Abstract
OBJECTIVE
The purpose of this study was to define the cholangiographic patterns of ischemic cholangiopathy and clinically silent nonanastomotic biliary strictures in donation-after-cardiac-death (DCD) liver grafts in a large single-institution series. We also examined the correlation of the radiologic findings with laboratory data and clinical outcomes.
MATERIALS AND METHODS
Data were collected for all DCD liver transplants at one institution from December 1998 to December 2011. Posttransplant cholangiograms were obtained during postoperative weeks 1 and 3 and when clinically indicated. Intrahepatic biliary strictures were classified by anatomic distribution and chronologic development. Radiologic findings were correlated with laboratory data and with 1-, 3-, and 5-year graft and patient survival rates.
RESULTS
A total of 231 patients received DCD grafts. Cholangiograms were available for 184 of these patients. Postoperative cholangiographic findings were correlated with clinical data and divided into the following three groups: A, normal cholangiographic findings with normal laboratory values; B, radiologic abnormalities and cholangiopathy according to laboratory values; and C, radiologic abnormalities without laboratory abnormalities. Group B had four distinct abnormal cholangiographic patterns that were predictive of graft survival. Group C had mild nonprogressive multifocal stenoses and decreased graft and patient survival rates, although cholangiopathy was not detected in these patients according to laboratory data.
CONCLUSION
Patterns and severity of nonanastomotic biliary abnormalities in DCD liver transplants can be defined radiologically and correlate with clinical outcomes. Postoperative cholangiography can depict the mild biliary abnormalities that occur in a subclinical manner yet cause a marked decrease in graft and patient survival rates in DCD liver transplants.
Keywords: biliary strictures, cholangiopathy, donation after cardiac death, liver transplant
There is a large imbalance between available donor livers and the number of patients waiting for transplants. Donation after brain death (DBD) accounts for most liver grafts, but efforts are underway to increase other donor sources, such as living donor transplants, split-liver transplants, and donation-after-cardiac-death (DCD) transplants. DCD can occur when declaration of death is based on cardiopulmonary criteria rather than cessation of brain and brainstem function. DCD donors are an important source of much-needed liver grafts and are increasingly being used. At our institution, DCD grafts account for approximately 11% of all liver transplants performed. However, biliary problems in DCD organs cause morbidity, mortality, and increased retransplant rates [1–3]. The reported outcomes with DCD grafts have been inferior in comparison with those from DBD grafts. In one study, Skaro et al. [4] found that the risk of failure of DCD grafts was 2.1 times as high as that for DBD, the risk of placing the patient back on the waiting list for a new transplant was 2.5 times as high, and the risk of retransplantation was 3.2 times as high. Pooled national data and reports from individual liver transplant programs show DCD liver grafts as high risk because of the overall increased rates of graft loss and morbidity, which are mostly related to the consequences of biliary complications.
The DCD procurement process involves a donor with irreversible brain injuries who does not meet the strict criteria for brain death. After the family is consulted and arrangements are made for organ donation, life support measures are removed, and the endotracheal tube is removed. An agonal phase is observed until asystole occurs. After this, there is an additional mandatory waiting period before declaration of death. The Society of Critical Care Medicine has stated 2 minutes after asystole is adequate, but some hospitals mandate a 5-minute waiting period [5]. Once death occurs, the donor is immediately taken to the operating room, the abdominal vasculature is accessed, and the organs are flushed with preservation solution and harvested. Therefore, in the DCD procurement process, the graft is subjected to a period of hypoperfusion from the removal of life support until asystole and a period of no perfusion from asystole until the thoracic aorta is cross-clamped. Then preservation solution is infused [6, 7]. The total time of ischemia related to hypoperfusion and absence of perfusion is termed warm ischemia time.
Nonanastomotic biliary strictures are most often associated with hepatic artery thrombosis. When they occur in the presence of a patent hepatic artery, they have been called ischemic cholangiopathy [8] and ischemic biliary lesions [9]. In a study comparing DCD and DBD recipients [4], DCD recipients had a 31.6% higher incidence of biliary complications and a 35.8% higher incidence of ischemic cholangiopathy. These conditions necessitated longer and more frequent hospitalizations and more frequent and invasive interventions. The predominance of biliary complications in the presence of a patent hepatic artery suggests that the bile ducts may be exquisitely sensitive to the ischemia-reperfusion injury that occurs during DCD procurement. It has been postulated that the warm ischemia time to which the graft is subjected during DCD procurement may result in increased rates of ischemic cholangiopathy, but the exact mechanism is not known [7, 10]. In most cases ischemic cholangiopathy appears irreversible, and treatment options are limited, although not all patients lose their grafts. Consequently, the cost of treating patients who receive DCD grafts is much higher than that of treating other liver transplant recipients [10].
Because ischemic cholangiopathy is an important aspect of DCD liver transplants and this type of graft can be expected to become more frequent as DCD livers gain favor as an organ source, radiologists need to become familiar with this pathologic condition. Previous studies have gone only as far as delineating the geographic pattern of ischemic cholangiopathy seen with imaging [11]. We undertook this study to gain an understanding of ischemic cholangiopathy in DCD grafts beyond the anatomic patterns by delineating the progression of ischemic cholangiopathy and correlating the cholangiographic findings with clinical outcomes. To our knowledge, this is the largest single cohort of DCD grafts evaluated with routine postoperative cholangiography in the literature to date.
Materials and Methods
This was a retrospective review of all DCD liver transplants performed over a 13-year period at our institution from December 1998 to December 2011. Approval was obtained from our institutional review board. The study was performed by chart review and radiologic review of biliary imaging studies. Information gathered about the liver recipients included age, sex, cause of liver disease, calculated model for end-stage liver disease (MELD) score at transplant, and follow-up time. Detailed information regarding the DCD donors was obtained from the procurement database at our institution. Donor information included donor risk index and individual components of the donor risk index. The donor risk index is a quantitative risk assessment of donor characteristics that includes age, sex, race, cause of death, partial or split-liver graft, DCD graft, hepatitis B surface antigen status, hepatitic C virus status, donor height, and electrolyte levels [12]. All DCD donors were classified as Maastricht type 3 (controlled awaiting cardiac death) [13].
