Abstract
Background: The 2007–2010 NHANES provides the first US nationally representative serum 25-hydroxyvitamin D [25(OH)D] concentrations measured by standardized liquid chromatography–tandem mass spectrometry.
Objective: We describe patterns for total 25(OH)D and individual metabolites in persons aged ≥1 y stratified by race-ethnicity and grouped by demographic, intake, physiologic, and lifestyle variables.
Methods: We measured 25-hydroxycholecalciferol [25(OH)D3], 25-hydroxyergocalciferol [25(OH)D2], and C3-epimer of 25(OH)D3 [C3-epi-25(OH)D3] in serum samples (n = 15,652) from the 2007–2010 cross-sectional NHANES [total 25(OH)D = 25(OH)D3 + 25(OH)D2].
Results: Concentrations (median, detection rate) of 25(OH)D3 (63.6 nmol/L, 100%) and C3-epi-25(OH)D3 (3.40 nmol/L, 86%) were generally detectable; 25(OH)D2 was detectable in 19% of the population. Total 25(OH)D, 25(OH)D3, and C3-epi-25(OH)D3 displayed similar demographic patterns and were strongly correlated (Spearman’s r > 0.70). Concentrations of 25(OH)D2 (90th percentile) were much higher in persons aged ≥60 y (17.3 nmol/L) than in younger age groups (≤4.88 nmol/L). We noted significant race-ethnicity differences in mean total 25(OH)D [non-Hispanic blacks (NHBs), Hispanics, and non-Hispanic whites (NHWs): 46.6, 57.2, and 75.2 nmol/L, respectively] and in the prevalence of total 25(OH)D <30 nmol/L overall (24% of NHBs, 6.4% of Hispanics, and 2.3% of NHWs) as well as stratified by season (winter months: 30% of NHBs, 7.5% of Hispanics, and 3.8% of NHWs; summer months: 17% of NHBs, 4.4% of Hispanics, and 1.6% of NHWs). Persons with higher vitamin D intakes (diet, supplements, or both) and those examined during May–October had significantly higher total 25(OH)D. Significant race-ethnicity interactions in a multiple linear regression model confirmed the necessity of providing race-ethnicity–specific estimates of total 25(OH)D.
Conclusions: Race-ethnicity differences in the prevalence of low total 25(OH)D remained strong even after adjustment for season to account for the NHANES design imbalance between season, latitude, and race-ethnicity. The strong correlation between C3-epi-25(OH)D3 and 25(OH)D3 may be because the epimer is a metabolite of 25(OH)D3. The presence of 25(OH)D2 mainly in older persons is likely a result of high-dose prescription vitamin D2.
Keywords: NHANES, 25-hydroxyvitamin D3, 25-hydroxyvitamin D2, C3-epimer of 25-hydroxyvitamin D3, LC-MS/MS
Introduction
Serum 25-hydroxyvitamin D [25(OH)D]9 is currently the biomarker of choice to assess vitamin D status, because it integrates dietary vitamin D intake from foods and supplements with endogenously produced cholecalciferol (also called vitamin D3) by the action of solar UV-B radiation on 7-dehydrocholesterol. A 2013 systematic review found that worldwide population data on vitamin D status are limited, both overall and within key subgroups (1). Data from contemporary national surveys are even more limited (2–4). In 2015, the US Preventive Services Task Force published a new recommendation, which concluded that the current evidence is insufficient to assess the balance of benefits and harms of screening for vitamin D deficiency in asymptomatic adults (5). The inadequate quality of 25(OH)D testing methods was mentioned as a limiting factor.
Between 1988 and 2006, the NHANES produced nationally representative 25(OH)D data for the US population aged ≥12 y [1988–1994 (6)], ≥6 y [2001–2002 (7)], and ≥1 y [2003–2006 (7)] by using an RIA. Various reports documented method bias and imprecision problems with existing assays, particularly if they were immunoassay-based (8, 9). As technology advanced, LC coupled with tandem MS (LC-MS/MS) became more accessible for routine, high-volume analyses (10), reference methods and materials for 25(OH)D became available (11, 12), and the Vitamin D Standardization Program developed protocols for standardizing data from national surveys (13). A 2009 expert roundtable on 25(OH)D in NHANES recommended LC-MS/MS as a superior analytical technique going forward from 2007–2008 onward (14). The UK Food Standards Agency also recommended LC-MS/MS for their future National Diet and Nutrition Survey (15). Previously generated immunoassay data from the 2008–2010 Irish National Adult Nutrition Survey (NANS) were standardized to LC-MS/MS equivalents and results were compared with a full reanalysis of survey samples using LC-MS/MS (2).
Consistent with these other national surveys, the CDC laboratory began to measure 25(OH)D in NHANES 2007–2008 with the use of an accurate LC-MS/MS method standardized to international reference materials (16). The main objective of this article is to describe the latest vitamin D status of the US population aged ≥1 y participating in the NHANES 2007–2010 with these newly available 25(OH)D data generated by LC-MS/MS. We explored demographic patterns in total 25(OH)D and individual metabolites: 25-hydroxycholecalciferol [25(OH)D3], 25-hydroxyergocalciferol [25(OH)D2], and C3-epimer of 25(OH)D3 [C3-epi-25(OH)D3]. We also assessed the associations between total 25(OH)D and selected intake, physiologic, and lifestyle variables. Because of known race-ethnicity differences in 25(OH)D, we present data separately for Hispanics, non-Hispanic blacks (NHBs), and non-Hispanic whites (NHWs).
Methods
Study participants and study design.
The NHANES, conducted by the National Center for Health Statistics at the CDC, collects cross-sectional data on the health and nutritional status of the civilian noninstitutionalized US population. The survey uses a stratified, multistage, probability-sample design. Survey participants are interviewed in their home, and sociodemographic, dietary supplement use, and health-related information is collected. The participants then visit a mobile examination center where they undergo various physical examinations, provide biological specimens, and complete a 24-h dietary recall (17). Although the sample design in 2007–2010 continued to oversample various population groups, oversampling for Hispanic persons such that one can create estimates for Mexican Americans and all Hispanics (including Mexican Americans and other Hispanics) was new (18, 19). Of the 25,205 participants aged ≥1 y selected to participate in NHANES 2007–2010, 19,775 (78.5%) were interviewed, 19,142 (75.9%) agreed to the examination, and 15,652 (62.1%) provided serum samples for 25(OH)D testing (81.8% of those examined) (20). Estimated characteristics of the US population based on the study population are shown in Supplemental Table 1. All of the respondents gave their informed consent, and the NHANES protocol was reviewed and approved by the National Center for Health Statistics Research Ethics Review Board.
Laboratory methods.
Serum samples from participants aged ≥1 y (n = 15,652) were analyzed by the CDC laboratory for 25(OH)D metabolites by use of LC-MS/MS: 25(OH)D3, n = 15,651; 25(OH)D2, n = 15,651; and C3-epi-25(OH)D3, n = 15,473 (16). Serum total 25(OH)D (n = 15,650) was calculated as the sum of 25(OH)D3 and 25(OH)D2, not including C3-epi-25(OH)D3. The epimer was chromatographically resolved from 25(OH)D3 to avoid misclassification bias. When metabolite results were below the limit of detection (LOD), the NHANES data release contained an imputed value (LOD divided by the square root of 2). We used the imputed value for 25(OH)D2 in our total 25(OH)D calculation when 25(OH)D2 was less than the LOD (2.05 nmol/L); no 25(OH)D3 result was less than the LOD (2.23 nmol/L). Serum total 25(OH)D was missing if the CDC laboratory was unable to obtain a valid result for either 25(OH)D3 or 25(OH)D2. Westgard-type quality-control multi-rules were used to judge assay performance (21). Long-term CVs for 3 serum quality-control pools analyzed in every assay were <4% for 25(OH)D3 (29.2–87.3 nmol/L), <6% for 25(OH)D2 (14.9–62.6 nmol/L), and ≤9% for C3-epi-25(OH)D3 (8.67–42.7 nmol/L) (Supplemental Table 2) (22, 23). Additional method performance information, such as bias relative to international reference materials, is also described in Supplemental Table 2.
Study variables.
We categorized the demographic variables as follows: age group based on standard NHANES reporting protocol (1–5, 6–11, 12–19, 20–39, 40–59, and ≥60 y), sex (male and female), and race-ethnicity [Hispanic (Mexican American + other Hispanic), NHB, and NHW; other racial-ethnic groups were included in overall estimates but estimates were not shown for them separately]. We also reported separate estimates (means and selected percentiles) for Mexican Americans as part of the supplemental tables to allow comparison to previous reports. Race-ethnicity was self-reported by the survey respondent. We examined selected physiologic and lifestyle variables previously shown to be associated with serum total 25(OH)D (6, 24–26), namely the following: inflammation as assessed by using serum C-reactive protein measured by latex-enhanced nephelometry [<10 mg/L (no inflammation) and ≥10 mg/L (inflammation)] (27), BMI (in kg/m2) based on measured height and weight [<18.5 (underweight), 18.5 to <25 (normal-weight), 25 to <30 (overweight), and ≥30 (obese); limited to adults ≥20 y] (28), practice of sun protection behaviors (very likely, somewhat likely, and unlikely; limited to adults 20–59 y) (26), season of blood draw [winter months (November–April) and summer months (May–October)], and latitude [southern (25–34°N) and northern (≥35°N)] where the specimen was collected (6). Last, we examined selected intake variables: any dietary supplement use (yes or no), dietary intake of vitamin D from food (<200 or ≥200 IU/d), supplemental intake of vitamin D (none, <400 IU/d, or ≥400 IU/d), and total vitamin D intake from diet and supplements (<200 or ≥200 IU/d) (for details, see Supplemental Methods).
Statistical methods.
