
- 31.National Climatic Data Center. Kisumu climate data [Internet]. [cited 2015 Aug 28]. Available from: http://www7.ncdc.noaa.gov/CDO/cdodata.cmd.
- 32.Gatei W, Wamae CN, Mbae C, Waruru A, Mulinge E, Waithera T, Gatika SM, Kamwati SK, Revathi G, Hart CA. Cryptosporidiosis: prevalence, genotype analysis, and symptoms associated with infections in children in Kenya. Am J Trop Med Hyg 2006;75:78–82. [PubMed] [Google Scholar]
- 33.Malakooti MA, Alaii J, Shanks GD, Phillips-Howard PA. Epidemic dysentery in western Kenya. Trans R Soc Trop Med Hyg 1997;91:541–3. [DOI] [PubMed] [Google Scholar]
- 34.Kang H, Kreuels B, Adjei O, Krumkamp R, May J, Small D. The causal effect of malaria on stunting: a Mendelian randomization and matching approach. Int J Epidemiol 2013;42:1390–8. [DOI] [PubMed] [Google Scholar]
- 35.Zambrano LD, Levy K, Menezes N, Freeman M. Human diarrhea infections associated with domestic animal husbandry: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg 2014;108:313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlaudecker EP, Steinhoff MC, Moore SR. Interactions of diarrhea, pneumonia, and malnutrition in childhood: recent evidence from developing countries. Curr Opin Infect Dis 2011;24:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]