The study population included patients who received a DCD graft during the study period. Patients were excluded if they had primary nonfunction or hepatic artery thrombosis, if they died or lost their grafts before undergoing cholangiography, if there was lack of or insufficient cholangiography as a result of biliary tube dislodgment, or if a tube was not placed at surgery.
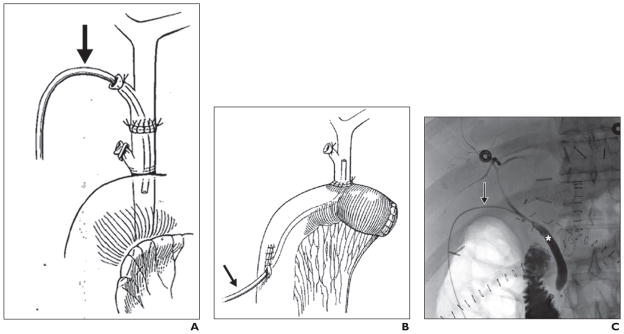
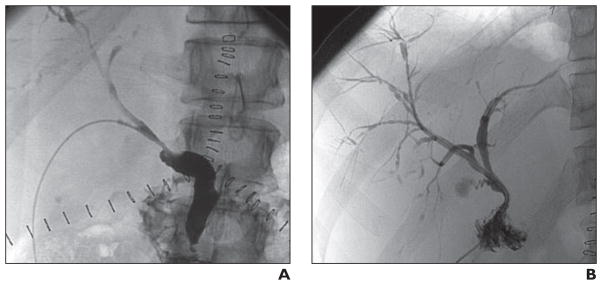
We have previously reported our surgical technique for the recovery of liver grafts from DCD donors and the interrelated events surrounding their recovery [7, 10]. Briefly, a rapid retrieval technique was followed in all procurements, and University of Wisconsin solution was used for aortic and portal flush. All liver transplants were performed with the piggyback technique without portocaval shunt, caval clamping, or venovenous bypass. All liver grafts were reperfused with portal flow followed by arterial flow. Duct-to-duct biliary reconstruction was used except in recipients with primary sclerosing cholangitis or when the surgeon operating on the recipient deemed biliary-enteric reconstruction necessary. In duct-to-duct biliary reconstruction, a 5-French ureteral stent (Bard polyurethane ureteral catheter, C. R. Bard) was placed through the cystic duct and secured to the stump with 5-0 polyglactin 910 suture (Vicryl, Ethicon) and a hemorrhoidal rubber band (Fig. 1). In Roux-en-Y biliary-enteric reconstruction, a 7-French soft silicone elastomer (Silastic, Dow Corning) tube (Jackson-Pratt, Cardinal Health) was placed through a Roux limb (Fig. 1). The biliary tube was then externalized through the abdominal wall, secured to the skin, and left to drain by gravity.
Fig. 1. Biliary tube placement.
A and B, Drawings show biliary tube placement (arrow) in duct-to-duct (A) and Roux-en-Y biliary-enteric (B) reconstruction. (By permission of Mayo Foundation for Medical Education and Research. All rights reserved.)
C, 58-year-old woman with normal cholangiogram after donation-after-cardiac-death lower transplant. Representative cholangiogram obtained through transcystic biliary tube (arrow) according to protocol on postoperative day 21. Asterisk indicates recipient common bile duct.
Posttransplant cholangiograms were obtained with a general guideline of performing cholangiography early during the first postoperative week and in the third postoperative week. The exact timing of cholangiography varied slightly owing to logistical factors, such as weekends, patient schedules, and travel arrangements. If the patients were doing well and the cholangiographic findings were normal, after the week 3 cholangiogram, the tube was removed. If there were laboratory abnormalities, radiographic abnormalities on cholangiograms, or patient conditions that suggested cholangiopathy (such as persistent right upper quadrant pain), the biliary tube was capped but not removed. The schedule of cholangiograms beyond the third postoperative week was highly variable and based on the clinical conditions of the individual patients.
Posttransplant cholangiograms were obtained by hand injection of 5–10 mL of iohexol (Omnipaque, GE Healthcare) into the indwelling biliary catheter under real-time fluoroscopic guidance until filling of the common bile duct and reflux into the intrahepatic ducts was obtained. Multiple radiographs were obtained in the anteroposterior and 30° oblique projections. The indwelling biliary catheters were flushed with 5–10 mL of sterile saline solution after completion of cholangiography.
Ischemic cholangiopathy is defined as intrahepatic or extrahepatic nonanastomotic biliary strictures in the absence of hepatic artery thrombosis [4, 9]. In our study, ischemic cholangiopathy was diagnosed cholangiographically by use of an intraoperatively placed transcystic duct–trans-Roux biliary tube, ERCP, or percutaneous transhepatic cholangiography (PTC). For this study, all cholangiograms were interpreted in consensus by an interventional radiology fellow and an attending interventional radiologist with more than 20 years’ experience who were blinded to the clinical outcome of the DCD liver graft recipients. The cholangiograms were graded according to severity, pattern, and progression of biliary ductal changes (narrowing, irregularity, and filling defects) in the intrahepatic and extrahepatic segments above the biliary anastomosis. Severity was subjectively graded as mild, moderate, or severe. Pattern was graded with a system modified from Buis et al. [11] whereby the nonanastomotic stenoses were classified the into four different zones within the biliary tree: hilar confluence, first-order bile ducts, second-order bile ducts, and peripheral bile ducts [14]. Time to the first appearance of the abnormalities and to progression of stenoses was recorded. The evolution of abnormalities related to progression, regression, or stability of the severity and geographic biliary involvement was also recorded with each subsequent cholangiogram.
