Abstract
Background: Breast milk vitamin B-12 concentration may be inadequate in regions in which animal-source food consumption is low or infrequent. Vitamin B-12 deficiency causes megaloblastic anemia and impairs growth and development in children.
Objective: We measured vitamin B-12 in breast milk and examined its associations with household hunger, recent animal-source food consumption, and vitamin B-12 intake.
Methods: In a cross-sectional substudy nested within a cluster-randomized trial assessing water, sanitation, hygiene, and nutrition interventions in Kenya, we sampled 286 women 1–6 mo postpartum. Mothers hand-expressed breast milk 1 min into a feeding after 90 min observed nonbreastfeeding. The Household Hunger Scale was used to measure hunger, food intake in the previous week was measured with the use of a food-frequency questionnaire (FFQ), and vitamin B-12 intake was estimated by using 24-h dietary recall. An animal-source food score was based on 10 items from the FFQ (range: 0–70). Breast milk vitamin B-12 concentration was measured with the use of a solid-phase competitive chemiluminescent enzyme immunoassay and was modeled with linear regression. Generalized estimating equations were used to account for correlated observations at the cluster level.
Results: Median (IQR) vitamin B-12 intake was 1.5 μg/d (0.3, 9.7 μg/d), and 60% of women consumed <2.4 μg/d, the estimated average requirement during lactation. Median (IQR) breast milk vitamin B-12 concentration was 113 pmol/L (61, 199 pmol/L); 89% had concentrations <310 pmol/L, the estimated adequate concentration. Moderate or severe hunger prevalence was 27%; the animal-source food score ranged from 0 to 30 item-d/wk. Hunger and recent animal-source food and vitamin B-12 intake were not associated with breast milk vitamin B-12 concentrations. Maternal age was negatively associated with breast milk vitamin B-12 concentrations.
Conclusion: Most lactating Kenyan women consumed less than the estimated average requirement of vitamin B-12 and had low breast milk vitamin B-12 concentrations. We recommend interventions that improve vitamin B-12 intake in lactating Kenyan women to foster maternal health and child development. The main trial was registered at clinicaltrials.gov as NCT01704105.
Keywords: animal-source foods, breast milk, lactation, vitamin B-12, Kenya, Africa, hunger, food security
Introduction
Vitamin B-12 deficiency in women and children is thought to be common, especially in low- and middle-income countries in which animal-source food consumption is limited (1–6). Vitamin B-12 deficiency has long been recognized in the etiology of megaloblastic anemia (7), and maternal deficiency is associated with offspring insulin resistance (8, 9). The risks for infants born to mothers with low plasma vitamin B-12 also include low vitamin B-12 stores at birth, as well as low dietary intake from breast milk (10, 11). Low vitamin B-12 status causes elevated plasma total homocysteine in all age groups (12, 13). In addition to metabolic disorders, severe vitamin B-12 deficiency is responsible for developmental delays, failure to thrive, and poor growth in infants (7, 14). Infants are particularly vulnerable to vitamin B-12 deficiency, because they are undergoing a period of rapid growth and development.
Breast milk vitamin B-12 concentration varies with maternal diet (11), and low breast milk vitamin B-12 concentrations have been reported in lactating women in Mexico (15), Guatemala (16), Kenya (17), Cameroon (18), and Bangladesh (19), where the animal-source food consumption is limited. Low vitamin B-12 concentrations in breast milk could put infants at risk of consuming inadequate vitamin B-12, which has been demonstrated in the case of maternal pernicious anemia (20). Breast milk is recommended as the only source of infant nutrition for the first 6 mo of life (21). The cutoff for adequate breast milk vitamin B-12 has been estimated at 362 pmol/L (22, 23), below which infant urinary methylmalonic acid concentrations increased (24). Although elevated methylmalonic acid is a biomarker of vitamin B-12 deficiency, some studies have shown that healthy breastfed infants have higher plasma methylmalonic acid than nonbreastfed infants (25). Calculated from data used to set the Institute of Medicine Adequate Intake (AI) for infants 0–6 mo old (AI = 0.4 μg vitamin B-12/d, based on milk concentrations of 0.42 μg/L and a consumption of 0.78 L breast milk/d) (26), we are considering concentrations of breast milk vitamin B-12 ≥310 pmol/L to be adequate. At present, there is no other basis for ascertaining breast milk vitamin B-12 adequacy.
Although animal-source foods are the only dietary source of vitamin B-12, to our knowledge, only 3 studies (16, 17, 22) have investigated the relation between maternal animal-source food intake and breast milk vitamin B-12 concentrations. Only one of these studies targeted women <6 mo postpartum, and all were conducted before improved assessment methods for breast milk vitamin B-12. There is an expected differential in breast milk vitamin B-12 concentration based on stage of lactation; the concentration is higher earlier in lactation than it is later (27). Furthermore, to our knowledge, there have been no studies that have examined the relation between breast milk vitamin B-12 and household hunger, which is a metric of food deprivation and may be a proxy for animal-source food consumption. Therefore, our objectives were to measure vitamin B-12 in breast milk and examine its associations with household hunger and recent animal-source food and vitamin B-12 intake in rural mothers 1–6 mo postpartum in western Kenya. We hypothesized that breast milk vitamin B-12 concentrations would be negatively associated with hunger and positively associated with animal-source food intake and vitamin B-12 intake.
Methods
Study overview and location.
Cross-sectional data on dietary intake and breast milk samples were collected from 300 women from June to November 2014. These women were all participants in the WASH Benefits Kenya study (NCT01704105) (28), an ongoing cluster-randomized trial designed to evaluate the efficacy of improved nutrition, water quality, sanitation, hygiene, or combined (water–sanitation–hygiene and water–sanitation–hygiene–nutrition) practices on child growth and development. Sampling for the breast milk substudy was done purposefully to ensure balance between intervention groups. A STrengthening Reporting of Observational studies in Epidemiology (STROBE) checklist has been included as Supplemental Table 1.
