Abstract
Background: A high dietary calcium intake with adequate vitamin D status has been linked to lower colorectal cancer risk, but the mechanisms of these effects are poorly understood.
Objective: The objective of this study was to elucidate the effects of a Western-style diet (WD) and supplemental calcium and/or 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] on the colorectal mucosa.
Design: We conducted 2 crossover trials to define molecular pathways in the human colorectum altered by 1) a 4-wk WD supplemented with and without 2 g calcium carbonate/d and 2) a 4-wk WD supplemented with 1,25(OH)2D3 (0.5 μg/d) with or without 2 g calcium carbonate/d. The primary study endpoint was genome-wide gene expression in biopsy specimens of the rectosigmoid colonic mucosa. Serum and urinary calcium concentrations were also measured.
Results: Changes in urinary calcium accurately reflected calcium consumption. The WD induced modest upregulation of genes involved in inflammatory pathways, including interferon signaling, and calcium supplementation reversed these toward baseline. In contrast, supplementation of the WD with 1,25(OH)2D3 induced striking upregulation of genes involved in inflammation, immune response, extracellular matrix, and cell adhesion. Calcium supplementation largely abrogated these changes.
Conclusions: Supplementing 1,25(OH)2D3 to a WD markedly upregulated genes in immune response and inflammation pathways, which were largely reversed by calcium supplementation. This study provides clinical trial evidence of global gene expression changes occurring in the human colorectum in response to calcium and 1,25(OH)2D3 intervention. One action of 1,25(OH)2D3 is to upregulate adaptive immunity. Calcium appears to modulate this effect, pointing to its biological interaction in the mucosa. This trial was registered at clinicaltrials.gov as NCT00298545. Trial protocol is available at http://clinicalstudies.rucares.org (protocol numbers PHO475 and PHO554).
Keywords: 1,25-dihydroxyvitamin D3; calcium; colon; gene expression; Western diet
See corresponding editorial on page 1195.
INTRODUCTION
Colorectal cancer (CRC)8 remains a major cause of cancer deaths in the Western world. Adoption of a Western lifestyle and diet, low in vitamin D and calcium, is a global problem leading to increased obesity and cancer incidence, including CRC (1). In addition to colonoscopic detection and removal of precancerous adenomas, safe chemoprevention is an adjunct measure for risk reduction.
Epidemiologic, observational, and randomized trials support the role of calcium and vitamin D in CRC chemoprevention (2–7). Although not all data are conclusive (8–10), moderate doses of calcium and vitamin D are both safe and modestly effective in reducing CRC incidence. However, it is difficult to separate the effects of vitamin D and calcium because vitamin D concentrations directly affect calcium concentrations through the effect of vitamin D in regulating calcium transport in the intestinal epithelium. Thus, the effects of calcium and vitamin D on reducing tumor incidence are likely linked. For example, a large randomized, placebo-controlled trial exploring effects of calcium supplementation and blood concentrations of 25-hydroxyvitamin D on adenoma recurrence found that calcium supplementation reduced adenoma recurrence (2). However, this finding was limited to those whose vitamin D blood concentrations were greater than the median level of the clinical trial population at baseline (73 nmol/L) (4). In those with vitamin D concentrations below this level, calcium supplementation did not reduce risk.
Both in vitro and animal studies show that calcium (11–13) and vitamin D (14) exert an impact on pathways involved in colonic neoplasia. However, these studies do not determine the direct effects of these compounds on the colon of subjects at risk of developing CRC. Furthermore, the effects of vitamin D and calcium on the human colorectum are largely unknown, and whole-genome expression responses have not been evaluated.
Our group has completed several studies of molecular events in rectosigmoid mucosal biopsy specimens that accompany administration of putative risk reduction agents such as estrogen and folic acid (15, 16). The present study aimed to identify the effects of calcium and/or 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] supplementation on gene expression profiles of the human colorectal mucosa of subjects at moderate risk of CRC consuming a Western-style diet (WD).
METHODS
For each of the 2 studies, 10 healthy subjects at modestly increased risk of colorectal neoplasia with a personal history of colorectal adenomas or a first-degree relative with a history of colorectal cancer or adenomas were recruited. Subjects included 9 men and 11 women (Table 1; detailed description is shown in Supplemental Table 1). Exclusion criteria included a history of cancer other than nonmelanoma skin cancer, previous intestinal surgery, a history suggestive of malabsorption, consumption of medications with anti-inflammatory properties such as nonsteroidal anti-inflammatory drugs or statins, and any contraindications to undergoing a sigmoidoscopy and biopsy, including any history of excessive bleeding. The study was approved by the institutional review board of the Rockefeller University (New York, New York), and written informed consent was obtained from each subject before participation in the study. Individuals performing the endpoint analyses were blinded to the diet details.
TABLE 1.
