Abstract
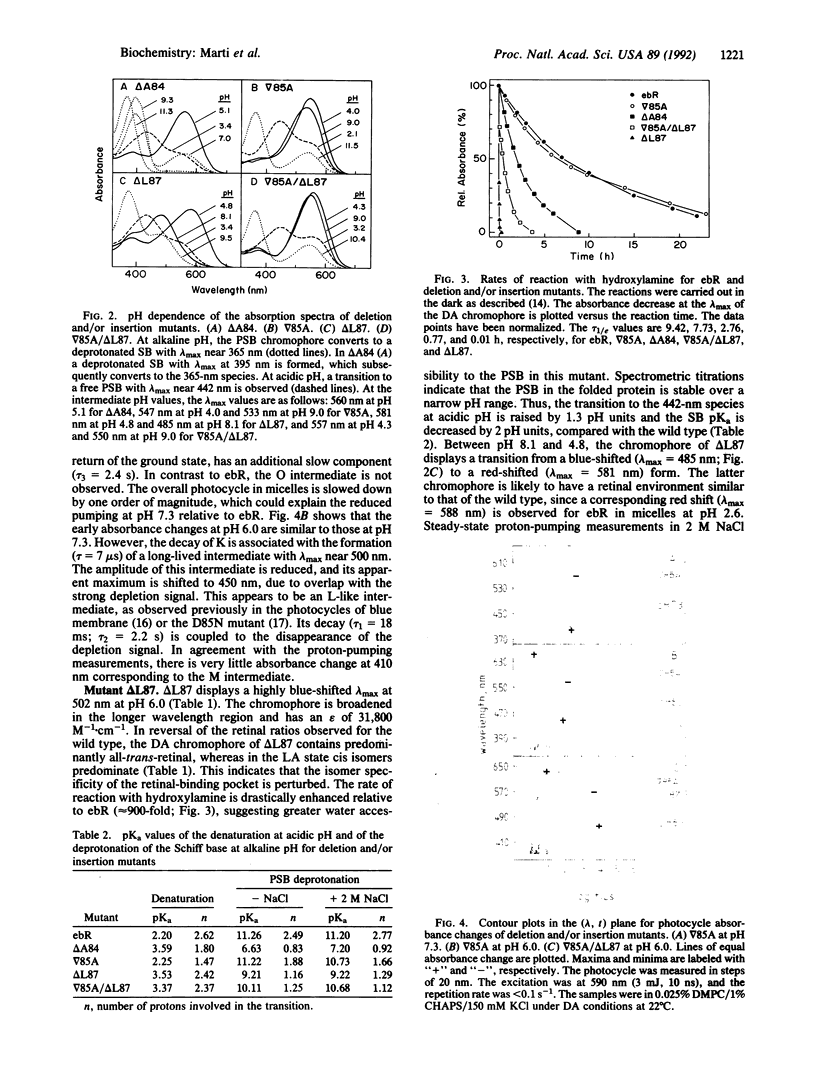
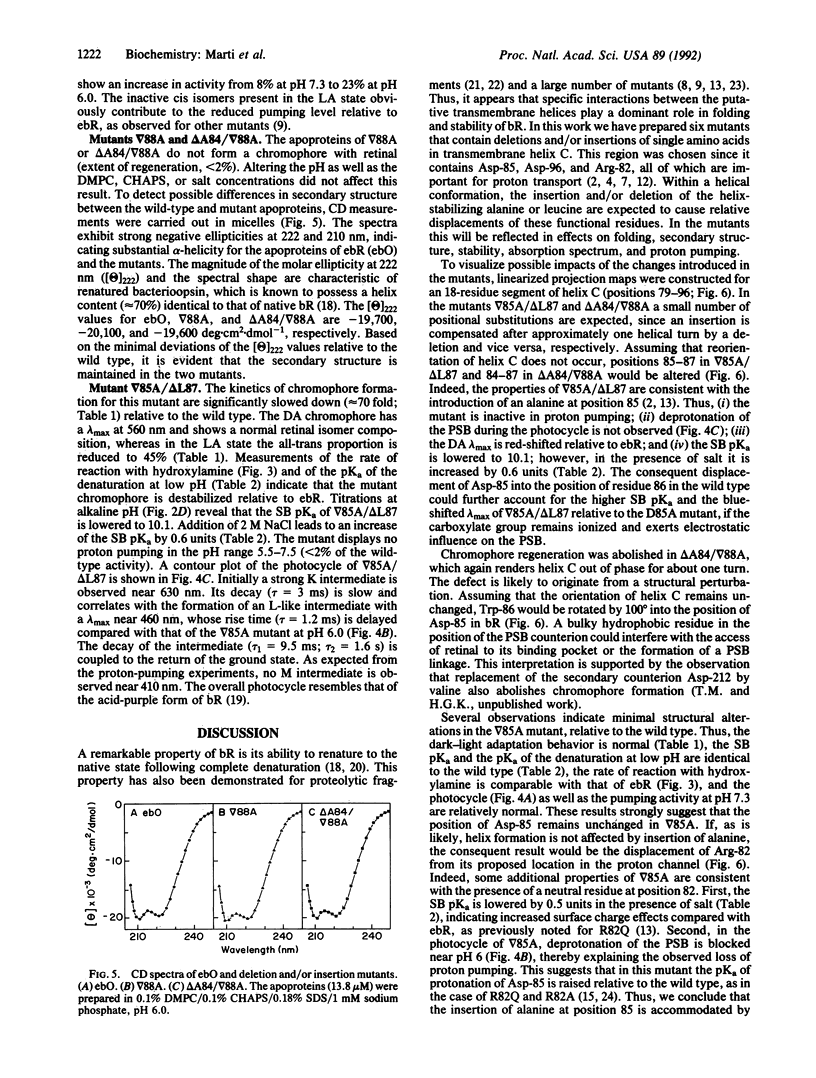
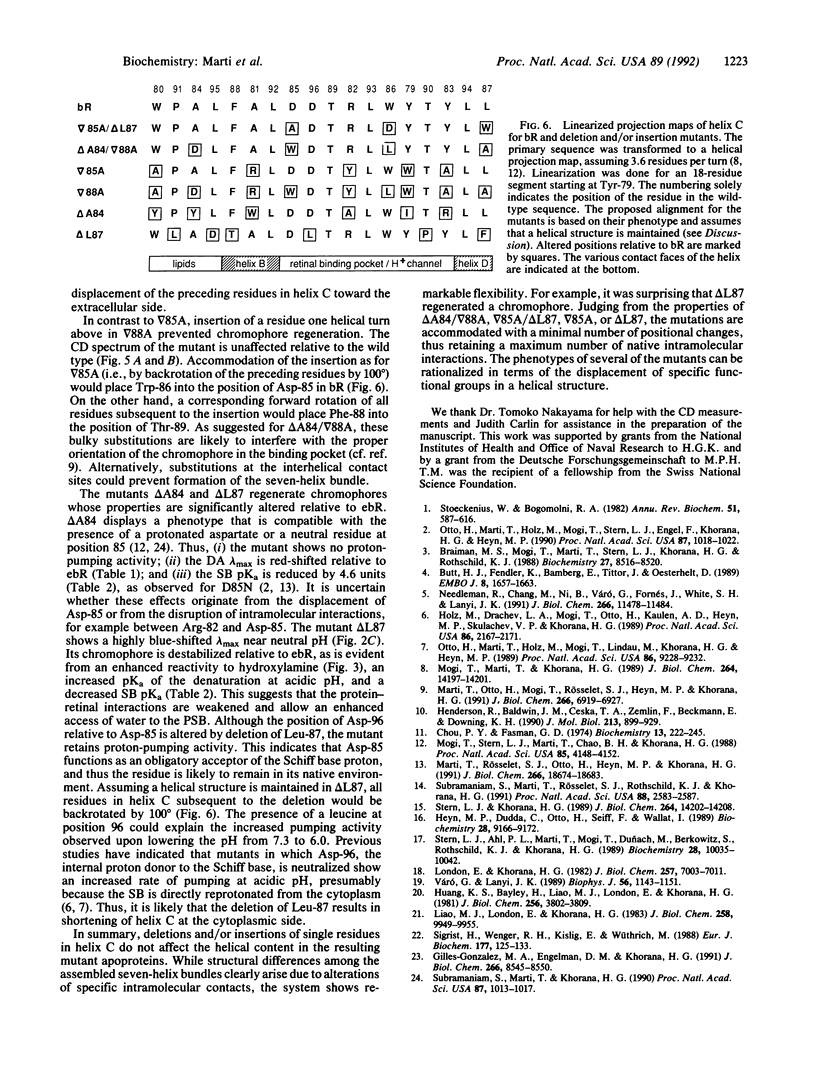
Six bacterioopsin mutants containing either single amino acid deletions (delta A84, delta L87), insertions (delta 85A, delta 88A), or both deletions and insertions (delta A84/delta 88A, delta 85A/delta L87) within the first two turns of transmembrane helix C, starting from the extracellular side, have been prepared. The mutant apoproteins refold in phospholipid/detergent micelles and display secondary structures similar to that of the wild type. However, the mutants delta 88A and delta A84/delta 88A do not form a chromophore with retinal. The regenerated chromophore of delta 85A displays absorption maxima and retinal isomer compositions in the dark- and light-adapted states similar to those of the wild type. In delta A84, delta L87, and delta 85A/delta L87 these chromophore properties are altered, and the structures are less stable than that of the wild type, as shown by an enhanced rate of reaction with hydroxylamine in the dark, an increased pKa of the denaturation at acidic pH, and a decreased pKa of Schiff base deprotonation. Proton translocation is abolished in the delta A84 and delta 85A/delta L87 mutants, whereas in delta 85A and delta L87 the activity is reduced to about 25% of the wild-type value at pH 6. The overall properties of the delta 85A, delta 85A/delta L87, and delta L87 mutants indicate that the deletions and/or insertions result in displacement of residues Arg-82, Asp-85, or Asp-96, respectively, which participate in proton translocation. The results are compatible with a helical structure for transmembrane segment C and emphasize the flexibility of intramolecular contacts in bacteriorhodopsin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Butt H. J., Fendler K., Bamberg E., Tittor