Details of the overall study design have been published elsewhere (28). In brief, the parent study enrolled pregnant women in their second and third trimesters. Sociodemographic data (marital and economic status and education) were collected during household interviews. Starting at 1–2 mo postenrollment, households were visited monthly by a health promoter who was selected by the community and trained in improved water–sanitation–hygiene and/or nutrition practices. Key nutrition messages were based on global maternal, infant, and young child feeding recommendations; locally tailored by formative research; and timed to match the life stage of the mother–infant dyad. Maternal nutrition during pregnancy and lactation, breastfeeding initiation within 1 h of birth, and exclusive breastfeeding for the first 6 mo of life were emphasized during these initial visits. Women in the control group received monthly visits without behavior change messaging. The breast milk outcomes are for a substudy nested within the parent trial and are not primary or secondary objectives of WASH Benefits Kenya.
Participants in the breast milk substudy.
Study mothers were recruited from 4 intervention groups: water–sanitation–hygiene, nutrition, water–sanitation–hygiene–nutrition, and a control that used convenience sampling. The eligibility criteria included being ≥18 y of age, having breastfed in the previous 24 h, having a 1- to 6-mo-old biological child enrolled in the main trial, and report of good breast health (no cracked or bleeding nipples or mastitis). There were 70 independent clusters sampled in this substudy.
A sample size of 300 was selected to detect a difference in mean breast milk vitamin B-12 concentration of ≥150 pmol/L between 2 groups of household hunger (little or none compared with moderate or severe), while accounting for a design effect of 1.2 (ρ = 0.1 and 3 women/cluster), 80% power, and α = 0.05. We expected a 30% prevalence of moderate or severe household hunger based on unpublished pilot data, and the SD of breast milk vitamin B-12 was estimated to be 200 pmol/L. The sample size of 300 is sufficient to detect a correlation of ≥0.2 between vitamin B-12 intake (micrograms per day) and breast milk vitamin B-12 concentration (picomoles per liter) and a minimum difference of 160 pmol/L between animal-source food consumption quartiles. A 10% buffer was included to account for specimen loss.
Data collection.
At ∼6 mo postenrollment, anthropometric and dietary data, as well as breast milk, were collected. Maternal height was recorded to the nearest 0.1 cm with the use of a stadiometer (ShorrBoard Infant/Child/Adult Portable Height–Length Measuring Board), and maternal weight was measured with the use of a digital floor scale (Seca 874) to the nearest 0.1 kg. For mothers who wore more clothing than cotton skirts and T-shirts during the measurement, 0.5 kg was subtracted from their weight. All measurements were done in triplicate; median height and weight were used to calculate BMI.
Dietary data and information on birth characteristics were collected during a household visit. Maternal dietary data were collected with the use of a 7-d FFQ and an interactive multipass 24-h recall (29). Food insecurity in the previous month was measured with the use of the Household Hunger Scale. The Household Hunger Scale has 3 questions that pertain to going to sleep hungry, having no food in the home because of a lack of resources, and passing a day and night without eating (30). Interview and anthropometric data were entered onto tablets with the use of SurveyCTO (Dobility).
Breast milk was collected while following an observed nonbreastfeeding period of ≥90 min. After 1 min of breastfeeding, study mothers hand-expressed 5 mL breast milk from the right breast into transparent plastic medicine cups while in an area of the home that had minimal light exposure. Collection was restricted to times between 0900 and 1200 to minimize potential diurnal variation (31, 32). Specimens were stored with ice packs in the field for ≤8 h, frozen at −4C° for ≤3 wk, and stored at −80C° until processed. International specimen transport to the USDA Agricultural Research Service Western Human Nutrition Research Center in Davis, California, was done on dry ice. Specimens were protected from sunlight: collection was restricted to dimly lit areas and cryovials were stored in opaque freezer boxes.
Breast milk vitamin B-12 analysis.
Breast milk samples were analyzed at the Western Human Nutrition Research Center as previously described (33). They were thawed at room temperature and centrifuged to separate the whey fraction, which then underwent heat treatment in the presence of dithiothreitol and potassium cyanide before analysis by solid-phase competitive chemiluminescent enzyme immunoassay with the use of a Siemens IMMULITE 1000 bioanalyzer. Breast milk vitamin B-12 inadequacy was defined as <310 pmol/L (34).
Variable creation.
Wealth scores were created from 16 questions related to household possessions (including livestock) and household material characteristics. Each question was scored such that higher numbers reflected more wealth and a composite score was generated with the use of factor analysis in which one factor was forced and the resultant continuous variable was divided into quartiles.
The FFQ queried how many days in the previous 7 d the following animal-source food items were eaten by the mother: 1) poultry, 2) meat (cow, goat, and pig), 3) organ meat, 4) egg, 5) yogurt, 6) tea with milk, 7) milk alone, 8) fresh fish, 9) dried fish, and 10) termites. An animal-source food score of item-days per week was created that summed all of the individual responses to these foods. The maximum animal-source food score from the FFQ was 70 if a respondent indicated daily consumption in the previous week of all 10 foods. The animal-source food score was divided into quartiles to reduce the influence of outliers.
The food composition table for Central and Eastern Uganda (HarvestPlus) was used to calculate nutrient intake from the 24-h dietary recall (35). Whole-day food records were deleted (n = 3) when implausible intake quantities were recorded (2 with >1800 g meat in one meal and 1 with 600 eggs in one meal). Energy ranges were not considered for exclusion. In order to include energy intake in our models, the estimated energy requirement for lactating women 0–6 mo postpartum was calculated by using the nonpregnant estimated energy requirement for women >19 y old, the milk energy output, and a reduction for weight loss (36).