The cholangiographic findings were correlated with retransplantation rate and with patient and graft survival. Categoric variables were examined with the chi-square test or Fisher exact test. Continuous variables were analyzed by t test or Mann-Whitney U test, as appropriate. Patient and graft survival were compared between groups by use of Kaplan-Meier plots and log-rank tests. For patient survival, time from liver transplant until death and, for graft survival, time from transplant to graft loss (retransplantation or death, whichever came first) were recorded. Censoring was done at the end of follow-up or on the date of the last correspondence for patients lost to follow-up. A value of p < 0.05 was considered significant. Statistical analysis was conducted with SPSS software (version 17.0, IBM-SPSS).
Results
During the study period a total of 231 patients received DCD liver grafts at our institution. All DCD liver graft recipients were retrospectively screened to identify those who underwent cholangiography after liver transplant. Cholangiograms were obtained for 184 DCD liver graft recipients (80%) during this time period. Forty-seven patients did not undergo cholangiography because the surgeons were technically unable to place a biliary tube at liver transplant or because the tube became dislodged before the initial postoperative day 3 cholangiogram. None of the 47 patients without cholangiograms had laboratory abnormalities suggestive of cholangiopathy; therefore, invasive cholangiography (ERCP or PTC) was not performed. Of the 184 patients with cholangiograms, 35 met the exclusion criteria (five patients had primary nonfunction, eight patients had hepatic artery thrombosis, four patients experienced early death or early death or graft loss without cholangiography, and 18 patients underwent only a single cholangiographic examination). Therefore, cholangiograms (including cholangiograms via an intraoperatively placed transcystic duct–trans-Roux biliary tube, PTC, or ERCP) from 149 DCD liver graft recipients were reviewed. The mean follow-up time was 59.8 months (median, 54 months; range, 1–167 months), and all follow-up was complete by November 2013. According to the postoperative cholangiographic findings, the patients were divided into three groups: those with no biliary abnormalities visible radiologically (normal cholangiograms) (n = 100); those with biliary abnormalities and clinical cholangiopathy as evidenced by abnormal laboratory values and symptoms (n = 31); and those with biliary abnormalities and no evidence of clinical cholangiopathy or abnormal laboratory values or symptoms (n = 18).
In our practice, the clinical diagnosis of ischemic cholangiopathy is usually made approximately 3–4 weeks after transplant. Therefore, we looked at the differences in total bilirubin and alkaline phosphatase (ALP) levels of all patients within the first 16 weeks after transplant (Table 1). During the first week, total bilirubin and ALP levels were not significantly different between study groups. At weeks 3–4, the patients with ischemic cholangiopathy had significantly higher ALP levels than did patients with normal cholangiograms (p = 0.02). Total bilirubin and ALP levels remained significantly higher in patients with ischemic cholangiopathy than in those with normal cholangiograms 8–16 weeks after transplant. These levels were slightly elevated in the patients who had no symptoms but had mild ischemic changes; the differences, however, did not reach statistical significance.
TABLE 1.
Posttransplant Total Bilirubin (mg/dL) and Alkaline Phosphatase (U/L) Levels in Recipients of Liver Grafts by Donation After Cardiac Death
| Level | Normal Cholangiogram Group (n = 100) | Biliary Abnormalities With Clinical Cholangiopathy (n = 31) | pa | Biliary Abnormalities Without Clinical Cholangiopathy (n = 18) | pa |
|---|---|---|---|---|---|
|
| |||||
| Week 1 | |||||
| Total bilirubin | 4.0 ± 4.8 (2.3; 0.3–24.6) | 3.0 ± 2.7 (1.9; 0.5–10) | 0.29 | 4.1 ± 4.5 (1.6; 0.6–14.7) | 0.72 |
| Alkaline phosphatase | 270.5 ± 259.5 (183; 47–1624) | 201.7 ± 184.4 (154; 44–944) | 0.18 | 228.2 ± 148.8 (214; 50–599) | 0.98 |
| Weeks 3–4 | |||||
| Total bilirubin | 2.1 ± 4.3 (1.1; 0.3–31.2) | 2.0 ± 3.8 (1.1; 0.4–9.7) | 0.92 | 1.8 ± 2.4 (0.3–8.5) | 0.59 |
| Alkaline phosphatase | 279.2 ± 376.4 (143; 37–2905) | 555.5 ± 593.6 (309; 21–2086) | 0.02 | 309.4 ± 272.0 (200; 52–952) | 0.38 |
| Week 8 | |||||
| Total bilirubin | 1.2 ± 2.4 (0.6; 0.1–19.6) | 2.6 ± 2.7 (1.6; 0.4–10) | 0.02 | 3.2 ± 4.7 (0.7; 0.4–15.5) | 0.44 |
| Alkaline phosphatase | 208.8 ± 264.9 (112; 30–1867) | 601.9 ± 548.0 (476; 60–2665) | < 0.01 | 267.5 ± 248.0 (174.5; 76–952) | 0.08 |
| Week 16 | |||||
| Total bilirubin | 1.2 ± 2.6 (0.6; 0.1–23.9) | 3.4 ± 4.5 (1.2; 0.2–17.7) | 0.02 | 2.3 ± 1.2 (0.6; 0.3–15.5) | 0.79 |
| Alkaline phosphatase | 206.8 ± 233.8 (132.5; 34–1428) | 685.8 ± 666.6 (471; 73–2471) | < 0.01 | 226.1 ± 156.2 (172; 70–630) | 0.15 |
Note—Continuous values presented as mean ± SD with median and minimum to maximum in parentheses.
Versus normal cholangiogram group.