We performed statistical analyses using SAS (version 9.3; SAS Institute) and SUDAAN (version 11.0.0; RTI) software to address the complex survey design and used the examination weights to account for differential nonresponse or noncoverage and to adjust for oversampling of some groups. We combined 2 periods (2007–2008 and 2009–2010) to provide estimates with greater statistical precision and to increase the sample size for multilevel stratifications. We used no exclusion criteria and pairwise deletion for missing values in bivariate analyses. The distributions of total 25(OH)D and 25(OH)D3 were reasonably symmetric, but the 25(OH)D2 and C3-epi-25(OH)D3 and distributions were right-skewed (Supplemental Figure 1). We calculated arithmetic or geometric means and selected percentiles (95% CIs) by demographic characteristics and calculated the contribution of 25(OH)D3 relative to total 25(OH)D and of C3-epi-25(OH)D3 relative to 25(OH)D3. Spearman coefficients were used to assess pairwise correlations among 25(OH)D metabolites. We expressed the prevalence of total 25(OH)D by season, age group, and sex at cutoff values defined by the Institute of Medicine [<30, <40, <50, and >125 nmol/L (29)] or recommended by the Endocrine Society [<75 nmol/L (30)]. Bivariate associations were evaluated between mean total 25(OH)D and selected intake, physiologic, or lifestyle variables; and means were compared across the categories by using the Wald F-test. We used a 2-sided significance level of α = 0.05 to indicate differences for statistical comparisons.
We developed a covariate-adjusted multiple linear regression model for total 25(OH)D using backward elimination. The initial model retained all study variables that had a P value < 0.25 for each of the bivariate associations with 25(OH)D for all the race-ethnicity groups. Variables that were significant (P < 0.05) remained in the model after each elimination step. Other variables were omitted if there was no evidence of data-based confounding (>30% change in the variable estimate). Pairwise interactions with race-ethnicity were explored in the final model, and we retained any significant interactions. To compare the fully adjusted model with the bivariate results, we constructed partially adjusted models for each of the covariates; these included race-ethnicity, a single variable (demographic, intake, lifestyle, or physiologic factor), and the corresponding pairwise interaction. To facilitate the interpretation of the race-ethnicity interactions, we held the level of race-ethnicity constant when reporting the β coefficients.
Results
Overall serum total 25(OH)D and metabolite concentrations and correlations.
Mean total 25(OH)D concentrations for 2007–2008 and 2009–2010 were similar (67.9 and 68.1 nmol/L; P = 0.89). We therefore combined data from both survey periods. The concentration ranges of total 25(OH)D, 25(OH)D3, 25(OH)D2, and C3-epi-25(OH)D3 were 5.41–262, 3.02–260, <LOD (2.05)−183, and <LOD (1.64)−37.8 nmol/L, respectively. We found detectable 25(OH)D3 and C3-epi-25(OH)D3 in 100% and 86% of the population, respectively, but detected 25(OH)D2 in only 19% of the population. We found strong positive and significant Spearman correlations between total 25(OH)D and 25(OH)D3 (r = 0.95, P < 0.0001), between total 25(OH)D and C3-epi-25(OH)D3 (r = 0.75, P < 0.0001), and between 25(OH)D3 and C3-epi-25(OH)D3 (r = 0.77, P < 0.0001). The positive correlation between total 25(OH)D and 25(OH)D2 (r = 0.13, P < 0.0001) was significant but weak. The correlation between 25(OH)D3 and 25(OH)D2 was not significant (P = 0.0542).
Serum total 25(OH)D and metabolite concentrations grouped by demographic variables.
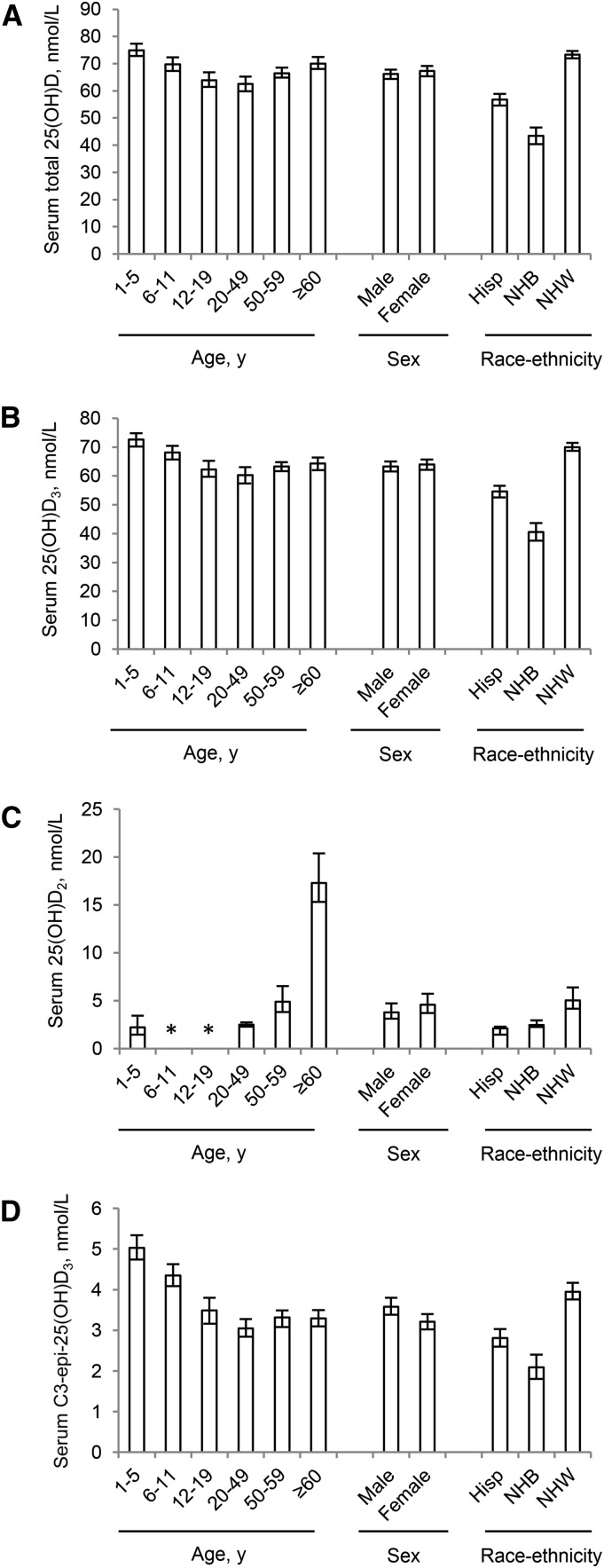
Visual inspection revealed similar demographic patterns of median total 25(OH)D (Figure 1A) and median 25(OH)D3 (Figure 1B), such as a U-shaped age pattern, similar concentrations in males and females, and pronounced race-ethnicity differences. Because 25(OH)D2 concentrations were detectable only in ∼20% of the population, we evaluated demographic patterns at the 90th percentile (Figure 1C). Persons aged ≥60 y showed several-fold higher 25(OH)D2 (17.3 nmol/L) than did younger age groups [<LOD (1.64)−4.88 nmol/L], whereas race-ethnicity differences in 25(OH)D2 were less pronounced (2.10–5.02 nmol/L). The median concentration of C3-epi-25(OH)D3 was small (3.40 nmol/L) compared with total 25(OH)D (66.7 nmol/L) or 25(OH)D3 (63.6 nmol/L). Concentrations of C3-epi-25(OH)D3 showed demographic patterns similar to 25(OH)D and 25(OH)D3, but age group differences were more pronounced (Figure 1D).
FIGURE 1.
Demographic patterns of total 25(OH)D (A), 25(OH)D3 (B), 25(OH)D2 (C), and C3-epi-25(OH)D3 (D) for persons aged ≥1 y: NHANES 2007–2010. Values are medians for total 25(OH)D, 25(OH)D3, and C3-epi-25(OH)D3 and 90th percentiles for 25(OH)D2; error bars denote 95% CIs. *25(OH)D2 concentrations were less than the limit of detection (i.e., <2.05 nmol/L) for participants aged 6–11 and 12–19 y. C3-epi-25(OH)D3, C3-epimer of 25-hydroxycholecalciferol; Hisp, Hispanic; NHB, non-Hispanic black; NHW, non-Hispanic white; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D2, 25-hydroxyergocalciferol; 25(OH)D3, 25-hydroxycholecalciferol.
Serum total 25(OH)D and metabolite concentrations stratified by race-ethnicity.
Overall, we noted a U-shaped age pattern for mean total 25(OH)D, with children (1–5 y) having significantly higher and adolescents (12–19 y) and adults (20–59 y) having significantly lower concentrations than older adults (≥60 y) (Table 1). However, when stratified by race-ethnicity, the U-shaped age pattern was not apparent in NHWs for whom total 25(OH)D declined from childhood to a relatively flat level throughout adulthood. Overall, females had higher total 25(OH)D than did males, but the sex pattern was also different by race-ethnicity: Hispanic females had lower, NHW females had higher, and NHB females had similar total 25(OH)D compared with their male counterparts. All pairwise race-ethnicity comparisons within a category of age group or sex were significant, with NHBs having the lowest, Hispanics having intermediate, and NHWs having the highest total 25(OH)D concentrations.
TABLE 1.