Statistical analysis.
The distribution of breast milk vitamin B-12 concentrations was skewed; thus, data were log-transformed for analysis. Correlates of breast milk vitamin B-12 concentrations were modeled by using linear regression, with generalized estimating equations that used robust SEs to account for outcome correlation at the cluster level (37). Covariates known to have a biological plausibility of influencing breast milk vitamin B-12 concentrations (maternal age and months postpartum) were included in the multivariable regression models. We considered adjusting for maternal energy intake (38), but no change was found in the point estimates or SEs in the model and therefore the energy term was excluded from the multivariable model. Similarly, wealth and the intervention arm were examined in the unadjusted regression models, because wealth is associated with our primary exposure variables (e.g., hunger), and data collection was nested within an intervention trial that could theoretically affect outcome and exposure variables, but both ultimately were dropped in the multivariable analyses because of nonsignificance. The quasi-likelihood information criterion was used to assess goodness of fit. Data analyses were performed in SAS version 9.3.
Ethical approval and informed consent.
The Kenya Medical Research Institute Scientific Steering Committee and Ethical Review Committee and the University of California, Berkeley, Committee for the Protection of Human Subjects approved this research. The University of California, Davis, has approval from the Institutional Review Board (IRB) reliance registry with Berkeley. Study mothers provided written consent for all study activities.
Results
Breast milk was collected from 300 study mothers between June and November 2014. Two milk samples were unavailable for analysis (one had insufficient milk and one sample was missing), 9 mothers who provided milk did not participate in the 24-h dietary recall, and 3 dietary recalls were implausible and dropped from the analysis, leaving complete data for 286 women. The majority of mothers were married or in a partnership and living with their spouse or partner (91%) (Table 1). Education beyond primary school was rare (20%). BMI (in kg/m2) was in a normal range (18.5–25.0) and 63% of women gave birth at a health facility. Antenatal care visits were common, and 16% of mothers reported that the study child was their first birth.
TABLE 1.
Characteristics of mothers 1–6 mo postpartum (n = 286) participating in a substudy of the WASH Benefits trial in rural western Kenya1
| Descriptive characteristics | Values |
| Maternal BMI, kg/m2 | 22.2 (20.5, 24.3) |
| Antenatal visits | 4 (3, 5) |
| Place of delivery | |
| Home | 105 (37) |
| Health facility | 182 (63) |
| Marital status | |
| Married, cohabitating | 260 (91) |
| Married, living apart | 24 (8) |
| Not married | 2 (1) |
| Maternal education | |
| None or primary | 228 (80) |
| Secondary or beyond | 58 (20) |
| Season2 | |
| Dry (harvest) | 220 (77) |
| Rainy | 66 (23) |
| Child sex | |
| M | 127 (44) |
| F | 159 (56) |
| Maternal age, y | 25 (22, 29) |
| Months postpartum | 3.6 (2.8, 4.4) |
| Maternal parity | |
| Primiparous | 46 (16) |
| 2–4 | 159 (56) |
| ≥5 | 81 (28) |
| Household hunger | |
| Little or none | 208 (73) |
| Moderate or severe | 78 (27) |
| Animal-source food score (item-d/wk) | |
| Q1 (0–7) | 80 (28) |
| Q2 (8–9) | 57 (20) |
| Q3 (10–13) | 79 (28) |
| Q4 (14–30) | 70 (24) |
| Vitamin B-12 intake, μg/d | |
| 0 | 54 (19) |
| <EAR and >0 | 117 (41) |
| ≥EAR | 115 (40) |
Values are medians (IQRs) or n (%). EAR, estimated average requirement; Q, quartile.
Season of milk collection. Dry season was June–September; rainy season was October and November.
Breast milk vitamin B-12 adequacy.
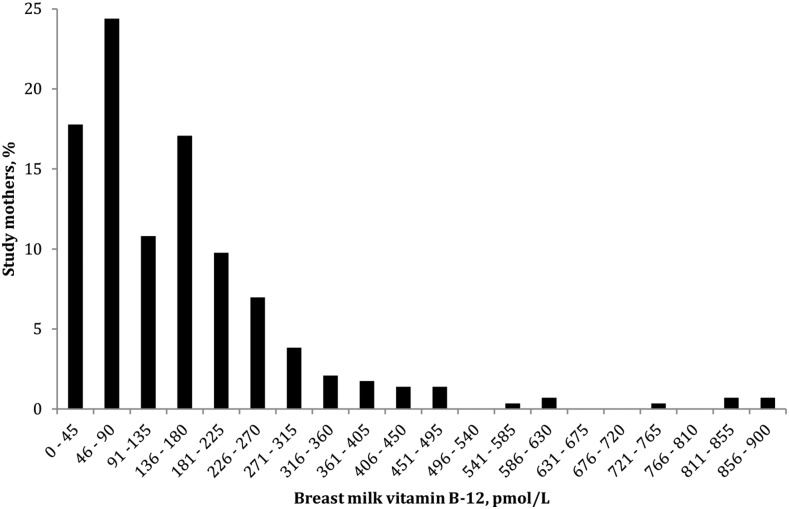
The median (IQR) concentration of breast milk vitamin B-12 was 113 pmol/L (61, 199 pmol/L). The majority (89%) of mothers had breast milk vitamin B-12 concentrations <310 pmol/L (Figure 1).
FIGURE 1.
Distribution of breast milk vitamin B-12 concentration in lactating mothers 1–6 mo postpartum (n = 286) participating in a substudy of the WASH Benefits trial in rural western Kenya.