Study subjects’ characteristics1
| Sex, n |
Ethnicity, n |
||||||||
| Age, y | Weight, kg | Height, cm | BMI, kg/m2 | M | F | W | AA | O | |
| Study A | 57.9 ± 7.82 | 80.76 ± 16.16 | 171.1 ± 10.71 | 27.35 ± 2.78 | 5 | 5 | 9 | 1 | 0 |
| Study B | 58.1 ± 5.84 | 90.49 ± 21.17 | 170 ± 12.62 | 28.79 ± 5.99 | 4 | 6 | 7 | 3 | 0 |
Subjects were excluded if they had a history of cancer other than nonmelanoma skin cancer, previous major intestinal surgery, malabsorption or bleeding disorders, estrogen and/or progesterone replacement, supplemental vitamin D or nonsteroidal anti-inflammatory drug intake, or systemic or bowel inflammatory disorder. For more details, refer to Supplemental Table 1. AA, African American; O, other; W, white.
Mean ± SD (all such values).
Subjects consumed a WD developed by the Nutrition Department at Rockefeller University. This ∼2200-kcal diet (40% derived from fat, 20% from protein) included 400 mg Ca and was relatively low in vitamin D content (Supplemental Table 2). After screening for exclusion criteria, volunteers underwent baseline testing, which included fasting blood tests, urine analysis, and evaluations of the nutritional composition of each subject’s prestudy diet by trained nutritionists by using The Food Processor Software Program, version 8.8.0 (ESHA Research) (Supplemental Table 2). A food-frequency questionnaire was administered once to assess the prestudy diets. For the duration of the studies, all subjects were admitted to the metabolic beds of the Rockefeller University hospital Center for Clinical and Translational Science Award and counseled throughout the study to maintain their usual physical activity. All main meals were consumed in the inpatient facility. The subjects were allowed to consume their research study lunch outside Rockefeller University Hospital. The prepared diets were considered part of the intervention, and all meals were consumed. Compliance was ensured by careful recruitment of the volunteers by the research nursing staff. Regular safety blood testing, which included measurement of serum calcium, phosphorus, and renal function tests, was performed.
Procedures and sample collections
Complete blood counts, serum electrolytes, renal function tests, and lipid profiles were performed by the clinical chemistry laboratory at Memorial Sloan-Kettering Cancer Center. Flexible sigmoidoscopies were performed after a 60-mL tap water enema between 0800 and 1000. Rectosigmoid mucosal biopsy specimens were taken ∼15 cm from the anal verge as described previously (15, 16). Six biopsy specimens for gene expression analysis were taken, immediately frozen in liquid nitrogen, and stored at −80°C.
Experimental study design
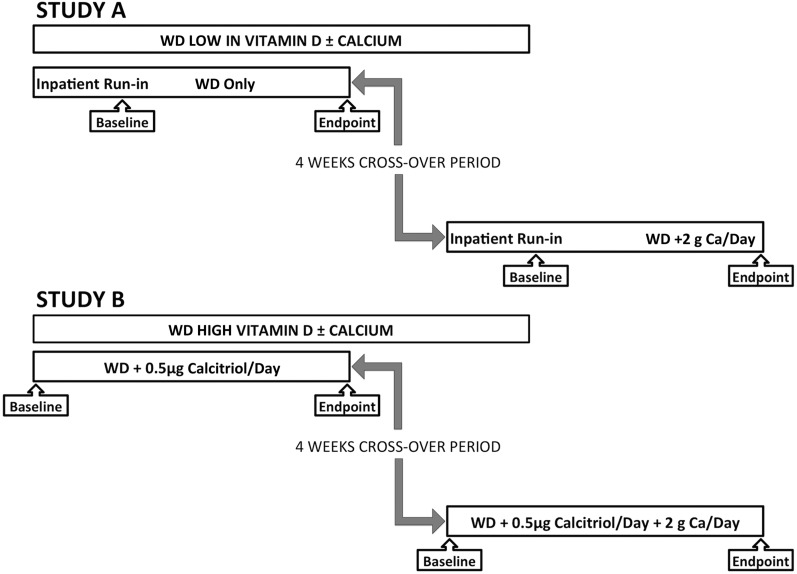
Two crossover studies of 4 wk in duration with a 4-wk crossover washout period were performed. In each study, 10 subjects were randomly allocated in 5 blocks of 2 by using a sequence of arrangement of AB, BA. The Rockefeller University Research Pharmacy did the randomization, and both the subjects and investigators were blinded to the calcium intervention. A summary of studies is shown in Figure 1. In all studies, subjects consumed the standard WD (detailed in Supplemental Table 2). In study A, the diet was supplemented with 2 g calcium carbonate/d. In study B, all subjects received 0.5 μg (or 20 IU) 1,25(OH)2D3/d (Roche) taken as 0.25 μg (10 IU) just before the morning and evening meals. After a 4-wk washout period during which subjects consumed their normal diet, the effect of adding 2 g calcium carbonate to the WD supplemented with 0.5 μg (20 IU) 1,25(OH)2D3/d was investigated for 1 mo (Supplemental Figure 1). To determine the effects of vitamin D action on the rectosigmoid mucosa, 1,25(OH)2D3 was used to obtain a rapid vitamin effect. Administration of oral vitamin D would not have permitted a reproducible increase in serum 25-hydroxyvitamin D3 [25(OH)D3] concentrations in a small group of subjects in this relatively short-term study. The primary study endpoint was genome-wide gene expression in biopsy specimens of the rectosigmoid colonic mucosa. Serum and urinary calcium concentrations also were measured.