Serum total 25(OH)D stratified by race-ethnicity and grouped by age or sex for persons aged ≥1 y: NHANES 2007–20101
| Total2 |
Hispanic3 |
Non-Hispanic black |
Non-Hispanic white |
|||||
| Group | n | nmol/L | n | nmol/L | n | nmol/L | n | nmol/L |
| All | 15,650 | 68.0 (66.2, 69.8) | 5138 | 57.2 (55.4, 59.0) | 2997 | 46.6 (43.8, 49.3) | 6711 | 75.2 (73.6, 76.8) |
| Age, y | ||||||||
| 1–5 | 1349 | 76.5* (74.4, 78.7) | 550 | 70.6* (68.8, 72.3) | 277 | 63.3* (60.2, 66.3) | 438 | 84.4* (81.1, 87.8) |
| 6–11 | 1855 | 72.2 (69.6, 74.7) | 782 | 63.0* (61.0, 65.1) | 409 | 57.4* (55.1, 59.6) | 558 | 80.3* (76.4, 84.3) |
| 12–19 | 2118 | 65.8* (63.1, 68.4) | 837 | 54.3* (51.8, 56.7) | 479 | 42.3* (39.1, 45.4) | 683 | 76.5 (73.2, 79.8) |
| 20–39 | 3349 | 64.6* (62.2, 66.9) | 1104 | 53.6* (51.2, 56.0) | 602 | 40.6* (37.1, 44.1) | 1433 | 74.4 (72.3, 76.6) |
| 40–59 | 3377 | 67.9* (66.0, 69.9) | 1043 | 56.4* (54.2, 58.7) | 625 | 45.4* (41.6, 49.2) | 1549 | 74.1 (72.1, 76.1) |
| ≥60 | 3602 | 71.0ref (68.9, 73.0) | 822 | 59.2ref (57.3, 61.2) | 605 | 52.0ref (48.6, 55.5) | 2050 | 74.4ref (72.2, 76.6) |
| Sex | ||||||||
| Male | 7802 | 66.8ref (65.0, 68.7) | 2499 | 58.7ref (56.9, 60.5) | 1486 | 47.0ref (44.1, 49.9) | 3411 | 73.0ref (71.2, 74.9) |
| Female | 7848 | 69.1* (67.3, 71.0) | 2639 | 55.6* (53.6, 57.6) | 1511 | 46.2 (43.4, 49.0) | 3300 | 77.3* (75.5, 79.2) |
Values are arithmetic means (95% CIs) unless otherwise indicated. All pairwise race-ethnicity comparisons within a category of age or sex differ, P < 0.0001 (t test). *Different from reference group for age or sex comparison within race-ethnicity subgroup, P < 0.05 (Wald F-test). ref, reference group for age or sex comparison within race-ethnicity subgroup; 25(OH)D, 25-hydroxyvitamin D.
Estimates for “other” race-ethnicity group (persons with multiethnic background) are not shown but are included in total estimates.
The Hispanic subgroup represents the sum of Mexican-American and other Hispanic ethnicities.
The central 95% reference intervals (2.5th–97.5th percentile) for total 25(OH)D and individual metabolites showed distinct race-ethnicity differences (Table 2). NHBs had the lowest, Hispanics had intermediate, and NHWs had the highest reference intervals for total 25(OH)D, 25(OH)D3, and C3-epi-25(OH)D3. Hispanics had the lowest reference interval for 25(OH)D2, whereas NHBs and NHWs had similar reference intervals. Detailed sets of percentiles categorized by age group and sex and stratified by race-ethnicity are available for total 25(OH)D, 25(OH)D3, 25(OH)D2, and C3-epi-25(OH)D3 (Supplemental Tables 3–6, respectively).
TABLE 2.
Central 95% reference intervals for serum concentrations of total 25(OH)D and metabolites stratified by race-ethnicity for persons aged ≥1 y: NHANES 2007–20101
| Total,2nmol/L |
Hispanic,3nmol/L |
Non-Hispanic black, nmol/L |
Non-Hispanic white, nmol/L |
|||||
| Analyte | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th |
| Total 25(OH)D | 23.5 (21.8, 25.1) | 124 (120, 128) | 24.2 (21.8, 25.6) | 95.1 (93.5, 98.0) | 15.4 (13.5, 17.0) | 94.6 (90.1, 99.8) | 30.6 (28.7, 32.3) | 130 (126, 135) |
| 25(OH)D3 | 20.7 (19.4, 22.1) | 120 (117, 124) | 22.3 (19.8, 24.0) | 92.9 (91.3, 96.2) | 13.6 (11.5, 14.9) | 89.6 (86.3, 94.2) | 26.2 (24.8, 27.6) | 126 (123, 131) |
| 25(OH)D2 | <LOD | 21.0 (19.0, 24.2) | <LOD | 9.15 (7.55, 12.4) | <LOD | 20.4 (16.7, 22.9) | <LOD | 22.6 (20.2, 27.6) |
| C3-epi-25(OH)D3 | <LOD | 10.7 (10.2, 11.7) | <LOD | 7.36 (6.91, 7.98) | <LOD | 6.45 (5.59, 7.83) | <LOD | 11.9 (10.8, 13.1) |
Values are percentiles (95% CIs); for sample sizes, see Supplemental Tables 3–6. LOD [2.05 nmol/L for 25(OH)D2 and 1.64 nmol/L for C3-epi-25(OH)D3]; total 25(OH)D, total 25-hydroxyvitamin D [sum of 25(OH)D3 and 25(OH)D2]. C3-epi-25(OH)D3, C3-epimer of 25-hydroxycholecalciferol; LOD, limit of detection; 25(OH)D2, 25-hydroxyergocalciferol; 25(OH)D3, 25-hydroxycholecalciferol.
Estimates for “other” race-ethnicity group (persons with multiethnic background) are not shown but are included in total estimates.
The Hispanic subgroup represents the sum of Mexican-American and other Hispanic ethnicities.
Contribution of metabolites to serum total 25(OH)D concentrations.
Serum 25(OH)D3 made up ∼95% of total 25(OH)D. Mean C3-epi-25(OH)D3 increased significantly in each quartile of 25(OH)D3, overall and by age group (1–19 y, ≥20 y) (Supplemental Table 7). The relative amount of C3-epi-25(OH)D3 compared with 25(OH)D3 was ∼6–7%, with a significantly higher proportion in the highest quartile than in the other 3 quartiles. Whereas C3-epi-25(OH)D3 was detected in the majority of the population, the detection frequency varied with age and race-ethnicity (Table 3). Children aged 1–5 y and 6–11 y showed the highest detection frequency (98% and 96%, respectively) compared with older age groups (83–88%). Among race-ethnicity groups, NHWs (91%) showed the highest and NHBs (64%) showed the lowest detection frequency. Regardless of the demographic subgroup, vitamin D supplement users showed higher detection frequencies than did nonusers. We detected 25(OH)D2 in <20% of the population overall, but the detection frequency varied with age (35% in persons ≥60 y, 21% in 20–39 y olds, and <15% in younger persons), race-ethnicity (22% in NHWs compared with 10–12% in Hispanics and NHBs), and supplement use (34% in users compared with 10% in nonusers) (Table 3).
TABLE 3.
Prevalence of detectable serum C3-epi-25(OH)D3 and serum 25(OH)D2 concentrations stratified by vitamin D–containing dietary supplement use and grouped by age, sex, or race-ethnicity for persons aged ≥1 y: NHANES 2007–20101
| C3-epi-25(OH)D3, % |
25(OH)D2, % |
|||||
| Vitamin D supplements |
Vitamin D supplements |
|||||
| Group | Overall | Nonusers | Users | Overall | Nonusers | Users |
| All | 85.6* (82.8, 88.1) | 82.7 (79.3, 85.7) | 91.1 (89.1, 92.7) | 18.9* (16.8, 21.1) | 10.5 (8.7, 12.6) | 34.4 (31.5, 37.4) |
| Age, y | ||||||
| 1–5 | 98.1* (96.6, 98.9) | 97.4 (95.5, 98.6) | 99.4 (97.8, 99.8) | 11.0* (8.7, 13.7) | 8.3 (6.3, 10.9) | 16.4 (11.8, 22.4) |
| 6–11 | 96.3* (94.5, 97.5) | 95.2 (93.0, 96.8) | 98.6 (96.9, 99.4) | 9.9* (7.3, 13.4) | 6.9 (4.1, 11.3) | 16.2 (12.5, 20.7) |
| 12–19 | 87.8* (84.3, 90.6) | 86.4 (82.5, 89.6) | 93.7 (90.0, 96.1) | 8.2* (5.5, 11.9) | 6.2 (4.0, 9.4) | 16.8 (11.1, 24.6) |
| 20–39 | 82.7* (78.8, 86.0) | 80.6 (76.2, 84.4) | 88.4 (84.1, 91.6) | 13.6* (11.9, 15.6) | 8.4 (6.7, 10.4) | 27.8 (23.9, 32.0) |
| 40–59 | 84.7* (81.5, 87.4) | 81.1 (77.2, 84.5) | 90.4 (87.5, 92.6) | 21.1* (17.9, 24.6) | 12.1 (9.6, 15.3) | 35.3 (31.0, 39.9) |
| ≥60 | 83.0* (80.1, 85.8) | 75.4 (70.4, 79.7) | 90.3 (88.1, 92.1) | 34.5* (31.4, 37.8) | 18.6 (16.2, 21.3) | 49.0 (44.7, 53.2) |
| Sex | ||||||
| Male | 88.6* (85.6, 91.0) | 87.0 (83.4, 89.9) | 92.2 (90.0, 94.0) | 18.2* (15.7, 20.9) | 10.0 (8.1, 12.3) | 36.9 (32.5, 41.5) |
| Female | 82.7* (79.8, 85.4) | 78.0 (74.3, 81.2) | 90.2 (88.0, 92.0) | 19.5* (17.5, 21.7) | 11.0 (9.1, 13.2) | 32.6 (29.6, 35.6) |
| Race-ethnicity | ||||||
| Hispanic2 | 82.4* (77.8, 86.2) | 81.0 (76.3, 85.0) | 87.8 (82.7, 91.5) | 10.2* (9.2, 11.4) | 6.4 (5.3, 7.6) | 25.1 (21.9, 28.6) |
| Non-Hispanic black | 64.2* (56.0, 71.7) | 61.7 (53.1, 69.6) | 72.4 (63.2, 80.0) | 12.2* (11.1, 13.5) | 7.5 (5.9, 9.6) | 27.4 (23.4, 31.8) |
| Non-Hispanic white | 91.0* (89.5, 92.2) | 89.1 (87.3, 90.7) | 93.7 (92.3, 94.9) | 21.7* (19.1, 24.6) | 12.0 (9.5, 15.0) | 36.1 (32.8, 39.5) |
Estimates are weighted percentages (95% CIs). *Supplement users and nonusers differ, P < 0.05 (Wald F-test). C3-epi-25(OH)D3, C3-epimer of 25-hydroxycholecalciferol; 25(OH)D2, 25-hydroxyergocalciferol.