Household hunger and dietary practice.
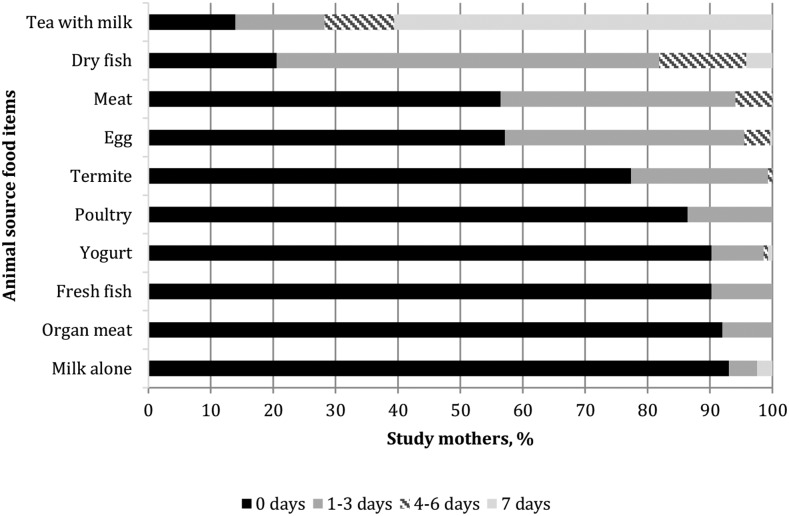
Little to no household hunger was reported by 73% of households. Generally, animal-source foods were rarely reported on the FFQ. The animal-source food score, which theoretically could range from 0 to 70, spanned from 0 to 30. Tea with milk and small dry fish (white bait) were the only 2 animal-source food items that were reported as consumed by more than one-half of mothers in the previous 7 d (Figure 2).
FIGURE 2.
Reported days of consumption of animal-source foods in the previous week by lactating mothers 1–6 mo postpartum (n = 286) participating in a substudy of the WASH Benefits Kenya trial.
Using data from the 24-h recall, we estimated women’s mean energy intake to be 2238 kcal/d (range: 156–5900 kcal/d) (Table 2). Based on the energy recommendation for this stage of lactation (based on age, height, weight, and energy expenditure), 30% of women met their estimated energy requirement. Consistent with the 7-d FFQ data, tea with milk was the most commonly reported animal-source food item, representing a mean ± SD consumption of 468 ± 472 g/d and 101 ± 127 kcal/d. The median (IQR) intake of vitamin B-12 was 1.45 μg/d (0.31, 9.65 μg/d). More than one-half (60%) of study mothers consumed less vitamin B-12 in the 24 h before breast milk sample collection than the estimated average requirement during lactation (2.4 μg/d) (26).
TABLE 2.
Mean daily intake of animal-source foods with accompanying energy and vitamin B-12 content in mothers 1–6 mo postpartum participating in a substudy of the WASH Benefits Kenya trial1
| Food group | Amount consumed, g | Energy, kcal | Vitamin B-12, μg |
| Tea with milk | 468 ± 472 | 101 ± 127 | 0.73 ± 0.92 |
| Dry fish | 45 ± 88 | 91 ± 178 | 7.49 ± 14.57 |
| Meat | 14 ± 54 | 42 ± 157 | 0.38 ± 1.34 |
| Eggs | 5 ± 22 | 7 ± 35 | 0.05 ± 0.24 |
| Termites | 1 ± 12 | 8 ± 72 | 0.04 ± 0.34 |
| Poultry | 1 ± 13 | 3 ± 28 | 0.01 ± 0.04 |
| Fresh fish | 13 ± 44 | 8 ± 33 | 0.14 ± 0.51 |
| Organ meat | 4 ± 32 | 3 ± 30 | 0.03 ± 0.23 |
| Milk | 4 ± 38 | 2 ± 23 | 0.01 ± 0.14 |
| Total animal-source foods | 555 ± 497 | 265 ± 289 | 1.41 (0.26, 9.13) |
| Overall total | 1889 ± 681 | 2238 ± 927 | 1.45 (0.31, 9.65) |
| <EAR,2 % | — | 71 | 60 |
Values are means ± SDs or medians (IQRs) unless otherwise indicated, n = 286. The EAR for lactating women 0–6 mo postpartum is 2.4 μg vitamin B-12 (36). Means ± SDs are presented, although data are not normally distributed because the median Q1 (and many Q3) values were all zero. EAR, estimated average requirement.
There is no recommendation by the Institute of Medicine for daily intake of food (grams).
Correlates of breast milk vitamin B-12.
In bivariate regression, higher breast milk vitamin B-12 concentration was associated with younger age, lower parity, and higher antenatal visit frequency. There were no significant relations between breast milk vitamin B-12 concentration and household hunger, vitamin B-12 intake in the previous 24 h, the animal-source food score, maternal BMI, birth location (home compared with health facility), season, or maternal education. In adjusted analyses, all significant associations became nonsignificant except for younger maternal age, which remained positively associated with breast milk vitamin B-12 (Table 3).
TABLE 3.