FIGURE 1.
Diagram of study organization. The figure represents an overview of the study design. All interventions were performed for 4 wk while subjects consumed a WD with or without supplemental calcium (as 2 g calcium carbonate/d) (study A) or a WD supplemented with vitamin D (as 0.5 μg calcitriol/d) with or without supplemental calcium (study B). There was a 4-wk “washout” period during which the subjects were advised by our nutritionists to return strictly to their prestudy diet. There was no run-in period for study B because the WD was found to be so similar to the subjects’ prestudy diets. WD, Western-style diet.
RNA extraction for expression analyses
Frozen human biopsy specimens were maintained in liquid nitrogen until total RNA was extracted by using the Trizol method (Invitrogen). Trizol-extracted RNA was further purified by using Qiagen RNEasy kits (Qiagen Inc.), yielding high-quality RNA suitable for microarray analyses. RNA quality was verified by capillary electrophoresis on an Agilent 2100 Bioanalyzer (Agilent Technologies), and RNA was quantified by NanoDrop (NanoDrop Technologies). For gene arrays, RNA was processed, amplified, labeled, and hybridized to arrays, and each array was scanned following standard recommended protocols as described previously by using 2 platforms: study A used 28,000 complementary DNA arrays produced at the Albert Einstein College of Medicine microarray facility as described previously. This consisted of the polymerase chain reaction–amplified products of ∼28,000 cloned sequences encompassing the annotated genome (17); all 10 subjects had a full set of gene array paired data available. Study B used commercial Affymetrix HG-U133 Plus2 arrays, with 8 subjects having a full set of paired gene expression data.
Gene array analyses
Albert Einstein College of Medicine arrays were generated and processed as described (17), and Affymetrix arrays were processed in accord with the manufacturer’s recommendations. Expression data were imported and normalized with GeneSpring v12 software (Agilent Technologies) by using quantile normalization. Data points for group analysis were averaged as done previously in our clinical trials (15, 16). Because 2 different gene array platforms were used, analyses of gene expression data were performed by using Gene Set Enrichment Analysis (18, 19), an approach better suited to directly compare changes in gene expression across different platforms by using gene set enrichment scores. Gene Set Enrichment Analysis is a computational method that determines whether an a priori defined set of genes shows statistically significant differences between 2 data sets. We ranked gene expression differences between intervention time points and baseline to identify gene sets that were statistically significantly enriched after intervention (http://www.broadinstitute.org/gsea). Gene Ontology, Canonical Pathways, and Transcriptional Factor Targets curated data sets were used to interpret the results. Version 4 of the Gene Set Enrichment Analysis Molecular Signature Database was used for this study. Gene sets that were positively or negatively enriched at P < 0.05 and a false discovery rate <5% (or false discovery rate <10% for Transcriptional Factor Targets) were considered significant. Enrichment heatmaps were used to visualize enriched categories across the interventional trials.
Statistics
The number of subjects was determined a priori by using sample size estimates derived from alterations in rectosigmoid gene expression observed in prior studies (15, 16), for the desired power of 0.8, per gene α of 0.05, and SD of 0.5. Detailed methods for statistical analysis of gene arrays are described above. Paired t test performed in Excel 2013 (Microsoft Corp.) was used to compare anthropometric measurements and biochemical variables. Significance was set at a value of P < 0.05.
RESULTS
All subjects successfully completed the study protocols and procedures without ill effects. Serum calcium and phosphorus concentrations did not change significantly with the interventions, but serum blood urea nitrogen rose marginally in response to calcium supplementation. Urine calcium output increased with calcium supplementation in both study A and study B (details in Supplemental Table 3).
The design of the 2 studies is summarized in Figure 1. The primary endpoint was a change in gene expression in biopsy specimens of the rectosigmoid mucosa. The goal of these studies was to investigate the interaction of calcium and of 1,25(OH)2D3 during consumption of a WD.
Study A: effects of the WD with or without calcium supplementation
For this study, baseline biopsy specimens were taken after a 2-wk inpatient run-in period to minimize the effect of variation in the subjects’ prestudy diets, followed by a 4-wk study of calcium supplementation. In Table 2, it is shown that the WD modestly upregulated pathways related to immune and/or inflammatory processes but significantly downregulated the nuclear factor of activated T cells pathway. Genes contributing most to the pathway’s enrichment were human leukocyte antigen (HLA) class genes ISG20, ISG15, IL1B, IL1A, FAS, TNF, IFNAR1, and STAT2 (for full details for pathway changes and individual genes involved, see Supplemental Figure 1). Calcium supplementation of the WD showed no significant enrichment of any gene pathway, but modest reversal of the effects of the WD was observed (Figure 2). Of interest, calcium administration with the WD was accompanied by upregulation of genes in the interferon signaling and the IL-23 pathways.
TABLE 2.