The Hispanic subgroup represents the sum of Mexican-American and other Hispanic ethnicities.
Prevalence of serum total 25(OH)D at selected cutoff values stratified by race-ethnicity.
When we applied the selected cutoff values, we found that overall, 5.9%, 13%, 24%, and 64% of the population had total 25(OH)D concentrations <30, <40, <50, and <75 nmol/L, respectively, whereas 2.3% had concentrations >125 nmol/L (Table 4). However, we observed a seasonal difference with prevalence estimates for low total 25(OH)D being higher during the winter months (November–April) than during the summer months (May–October). Twenty-four percent of NHBs (30% in winter compared with 17% in summer months) compared with only 6.4% of Hispanics (7.5% in winter compared with 4.4% in summer months) and 2.3% of NHWs (3.8% in winter compared with 1.6% in summer months) had total 25(OH)D concentrations <30 nmol/L. On the other hand, 3.4% of NHWs compared with <1% of Hispanics and <1% of NHBs had total 25(OH)D concentrations >125 nmol/L, with little difference between winter and summer months.
TABLE 4.
Prevalence of serum total 25(OH)D concentrations at various cutoff values stratified by race-ethnicity and grouped by season, age, or sex for persons aged ≥1 y: NHANES 2007–20101
| Cutoff value and group | Total2 | Hispanic3 | Non-Hispanic black | Non-Hispanic white |
| Less than 30 nmol/L | ||||
| All | 5.9 (4.9, 7.1) | 6.4 (5.1, 8.2) | 24 (19, 29) | 2.3 (1.7, 2.9) |
| Winter months4 | 9.9 (8.3, 12) | 7.5 (5.6, 10) | 30 (24, 36) | 3.8 (2.7, 5.4) |
| Summer months5 | 3.3 (2.8, 3.9) | 4.4 (3.2, 6.1) | 17 (12, 23) | 1.6 (1.2, 2.2) |
| Age, y | ||||
| 1–11 | 0.7 (0.5, 1.1) | 0.8 (0.4, 1.6)6 | 3.2 (2.2, 4.7) | 0.0 (0.0, 0.3)7 |
| 12–19 | 5.9 (4.4, 7.7) | 6.7 (4.2, 10) | 25 (19, 32) | 1.0 (0.5, 1.9)6 |
| 20–39 | 8.2 (6.6, 10) | 9.7 (7.3, 13) | 32 (26, 40) | 1.7 (1.0, 2.9) |
| 40–59 | 5.9 (4.7, 7.6) | 6.3 (4.4, 8.9) | 26 (19, 34) | 2.5 (1.8, 3.6) |
| ≥60 | 5.7 (4.6, 7.1) | 5.6 (4.1, 7.6) | 22 (17, 28) | 3.9 (2.8, 5.5) |
| Sex | ||||
| Male | 4.9 (3.9, 6.2) | 4.6 (3.4, 6.1) | 21 (16, 27) | 1.7 (1.1, 2.4) |
| Female | 6.9 (5.7, 8.2) | 8.4 (6.5, 11) | 26 (21, 31) | 2.8 (2.2, 3.6) |
| Less than 40 nmol/L | ||||
| All | 13 (11, 15) | 18 (15, 22) | 43 (38, 49) | 5.8 (4.9, 7.0) |
| Winter months4 | 20 (18, 23) | 21 (18, 25) | 49 (44, 55) | 9.3 (7.1, 12) |
| Summer months5 | 8.6 (7.5, 9.8) | 13 (9.5, 18) | 36 (29, 43) | 4.4 (3.7, 5.3) |
| Age, y | ||||
| 1–11 | 3.0 (2.2, 4.1) | 3.2 (2.2, 4.6) | 12 (8.2, 16) | 0.5 (0.2, 1.6)7 |
| 12–19 | 14 (11, 17) | 20 (15, 27) | 47 (39, 56) | 2.8 (1.6, 4.7) |
| 20–39 | 18 (15, 20) | 24 (20, 29) | 56 (48, 63) | 5.9 (4.4, 8.0) |
| 40–59 | 13 (11, 16) | 19 (16, 23) | 47 (40, 55) | 5.9 (4.5, 7.9) |
| ≥60 | 13 (11, 15) | 19 (16, 24) | 39 (33, 45) | 9.1 (7.5, 11) |
| Sex | ||||
| Male | 11 (9.4, 14) | 14 (12, 17) | 41 (34, 48) | 4.5 (3.4, 5.9) |
| Female | 15 (13, 17) | 23 (19, 27) | 45 (40, 50) | 7.2 (5.8, 8.8) |
| Less than 50 nmol/L | ||||
| All | 24 (21, 27) | 36 (32, 40) | 62 (56, 67) | 13 (12, 15) |
| Winter months4 | 35 (31, 38) | 40 (36, 44) | 68 (64, 71) | 19 (17, 23) |
| Summer months5 | 17 (15, 19) | 29 (23, 36) | 55 (46, 63) | 11 (9.2, 13) |
| Age, y | ||||
| 1–11 | 9.7 (7.8, 12) | 13 (10, 16) | 29 (24, 34) | 2.8 (1.8, 4.3) |
| 12–19 | 25 (21, 30) | 41 (34, 47) | 70 (63, 76) | 7.7 (5.4, 11) |
| 20–39 | 30 (26, 34) | 45 (39, 51) | 75 (66, 82) | 14 (12, 18) |
| 40–59 | 24 (22, 28) | 38 (33, 42) | 65 (57, 72) | 15 (12, 18) |
| ≥60 | 22 (20, 24) | 35 (31, 40) | 53 (47, 59) | 17 (15, 19) |
| Sex | ||||
| Male | 22 (19, 25) | 31 (27, 36) | 60 (53, 66) | 12 (9.9, 14) |
| Female | 26 (23, 29) | 41 (36, 45) | 64 (58, 69) | 15 (13, 17) |
| Less than 75 nmol/L | ||||
| All | 64 (61, 66) | 83 (81, 86) | 91 (89, 93) | 53 (50, 55) |
| Winter months4 | 75 (72, 78) | 86 (83, 88) | 93 (91, 95) | 61 (58, 65) |
| Summer months5 | 57 (54, 59) | 79 (75, 83) | 88 (85, 91) | 49 (46, 52) |
| Age, y | ||||
| 1–11 | 56 (52, 61) | 73 (69, 77) | 83 (77, 87) | 40 (32, 47) |
| 12–19 | 70 (66, 73) | 89 (85, 92) | 97 (94, 99) | 54 (48, 60) |
| 20–39 | 69 (66, 73) | 87 (84, 90) | 96 (93, 97) | 56 (52, 60) |
| 40–59 | 64 (61, 66) | 84 (81, 87) | 92 (87, 94) | 54 (52, 57) |
| ≥60 | 56 (53, 60) | 79 (76, 82) | 82 (78, 86) | 51 (47, 55) |
| Sex | ||||
| Male | 66 (63, 69) | 82 (79, 85) | 92 (89, 94) | 57 (54, 60) |
| Female | 61 (59, 64) | 84 (82, 87) | 90 (88, 92) | 49 (46, 51) |
| Greater than 125 nmol/L | ||||
| All | 2.3 (1.9, 2.9) | 0.1 (0.1, 0.3)6 | 0.4 (0.2, 0.7)6 | 3.4 (2.7, 4.2) |
| Winter months4 | 1.5 (1.0, 2.4) | <17 | 0.4 (0.2, 0.9)6 | 2.9 (1.8, 4.7) |
| Summer months5 | 2.9 (2.2, 3.7) | <17 | <17 | 3.5 (2.7, 4.6) |
| Age, y | ||||
| 1–11 | 1.2 (0.6, 2.7)6 | <17 | <17 | 2.3 (1.1, 5.0)6 |
| 12–19 | 2.3 (1.4, 3.8) | <17 | <17 | 3.8 (2.3, 6.2) |
| 20–39 | 2.9 (2.1, 4.0) | <17 | <17 | 4.6 (3.2, 6.6) |
| 40–59 | 2.1 (1.4, 3.1) | <17 | <17 | 2.7 (1.9, 4.0) |
| ≥60 | 2.6 (1.7, 4.0) | <17 | 2.1 (1.1, 4.0)6 | 3.0 (2.0, 4.6) |
| Sex | ||||
| Male | 1.0 (0.6, 1.7) | <17 | <17 | 1.4 (0.8, 2.5) |
| Female | 3.6 (3.0, 4.4) | 0.2 (0.1, 0.4)6 | 0.6 (0.3, 1.2)6 | 5.2 (4.3, 6.4) |
Estimates are weighted percentages (95% CIs). The Institute of Medicine Dietary Reference Intake Report defines cutoff values for 25(OH)D as follows: <30 nmol/L, at risk for deficiency; 30 to <50 nmol/L, at risk for inadequacy; ≥50, sufficient; >125 nmol/L, may be reason for concern; and 40 nmol/L, targeted level for median dietary requirements (29). The Endocrine Society recommends concentrations of at least 75 nmol/L (30). 25(OH)D, 25-hydroxyvitamin D.
Estimates for “other” race-ethnicity group (persons with multiethnic background) are not shown but are included in total estimates.
The Hispanic subgroup represents sum of Mexican-American and other Hispanic ethnicities.
Participants sampled from 1 November through 30 April.
Participants sampled from 1 May through 31 October.
30% ≤ relative SE < 40%.
Estimate suppressed, relative SE ≥40%.
Associations between serum total 25(OH)D and selected intake, physiologic, or lifestyle variables stratified by race-ethnicity.