Correlates of log10 breast milk vitamin B-12 concentration (picomoles per liter) in lactating mothers 1–6 mo postpartum participating in a substudy of the WASH Benefits trial in rural western Kenya1
| β (95% CI) |
||||
| Exposure characteristics | Unadjusted model | Adjusted model 1 | Adjusted model 2 | Adjusted model 3 |
| Household hunger | ||||
| Little/none | Ref | |||
| Moderate/severe | 0.00 (−0.13, 0.13) | −0.01 (−0.14, 0.12) | ||
| Animal-source food score, item-d/wk | ||||
| Q1 (0–7) | Ref | Ref | ||
| Q2 (8–9) | −0.01 (−0.21, 0.18) | 0.02 (−0.16, 0.19) | ||
| Q3 (10–13) | 0.01 (−0.13, 0.14) | 0.01 (−0.14, 0.15) | ||
| Q4 (14 – 30) | 0.08 (−0.04, 0.20) | 0.09 (−0.05, 0.22) | ||
| Vitamin B-12 intake, μg/d | −0.01 (−0.03, 0.01) | −0.01 (−0.03, 0.02) | ||
β coefficients and Wald 95% CIs were computed with linear regression with the use of generalized estimating equations and robust SEs to account for correlation at the cluster level. Adjusted model 1 includes household hunger, maternal age, and months postpartum. Adjusted model 2 includes animal-source food score, maternal age, and months postpartum. Adjusted model 3 includes vitamin B-12 intake, maternal age, and months postpartum. Q, quartile; Ref, reference.
Discussion
Within this study population in western Kenya, 60% of women consumed less than the estimated average requirement of vitamin B-12 during lactation (2.4 μg/d) (26) and the vast majority (89%) of women had breast milk vitamin B-12 concentrations <310 pmol/L (23). We found that ∼12% of energy was from animal sources, primarily derived from tea with milk or dishes with small dried fish. Although Western Province is a fertile region in Kenya, it has seasonal food shortages (39), and 27% of households reported moderate or severe hunger in the previous month.
The low concentration of vitamin B-12 in breast milk that we found is consistent with evidence from a variety of low- and middle- income countries. Two studies from Guatemala found a high prevalence of women with low vitamin B-12 concentrations in breast milk (16, 22). The first found that 31.5% of women who were 3 mo postpartum had breast milk vitamin B-12 concentrations <362 pmol/L (22); among women who were 12 mo postpartum, 65% had breast milk vitamin B-12 concentrations <50 pmol/L (16). In Cameroon, the median breast milk vitamin B-12 concentration was 180 pmol/L in a nationally representative survey, but it ranged from 47 pmol/L (north) to 287 pmol/L (city) regionally (18). A trial in eastern Kenya described 95% of women 1–6 mo postpartum as having breast milk vitamin B-12 concentrations <362 pmol/L (17). Those data from Kenya were collected in the mid-1990s, indicating that vitamin B-12 deficiency likely has been endemic in regions of Kenya for decades.
To our knowledge, there have been 2 efficacy trials of maternal supplementation during pregnancy and lactation to improve breast milk vitamin B-12 concentrations in women with low animal-source food intake. In India, women randomly assigned to receive 50 μg/d of vitamin B-12 during pregnancy until 6 wk postpartum had median breast milk vitamin B-12 concentrations of 136 pmol/L, compared with 87 pmol/L in the placebo group. The differential was not sustained when supplementation ceased, and there was no difference in breast milk concentrations between those groups at 6 mo postpartum (40). Bangladeshi women who received 250 μg/d of vitamin B-12 from 11–14 wk gestation until 6 mo postpartum had a median breast milk vitamin B-12 concentration of 235 pmol/L, compared with 170 pmol/L in the control group (19). Although these supplementation trials did not raise median breast milk vitamin B-12 concentrations to 310 pmol/L, they did reduce infant vitamin B-12 deficiency, as evidenced by lower concentrations of methylmalonic acid and total homocysteine in infants. This suggests that the cutoff for adequate breast milk vitamin B-12 is a priority research question.
Lack of associations between household hunger, recent animal-source food intake, or vitamin B-12 intake and breast milk vitamin B-12 could be due to biological processes or limitations in exposure measurements. Biologically, low breast milk vitamin B-12 concentrations could be due to a marked underlying vitamin B-12 deficiency in the majority of study mothers. Physiologically, lactating women deficient in vitamin B-12 may replenish liver stores before vitamin B-12 can be recruited into milk by the mammary tissue (41). Maternal age was the only significant correlate of breast milk vitamin B-12 concentration in our adjusted analyses, which is plausible because nutrient stores may become depleted with multiple births (42) and suboptimal birth spacing (43, 44), but this was an incidental finding. The animal-source food score had limitations because it did not measure the amount of animal-source food, only frequency, and the amounts consumed in, e.g., tea were very small. The score was also restricted to a small range, thereby reducing variation and the chance for finding associations with the breast milk vitamin B-12 outcome. Household hunger, a binary variable, has similar limitations and could be too imprecise a measure when looking for associations with biological outcomes. Quantitative dietary data from 24-h recall is prone to measurement errors such as recall bias and misclassification of serving sizes (45), which leads to data with a large amount of variation; this could therefore make finding associations that may exist between recent vitamin B-12 intake and breast milk vitamin B-12 more challenging. Similarly, having a single day of 24-h recall intake would make it more difficult to find a correlation between vitamin B-12 intake and breast milk vitamin B-12 concentration.
The primary protein source for pregnant women in Africa is cereal and grains (46). Low dietary diversity and limited intake of animal-source foods have been quantified in Burkina Faso (47), Bangladesh (48), and Nepal (49) in women of reproductive age. Low animal-source food intake has been reported in Kenya in women (17, 50) and children (51). The most commonly consumed animal-source food was tea with milk among pregnant women in Nakuru, Kenya (50). We found similar patterns in our data, in which the primary protein source was also cereal and grains (data not shown), and the most common animal-source food consumed by lactating mothers was tea with milk. Seasonal data on maternal diet in northern Kenya found animal-source food to be a very small component of the diet in rural households (52). The phenomenon of low maternal dietary diversity and limited animal-source food intake is evident where data are available (53). Evidence of low intake of animal-source food in lactating women in Bangladesh indicated that although 84% of women had consumed flesh foods at least 1 of 2 d sampled, approximately one-half consumed <10 g/d (48). Our results have implication for populations with similarly low animal-source food intake in lactating women.