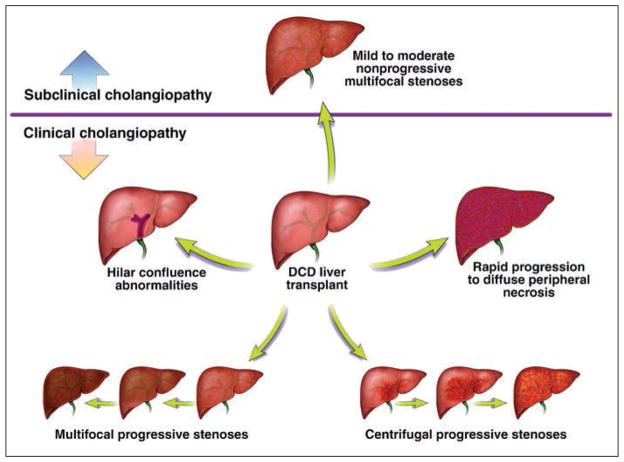
In patients with clinically evident ischemic cholangiopathy and abnormal laboratory values (n = 31), we identified the following four distinct radiologic patterns of intrahepatic biliary changes: hilar confluence abnormalities, multifocal progressive stenoses, centrifugal progressive stenoses, and rapid progression to diffuse peripheral duct disruption consistent with necrosis.
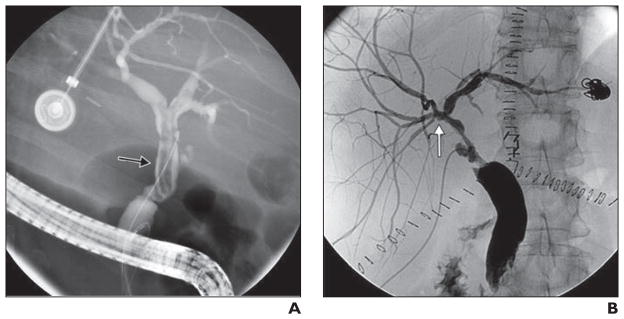
The six patients with hilar confluence abnormalities had intraluminal filling defects (presumed to represent sloughed mucosa) or strictures related to adhesion of the submucosa confined to the biliary confluence with varying degrees of severity and preservation of the first-order, second-order, and peripheral bile ducts. In this pattern, the biliary abnormalities progressed in severity over time but geographically never expanded beyond the biliary hilar confluence to involve intrahepatic ducts (Fig. 2). In this subgroup, ischemic changes were detected 20–178 days after transplant. The graft survival rate at 1 year in this group was 66%. Two patients lost their grafts within 8 months (one patient underwent repeat transplant, and one patient died of complications related to ischemic cholangiopathy). Another patient underwent repeat transplant 54 months after the initial transplant.
Fig. 2. Hilar confluence abnormalities after donation-after-cardiac-death liver transplants.
A, 65-year-old woman with filling defect of common bile duct with minimal extension into first-order duct (arrow) 21 days after transplant.
B, 58-year-old man with mild stricturing (arrow) confined to biliary hilum 20 days after transplant.
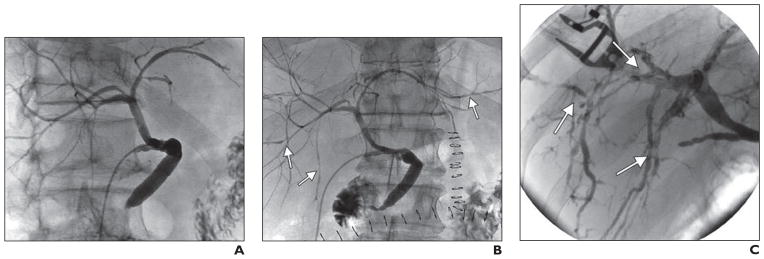
The multifocal progressive stenoses pattern (nine of the 31 patients) began with mild to moderate stenoses of the second-order and peripheral bile ducts and progressively worsened until severe necrosis of the peripheral ducts evidenced by mucosal sloughing and subsequent stricturing was found (Fig. 3). Ischemic changes in this group were initially detected 3–121 days after the transplant. The graft survival rate at 1 year in this group was 66%. Two patients underwent repeat transplants within 6 months after the initial transplant. Another patient died of complications of ischemic cholangiopathy 6 months after transplant. One patient underwent retransplant 14 months after the initial transplant. One patient died of a stroke 30 months after transplant, and another patient died of recurrent hepatocellular cancer 47 months after transplant.
Fig. 3. 55-year-old man with multifocal progressive stenoses after donation-after-cardiac-death liver transplant.
A, Initial cholangiogram shows normal findings 5 days after transplant.
B and C, Follow-up cholangiograms show multifocal stenoses (arrows) on postoperative day 14 (B), which have worsened by postoperative day 36 (C).
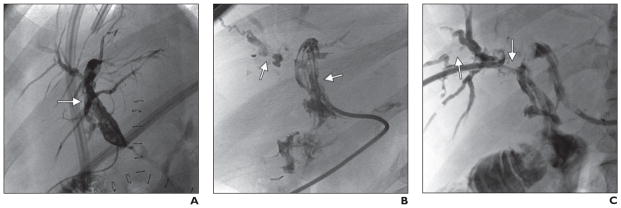
In patients with centrifugal progressive stenoses (five of the 31), abnormalities at the biliary confluence or the first-order ducts were noted initially, but over time the second-order ducts and eventually the peripheral ducts became affected. The abnormalities of this centrifugal pattern worsened at a slower rate than did those of the other patterns (Fig. 4). Ischemic changes were detected 14–23 days after transplant. The 1-year graft survival rate in this group was 100%. One patient underwent retransplant 28 months after the initial transplant because of ischemic cholangiopathy, and one patient died of complications related to ischemic cholangiopathy 37 months after transplant.
Fig. 4. 58-year-old man 34 days after donation-after-cardiac-death liver transplant.
A, Cholangiogram shows stenoses (arrow) involving proximal bile ducts with sparing of peripheral bile ducts.
B, Follow-up cholangiogram 20 months after transplant shows extension (arrows) into second- and third-order ducts.
C, Cholangiogram 27 months after transplant shows stenoses (arrows) have further extended peripherally.
Eleven of the 31 patients had rapid progression to diffuse peripheral duct disruption consistent with necrosis. These severe abnormalities of nearly the entire biliary system were identified soon after transplant. The postoperative day 3 cholangiogram was often normal, but close follow-up examination revealed severe diffuse biliary abnormalities (Fig. 5). Initial ischemic changes were detected 2–36 days after liver transplant in this group. The 1-year graft survival rate in this group was 36%. Seven patients lost their grafts within 1 year of transplant: six patients underwent retransplant, and one patient died of complications related to ischemic cholangiopathy. An additional patient underwent retransplant 15 months after the initial transplant, and one patient died of complications related to ischemic cholangiopathy 35 months after transplant.