Canonical pathways and gene ontology groups modulated by administration of the WD with or without calcium supplementation1
| FDR-q val | ↑ or ↓ | Database | |
| WD | |||
| ANTIGEN_PROCESSING_AND_PRESENTATION | 0.013 | ↑ | KEGG |
| INTERFERON_ALPHA_BETA_SIGNALING | 0.014 | ↑ | REACTOME |
| TYPE_I_DIABETES_MELLITUS | 0.023 | ↑ | KEGG |
| GRAFT_VERSUS_HOST_DISEASE | 0.052 | ↑ | KEGG |
| INTERFERON_SIGNALING | 0.064 | ↑ | REACTOME |
| NFAT_TFPATHWAY | 0.031 | ↓ | PID |
| WD + 2 g Ca/d | |||
| No category or pathway enriched at significance criteria (FDR ≤ 0.05) | |||
The WD was high in fat content (40% of calories) and low in vitamin D (estimated as ∼150–200 IU/d) and calcium (∼450 mg/d). To determine effects of a high calcium intake, 2 g calcium carbonate/d was added to the WD. All of the pathways upregulated are related to immune or inflammatory processes. FDR, false discovery rate; FDR-q val, false discovery rate adjusted significance (q value); WD, Western-style diet; ↑, increase; ↓, decrease.
FIGURE 2.
Heatmap of Gene Set Enrichment Analysis of canonical pathways. The WD induced small but significant upregulation of genes involved in immunity-modulated pathways such as interferon signaling or antigen processing and presentation. Supplementation with calcium partially reversed these changes toward baseline. The WD supplemented with vitamin D induced striking upregulation of genes involved in immune response and inflammation, extracellular matrix, and cell adhesion and cell cycle. Addition of calcium largely reversed these changes. Only the top significant pathways and categories are presented in the figure (FDR-q val < 0.005). For a full list of significantly enriched pathways and categories, please refer to Table 2 and Supplemental Table 4. FDR-q val, false discovery rate adjusted significance (q value); WD, Western-style diet.
Study B: effects of the WD supplemented with vitamin D with or without calcium
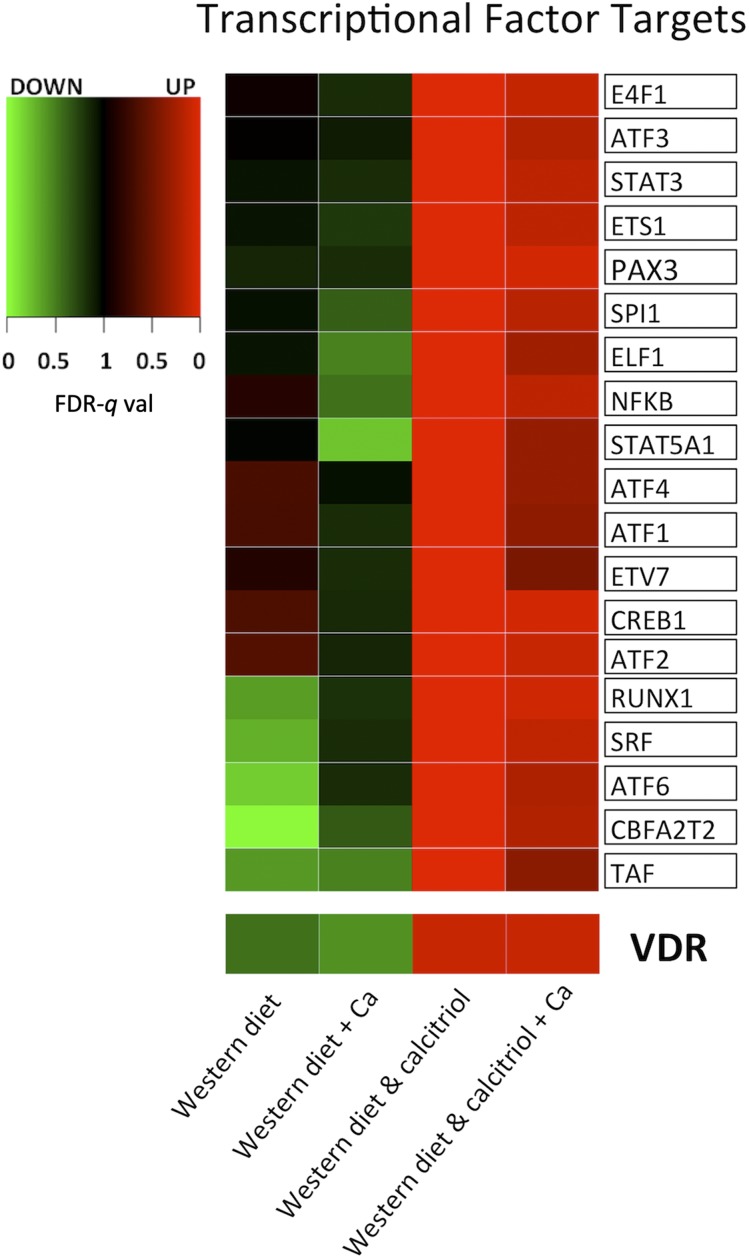
To induce a rapid systemic active vitamin D effect, 1,25(OH)2D3 was administered in pharmacotherapeutic doses. The 1,25(OH)2D3 upregulated vitamin D receptor (VDR) transcription factor targets (Figure 3, bottom), indicating that colorectal mucosal genes known to be activated by vitamin D responded to this intervention. The 1,25(OH)2D3 induced striking upregulation of genes involved in immune and inflammatory response processes such as chemokines and cytokines, complement system genes and HLA genes, and the cell cycle pathway genes such as DNA polymerases, cyclin-dependent kinases, and mini chromosome maintenance genes. Furthermore, 1,25(OH)2D3 induced significant upregulation of cell adhesion and extracellular matrix genes such as collagens, matrix metalloproteinases, integrins, and serine protease inhibitors (Table 3, Figure 2, and Supplemental Table 4). The 1,25(OH)2D3 also induced striking upregulation of genes that are targets of transcriptional factors known to play key roles in inflammation, immune response, proliferation, and extracellular matrix function (Figure 3 and Supplemental Table 5). Calcium administration largely reversed the changes associated with 1,25(OH)2D3 supplementation (Figures 1 and 2 and Supplemental Table 4). For full details on significant pathways contributing to enrichment underlying the effect of calcium, see Supplemental Table 6.