Strengths of this study included providing, to our knowledge, some of the first data on vitamin B-12 breast milk concentrations in women <6 mo postpartum by using a recently validated method. Study limitations included the lack of additional biomarkers (serum vitamin B-12, methylmalonic acid, and total homocysteine) from mothers and infants to corroborate the interpretation of vitamin B-12 deficiency and the self-reported nature of the dietary data, which is susceptible to reporting bias. Another limitation was the lack of dietary replicates, which prevents estimation of day-to-day variability within people and can lead to an underestimation of infrequently consumed foods. However, for the purpose of this study, we were interested primarily in the recent intake of vitamin B-12, and it was impractical to carry out the number of repeat 24-h recalls required to sufficiently estimate usual vitamin B-12 intake.
We observed a high prevalence of markedly low breast milk vitamin B-12 concentration in this population, which is not only suggestive of maternal vitamin B-12 deficiency, but of infant vitamin B-12 deficiency. The median breast milk vitamin B-12 concentration of 113 pmol/L would have provided an exclusively breastfed infant with 0.12 μg vitamin B-12/d based on 780 mL breast milk consumed daily (the volume used to calculate AI for exclusively breastfed infants), which is far below the AI of 0.4 μg/d for infants 0–6 mo old (26). Children >6 mo old often experience poor micronutrient intake because thin porridge is the predominant complementary food (3), and this increases the importance of breast milk as a rich source of micronutrients in the infant’s diet. Animal-source food purchasing power is low in many rural populations (54); therefore, food-based dietary recommendations for pregnant and lactating women in Kenya coupled with nutrition education may be insufficient to adequately improve dietary quality. For women experiencing hunger, effective supplementation and/or fortification strategies are likely necessary to improve vitamin B-12 status (55). We recommend investigating interventions to address breast milk vitamin B-12 inadequacies because vitamin B-12 deficiency has metabolic consequences in all age groups, is associated with impaired infant development (56, 57), and is associated with poor educational performance in school-aged children (58, 59).
Acknowledgments
AMW, CJC, SLY, and CPS designed the substudy; AMW, BSA, JMC, HND, MCK, AL, CAN, GMN, and CPS were involved in the design of the main study and collection of data; LHA, DH, and SS-F were involved in the laboratory analysis and interpretation of vitamin B-12 data; AMW and CPS analyzed the data with input from LHA, BFA, DH, and SS-F; and AMW and CPS were responsible for the final content of the manuscript. All authors read and approved the final manuscript.
References
- 1.Bwibo NO, Neumann CG. The need for animal source foods by Kenyan children. J Nutr 2003;133:3936S–40S. [DOI] [PubMed] [Google Scholar]
- 2.Murphy SP, Allen LH. Nutritional importance of animal source foods. J Nutr 2003;133:3932S–5S. [DOI] [PubMed] [Google Scholar]
- 3.Dewey KG, Brown KH. Update on technical issues concerning complementary feeding of young children in developing countries and implications for intervention programs. Food Nutr Bull 2003;24:5–28. [DOI] [PubMed] [Google Scholar]
- 4.Allen LH. How common is vitamin B-12 deficiency? Am J Clin Nutr 2009;89:693S–6S. [DOI] [PubMed] [Google Scholar]
- 5.Lubree HG, Katre PA, Joshi SM, Bhat DS, Deshmukh US, Memane NS, Otiv SR, Rush EC, Yajnik CS. Child’s homocysteine concentration at 2 years is influenced by pregnancy vitamin B12 and folate status. J Dev Orig Health Dis 2012;3:32–8. [DOI] [PubMed] [Google Scholar]
- 6.Ulak M, Chandyo RK, Adhikari RK, Sharma PR, Sommerfelt H, Refsum H, Strand TA. Cobalamin and folate status in 6 to 35 months old children presenting with acute diarrhea in Bhaktapur, Nepal. PLoS One 2014;9:e90079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dror DK, Allen LH. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev 2008;66:250–5. [DOI] [PubMed] [Google Scholar]
- 8.Stewart CP, Christian P, Schulze KJ, Arguello M, LeClerq SC, Khatry SK, West KP Jr. Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J Nutr 2011;141:1912–7. [DOI] [PubMed] [Google Scholar]
- 9.Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, Bhat DS, Naik SS, Coyaji KJ, Joglekar CV, et al. . Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia 2008;51:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen LH. Maternal micronutrient malnutrition: effects on breast milk and infant nutrition, and priorities for intervention. SCN News 1994;11:21–4. [PubMed] [Google Scholar]
- 11.Allen LH. B vitamins in breast milk: relative importance of maternal status and intake, and effects on infant status and function. Adv Nutr 2012;3:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green R, Miller JW. Vitamin B12 deficiency is the dominant nutritional cause of hyperhomocysteinemia in a folic acid-fortified population. Clin Chem Lab Med 2005;43:1048–51. [DOI] [PubMed] [Google Scholar]
- 13.Yajnik CS, Deshpande SS, Lubress HG, Naik SS, Bhat DS, Uradey BS, Deshpande JA, Rege SS, Refsum H, Yudkin JS. Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J Assoc Physicians India 2006;54:775–82. [PubMed] [Google Scholar]