Fig. 5. Cholangiograms show severe stenoses involving nearly entire biliary system after donation-after-cardiac-death liver transplant. Initial cholangiograms on day 3 were normal.
A, 48-year-old man 11 days after transplant.
B, 65-year-old woman 19 days after transplant.
During the retrospective evaluation of patients who underwent cholangiography, subtle biliary abnormalities were identified radiologically on postoperative cholangiograms in a subset of 18 of the 118 patients without clinically evident ischemic cholangiopathy or laboratory abnormalities. In this subset of patients, the pattern of biliary abnormalities was mild to moderate nonprogressive multifocal stenoses affecting second-order or peripheral bile ducts (Fig. 6). These subtle abnormalities, which were seen on the routine day 3 or day 21 cholangiograms, remained stable in some of the patients and resolved over time in others without any intervention.
Fig. 6.
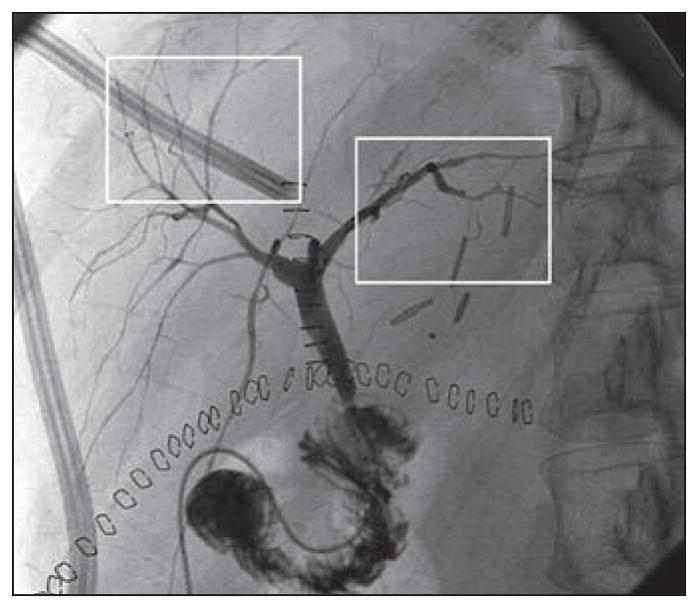
69-year-old man 4 days after donation-after-cardiac-death liver transplant with no laboratory evidence of cholangiopathy. Cholangiogram shows mild multifocal stenoses (boxes), which remained stable over multiple follow-up examinations.
Recipient, graft, and perioperative and postoperative factors were similar among the groups (Table 2). Specifically, there were no differences between the groups in incidences of moderate to severe acute cellular rejection or cytomegalovirus infection within the first year after liver transplant.
TABLE 2.
Comparison of Recipient, Donor, Perioperative, and Postoperative Factors in Recipients of Liver Grafts by Donation After Cardiac Death
| Factor | Normal Cholangiogram Group (n = 100) | Biliary Abnormalities With Clinical Cholangiopathy (n = 31) | pa | Biliary Abnormalities Without Clinical Cholangiopathy (n = 18) | pa,b |
|---|---|---|---|---|---|
|
| |||||
| Recipient factors | |||||
| Age (y) | 55.8 ± 8.4 (55.5; 32–75) | 57.2 ± 9.6 (57; 35–72) | 0.43 | 54.0 ± 10.1 (53; 33–73) | 0.40 |
| Sex, male | 74 (74) | 21 (67.7) | 0.82 | 14 (77.7) | 0.39 |
| Race, white vs nonwhite | 95 (95) | 23 (74.1) | 0.01 | 17 (94.4) | 0.61 |
| Biologic MELD score | 17.9 ± 7.8 (17; 6–44) | 14.4 ± 6.5 (17; 6–34) | 0.78 | 19.5 ± 5.9 (18; 10–31) | 0.17 |
| Hepatitis C virus infection | 49 (49) | 20 (64.5) | 0.06 | 5 (27.7) | 0.29 |
| Hepatocellular carcinoma | 26 (26) | 8 (25.8) | 0.92 | 1 (5.5) | 0.12 |
| Primary sclerosing cholangitis | 3 (3) | 1 (3.2) | 0.99 | 0 (0) | 0.99 |
| Graft factors | |||||
| Age (y) | 39.7 ± 15.8 (38.5; 7–81) | 42.6 ± 18.8 (48; 11–74) | 0.39 | 36.4 ± 12.2 (42; 8–49) | 0.40 |
| Sex, male | 78 (78) | 19 (61.2) | 0.53 | 10 (55.5) | 0.33 |
| Organ sharing area | 0.98c | 0.75c | |||
| Local | 60 (60) | 18 (58) | 9 (50) | ||
| Regional | 39 (39) | 3 (9.6) | 8 (44.4) | ||
| National | 7 (7) | 8 (25.8) | 0 (0) | ||
| Donor warm ischemia time (min) | 24.0 ± 8.4 (23.5; 5–57) | 25.1 ± 6.9 (24; 13–39) | 0.52 | 21.4 ± 5.7 (21; 12–33) | 0.21 |
| Asystole to cross-clamp time (min) | 8.7 ± 3.1 (7; 1–16) | 10.0 ± 4.0 (9; 5–24) | 0.07 | 9.5 ± 3.9 (9; 2–16) | 0.63 |
| Donor risk index | 2.0 ± 0.5 (1.8; 1.3–4.2) | 2.1 ± 0.5 (2.1; 1.3–3.0) | 0.26 | 1.8 ± 0.3 (1.8; 1.4–2.6) | 0.68 |
| Preservation injury | 0.68 | 0.14 | |||
| Mild | 51 (51) | 15 (48.3) | 7 (38.8) | ||
| Moderate | 12 (12) | 5 (16.1) | 0 (0) | ||
| Perioperative factors | |||||
| International normalized ratio | 1.7 ± 1.0 (1.6; 1–10) | 1.7 ± 0.5 (1.6; 1–3) | 0.67 | 1.6 ± 0.2 (1.5; 1.4–1.9) | 0.57 |
| Total bilirubin level (mg/dL) | 5.5 ± 9.8 (2.8; 0.2–62.7) | 4.7 ± 6.8 (2.2; 0.4–31.4) | 0.67 | 4.2 ± 2.9 (3.1; 1.5–11.4) | 0.22 |
| Creatinine level (mg/dL) | 1.2 ± 1.1 (0.8; 0.3–7.4) | 1.3 ± 0.8 (1.1; 0.5–4.4) | 0.47 | 1.2 ± 0.6 (1.1; 0.6–2.7) | 0.28 |
| Cold ischemia time (h) | 5.9 ± 1.4 (5.7; 3.0–10.1) | 6.1 ± 1.7 (5.8; 3.1–10.5) | 0.50 | 6.0 ± 1.4 (5.9; 4.3–5.8) | 0.99 |
| Warm ischemia time (min) | 33.5 ± 10.5 (32; 19–71) | 34.9 ± 16.8 (33; 18–106) | 0.87 | 33.2 ± 7.8 (33; 17–46) | 0.77 |
| Packed RBC transfusion (mL) | 4000.6 ± 3894.1 (2975; 0–21700) | 4019 ± 2296.5 (3150; 700–9800) | 0.98 | 3075.6 ± 2366.7 (2500; 0–8750) | 0.39 |