J., Oesterhelt D. Aspartic acids 96 and 85 play a central role in the function of bacteriorhodopsin as a proton pump. EMBO J. 1989 Jun;8(6):1657–1663. doi: 10.1002/j.1460-2075.1989.tb03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Gilles-Gonzalez M. A., Engelman D. M., Khorana H. G. Structure-function studies of bacteriorhodopsin XV. Effects of deletions in loops B-C and E-F on bacteriorhodopsin chromophore and structure. J Biol Chem. 1991 May 5;266(13):8545–8550. [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Dudda C., Otto H., Seiff F., Wallat I. The purple to blue transition of bacteriorhodopsin is accompanied by a loss of the hexagonal lattice and a conformational change. Biochemistry. 1989 Nov 14;28(23):9166–9172. doi: 10.1021/bi00449a031. [DOI] [PubMed] [Google Scholar]
- Holz M., Drachev L. A., Mogi T., Otto H., Kaulen A. D., Heyn M. P., Skulachev V. P., Khorana H. G. Replacement of aspartic acid-96 by asparagine in bacteriorhodopsin slows both the decay of the M intermediate and the associated proton movement. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2167–2171. doi: 10.1073/pnas.86.7.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. S., Bayley H., Liao M. J., London E., Khorana H. G. Refolding of an integral membrane protein. Denaturation, renaturation, and reconstitution of intact bacteriorhodopsin and two proteolytic fragments. J Biol Chem. 1981 Apr 25;256(8):3802–3809. [PubMed] [Google Scholar]
- Liao M. J., London E., Khorana H. G. Regeneration of the native bacteriorhodopsin structure from two chymotryptic fragments. J Biol Chem. 1983 Aug 25;258(16):9949–9955. [PubMed] [Google Scholar]
- London E., Khorana H. G. Denaturation and renaturation of bacteriorhodopsin in detergents and lipid-detergent mixtures. J Biol Chem. 1982 Jun 25;257(12):7003–7011. [PubMed] [Google Scholar]