Persons with higher intakes of vitamin D (diet, supplements, or both) and those who reported consuming any supplements had significantly higher total 25(OH)D (Table 5) and C3-epi-25(OH)D3 (Supplemental Table 8), overall and within each race-ethnicity subgroup. All pairwise race-ethnicity comparisons within a category of intake were significant. We observed inverse associations between total 25(OH)D or C3-epi-25(OH)D3 and inflammation as measured by elevated serum C-reactive protein concentrations and between total 25(OH)D or C3-epi-25(OH)D3 and BMI, both overall and in each race-ethnicity subgroup. Persons who were examined during May–October had significantly higher total 25(OH)D or C3-epi-25(OH)D3 than did those examined during November–April, both overall and in each race-ethnicity subgroup. We did not find significant differences in total 25(OH)D when participants were grouped by latitude (except for NHWs) or by practice of sun protection behaviors (except for Hispanics) [not tested for C3-epi-25(OH)D3]. All pairwise race-ethnicity comparisons within a physiologic or lifestyle variable category were significant.
TABLE 5.
Concentrations of serum total 25(OH)D stratified by race-ethnicity and grouped by selected intake, physiologic, or lifestyle variables for persons aged ≥1 y: NHANES 2007–20101
| Total2 |
Hispanic3 |
Non-Hispanic black |
Non-Hispanic white |
|||||
| Variable | n | nmol/L | n | nmol/L | n | nmol/L | n | nmol/L |
| Dietary intake,4 IU/d | ||||||||
| <200 | 9304 | 66.2 (64.4, 68.0) | 2903 | 54.9 (52.7, 57.1) | 2008 | 44.3 (41.6, 47.0) | 3929 | 74.0 (72.6, 75.5) |
| ≥200 | 5723 | 71.4 (69.6, 73.2) | 2016 | 60.6 (59.1, 62.2) | 877 | 52.2 (48.8, 55.6) | 2551 | 77.3 (75.5, 79.2) |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Supplemental intake, IU/d | ||||||||
| 0 | 10,926 | 62.5 (60.4, 64.5) | 4001 | 55.2 (53.3, 57.0) | 2305 | 42.9 (40.0, 45.8) | 4078 | 70.1 (68.1, 72.1) |
| <400 | 1889 | 72.0 (70.2, 73.7) | 534 | 61.6 (59.9, 63.2) | 288 | 51.2 (47.6, 54.8) | 954 | 77.0 (75.0, 79.1) |
| ≥400 | 2726 | 82.0 (80.2, 83.7) | 559 | 67.7 (65.1, 70.3) | 382 | 63.3 (59.8, 66.7) | 1644 | 85.7 (84.1, 87.4) |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Total intake,5 IU/d | ||||||||
| <200 | 7001 | 60.8 (58.7, 63.0) | 2418 | 53.1 (50.9, 55.2) | 1629 | 40.9 (37.9, 43.9) | 2608 | 68.9 (66.9, 71.0) |
| ≥200 | 8026 | 73.9 (72.3, 75.5) | 2501 | 61.5 (59.8, 63.2) | 1256 | 54.1 (51.2, 57.1) | 3872 | 79.6 (78.2, 81.1) |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Any supplement use | ||||||||
| No | 9413 | 62.1 (59.8, 64.3) | 3549 | 55.2 (53.3, 57.1) | 2042 | 42.8 (39.8, 45.9) | 3366 | 70.1 (67.8, 72.3) |
| Yes | 6228 | 75.1 (73.5, 76.6) | 1586 | 61.8 (60.0, 63.7) | 954 | 54.0 (51.4, 56.7) | 3343 | 80.1 (78.6, 81.6) |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Infection/ inflammation6 | ||||||||
| CRP <10 mg/L | 12,978 | 68.2 (66.4, 69.9) | 4134 | 56.7 (54.8, 58.6) | 2376 | 46.1 (43.3, 48.9) | 5767 | 75.3 (73.8, 76.9) |
| CRP ≥10 mg/L | 1190 | 62.4 (59.1, 65.6) | 392 | 52.4 (50.2, 54.7) | 278 | 41.6 (37.1, 46.2) | 494 | 70.6 (66.5, 74.7) |
| P | <0.0001 | 0.0001 | 0.0184 | 0.0153 | ||||
| BMI7,8 | ||||||||
| Underweight | 163 | 72.2 (66.0, 78.5) | 15 | —9 | 39 | 50.3 (28.8, 71.8) | 92 | 79.8 (72.6, 87.1) |
| Normal-weight | 2711 | 73.6 (71.4, 75.8) | 611 | 59.3 (56.5, 62.1) | 416 | 46.4 (43.7, 49.0) | 1465 | 81.2 (79.4, 83.0) |
| Overweight | 3502 | 69.2 (67.3, 71.0) | 1143 | 56.6 (54.7, 58.5) | 525 | 46.4 (42.8, 50.1) | 1687 | 75.7 (73.7, 77.6) |
| Obese | 3831 | 60.6 (58.7, 62.5) | 1173 | 51.8 (49.8, 53.7) | 832 | 42.5 (39.4, 45.7) | 1718 | 66.8 (65.0, 68.6) |
| P | <0.0001 | <0.0001 | 0.0006 | <0.0001 | ||||
| Sun protection behavior10 | ||||||||
| Very likely | 3153 | 65.6 (63.5, 67.7) | 1024 | 53.9 (51.7, 56.1) | 567 | 42.7 (39.2, 46.2) | 1362 | 73.8 (71.8, 75.8) |
| Somewhat likely | 2120 | 67.6 (65.4, 69.7) | 528 | 57.5 (55.0, 60.0) | 424 | 43.2 (39.7, 46.7) | 1054 | 74.2 (72.2, 76.1) |
| Unlikely | 1135 | 68.5 (65.6, 71.3) | 355 | 56.7 (53.6, 59.8) | 196 | 44.0 (40.1, 48.0) | 542 | 75.8 (72.8, 78.9) |
| P | 0.22 | 0.011 | 0.68 | 0.43 | ||||
| Season | ||||||||
| Winter months11 | 7261 | 60.6 (58.5, 62.7) | 3371 | 55.4 (53.5, 57.3) | 1597 | 43.3 (41.0, 45.6) | 1865 | 70.1 (67.8, 72.5) |
| Summer months12 | 8389 | 72.8 (71.2, 74.3) | 1767 | 60.5 (58.0, 62.9) | 1400 | 50.5 (46.6, 54.3) | 4846 | 77.3 (75.4, 79.2) |
| P | <0.0001 | 0.0016 | 0.0007 | <0.0001 | ||||
| Latitude | ||||||||
| Southern13 | 5049 | 60.5 (57.5, 63.5) | 2905 | 56.3 (53.9, 58.7) | 946 | 45.3 (41.2, 49.4) | 981 | 70.4 (66.7, 74.2) |
| Northern14 | 10,600 | 70.5 (68.7, 72.3) | 2232 | 58.4 (56.1, 60.7) | 2051 | 47.2 (43.5, 50.9) | 5730 | 76.1 (74.5, 77.8) |
| P | 0.59 | 0.20 | 0.51 | 0.008 | ||||
Values are arithmetic means (95% CIs). P values are based on the overall Wald F-test, which tests whether at least 1 of the means among the categories is significantly different; all pairwise comparisons across race-ethnic groups within the same category of each variable were significant at P < 0.0001. CRP, C-reactive protein; 25(OH)D, 25-hydroxyvitamin D.
Estimates for “other” race-ethnicity group (persons with multiethnic background) are not shown but are included in total estimates.
The Hispanic subgroup represents the sum of Mexican-American and other Hispanic ethnicities.
1 μg cholecalciferol = 40 IUs.
Total intake represents the sum of dietary and supplemental vitamin D intake.
CRP concentrations in serum were used to assess inflammation status (<10 mg/L, no inflammation; ≥10 mg/L, inflammation).
BMI definitions (in kg/m2): <18.5 (underweight), 18.5 to <25 (normal-weight), 25 to <30 (overweight), and ≥30 (obese).
Limited to adults aged ≥20 y.
Estimate suppressed because considered statistically unreliable (n < 30).
Limited to adults aged 20–59 y.
Participants sampled from 1 November through 30 April.
Participants sampled from 1 May through 31 October.
Participants sampled at latitudes between 25°N and 34°N.
Participants sampled at latitudes ≥35°N.
Covariate-adjusted serum total 25(OH)D concentrations.
We developed a regression model for total 25(OH)D and examined pairwise interactions with race-ethnicity to assess the necessity of providing race-ethnicity–specific estimates and to assess the robustness of any conclusions drawn from the stratified results presented in Tables 1 and 5. The final fully adjusted regression model included age, sex, race-ethnicity, total vitamin D intake (from food and supplements), any supplement use, season, and BMI as well as significant interactions between race-ethnicity and age, sex, total vitamin D intake, or BMI (Table 6). When we compared this model with partially adjusted models that corresponded to stratified bivariate results, we noted that both models arrived at similar conclusions for sex, total vitamin D intake, any supplement use, season, and BMI, although inflammation was no longer significantly associated with total 25(OH)D. However, the impact of the association of age with total 25(OH)D within each race-ethnicity group was different after simultaneously controlling for total vitamin D intake (dietary and supplements at 200 IU), any supplement use (no use), season (May–October), and BMI (25 kg/m2) (Table 6, Supplemental Figure 2). We found no significant interaction with race-ethnicity for the effects of any supplement use or season in the fully adjusted model, suggesting that these effects were not modified by race-ethnicity, although the effect of any supplement use was attenuated by at least 50% after controlling for the other variables.
TABLE 6.
