Abstract
Clopidogrel and aspirin are commonly prescribed anti-platelet medications indicated for patients who have experienced, or are at risk for, ischemic cardiovascular events. The Pharmacogenomics of Anti-Platelet Intervention (PAPI) Study was designed to characterize determinants of clopidogrel and dual anti-platelet therapy (DAPT) response in a healthy cohort of Old Order Amish from Lancaster, PA. Following a loading dose, clopidogrel was taken once a day for 7 days. One hour after the last dose of clopidogrel, 325 mg of aspirin was given. Ex vivo platelet aggregometry was performed at baseline, post-clopidogrel, and post-DAPT. Platelet aggregation measurements were significantly lower after both interventions for all agonists tested (p <0.05), although there was large inter-individual variation in the magnitude of anti-platelet response. Female sex and older age were associated with higher platelet aggregation at all three time-points. Change in aggregation was correlated among the various agonists at each time point. Heritability (h2) of change in platelet aggregation was significant for most traits at all time-points (range h2=0.14–0.57). Utilization of a standardized, short-term intervention provided a powerful approach to investigate sources of variation in platelet aggregation response due to drug therapy. Further, this short-term intervention approach may provide a useful paradigm for pharmacogenomics studies.
Keywords: Anti-platelet therapies, aspirin, clopidogrel, pharmacogenomics, platelet aggregation, short-term intervention
INTRODUCTION
Clopidogrel and aspirin are used to improve cardiovascular (CV) outcomes in patients after acute coronary syndrome and/or percutaneous coronary intervention (PCI) by inhibiting platelet function and reducing recurrent arterial thrombotic events [1]. Clopidogrel is widely used either alone or in combination with aspirin as part of dual anti-platelet therapy (DAPT) regimen. There is a wide variability in drug response for both clopidogrel [2] and aspirin [3, 4], and patients who exhibit high on-treatment ex vivo platelet activity are at an increased risk of secondary ischemic events [5]. A better understanding of the factors that influence response to clopidogrel, both alone or in combination with aspirin, could improve treatment outcomes and reduce recurrent CV events. Many pharmacoepidemiologic and pharmacogenomic studies that seek to answer such questions utilize medical-record databases, biobanks or recruitment from tertiary care facilities, however, a challenge of these studies is that they are often insufficiently powered due to small sample size and cannot adequately control for co-morbidities and polypharmacy. In contrast, short-term intervention studies in healthy individuals can be a powerful tool to understand variations in drug response provided there is an appropriate sub-clinical endpoint and that the medication is appropriate for short-term use in healthy individuals. With this in mind, we conducted the Pharmacogenomics of Anti-Platelet Intervention (PAPI) Study to identify factors associated with response to anti-platelet therapy.
In this report, we describe the design and unique characteristics of the PAPI Study and then address the following specific questions: (1) What is the magnitude of variation in the platelet aggregation response to standardized clopidogrel and/or DAPT in this short-term intervention? (2) What baseline participant characteristics are associated with platelet aggregation response? (3) Is response to clopidogrel or DAPT correlated among different agonists used to stimulate platelet aggregation? (4) To what extent are genes predicted to contribute to variation in platelet aggregation response? Finally, we discuss the unique attributes of the PAPI study design and how this study may serve as a model for pharmacogenomics research to reduce non-genetic confounders and enhance genetic factors underlying variation in drug response.
METHODS
Study Overview and Population
The PAPI study was initiated in August 2006 and successfully recruited 687 healthy Amish adults to participate in a two-phase intervention consisting of: (1) a one week clopidogrel-only intervention (300 mg loading dose + 75 mg/day), and, (2) addition of 325 mg of aspirin after the last 75 mg dose of clopidogrel. Ex vivo platelet aggregation was assessed using optical aggregometry performed at baseline and after each phase of the intervention to evaluate response to clopidogrel alone or clopidogrel and aspirin in combination (i.e. DAPT). An overview of the study design is provided in Fig. (1).
Fig. 1. Overview of the PAPI Study Design.