- 14.Black MM. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr Bull 2008;29:S126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen LH, Rosado JL, Casterline JE, Martinez H, Lopez P, Munoz E, Black AK. Vitamin B-12 deficiency and malabsorption are highly prevalent in rural Mexican communities. Am J Clin Nutr 1995;62:1013–9. [DOI] [PubMed] [Google Scholar]
- 16.Deegan KL, Jones KM, Zuleta C, Ramirez-Zea M, Lidballe DL, Nexo E, Allen LH. Breast milk vitamin B-12 concentrations in Guatemalan women are correlated with maternal but not infant vitamin B-12 status at 12 months postpartum. J Nutr 2012;142:112–6. [DOI] [PubMed] [Google Scholar]
- 17.Neumann CG, Oace SM, Chaparro MP, Herman D, Drorbaugh N, Bwibo NO. Low vitamin B12 intake during pregnancy and lactation and low breast milk vitamin 12 content in rural Kenyan women consuming predominantly maize diets. Food Nutr Bull 2013;34:151–9. [DOI] [PubMed] [Google Scholar]
- 18.Shahab-Ferdows S, Engle-Stone R, Hampel D, Ndjebayi AO, Nankap M, Brown KH, Allen LH. Regional, socioeconomic, and dietary risk factors for vitamin B-12 deficiency differ from those for folate deficiency in cameroonian women and children. J Nutr 2015;145:2587–95. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqua TJ, Ahmad SM, Ahsan KB, Rashid M, Roy A, Rahman SM, Shahab-Ferdows S, Hampel D, Ahmed T, Allen LH, et al. . Vitamin B12 supplementation during pregnancy and postpartum improves B12 status of both mothers and infants but vaccine response in mothers only: a randomized clinical trial in Bangladesh. Eur J Nutr 2016;55:281–93. [DOI] [PubMed] [Google Scholar]
- 20.McPhee AJ, Davidson GP, Leahy M, Beare T. Vitamin B12 deficiency in a breast fed infant. Arch Dis Child 1988;63:921–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO. World Health Assembly Nutrition Targets [Internet]. 2015 [cited 2015 Feb 10]. Available from: http://www.who.int/nutrition/topics/nutrition_globaltargets2025/en/..
- 22.Casterline JE, Allen LH, Ruel MT. Vitamin B-12 deficiency is very prevalent in lactating Guatemalan women and their infants at three months postpartum. J Nutr 1997;127:1966–72. [DOI] [PubMed] [Google Scholar]
- 23.Specker BL, Black A, Allen L, Morrow F. Vitamin B-12: low milk concentrations are related to low serum concentrations in vegetarian women and to methylmalonic aciduria in their infants. Am J Clin Nutr 1990;52:1073–6. [DOI] [PubMed] [Google Scholar]
- 24.Specker BL, Brazerol W, Ho ML, Norman EJ. Urinary methylmalonic acid excretion in infants fed formula or human milk. Am J Clin Nutr 1990;51:209–11. [DOI] [PubMed] [Google Scholar]
- 25.Hay G, Johnston C, Whitelaw A, Trygg K, Refsum H. Folate and cobalamin status in relation to breastfeeding and weaning in healthy infants. Am J Clin Nutr 2008;88:105–14. [DOI] [PubMed] [Google Scholar]
- 26.Institute of Medicine. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, patothenic acid, biotin, and choline. In: Standing committee on the scientific evaluation of dietary reference intakes and its panel on folate, other B vitamins, and choline and subcommittee on upper reference levels of nutrients. 2011, National Academy Press: Washington, DC. [Google Scholar]
- 27.Greibe E, Lidballe DL, Streym S, Vestergaard P, Rejnmark L, Mosekilde L, Nexo E. Cobalamin and haptocorrin in human milk and cobalamin-related variables in mother and child: a 9-mo longitudinal study. Am J Clin Nutr 2013;98:389–95. [DOI] [PubMed] [Google Scholar]
- 28.Arnold BF, Null C, Luby SP, Unicomb L, Stewart CP, Dewey KG, Ahmed T, Ashraf S, Christensen G, Clasen T, et al. . Cluster-randomised controlled trials of individual and combined water, sanitation, hygiene and nutritional interventions in rural Bangladesh and Kenya: the WASH Benefits study design and rationale. BMJ Open 2013;3:e003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibson RS, Ferguson EL. An interactive 24-hour recall for assessing the adequacy of iron and zinc intakes in developing countries. HarvestPlus Technical Monograph 8: Washington, DC and Cali, Colombia: International Food Policy Research Institute and International Center for Tropical Agriculture. 2008. [Google Scholar]
- 30.Deitchler MB, Ballard T, Swindale A, Coates J. Introducing a simple measure of household hunger for cross-cultural use. Technical note no. 12: Food and nutrition technical assistance II: Washington, D.C. 2011. [Google Scholar]
- 31.Ruel MT, Dewey KG, Martinez C, Flores R, Brown KH. Validation of single daytime samples of human milk to estimate the 24-h concentration of lipids in urban Guatemalan mothers. Am J Clin Nutr 1997;65:439–44. [DOI] [PubMed] [Google Scholar]
- 32.Brown KH, Black RE, Robertson AD, Akhtar NA, Ahmed G, Becker S. Clinical and field studies of human lactation: methodological considerations. Am J Clin Nutr 1982;35:745–56. [DOI] [PubMed] [Google Scholar]
- 33.Hampel D, Shahab-Ferdows S, Domek JM, Siddiqua T, Raqib R, Allen LH. Competitive chemiluminescent enzyme immunoassay for vitamin B12 analysis in human milk. Food Chem 2014;153:60–5. [DOI] [PubMed] [Google Scholar]
- 34.Institute of Medicine. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B-12, pantothenic acid, biotin, and choline. 1998, National Academy Press: Washington, DC. [PubMed] [Google Scholar]