Race-ethnicity–specific β coefficients from covariate-adjusted multiple linear regression models for serum total 25(OH)D grouped by selected demographic, intake, physiologic, or lifestyle variables for persons aged ≥1 y: NHANES 2007–20101
| Partially adjusted model |
Fully adjusted model |
|||||
| Hispanic2 | Non-Hispanic black | Non-Hispanic white | Hispanic2 | Non-Hispanic black | Non-Hispanic white | |
| Age, y | ||||||
| 1–19 | 1.77 (–0.39, 3.94) | −0.69 (−4.07, 2.69) | 4.66* (0.65, 8.67) | −4.41* (−6.9, −1.92) | −3.28* (−6.33, −0.23) | 0.44 (−2.94, 3.82) |
| 20–59 | −4.53* (−6.51, −2.54) | −9.09* (−12.2, −6.04) | −0.18 (−2.10, 1.75) | −4.65* (−6.37, −2.94) | −7.18* (−9.85, −4.51) | 2.68* (0.88, 4.48) |
| ≥60 | Ref | Ref | Ref | Ref | Ref | Ref |
| Sex | ||||||
| Male | 3.10* (1.75, 4.46) | 0.81 (−0.64, 2.27) | −4.28* (−6.16, −2.41) | 3.19* (1.93, 4.44) | 0.05 (−1.28, 1.39) | −3.34* (−4.91, −1.78) |
| Female | Ref | Ref | Ref | Ref | Ref | Ref |
| Log10 total intake3 | 14.2* (12.1, 16.3) | 20.9* (17.7, 24.0) | 17.0* (15.2, 18.9) | 10.5* (8.31, 12.7) | 17.7* (14.4, 20.9) | 14.9* (13.0, 16.8) |
| Any supplement use | ||||||
| No | −6.62* (−8.22, −5.01) | −11.2* (−13.8, −8.55) | −10.1* (−12.1, −8) | −3.59* (−5.04, −2.15) | −3.59* (−5.04, −2.15) | −3.59* (−5.04, −2.15) |
| Yes | Ref | Ref | Ref | Ref | Ref | Ref |
| Season | ||||||
| Winter months4 | −5.04* (−8.03, −2.06) | −7.2* (−11.1, −3.27) | −7.19* (−10.3, −4.09) | −6.91* (−9.26, −4.56) | −6.91* (−9.26, −4.56) | −6.91* (−9.26, −4.56) |
| Summer months5 | Ref | Ref | Ref | Ref | Ref | Ref |
| Log10 BMI | −41.6* (−45.5, −37.8) | −37.6* (−43.7, −31.5) | −53.8* (−62.9, −44.6) | −38.7* (−43.4, −33.9) | −30.6* (−38.7, −22.5) | −54.6* (−62.1, −47.1) |
| Inflammation6 | ||||||
| CRP <10 mg/L | 4.27* (2.28, 6.27) | 4.47* (0.80, 8.13) | 4.72* (0.97, 8.48) | NS | NS | NS |
| CRP ≥10 mg/L | Ref | Ref | Ref | Ref | Ref | Ref |
Values are β coefficients (95% CIs). Each partially adjusted model includes race-ethnicity, a single variable (demographic, intake, lifestyle, or physiologic factor), and the corresponding pairwise interaction (race-ethnicity × age, race-ethnicity × sex, etc.); all interactions with race-ethnicity in the partially adjusted model were significant (P < 0.05, Satterthwaite F-test), except for inflammation (race-ethnicity adjusted β = 4.6) and season (race-ethnicity adjusted β = −6.81). The fully adjusted model was derived from backward elimination; variables in Tables 1 and 5 with a P value <0.25 for all race-ethnicity groups were included and all significant pairwise interactions with race-ethnicity were retained; persons who reported to be of other or multiple race-ethnicity groups were omitted from this analysis. To facilitate the interpretation of the race-ethnicity interactions, we held the level of race-ethnicity constant when reporting the β coefficients. *Significant β coefficient, P < 0.05 (Satterthwaite F-test). CRP, C-reactive protein; Ref, reference group; 25(OH)D, 25-hydroxyvitamin D.
The Hispanic subgroup represents the sum of Mexican-American and other Hispanic ethnicities.
Total intake represents the sum of dietary and supplemental vitamin D intake.
Participants sampled from 1 November through 30 April.
Participants sampled from 1 May through 31 October.
CRP concentrations in serum were used to assess inflammation status (<10 mg/L, no inflammation; ≥10 mg/L, inflammation).
Discussion
To our knowledge, this article presents the first and most current US national data for total 25(OH)D obtained by a standardized LC-MS/MS method. Previous reports from NHANES relied on data from an RIA and focused on earlier time periods. To our knowledge, the present study also provides the first opportunity to examine 25(OH)D metabolites, including C3-epi-25(OH)D3, in a representative sample of the US population aged ≥1 y. Although the interest in this epimer has grown considerably, its biological significance is not yet clear. Some data suggest that the activated form, C3-epi-1,25-dihydroxyvitamin D3, has less effect on vitamin D–related gene activation and calcemic activity than 1,25-dihydroxyvitamin D3 (31). Other data suggest that the C3-epimer form potently suppresses parathyroid hormone secretion (32) and inhibits keratinocyte growth (31).
Previous information on individual metabolites of 25(OH)D came from studies that used convenience samples and were mostly small in size. However, 2 large cross-sectional population studies, the US Atherosclerosis Risk in Communities Study [ARIC; n = 13,108 (33)] and the Irish NANS [n = 1122 (34, 35)], recently published vitamin D metabolite data. We found a strong correlation between 25(OH)D3 and C3-epi-25(OH)D3 consistent with previous findings (34, 36, 37), although some studies, including ARIC, showed weaker (33, 38, 39) or no correlation (40). The metabolites 25(OH)D3 and C3-epi-25(OH)D3 showed similar demographic patterns compared with total 25(OH)D. Concentrations of C3-epi-25(OH)D3 were detected in 86% of the population, but its detection frequency and concentrations were higher in children than in older age groups. With the exception of ARIC (33), other studies reported that the majority of adults and children showed detectable C3-epi-25(OH)D3 (31, 41–43) and concentrations were usually higher in children than in adults (31, 44, 45). Similar to the Irish NANS (34), we found that the C3-epi-25(OH)D3 proportion was ∼6% of 25(OH)D3.
We found the same significant associations between C3-epi-25(OH)D3 or total 25(OH)D and intake or physiologic variables overall and within each race-ethnicity group—namely, positive associations with vitamin D intake, inverse associations with inflammation and BMI, and higher concentrations for persons examined during May–October. Cashman et al. (34) in the NANS also reported similar variables to be predictive of C3-epi-25(OH)D3. Cholecalciferol from foods, supplements, and endogenous production in the skin are likely the common origins of 25(OH)D3 and C3-epi-25(OH)D3. Dose-response studies showed that C3-epi-25(OH)D3 increased with dietary or supplemental cholecalciferol (46, 47). Skin cells showed the C3-epimerization pathway (48), and the skin of mice fed diets containing cholecalciferol contained up to 20% of 25(OH)D3 as the C3-epimer (49). To date, little to no C3-epimer has been found in vitamin D supplements (36, 39, 45), and C3-epimers of cholecalciferol or its metabolites have not been reported in foods.
If C3-epi-25(OH)D3 is captured as part of 25(OH)D3, participants could be misclassified as not being deficient. The misclassification bias was reported to be small (<5%) in adults (33, 34, 50). We also found a small misclassification bias at total 25(OH)D concentrations <30 nmol/L [prevalence of 5.0% (included) compared with 5.9% (not included)] and <40 nmol/L [prevalence of 12.0% (included) compared with 13% (not included)] (data not shown). In our experience, C3-epi-25(OH)D3 more often than the other metabolites shows mass spectrometric interference and is measured with less precision. For the most accurate assessment, this metabolite should be chromatographically separated from 25(OH)D3.
We detected 25(OH)D2 in <20% of the population, but the detection frequency varied with age and supplement use. In fact, 49% of persons aged ≥60 y who reported taking vitamin D supplements had detectable 25(OH)D2 compared with only 6–8% of children and young adults who were non–supplement users. Approximately one-fifth of adults aged ≥20 y (22%) with detectable 25(OH)D2 reported taking vitamin D supplements at an average dose of 517 IU/d (median: 344 IU/d) (data not shown). This dose was higher than the average dose of supplements in participants with 25(OH)D2 less than the LOD (220 IU/d; median: 0 IU/d; P = 0.0013), suggesting that the presence of 25(OH)D2 was likely a result of high-dose prescription ergocalciferol. Furthermore, 25% of those with high total 25(OH)D (>125 nmol/L) had detectable 25(OH)D2, suggesting supplementation with ergocalciferol (data not shown). However, we could not ascertain whether participants were using ergocalciferol because the NHANES dietary supplements files do not distinguish between cholecalciferol and ergocalciferol. Cashman et al. (34) reported age and vitamin D supplement use to be positive predictors of 25(OH)D2. The 90th percentile of 25(OH)D2 for Irish adults aged ≥18 y was 6.36 nmol/L (35). However, due to the lower LOD (0.44 nmol/L) compared with NHANES (2.05 nmol/L), 99% of Irish survey participants had detectable 25(OH)D2 (34).
Older data from surveys that used immunoassays showed a positive relation of total 25(OH)D with sun exposure (3, 4, 51–54) and vitamin D intake from diet (52) or supplement use (3, 26, 51, 52) and a negative relation with BMI (3, 26, 52, 53) and inflammation (26). Our new LC-MS/MS data on total 25(OH)D showed similar associations. We confirmed that age, sex, race-ethnicity, total vitamin intake, any supplement use, season, and BMI were determinants of vitamin D status before and after covariate adjustment, whereas inflammation was no longer a determinant after controlling for the other covariates examined in the present study. This is likely due to the known correlation between BMI and inflammation (55). The relation between latitude and total 25(OH)D is confounded by the NHANES seasonal design of sampling the lower latitudes during the winter months and the higher latitudes during the summer months (6). With the use of the results from the bivariate analyses for season or latitude stratified by race-ethnicity, we concluded that the season variable (which includes the latitude imbalance and is provided in the publicly released data) was a better predictor of 25(OH)D across race-ethnicity groups than the latitude variable (which includes the season imbalance but can only be derived by using the CDC Research Data Center).