FIGURE 3.
Heatmap of Gene Set Enrichment Analysis of Transcriptional Factor targets. The Western-style diet supplemented with vitamin D induced significant upregulation of VDR target genes (VDR, FDR-q val = 0.09) and target genes of other transcription factors that have key roles in immune response, proliferation, and extracellular matrix function. Addition of calcium reversed these changes toward baseline. Note that only the top significant pathways and categories are presented in the figure (FDR-q val < 0.01). For a full list of significantly enriched pathways, please refer to Supplemental Table 5. FDR-q val, false discovery rate adjusted significance (q value); VDR, vitamin D receptor.
TABLE 3.
Selected canonical pathways and gene ontology groups modulated by administration of vitamin D with or without calcium supplementation1
| FDR-q val | ↑ or ↓ | Database | |
| WD + vitamin D | |||
| CHEMOKINE_SIGNALING_PATHWAY | <0.000 | ↑ | REACTOME |
| LEISHMANIA_INFECTION | <0.000 | ↑ | KEGG |
| INTERFERON_ALPHA_BETA_SIGNALING | <0.000 | ↑ | REACTOME |
| CYTOKINE_CYTOKINE_RECEPTOR_INTERACTION | <0.000 | ↑ | KEGG |
| NOD_LIKE_RECEPTOR_SIGNALING_PATHWAY | <0.000 | ↑ | KEGG |
| ASTHMA | <0.000 | ↑ | KEGG |
| IL12_2PATHWAY | <0.000 | ↑ | PID |
| G1_S_TRANSITION | <0.000 | ↑ | REACTOME |
| INTEGRIN1_PATHWAY | <0.000 | ↑ | PID |
| INTERFERON_GAMMA_SIGNALING | <0.000 | ↑ | REACTOME |
| CELL_ADHESION_MOLECULES_CAMS | <0.000 | ↑ | KEGG |
| ANTIGEN_PROCESSING_AND_PRESENTATION | <0.001 | ↑ | KEGG |
| MITOTIC_G1_G1_S_PHASES | <0.001 | ↑ | REACTOME |
| IL23PATHWAY | <0.001 | ↑ | PID |
| CHEMOKINE_ACTIVITY | <0.000 | ↑ | GO |
| CYTOKINE_ACTIVITY | <0.000 | ↑ | GO |
| INFLAMMATORY_RESPONSE | <0.000 | ↑ | GO |
| IMMUNE_RESPONSE | <0.000 | ↑ | GO |
| EXTRACELLULAR_REGION | <0.001 | ↑ | GO |
| REGULATION_OF_CELL_CYCLE | <0.001 | ↑ | GO |
| WD + vitamin D + 2 g Ca/d | |||
| CHEMOKINE_RECEPTORS_BIND_CHEMOKINES | <0.001 | ↑ | REACTOME |
| CHEMOKINE_ACTIVITY | 0.011 | ↑ | GO |
| IMMUNE_RESPONSE | 0.049 | ↑ | GO |
| PEROXISOME | <0.001 | ↓ | KEGG |
| EXCRETION | <0.001 | ↓ | GO |
| PEROXISOME | 0.005 | ↓ | GO |
| SECRETION | 0.012 | ↓ | GO |
The full list is shown in Supplemental Table 4. The WD was designed as high in fat content (40% of calories) and low in vitamin D (estimated as ∼150–200 IU/d) and calcium (∼400 mg/d). We determined the effects of vitamin D (0.5 μg calcitriol) with or without 2 g supplemental Ca/d on rectosigmoid mucosal gene expression. Note that most of the pathways or processes changed by vitamin D occurred in immune or inflammatory genes with some contribution of cell cycle genes and extracellular matrix protein genes. Most of these effects of vitamin D were abrogated by calcium supplementation. FDR-q val, false discovery rate adjusted significance (q value); WD, Western-style diet; ↑, increase; ↓, decrease.