| Postoperative course | |||||
| Cytomegalovirus infection | 10 (10) | 4 (12.9) | 0.50 | 2 (11.1) | 0.67 |
| Moderate to severe rejection 1 wk after transplant | 19 (19) | 5 (16.1) | 0.99 | 3 (16.6) | 0.99 |
| Moderate to severe rejection 1 y after transplant | 23 (23) | 10 (32.2) | 0.24 | 5 (27.7) | 0.53 |
Note—Categoric values are number with percentages in parentheses. Continuous values are mean ± SD with median and minimum to maximum in parentheses. MELD = model for end-stage liver disease.
Versus normal cholangiogram group.
Mann-Whitney test.
Local versus nonlocal.
Graft survival rates 1, 3, and 5 years after transplant are shown in Table 3. At all time points, a statistically significant difference in graft survival rates was found between recipients who had clinical ischemic cholangiopathy and those who had normal cholangiographic findings (p < 0.001). There was also a significant difference in graft survival rates between the patients who had mild ischemic changes radiologically and patients with normal cholangiograms at the 3-year (p = 0.03) and 5-year (p = 0.04) time points. The graft survival difference also approached statistical significance at 1 year (p = 0.06).
TABLE 3.
Graft Survival Rates (%) Among Recipients of Donation After Cardiac Death Liver Grafts
| Time After Transplant (y) | Normal Cholangiogram Group (n = 100) | Biliary Abnormalities With Clinical Cholangiopathy (n = 31) | pa | Biliary Abnormalities Without Clinical Cholangiopathy (n = 18) | pa |
|---|---|---|---|---|---|
|
| |||||
| 1 | 90.6 | 58.6 | < 0.001 | 76.5 | 0.06 |
| 3 | 84.3 | 41.1 | < 0.001 | 61.3 | 0.03 |
| 5 | 79.8 | 27.2 | < 0.001 | 61.3 | 0.04 |
Versus normal cholangiogram group.
Patient survival rates 1, 3, and 5 years after transplant are shown in Table 4. No statistically significant difference in patient survival rates was found between recipients who had clinical ischemic cholangiopathy and patients with normal cholangiograms 1 and 3 years after transplant (p = 0.16); there was a statistical difference at 5 years (p = 0.03). No patient survival difference was found between the recipients who had mild ischemic changes radiologically and recipients with normal cholangiograms 3 years (p = 0.05) and 5 years (p = 0.07) after transplant.
TABLE 4.
Patient Survival Rate (%) Among Recipients of Donation After Cardiac Death Liver Grafts
| Time After Transplant (y) | Normal Cholangiogram Group (n = 100) | Biliary Abnormalities With Clinical Cholangiopathy (n = 31) | pa | Biliary Abnormalities Without Clinical Cholangiopathy (n = 18) | pa |
|---|---|---|---|---|---|
|
| |||||
| 1 | 92.5 | 89.7 | 0.61 | 88.2 | 0.52 |
| 3 | 87.2 | 75.7 | 0.16 | 66.5 | 0.05 |
| 5 | 84.2 | 64.5 | 0.03 | 66.5 | 0.07 |
Versus normal cholangiogram group.
Discussion
The use of DCD grafts for liver transplants has been regarded as high risk because of lower rates of graft and patient survival in comparison with those associated with liver transplants with DBD grafts [1]. Development of ischemic cholangiopathy in DCD livers has been a major obstacle to the successful use of these grafts. Ischemic cholangiopathy is thought to be due to ischemia at DCD liver graft procurement and reperfusion injury at implantation [15, 16]. Ischemic damage to the bile ducts results in varying degrees of strictures, which then lead to intrahepatic bile stasis, intrahepatic abscesses, and systemic sepsis.
Our liver transplant program began using DCD grafts in 1998. In our experience, among 231 DCD recipients, 31 patients had clinically and radiologically proven ischemic cholangiopathy. In this study, we retrospectively examined all routine and clinically indicated cholangiograms for the pattern and severity of intrahepatic bile duct changes. The findings were then correlated with the recipients’ clinical outcomes. We have observed that not all of these patients with ischemic cholangiopathy needed a repeat transplant. In the DCD literature, ischemic cholangiopathy is often considered a single entity resulting in poor outcomes [4, 17, 18]. Our results suggest that this may not be the case and that ischemic cholangiopathy may encompass a wide spectrum of disease.