The race-ethnicity patterns of total 25(OH)D seen in previous survey periods in which the RIA was used were similar to current periods in which the LC-MS/MS was used: NHWs > Hispanics > NHBs. We observed for each race-ethnicity group higher prevalence estimates of low total 25(OH)D during winter compared with summer months. However, even after we adjusted for season through stratification, the race-ethnicity differences in prevalence remained. This emphasizes the importance of reporting race-specific estimates of total 25(OH)D, which is still the accepted biomarker for vitamin D status. Studies investigating whether black Americans have lower vitamin D–binding protein concentrations than whites arrived at discordant results (56, 57), possibly due to technical issues with immunoassays. The ratio of 24,25-dihydroxyvitamin D to total 25(OH)D was shown to be positively associated with 25(OH)D3, negatively correlated with parathyroid hormone, and to be similar in blacks and whites, suggesting that the ratio may have utility as biomarker for vitamin D status (58, 59).
Our study has limitations. The vitamin D intake data are likely underestimated because of underreporting of total intakes, underestimation of fortificant amounts in fortified foods and supplements as compared with label values, and failure of composition databases to account for naturally occurring food content of 25(OH)D (59–62). On the other hand, vitamin D status may be slightly overestimated because, by design, the NHANES visits northern states in the summer and southern states in the winter. Thus, stratifying by season in addition to race-ethnicity provides improved prevalence estimates to assess the current vitamin D status in the US population. We did not estimate usual intake, which adjusts for within-person variability and is preferred to describe the proportion of the population with intakes above or below threshold values. We instead assessed mean intake data collected at the time of the blood draw. However, measures of central tendency are generally not affected by within-person variability (63). The appropriateness of using RIA-derived cutoff values for total 25(OH)D data generated by LC-MS/MS has been questioned (64), but this issue cannot be addressed in the current study. Investigating various dietary sources of vitamin D and their relations with vitamin D metabolite concentrations was outside the scope of our analysis. Assessing long-term (1988–2010) temporal trends in vitamin D status of the US population aged ≥12 y is the focus of a separate report that used RIA data to predict standardized LC-MS/MS equivalents for NHANES 1988–2006 (unpublished results, 2016).
The strengths of our study include the use of data from 2 survey periods measured with a new standardized LC-MS/MS method. This helps ensure the accuracy of the measurements as well as allowing comparability with other studies that use standardized laboratory methods. The high quality of the laboratory measurements was ensured by adhering to multiple levels of quality assurance and by following a strict traceability chain to international reference materials. To our knowledge, this study also presents the first nationally representative data on vitamin D metabolites for the US population aged ≥1 y and for individual race-ethnicity groups. In summary, we confirmed that race-ethnicity patterns of total 25(OH)D observed earlier and with a less specific laboratory method are still present. The similar patterns of C3-epi-25(OH)D3 and 25(OH)D3 relative to selected demographic, intake, physiologic, or lifestyle variables are not surprising given the probable common origin of these 2 compounds. The presence of 25(OH)D2 mainly in older supplement users is likely a result of high-dose prescription vitamin D2.
Acknowledgments
We acknowledge contributions from the following laboratory members: Sara Encisco, Paul Yoon, and Huiping Chen (CDC National Center for Environmental Health). We thank Te-Ching Chen (CDC National Center for Health Statistics) for some specialized data analyses. We also thank Paul Coates (NIH Office of Dietary Supplements) for his valuable scientific input and support. RLS, MRS, and CMP designed the overall research project; RLS, MRS, KLM, MC-W, and CMP conducted most of the research; RLS and MRS analyzed most of the data; RLS and CMP wrote the initial draft, which was modified based on critical review from all coauthors including MRS, ACL, EAY, DAL, CTS, CLT, RAD-A, KLM, MC-W, and CLJ; and CMP had primary responsibility for all content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ARIC, Atherosclerosis Risk in Communities Study; C3-epi-25(OH)D3, C3-epimer of 25-hydroxycholecalciferol; LC-MS/MS, liquid chromatography–tandem mass spectrometry; LOD, limit of detection; NANS, National Adult Nutrition Survey; NHB, non-Hispanic black; NHW, non-Hispanic white; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D2, 25-hydroxyergocalciferol; 25(OH)D3, 25-hydroxycholecalciferol.
References
- 1.Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, Pierroz DD, Weber P, Hoffmann K. A systematic review of vitamin D status in populations worldwide. Br J Nutr 2014;111:23–45. [DOI] [PubMed] [Google Scholar]
- 2.Cashman KD, Kiely M, Kinsella M, Durazo-Arvizu R, Tian L, Zhang Y, Lucey A, Flynn A, Gibney MJ, Vesper HW. Evaluation of vitamin D standardization program protocols for standardizing serum 25-hydroxyvitamin D data: a case study of the program’s potential for national nutrition and health surveys. Am J Clin Nutr 2013;97:1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janz T, Pearson C. Vitamin D blood levels of Canadians. Statistics Canada. Catalog 82–624-X [cited 2015 Nov 20]. Available from: www.statcan.gc.ca.
- 4.UK Food Standards Agency. National Diet and Nutrition Survey: results from years 1–4 (combined) of the Rolling Programme (2008–2009–2011/12): executive summary. London: Public Health England; 2014.
- 5.LeFevre ML. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2015;162:133–40. [DOI] [PubMed] [Google Scholar]
- 6.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 2002;30:771–7. [DOI] [PubMed] [Google Scholar]
- 7.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001–2006. NCHS Data Brief 2011;59:1–8. [PubMed] [Google Scholar]
- 8.Carter GD, Jones JC, Berry JL. The anomalous behavior of exogenous 25-hydroxyvitamin D in competitive binding assays. J Steroid Biochem Mol Biol 2007;103:480–2. [DOI] [PubMed] [Google Scholar]
- 9.Roth HJ, Schmidt-Gayk H, Weber H, Niederau C. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem 2008;45:153–9. [DOI] [PubMed] [Google Scholar]
- 10.Vogeser M, Seger C. A decade of HPLC-MS/MS in the routine clinical laboratory—goals for further developments. Clin Biochem 2008;41:649–62. [DOI] [PubMed] [Google Scholar]
- 11.Tai SS-C, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem 2010;82:1942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phinney KW, Bedner M, Tai S-C, Vamathevan VV, Sander LC, Sharpless KE, Wise SA, Yen JH, Schleicher RL, Chaudhary-Webb M, et al. Development and certification of a Standard Reference Material for vitamin D metabolites in human serum. Anal Chem 2012;84:956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl 2012;243:32–40. [DOI] [PubMed] [Google Scholar]
- 14.Yetley EA, Pfeiffer CM, Schleicher RL, Phinney KW, Lacher DA, Christakos S, Eckfeldt JH, Fleet JC, Howard G, Hoofnagle AN, et al. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. J Nutr 2010;140(Suppl):2030S–45S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de la Hunty A, Wallace AM, Gibson S, Viljakainen H, Lamberg-Allardt C, Ashwell M. UK Food Standards Agency Workshop Consensus Report: the choice of method for measuring 25-hydroxyvitamin D to estimate vitamin D status for the UK National Diet and Nutrition Survey. Br J Nutr 2010;104:612–9. [DOI] [PubMed] [Google Scholar]
- 16.Schleicher RL, Encisco SE, Chaudhary-Webb M, Paliakov E, McCoy LF, Pfeiffer CM. Isotope dilution ultra performance liquid chromatography-tandem mass spectrometry method for simultaneous measurement of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 in human serum. Clin Chim Acta 2011;412:1594–9. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey, 2007–2008 [cited 2015 Nov 20]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/overviewbrochure_0708.pdf.
- 18.Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, Hirsch R, Burt VL, Johnson CL. National Health and Nutrition Examination Survey: sample design, National Center for Health Statistics. Vital Health Stat 2 2007–2010;160:2013. [PubMed] [Google Scholar]
- 19.Mirel LB, Mohadjer LK, Dohrmann SM, Clark J, Burt VL, Johnson CL, Curtin LR. National Health and Nutrition Examination Survey: estimation procedures, National Center for Health Statistics. Vital Health Stat 2 2007–2010;159:2013. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES response rates and population totals [cited 2015 Nov 20]. Available from: http://www.cdc.gov/nchs/nhanes/response_rates_CPS.htm.
- 21.Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med 2008;27:4094–106. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention, National Center for Health Statistics. Laboratory procedure manual: 25-hydroxivitamin D3, C3-epi-25-hydroxyvitamin D3, and 25-hydroxyvitamin D2 in serum by ultra high performance liquid chromatography-tandem mass spectrometry [cited 2015 Nov 20]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/VID_E_met_Vitamin_D.pdf.
- 23.Centers for Disease Control and Prevention, National Center for Health Statistics. Laboratory procedure manual: 25-hydroxivitamin D3, C3-epi-25-hydroxyvitamin D3, and 25-hydroxyvitamin D2 in serum by ultra high performance liquid chromatography-tandem mass spectrometry [cited 2015 Nov 20]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/VID_F_met_Vitamin_D.pdf.
- 24.Haynes BMH, Pfeiffer CM, Sternberg MR, Schleicher RL. Selected pre-analytical and physiological factors are weakly to moderately associated with twenty-nine biomarkers of diet and nutrition, NHANES 2003–2006. J Nutr 2013;143(Suppl):1001S–10S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schleicher RL, Sternberg MR, Pfeiffer CM. Race-ethnicity is a strong correlate of circulating fat-soluble nutrient concentrations in a representative sample of the U.S. population. J Nutr 2013;143(Suppl):966S–76S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared to 2000–2004. Am J Clin Nutr 2008;88:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention, National Center for Health Statistics. Laboratory procedure manual: C-reactive protein in serum by nephelometry [cited 2015 Nov 20]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/CRP_F_met.pdf.