DISCUSSION
Epidemiologic evidence suggests that higher calcium and/or high vitamin D intake reduces colorectal neoplasia risk. Randomized clinical trials have shown that calcium lowers adenoma recurrence, possibly linked to circulating vitamin D concentrations (2, 4). Mechanisms suggested as responsible for the beneficial effects of calcium supplementation in WD-consuming subjects (11–13) include reduced solubility of damaging bile acids (20), fatty acids (21), or heme (22) in the colonic lumen and lower proliferation of colonic epithelial cells (11). Nevertheless, it has been difficult to separate the relative actions of vitamin D and calcium because of their biological interactions and our incomplete understanding of their effects in the human colon.
To clarify interactions of calcium and vitamin D, we conducted 2 randomized clinical trials investigating the effects of supplementing calcium with or without supplementation with 1,25(OH)2D3 in subjects at increased risk of colorectal neoplasia consuming a WD. Because little is known about their actions in the human colon, we used an unbiased approach studying altered expression of canonical gene sequences in colorectal biopsy specimens associated with calcium supplementation or by 1,25(OH)2D3 with or without calcium supplementation.
It was surprising that providing a WD alone induced only small changes in gene expression. In contrast, 1,25(OH)2D3 produced striking upregulation of genes involved in immune responses, inflammation, extracellular matrix, and cell adhesion. Remarkably, upregulation of these gene pathways was almost completely eliminated by the addition of 2 g calcium carbonate/d to the WD that contained ∼400 g Ca/d. To our knowledge, ours is the first report to examine the effects of 1,25(OH)2D3 with or without supplemental calcium on global gene expression in the human colorectum.
Others have investigated the effects of calcium and vitamin D on the colon in human subjects. For example, a randomized study showed that vitamin D or calcium administration, but not their combination, reduced an inflammatory marker score (23). Semiquantitative immunohistochemical studies of the distribution in colonic crypts of the calcium receptor, VDR, and CYP27B1 and CYP24A1 proteins suggested that several effects of vitamin D, such as an upregulated level of CYP27B1 or the calcium receptor, were also reduced when calcium carbonate was added (24). We also observed a similar effect on the whole-genome scale as the 1,25(OH)2D3 regimen greatly increased the expression of genes in immune and inflammatory, cell cycle, and matrix function pathways, and this effect was abrogated by calcium supplementation. Importantly, gene enrichment analysis showed that 1,25(OH)2D3 upregulated several vitamin D responsive genes, thus showing a direct effect of the vitamin on the colonic epithelium. In addition, we also observed that calcium supplementation reversed the modest upregulation of genes involved in immune function and inflammation induced by the WD containing only 200 IU of vitamin D content. These results together imply that calcium markedly modulates the effects of 1,25(OH)2D3 in the colorectal mucosa.
Striking upregulation of immune and inflammatory gene pathways induced by the 1,25(OH)2D3 regimen was unexpected, although we are unaware of other whole-genome gene expression data for the human colon. However, several lines of experimental evidence point to a strong immunomodulatory effect of 1,25(OH)2D3 on both the innate and adaptive immune systems (25). VDR is expressed in activated T cells and antigen-presenting cells, and 25-α-hydroxylase, 1α-hydroxylase, and 24,25-hydroxylase enzymes involved in vitamin D metabolism are expressed by cells of the immune system (26). Active vitamin D also exerts pronounced effect on dendritic cells (27). The immune system of VDR null or vitamin D–deficient mice is grossly normal but shows increased sensitivity to autoimmune diseases such as inflammatory bowel disease or type 1 diabetes (28). VDR-deficient mice do not have a spontaneous increase in cancer but are more prone to oncogene- or chemocarcinogen-induced tumors (28). One randomized double-blind placebo-controlled study showed that high-dose cholecalciferol 50,000 IU/wk for 12 wk, followed by 50,000 IU every other week for 40 wk, did not significantly alter circulating inflammatory markers at the end of the study, confirmed by 3 other studies (29–33). On the other hand, low concentrations of vitamin D are associated with a poorer clinical outcome in some autoimmune disorders such as Crohn disease (34). Thus, the immune-modulating effects of the vitamin may only be clinically apparent in states of abnormal immune activation.
A further finding was the effect of 1,25(OH)2D3 on the expression of genes involved in the cell cycle and in proliferation. Vitamin D has been reported to stimulate intestinal epithelial cell turnover after a large small bowel resection in rats (35) and also modulated CDK4 and CDK6 expression and localization in muscle tissue (36). The effects of 1,25(OH)2D3 on the cell cycle, extracellular matrix, and cell adhesion gene pathways that we observed in our study have been also described in cell culture studies by others (37). The biological mechanism for this observation in human colon is not clear, and further studies are needed to clarify.