Examination of postoperative cholangiograms by radiologists blinded to patient and graft outcomes revealed four distinct patterns of ischemic cholangiopathy (Fig. 7). Graft survival was different with each distinct pattern. Among the four identifiable groups, patients with rapid progression to diffuse peripheral duct necrosis fared worst, having a graft survival rate of 36% at 1 year. This entity likely represents overwhelming ischemic damage to the entire intrahepatic biliary system, resulting in rapid deterioration of the recipient’s clinical condition. In contrast, recipients with a pattern of centrifugal progressive stenoses fared better clinically, because the progression of the disease was slower and less severe. Patients with hilar confluence abnormalities and multifocal progressive stenoses had intermediate graft survival rates and clinical outcomes.
Fig. 7.
Schematic shows multiple patterns of cholangiographic abnormalities in liver grafts obtained by donation after cardiac death (DCD). (By permission of Mayo Foundation for Medical Education and Research. All rights reserved.)
The decision to categorize a DCD liver graft as having failed and necessitating retransplant is subject to center-specific strategies of management and to local availability of organs for retransplant. The poor quality of life and the need for repeat endoscopic or percutaneous interventions in recipients of DCD liver grafts with fairly well preserved synthetic function but with cholestasis of variable progression should not be viewed as a successful outcome despite the benefit of avoiding waiting list dropout. Use of the graft failure metric alone may result in an underestimate of the true disease burden of biliary complications and ischemic cholangiopathy.
Transplant center–specific management practices in the care of recipients of DCD liver grafts may also allow the introduction of bias. In our study, we used routine cholangiograms and blinded assessment to standardize the detection and evaluation of ischemic cholangiopathy. Understanding the severity and progression of biliary ductal damage can help with future medical and surgical planning for individual recipients. Although the small number of patients in our study with both clinical and radiologic ischemic cholangiopathy precluded statistical significance, we believe this classification will provide meaningful prognostic information for individual patients if these trends are confirmed at other transplant centers. This may allow a liver transplant program to determine whether a patient will likely have to be considered for retransplant or whether interventions short of retransplant can be successful in the long term. In the future, as more data on patterns of ischemic cholangiopathy are reported, it may be possible to develop a risk index to help transplant programs predict prognosis for graft survival.
The results of our study provide a nuanced framework for evaluating the spectrum and degree of ischemic cholangiopathy through classification of the cholangiographic pattern and for guiding planning of future interventions. In patients with hilar confluence abnormalities or centrifugal progressive stenoses, endoscopic or percutaneous biliary stenting may be reasonable first-line management, whereas in patients with rapid progression to peripheral duct necrosis, planning for retransplant would appear to be appropriate. The development of a potential MELD exception policy for retransplant for patients with ischemic cholangiopathy could also be based on the prognosis associated with different types of ischemic cholangiopathy [19]. Therefore, better understanding of the spectrum of ischemic cholangiopathy and prognosis may help transplant programs achieve better long-term outcomes.
One major yet unexpected finding of the current study is the identification of a subset of patients without symptoms and without cholangiopathy according to laboratory values who had mild ischemic changes in the intrahepatic bile ducts. These patients did not have an initial clinical or radiologic diagnosis of ischemic cholangiopathy. Although their total bilirubin and ALP levels remained slightly elevated for the first 8 weeks after transplant, these values normalized by postoperative week 16 (Table 1). On retrospective review, these patients had findings of mild to moderate nonprogressive multifocal stenoses at postoperative cholangiography. Correlation with clinical outcomes showed that this subset of patients with isolated radiologic abnormalities and no overt laboratory evidence of clinical cholangiopathy had intermediate graft survival 1, 3, and 5 years after transplant (Table 3). Patient survival paralleled graft survival; patients with normal cholangiograms did well, and patients with clinical cholangiopathy did poorly. The patients with abnormalities on cholangiograms and no signs of clinical cholangiopathy had a poor survival rate, which was worse than that among patients with overt ischemic cholangiopathy at 3 years and was nearly identical to that at 5 years (Table 4). These findings clearly show that these patients were not identified as having serious biliary problems.
In an effort to exclude confounding factors, a chart review of the cause of graft loss in the patients with abnormalities on cholangiograms and no signs of clinical cholangiopathy was undertaken. The review revealed that in four of seven patients, graft loss was related to graft failure or biliary complications. One patient died of myocardial infarction and two died of undetermined causes because they were lost to follow-up. Although these patients may have benefited from closer follow-up, the mild ischemic changes and overall normal liver function would have disqualified them for retransplant. At some point beyond postoperative week 16, these patients experienced biliary problems, but the timeline for the development of these problems is different from that for most patients with ischemic cholangiopathy. Where this inflection point exists is unknown. Therefore, the best management strategy for this subset of patients is unclear. Earlier or more aggressive intervention may be necessary to prevent progression to graft loss.
Given the wide range and degree of overlap of laboratory values in normal grafts, it is difficult to apply strict laboratory criteria to a single patient for the diagnosis of ischemic cholangiopathy. Routine postoperative cholangiography adds an element to aid in the diagnosis and treatment of individual patients in whom ischemic cholangiopathy may or may not be suspected. The findings of different patterns of ischemic cholangiopathy and the mild ischemic changes without clinical cholangiopathy found in a subset of patients suggest that the development of ischemic cholangiopathy is not an all-or-none phenomenon. A wide spectrum of ischemic changes ranging from mild nonprogressive stenoses to frank biliary necrosis may be present in DCD recipients (Fig. 7). With further delineation of this spectrum, specific management recommendations may be tailored to the degree and pattern of cholangiopathy.
It is clear from the published data that patients with ischemic cholangiopathy have the worst outcomes [2–4, 10]. At our center, however, where the overall rate of ischemic cholangiopathy is low, many patients have benefited from receiving DCD grafts. The patients who did not experience ischemic cholangiopathy had graft and patient survival rates similar to those of DBD graft recipients [10].