- 28. World Health Organization Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. World Health Organ Tech Rep Ser 2000;894:1–253. [PubMed]
- 29.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for calcium and vitamin D. Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- 30.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- 31.Norman AW, Bouillon R, Farach-Carson MC, Bishop JE, Zhou LX, Nemere I, Zhao J, Muralidharan KR, Okamura WH. Demonstration that 1 beta,25-dihydroxyvitamin D3 is an antagonist of the nongenomic but not genomic biological responses and biological profile of the three A-ring diastereomers of 1 alpha,25-dihydroxyvitamin D3. J Biol Chem 1993;268:20022–30. [PubMed] [Google Scholar]
- 32.Brown AJ, Ritter C, Slatopolsky E, Muralidharan KR, Okamura WH, Reddy GS. 1α,25-Dihydroxy-3-epi-vitamin D3, a natural metabolite of 1α,25-dihydroxyvitamin D3, is a potent suppressor of parathyroid hormone secretion. J Cell Biochem 1999;73:106–13. [PubMed] [Google Scholar]
- 33.Lutsey PL, Eckfeldt JH, Ogagarue ER, Folsom AR, Michos ED, Gross M. The 25-hydroxyvitamin D3 C-3 epimer: distribution, correlates, and reclassification of 25-hydroxyvitamin D status in the population-based Atherosclerosis Risk in Communities Study (ARIC). Clin Chim Acta 2015;442:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cashman KD, Kinsella M, Walton J, Flynn A, Hayes A, Lucey AJ, Seamans KM, Kiely M. The 3 epimer of 25-hydroxycholecalciferol is present in the circulation of the majority of adults in a nationally representative sample and has endogenous origins. J Nutr 2014;144:1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cashman KD, Kinsella M, McNulty BA, Walton J, Gibney MJ, Flynn A, Kiely M. Dietary vitamin D2—a potentially underestimated contributor to vitamin D nutritional status of adults. Br J Nutr 2014;112:193–202. [DOI] [PubMed] [Google Scholar]
- 36.Bailey D, Perumal N, Yazdanpanah M, Al Mahmud A, Baqui AH, Adeli K, Roth DE. Maternal-fetal-infant dynamics of the C3-epimer of 25-hydroxyvitamin D. Clin Biochem 2014;47:816–22. [DOI] [PubMed] [Google Scholar]
- 37.Engelman CD, Bo R, Zuelsdorff M, Steltenpohl H, Kirby T, Nieto FJ. Epidemiologic study of the C-3 epimer of 25-hydroxyvitamin D3 in a population-based sample. Clin Nutr 2014;33:421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke MW, Tuckey RC, Gorman S, Holt B, Hart PH. Optimized 25-hydroxyvitamin D analysis using liquid-liquid extraction with 2D separation with LC/MS/MS detection, provides superior precision compared to conventional assays. Metabolomics 2013;9:1031–40. [Google Scholar]
- 39.Granado-Lorencio F, Blanco-Navarro I, Pérez-Sacristán B, Donoso-Navarro E, Silvestre-Mardomingo R. Serum levels of 3-epi-25-OH-D3 during hypervitaminosis D in clinical practice. J Clin Endocrinol Metab 2012;97:E2266–70. [DOI] [PubMed] [Google Scholar]
- 40.Karras SN, Shah I, Petroczi A, Goulis DG, Bili H, Papadopoulou F, Harizopoulou V, Tarlatzis BC, Naughton DP. An observational study reveals that neonatal vitamin D is primarily determined by maternal contributions: implications of a new assay on the roles of vitamin D forms. Nutr J 2013;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Ouweland JM, Beijers Henny Van Daal AM. Fast separation of 25-hydroxyvitamin D3 from 3-epi-25-hydroxyvitamin D3 in human serum by liquid chromatography-tandem mass spectrometry: variable prevalence of 3-epi-25-hydroxyvitamin D3 in infants, children, and adults. Clin Chem 2011;57:1618–9. [DOI] [PubMed] [Google Scholar]
- 42.Lensmeyer G, Poquette M, Wiebe D, Binkley N. The C-3 epimer of 25-hydroxyvitamin D3 is present in adult serum. J Clin Endocrinol Metab 2012;97:163–8. [DOI] [PubMed] [Google Scholar]
- 43.Liebisch G, Matysik S. Accurate and reliable quantification of 25-hydroxyvitamin D species by liquid chromatography high-resolution tandem mass spectrometry. J Lipid Res 2015;56:1234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stepman HCM, Vanderroost A, Van Uytfanghe K, Thienpont LM. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem 2011;57:441–8. [DOI] [PubMed] [Google Scholar]
- 45.Yazdanpanah M, Bailey D, Walsh W, Wan B, Adeli K. Analytical measurement of serum 25-OH-vitamin D3, 25-OH-vitamin D2 and their C3-epimers by LC-MS/MS in infant and pediatric specimens. Clin Biochem 2013;46:1264–71. [DOI] [PubMed] [Google Scholar]
- 46.Bianchini C, Lavery P, Agellon S, Weiler HA. The generation of C-3α epimer of 25-hydroxyvitamin D and its biological effects on bone mineral density in adult rodents. Calcif Tissue Int 2015;96:453–64. [DOI] [PubMed] [Google Scholar]
- 47.Gallo S, Comeau K, Vanstone C, Agellon S, Sharma A, Jones G, L'Abbé M, Khamessan A, Rodd C, Weiler H. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA 2013;309:1785–92. [DOI] [PubMed] [Google Scholar]
- 48.Reddy GS, Muralidharan KR, Okamura WH, Tserng KY, McLane JA. Metabolism of 1α,25-dihydroxyvitamin D3 and its C-3 epimer 1α,25-dihydroxy-3-epi-vitamin D3 in neonatal human keratinocytes. Steroids 2001;66:441–50. [DOI] [PubMed] [Google Scholar]
- 49.Teegarden MD, Riedl KM, Schwartz SJ. Chromatographic separation of PTAD-derivatized 25-hydroxyvitamin D3 and its C-3 epimer from human serum and murine skin. J Chromatogr B Analyt Technol Biomed Life Sci 2015;991:118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright MJP, Halsall DJ, Keevil BG. Removal of 3-epi-25-hydroxyvitamin D3 interference by liquid chromatography-tandem mass spectrometry is not required for the measurement of 25-hydroxyvitamin D3 in patients older than 2 years. Clin Chem 2012;58:1719–20. [DOI] [PubMed] [Google Scholar]
- 51.Cashman KD, Muldowney S, McNulty B, Nugent A, FitzGerald AP, Kiely M, Walton J, Gibney MJ, Flynn A. Vitamin D status of Irish adults: findings from the National Adult Nutrition Survey. Br J Nutr 2013;109:1248–56. [DOI] [PubMed] [Google Scholar]
- 52.Hintzpeter B, Mensink GBM, Thierfelder W, Mueller JM, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr 2008;62:1079–89. [DOI] [PubMed] [Google Scholar]
- 53.Rockell JEP, Skeaff CM, Williams SM, Green TJ. Serum 25-hydroxyvitamin D concentrations of New Zealanders aged 15 years and older. Osteoporos Int 2006;17:1382–9. [DOI] [PubMed] [Google Scholar]
- 54.Briefel RR, Sempos CT, McDowell MA, Chien S, Alaimo K. Dietary methods research in the third National Health and Nutrition Examination Survey: underreporting of energy intake. Am J Clin Nutr 1997;65(Suppl):1203S–9S. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen X-MT, Lane J, Smith BR, Nguyen NT. Changes in inflammatory biomarkers across weight classes in a representative US population: a link between obesity and inflammation. J Gastrointest Surg 2009;13:1205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 2013;369:1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aloia J, Mikhail M, Rhaliwal R, Shieh A, Usera G, Stolberg A, Ragolia L, Islam S. Free 25(OH)D and the vitamin D paradox in African Americans. J Clin Endocrinol Metab 2015;100:3356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cashman KD, Hayes A, Galvin K, Merkel J, Jones G, Kaufmann M, Hoofnagle A, Carter GD, Durazo-Arvizu RA, Sempos CT. Significance of serum 24,25-dihydroxyvitamin D in the assessment of vitamin D status: a double-edged sword? Clin Chem 2015;61:636–45. [DOI] [PubMed] [Google Scholar]
- 59.Berg AH, Powe CE, Evans MK, Wenger J, Ortiz G, Zonderman AB, Sutharalingam P, Lucchesi K, Powe NR, Karumanchi SA, et al. 24,25-Dihydroxyvitamin D3 and vitamin D status of community-dwelling black and white Americans. Clin Chem 2015;61:877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patterson KY, Phillips KM, Horst RL, Byrdwell WC, Exler J, Lemar LE, Holden JM. Vitamin D content and variability in fluid milks from a US Department of Agriculture nationwide sampling to update values in the National Nutrient Database for Standard Reference. J Dairy Sci 2010;93:5082–90. [DOI] [PubMed] [Google Scholar]
- 61.Taylor CL, Patterson KY, Roseland JM, Wise SA, Merkel JM, Pehrsson PR, Yetley EA. Including food 25-hydroxyvitamin D in intake estimates may reduce the discrepancy between dietary and serum measures of vitamin D status. J Nutr 2014;144:654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ovesen L, Brot C, Jakobsen J. Food contents and biological activity of 25-hydroxyvitamin D: a vitamin D metabolite to be reckoned with? Ann Nutr Metab 2003;47:107–13. [DOI] [PubMed] [Google Scholar]
- 63.Subcommittee on Criteria for Dietary Evaluation, Food and Nutrition Board. Nutrient adequacy: assessment using food consumption surveys. Washington (DC): National Academies Press; 1986. [PubMed] [Google Scholar]
- 64.Hollis BW. Assessment of vitamin D status and definition of a normal circulating range of 25-hydroxyvitamin D. Curr Opin Endocrinol Diabetes Obes 2008;15:489–94. [DOI] [PubMed] [Google Scholar]