Although our study was conducted in a tightly controlled environment in an inpatient metabolic unit, several limitations could affect interpretation of the results. We used 1,25(OH)2D3 to induce a rapid vitamin D effect on the colorectal epithelium, which differs from the effect of vitamin D3 in the diet or 25(OH)D3. To evaluate vitamin D actions on the colorectal epithelium, we needed to achieve a rapid and reproducible local vitamin D milieu. Oral vitamin D, even in large doses, results in variable changes in 25(OH)D3 concentrations in individual subjects, especially in a relatively short-term trial in small numbers of subjects (38). The more physiologic preparation of 25(OH)D3 medication is not available in the United States, so we elected to use 1,25(OH)2D3. It is possible that administration of 1,25(OH)2D3 might have resulted in transcriptional changes that might differ from those induced by oral vitamin D. However, 1,25(OH)2D3 did induce VDR transcriptional targets at the level of the colorectal mucosa. In addition, it was shown in a previous study that tissue levels of 1,25(OH)2D3 correlated closely with serum concentrations (39). Nevertheless, it is uncertain whether colonic epithelial 1α hydroxylation of circulating 25(OH)D3 would parallel gene expression signatures in the colon that occurred with the administration of 1,25(OH)2D3 in this trial.
In summary, this randomized clinical study describes changes in gene expression profiles in the colorectum in response to calcium and/or 1,25(OH)2D3 supplementation in subjects at elevated risk of colorectal neoplasia who were consuming a WD. The genes that are VDR transcriptional targets were significantly upregulated when 1,25-dihydroxyvitamin was provided, confirming this intervention had an effect on the colon. We show that calcium supplementation of a commonly consumed WD that is generally low in calcium and vitamin D content had only modest effects on gene expression. In contrast, 1,25(OH)2D3 induced gene expression from several immune and inflammatory pathways, an effect that was almost totally eliminated by addition of calcium to the WD. To our knowledge, this is the first human trial exploring the interaction between calcium, 1,25(OH)2D3, and whole-genome gene expression in the colorectal tissue. Our data raise questions that have considerable implications for better understanding of the chemoprevention of CRC by these agents.
Acknowledgments
The authors’ responsibilities were as follows—PP, SP, LHA, ML, and PRH: designed the research (project conception, development of overall research plan, and study oversight); SP, CN, LHA, and PRH: conducted the research (hands-on conduct of the experiments and data collection); PP, LHA, ML, and PRH: provided essential reagents or essential materials and wrote the manuscript; and PP, LHA, and PRH: analyzed the data or performed the statistical analysis and had primary responsibility for the final content. None of the authors reported any conflicts of interest.
Footnotes
Abbreviations used: CRC, colorectal cancer; VDR, vitamin D receptor; WD, Western-style diet; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3.
REFERENCES
- 1.Vargas AJ, Thompson PA. Diet and nutrient factors in colorectal cancer risk. Nutr Clin Pract 2012;27:613–23. [DOI] [PubMed] [Google Scholar]
- 2.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, Rothstein R, Summers RW, Snover DC, Beck GJ, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med 1999;340:101–7. [DOI] [PubMed] [Google Scholar]
- 3.Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst 2007;99:1594–602. [DOI] [PubMed] [Google Scholar]
- 4.Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML, Church TR, Heber D. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst 2003;95:1765–71. [DOI] [PubMed] [Google Scholar]
- 5.Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, Jansen EH, Slimani N, Byrnes G, Rinaldi S, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. BMJ 2010;340:b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieberman DA, Prindiville S, Weiss DG, Willett W, Group VACS. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 2003;290:2959–67. [DOI] [PubMed] [Google Scholar]
- 7.McCullough ML, Robertson AS, Rodriguez C, Jacobs EJ, Chao A, Carolyn J, Calle EE, Willett WC, Thun MJ. Calcium, vitamin D, dairy products, and risk of colorectal cancer in the Cancer Prevention Study II Nutrition Cohort (United States). Cancer Causes Control 2003;14:1–12. [DOI] [PubMed] [Google Scholar]
- 8.Hartman TJ, Albert PS, Snyder K, Slattery ML, Caan B, Paskett E, Iber F, Kikendall JW, Marshall J, Shike M, et al. The association of calcium and vitamin D with risk of colorectal adenomas. J Nutr 2005;135:252–9. [DOI] [PubMed] [Google Scholar]
- 9.Huncharek M, Muscat J, Kupelnick B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: a meta-analysis of 26,335 cases from 60 observational studies. Nutr Cancer 2009;61:47–69. [DOI] [PubMed] [Google Scholar]
- 10.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006;354:684–96. [DOI] [PubMed] [Google Scholar]
- 11.Lipkin M, Newmark H. Calcium and the prevention of colon cancer. J Cell Biochem Suppl 1995;22:65–73. [DOI] [PubMed] [Google Scholar]
- 12.Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis 2009;30:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang K, Lamprecht SA, Shinozaki H, Fan K, Yang W, Newmark HL, Kopelovich L, Edelmann W, Jin B, Gravaghi C, et al. Dietary calcium and cholecalciferol modulate cyclin D1 expression, apoptosis, and tumorigenesis in intestine of adenomatous polyposis coli1638N/+ mice. J Nutr 2008;138:1658–63. [DOI] [PubMed] [Google Scholar]