Although single-center analysis has the advantage of homogeneous donor and recipient selection and perioperative and postoperative clinical management, we acknowledge the weaknesses associated with the retrospective nature of clinical practice. Transplant center–specific management practices, such as a decision to perform retransplant, may also allow the introduction of bias. Even though this is the largest, to our knowledge, reported single-center experience with routine and clinically indicated cholangiograms, the number of DCD recipients who had ischemic cholangiopathy remains fairly small, which limits the ability to detect differences in disease type and progression. The findings in our analysis should be confirmed, if possible, in a larger cohort from multiple institutions using DCD liver grafts.
Conclusion
We report that routine postoperative cholangiography yields enough information for early diagnosis of biliary abnormalities, before they are clinically evident. Our findings suggest that ischemic cholangiopathy associated with DCD grafts is not an all-or-none phenomenon but presents as a spectrum of disease with a variable prognosis. The severity and pattern of cholangiographic abnormalities correlate with clinical outcomes. We also found that early subclinical mild cholangiographic abnormalities seen in some DCD liver grafts caused poorer graft and patient outcomes at 3 and 5 years. Although the goal of using DCD liver grafts should be to minimize development of ischemic cholangiopathy, it is also essential that physicians participating in liver transplant programs understand the implications of various patterns of ischemic cholangiopathy for diagnosis, prognosis, clinical management, follow-up, and patient counseling.
References
- 1.Mathur AK, Heimbach J, Steffick DE, Sonnenday CJ, Goodrich NP, Merion RM. Donation after cardiac death liver transplantation: predictors of outcome. Am J Transplant. 2010;10:2512–2519. doi: 10.1111/j.1600-6143.2010.03293.x. [DOI] [PubMed] [Google Scholar]
- 2.Foley DP, Fernandez LA, Leverson G, et al. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253:817–825. doi: 10.1097/SLA.0b013e3182104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vera ME, Lopez-Solis R, Dvorchik I, et al. Liver transplantation using donation after cardiac death donors: long-term follow-up from a single center. Am J Transplant. 2009;9:773–781. doi: 10.1111/j.1600-6143.2009.02560.x. [DOI] [PubMed] [Google Scholar]
- 4.Skaro AI, Jay CL, Baker TB, et al. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: the untold story. Surgery. 2009;146:543–552. doi: 10.1016/j.surg.2009.06.052. discussion, 552–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan EY, Olson LC, Kisthard JA, et al. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transpl. 2008;14:604–610. doi: 10.1002/lt.21361. [DOI] [PubMed] [Google Scholar]
- 6.Abou Abbass A, Abouljoud M, Yoshida A, et al. Biliary complications after orthotopic liver transplantation from donors after cardiac death: broad spectrum of disease. Transplant Proc. 2010;42:3392–3398. doi: 10.1016/j.transproceed.2010.07.099. [DOI] [PubMed] [Google Scholar]
- 7.Taner CB, Bulatao IG, Perry DK, et al. Asystole to cross-clamp period predicts development of biliary complications in liver transplantation using donation after cardiac death donors. Transpl Int. 2012;25:838–846. doi: 10.1111/j.1432-2277.2012.01508.x. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Stratta RJ, Langnas AN, Wood RP, Marujo W, Shaw BW., Jr Diffuse biliary tract injury after orthotopic liver transplantation. Am J Surg. 1992;164:536–540. doi: 10.1016/s0002-9610(05)81196-1. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Urdazpal L, Gores GJ, Ward EM, et al. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology. 1992;16:49–53. doi: 10.1002/hep.1840160110. [DOI] [PubMed] [Google Scholar]
- 10.Taner CB, Bulatao IG, Willingham DL, et al. Events in procurement as risk factors for ischemic cholangiopathy in liver transplantation using donation after cardiac death donors. Liver Transpl. 2012;18:100–111. doi: 10.1002/lt.22404. [DOI] [PubMed] [Google Scholar]
- 11.Buis CI, Verdonk RC, Van der Jagt EJ, et al. Non-anastomotic biliary strictures after liver transplantation. Part 1. Radiological features and risk factors for early vs. late presentationf. Liver Transpl. 2007;13:708–718. doi: 10.1002/lt.21166. [DOI] [PubMed] [Google Scholar]
- 12.Feng S, Goodrich NP, Bragg-GreshAm JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 13.Potts J, Herdman R. Non-heart-beating organ transplantation: medical and ethical issues in procurement. Washington, DC: National Academies Press; 1997. [PubMed] [Google Scholar]
- 14.Verdonk RC, Buis CI, van der Jagt EJ, et al. Non-anastomotic biliary strictures after liver transplantation. Part 2. Management, outcome, and risk factors for disease progression. Liver Transpl. 2007;13:725–732. doi: 10.1002/lt.21165. [DOI] [PubMed] [Google Scholar]
- 15.Rhee JY, Alroy J, Freeman RB. Characterization of the withdrawal phase in a porcine donation after the cardiac death model. Am J Transplant. 2011;11:1169–1175. doi: 10.1111/j.1600-6143.2011.03567.x. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto K, Eghtesad B, Gunasekaran G, et al. Use of tissue plasminogen activator in liver transplantation from donation after cardiac death donors. Am J Transplant. 2010;10:2665–2672. doi: 10.1111/j.1600-6143.2010.03337.x. [DOI] [PubMed] [Google Scholar]
- 17.Jay CL, Lyuksemburg V, Kang R, et al. The increased costs of donation after cardiac death liver transplantation: caveat emptor. Ann Surg. 2010;251:743–748. doi: 10.1097/SLA.0b013e3181d3d3da. [DOI] [PubMed] [Google Scholar]
- 18.Hong JC, Yersiz H, Kositamongkol P, et al. Liver transplantation using organ donation after cardiac death: a clinical predictive index for graft failure-free survival. Arch Surg. 2011;146:1017–1023. doi: 10.1001/archsurg.2011.240. [DOI] [PubMed] [Google Scholar]
- 19.Skaro AI, Wang E, Lyuksemburg V, Abecassis M. Donation after cardiac death liver transplantation: time for policy to catch up with practice. Liver Transpl. 2012;18:5–8. doi: 10.1002/lt.22478. [DOI] [PubMed] [Google Scholar]