- 35.Hotz C, Lubowa A, Sison C, Moursi M, Loechl C. A food composition table for Central and Eastern Uganda. HarvestPlus technical monograph 9: Washington, DC. 2012. [Google Scholar]
- 36.Institute of Medicine. Dietary reference intakes: the essential guide to nutrient requirements. 2006, National Academy of Sciences: Washington, DC. p. 1344. [Google Scholar]
- 37.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–30. [PubMed] [Google Scholar]
- 38.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–8S. [DOI] [PubMed] [Google Scholar]
- 39.Kigutha HN, van Staveren WA, Wijnhoven TM, Hautvast JG. Maternal nutritional status may be stressed by seasonal fluctuations in food availability: evidence from rural women in Kenya. Int J Food Sci Nutr 1995;46:247–55. [DOI] [PubMed] [Google Scholar]
- 40.Duggan C, Srinivasan K, Thomas T, Samuel T, Rajendran R, Muthayya S, Finkelstein JL, Lukose A, Fawzi W, Allen LH, et al. . Vitamin B-12 supplementation during pregnancy and early lactation increases maternal, breast milk, and infant measures of vitamin B-12 status. J Nutr 2014;144:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luhby AL, Cooperman JM, Donnefeld AM, Herreo JM, Teller DN, Wenig JB. Observations on transfer of Vitamin B12 from mother to fetus and newborn. Am J Dis Child 1958;96:532–3. [Google Scholar]
- 42.Conde-Agudelo A, Rosas-Bermudez A, Castano F, Norton MH. Effects of birth spacing on maternal, perinatal, infant, and child health: a systematic review of causal mechanisms. Stud Fam Plann 2012;43:93–114. [DOI] [PubMed] [Google Scholar]
- 43.King JC. The risk of maternal nutritional depletion and poor outcomes increases in early or closely spaced pregnancies. J Nutr 2003;133:1732S–6S. [DOI] [PubMed] [Google Scholar]
- 44.WHO. Report of a WHO technical consultation on birth spacing. 2006: Geneva, Switzerland, 13–15 June 2005. p. 44. [Google Scholar]
- 45.Alemayehu AA, Abebe Y, Gibson RSA. 24-h recall does not provide a valid estimate of absolute nutrient intakes for rural women in southern Ethiopia. Nutrition 2011;27:919–24. [DOI] [PubMed] [Google Scholar]
- 46.Lee SE, Talegawkar SA, Merialdi M, Caulfield LE. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr 2013;16:1340–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becquey E, Martin-Prevel Y. Micronutrient adequacy of women’s diet in urban Burkina Faso is low. J Nutr 2010;140:2079S–85S. [DOI] [PubMed] [Google Scholar]
- 48.Arsenault JE, Yakes EA, Islam MM, Hossain MB, Ahmed T, Hotz C, Lewis B, Rahman AS, Jamil KM, Brown KH. Very low adequacy of micronutrient intakes by young children and women in rural Bangladesh is primarily explained by low food intake and limited diversity. J Nutr 2013;143:197–203. [DOI] [PubMed] [Google Scholar]
- 49.Campbell RK, Talegawkar SA, Christian P, LeClerq SC, Khatry SK, Wu LS, West KP Jr. Seasonal dietary intakes and socioeconomic status among women in the Terai of Nepal. J Health Popul Nutr 2014;32:198–216. [PMC free article] [PubMed] [Google Scholar]
- 50.Kamau-Mbuthia E, Elmadfa I. Diet quality of pregnant women attending an antenatal clinic in Nakuru, Kenya. Ann Nutr Metab 2007;51:324–30. [DOI] [PubMed] [Google Scholar]
- 51.McLean ED, Allen LH, Neumann CG, Peerson JM, Siekmann JH, Murphy SP, Bwibo NO, Demment MW. Low plasma vitamin B-12 in Kenyan school children is highly prevalent and improved by supplemental animal source foods. J Nutr 2007;137:676–82. [DOI] [PubMed] [Google Scholar]
- 52.M’Kaibi FK, Steyn NP, Ochola S, Du Plessis L. Effects of agricultural biodiversity and seasonal rain on dietary adequacy and household food security in rural areas of Kenya. BMC Public Health 2015;15:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arimond M, Wiesmann D, Becquey E, Carriquiry A, Daniels MC, Deitchler M, Fanou-Fogny N, Joseph ML, Kennedy G, Martin-Prevel Y, et al. . Simple food group diversity indicators predict micronutrient adequacy of women’s diets in 5 diverse, resource-poor settings. J Nutr 2010;140:2059S–69S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allen LH, Dror DK. Effects of animal source foods, with emphasis on milk, in the diet of children in low-income countries. Nestle Nutr Workshop Ser Pediatr Program 2011;67:113–30. [DOI] [PubMed] [Google Scholar]
- 55.Allen LH. Micronutrient research, programs, and policy: From meta-analyses to metabolomics. Adv Nutr 2014;5:344S–51S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS, and Maternal Child Undernutrition Study Group. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008;371:340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mendez MA, Adair LS. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J Nutr 1999;129:1555–62. [DOI] [PubMed] [Google Scholar]
- 58.Duong MC, Mora-Plazas M, Marin C, Villamor E. Vitamin B-12 deficiency in children is associated with grade repetition and school absenteeism, independent of folate, iron, zinc, or vitamin a status biomarkers. J Nutr 2015;145:1541–8. [DOI] [PubMed] [Google Scholar]
- 59.Hulett JL, Weiss RE, Bwibo NO, Galal OM, Drorbaugh N, Neumann CG. Animal source foods have a positive impact on the primary school test scores of Kenyan schoolchildren in a cluster-randomised, controlled feeding intervention trial. Br J Nutr 2014;111:875–86. [DOI] [PubMed] [Google Scholar]