- 14.Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J 2012;441:61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Protiva P, Cross HS, Hopkins ME, Kallay E, Bises G, Dreyhaupt E, Augenlicht L, Lipkin M, Lesser M, Livote E, et al. Chemoprevention of colorectal neoplasia by estrogen: potential role of vitamin D activity. Cancer Prev Res (Phila) 2009;2:43–51. [DOI] [PubMed] [Google Scholar]
- 16.Protiva P, Mason JB, Liu Z, Hopkins ME, Nelson C, Marshall JR, Lambrecht RW, Pendyala S, Kopelovich L, Kim M, et al. Altered folate availability modifies the molecular environment of the human colorectum: implications for colorectal carcinogenesis. Cancer Prev Res (Phila) 2011;4:530–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mariadason JM, Corner GA, Augenlicht LH. Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res 2000;60:4561–72. [PubMed] [Google Scholar]
- 18.Subramanian A, Kuehn H, Gould J, Tamayo P, Mesirov JP. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics 2007;23:3251–3. [DOI] [PubMed] [Google Scholar]
- 19.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernstein C, Holubec H, Bhattacharyya AK, Nguyen H, Payne CM, Zaitlin B, Bernstein H. Carcinogenicity of deoxycholate, a secondary bile acid. Arch Toxicol 2011;85:863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newmark HL, Wargovich MJ, Bruce WR. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J Natl Cancer Inst 1984;72:1323–5. [PubMed] [Google Scholar]
- 22.Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R. Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme. Cancer Res 1999;59:5704–9. [PubMed] [Google Scholar]
- 23.Hopkins MH, Owen J, Ahearn T, Fedirko V, Flanders WD, Jones DP, Bostick RM. Effects of supplemental vitamin D and calcium on biomarkers of inflammation in colorectal adenoma patients: a randomized, controlled clinical trial. Cancer Prev Res (Phila) 2011;4:1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahearn TU, McCullough ML, Flanders WD, Long Q, Sidelnikov E, Fedirko V, Daniel CR, Rutherford RE, Shaukat A, Bostick RM. A randomized clinical trial of the effects of supplemental calcium and vitamin D3 on markers of their metabolism in normal mucosa of colorectal adenoma patients. Cancer Res 2011;71:413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun J. Vitamin D and mucosal immune function. Curr Opin Gastroenterol 2010;26:591–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Etten E, Stoffels K, Gysemans C, Mathieu C, Overbergh L. Regulation of vitamin D homeostasis: implications for the immune system. Nutr Rev 2008;66(Suppl 2):S125–34. [DOI] [PubMed] [Google Scholar]
- 27.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 2000;164:2405–11. [DOI] [PubMed] [Google Scholar]
- 28.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008;29:726–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez JA, Zughaier SM, Law J, Hao L, Wasse H, Ziegler TR, Tangpricha V. Effects of high-dose cholecalciferol on serum markers of inflammation and immunity in patients with early chronic kidney disease. Eur J Clin Nutr 2013;67:264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes MS, Horigan G, Cashman KD, Hill TR, Forsythe LK, Lucey AJ, McSorley EM, Kiely M, Bonham MP, Magee PJ, et al. Maintenance of wintertime vitamin D status with cholecalciferol supplementation is not associated with alterations in serum cytokine concentrations among apparently healthy younger or older adults. J Nutr 2011;141:476–81. [DOI] [PubMed] [Google Scholar]
- 31.Belenchia AM, Tosh AK, Hillman LS, Peterson CA. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr 2013;97:774–81. [DOI] [PubMed] [Google Scholar]
- 32.Carrillo AE, Flynn MG, Pinkston C, Markofski MM, Jiang Y, Donkin SS, Teegarden D. Vitamin D supplementation during exercise training does not alter inflammatory biomarkers in overweight and obese subjects. Eur J Appl Physiol 2012;112:3045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, Simpson WG, Fraser WD, Reid DM, Macdonald HM. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab 2012;97:3557–68. [DOI] [PubMed] [Google Scholar]
- 34.Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, Richter JM, Fuchs CS, Chan AT. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology 2012;142:482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadjittofi C, Coran AG, Mogilner JG, Pollak Y, Matter I, Sukhotnik I. Dietary supplementation with vitamin D stimulates intestinal epithelial cell turnover after massive small bowel resection in rats. Pediatr Surg Int 2013;29:41–50. [DOI] [PubMed] [Google Scholar]
- 36.Irazoqui AP, Heim NB, Boland RL, Buitrago CG. 1alpha,25 dihydroxi-vitamin D(3) modulates CDK4 and CDK6 expression and localization. Biochem Biophys Res Commun 2015;459:137–42. [DOI] [PubMed] [Google Scholar]
- 37.Pálmer HG, Sanchez-Carbayo M, Ordonez-Moran P, Larriba MJ, Cordon-Cardo C, Munoz A. Genetic signatures of differentiation induced by 1alpha,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res 2003;63:7799–806. [PubMed] [Google Scholar]
- 38.Ponda MP, Dowd K, Finkielstein D, Holt PR, Breslow JL. The short-term effects of vitamin D repletion on cholesterol: a randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol 2012;32:2510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner D, Dias AG, Schnabl K, Van der Kwast T, Vieth R. Determination of 1,25-dihydroxyvitamin D concentrations in human colon tissues and matched serum samples. Anticancer Res 2012;32:259–63. [PubMed] [Google Scholar]