- Marti T., Otto H., Mogi T., Rösselet S. J., Heyn M. P., Khorana H. G. Bacteriorhodopsin mutants containing single substitutions of serine or threonine residues are all active in proton translocation. J Biol Chem. 1991 Apr 15;266(11):6919–6927. [PubMed] [Google Scholar]
- Marti T., Rösselet S. J., Otto H., Heyn M. P., Khorana H. G. The retinylidene Schiff base counterion in bacteriorhodopsin. J Biol Chem. 1991 Oct 5;266(28):18674–18683. [PubMed] [Google Scholar]
- Mogi T., Marti T., Khorana H. G. Structure-function studies on bacteriorhodopsin. IX. Substitutions of tryptophan residues affect protein-retinal interactions in bacteriorhodopsin. J Biol Chem. 1989 Aug 25;264(24):14197–14201. [PubMed] [Google Scholar]
- Mogi T., Stern L. J., Marti T., Chao B. H., Khorana H. G. Aspartic acid substitutions affect proton translocation by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4148–4152. doi: 10.1073/pnas.85.12.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman R., Chang M., Ni B., Váró G., Fornés J., White S. H., Lanyi J. K. Properties of Asp212----Asn bacteriorhodopsin suggest that Asp212 and Asp85 both participate in a counterion and proton acceptor complex near the Schiff base. J Biol Chem. 1991 Jun 25;266(18):11478–11484. [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Lindau M., Khorana H. G., Heyn M. P. Aspartic acid-96 is the internal proton donor in the reprotonation of the Schiff base of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9228–9232. doi: 10.1073/pnas.86.23.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Stern L. J., Engel F., Khorana H. G., Heyn M. P. Substitution of amino acids Asp-85, Asp-212, and Arg-82 in bacteriorhodopsin affects the proton release phase of the pump and the pK of the Schiff base. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1018–1022. doi: 10.1073/pnas.87.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist H., Wenger R. H., Kislig E., Wüthrich M. Refolding of bacteriorhodopsin. Protease V8 fragmentation and chromophore reconstitution from proteolytic V8 fragments. Eur J Biochem. 1988 Oct 15;177(1):125–133. doi: 10.1111/j.1432-1033.1988.tb14352.x. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Ahl P. L., Marti T., Mogi T., Duñach M., Berkowitz S., Rothschild K. J., Khorana H. G. Substitution of membrane-embedded aspartic acids in bacteriorhodopsin causes specific changes in different steps of the photochemical cycle. Biochemistry. 1989 Dec 26;28(26):10035–10042. doi: 10.1021/bi00452a023. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Khorana H. G. Structure-function studies on bacteriorhodopsin. X. Individual substitutions of arginine residues by glutamine affect chromophore formation, photocycle, and proton translocation. J Biol Chem. 1989 Aug 25;264(24):14202–14208. [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Marti T., Khorana H. G. Protonation state of Asp (Glu)-85 regulates the purple-to-blue transition in bacteriorhodopsin mutants Arg-82----Ala and Asp-85----Glu: the blue form is inactive in proton translocation. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1013–1017. doi: 10.1073/pnas.87.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S., Marti T., Rösselet S. J., Rothschild K. J., Khorana H. G. The reaction of hydroxylamine with bacteriorhodopsin studied with mutants that have altered photocycles: selective reactivity of different photointermediates. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2583–2587. doi: 10.1073/pnas.88.6.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Photoreactions of bacteriorhodopsin at acid pH. Biophys J. 1989 Dec;56(6):1143–1151. doi: 10.1016/S0006-3495(89)82761-4. [DOI] [PMC free article] [PubMed] [Google Scholar]