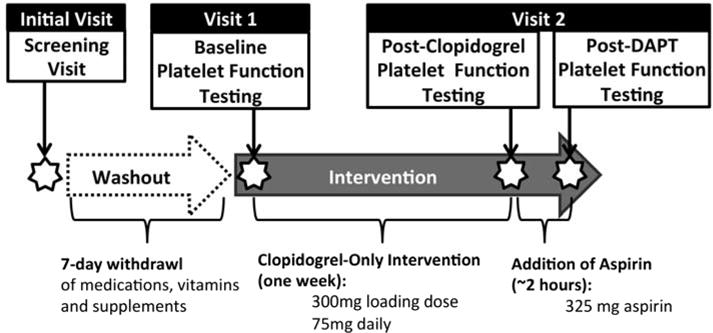
After the initial screening visit, subjects underwent a seven day washout period followed by baseline aggregation measurements. All participants were then given a 300 mg loading dose of clopidogrel followed by 75 mg daily for 7 days. After their last dose of clopidogrel, platelet aggregation measurements were taken, followed by the administration of 325 mg of aspirin. Final aggregation measurements were taken 2 h post-aspirin dose.
PAPI Study participants were recruited from the Old Order Amish (OOA) community of Lancaster County, PA. In the 18th century, approximately 550 OOA fled Switzerland to escape religious persecution and settled in Pennsylvania [6]. Currently, the OOA population in Lancaster County consists of approximately 30,000 individuals; nearly all of whom are descendants of the original set of 550 immigrants. Extensive genealogical records are available for the OOA, enabling PAPI study participants to be linked to a single, 14-generation pedigree [6, 7]. The relatively homogeneous lifestyle and genetic architecture of the OOA make them an ideal population for identifying complex trait genes through minimization of potentially confounding variables.
Eligibility Criteria and Recruitment
A total of 800 individuals were approached for the PAPI Study between August 2006 and January 2012, of whom 717 expressed interest in participating and met initial eligibility criteria. Among these, 687 subjects completed at least the baseline exam (Suppl Fig. 1). Participants were generally healthy and not recruited based on known CV disease (CVD) risk or drug response. Many of the PAPI Study participants had participated in previous studies; others were identified by word of mouth, and regular newsletter mailings. Eligible family members were also encouraged to enroll.
Participants had to be at least 20 years of age to be eligible for the study and free of the following conditions: severe hypertension (blood pressure > 160/95 mmHg); current pregnancy or lactation; use of prescription medications that could not be discontinued and would impact outcomes of the study; unwillingness to discontinue over-the-counter remedies (including vitamins and supplements) one week prior to study; coexisting malignancy; thrombocytosis (platelet count >500,000/μl) or thrombocytopenia (platelet count <75,000/μl), anemia (hematocrit < 32%); abnormal thyroid stimulating hormone (TSH) levels (<0.5 or >5.5 mU/L); elevated liver enzymes (aspartate transaminase or alanine transaminase >2 times the upper normal limit); renal insufficiency (creatinine >2.0 mg/dl); allergies to clopidogrel or aspirin; surgery in the 6 months prior to the study; history of gastrointestinal bleeding or other bleeding disorders; and current aspirin or clopidogrel use. Also, those on anti-platelet therapy with preexisting conditions such as a history of angioplasty (including stent placement), atrial fibrillation, coronary artery bypass surgery, deep vein thrombosis or other thrombosis, myocardial infarction, stroke or transient ischemic attacks, type 2 diabetes, unstable angina, or other conditions where anti-platelet therapy withdrawal 14 days before study initiation would cause increased risk, were excluded from the study. A detailed listing of the exclusion factors is available in Suppl Table 1.
The PAPI study was approved by the Institutional Review Board at the University of Maryland, Baltimore and an external Data Safety and Monitoring Board supervised the conduct of the study. Written informed consent (including permission to contact relatives) was obtained from each subject. The study was conducted under an investigational new drug protocol (IND #74,600) and registered at Clinicaltrials.gov (#NCT00799396).
Study Protocol and Interventions
Prior to the subject’s enrollment, each potential participant was visited at home by a study nurse who performed a screening examination. This exam included a thorough review of personal and family history, complete blood count, kidney and liver function tests, and measurement of TSH levels. After assessment of other exclusion criteria (see Suppl Table 1), eligible participants were scheduled for their first clinic visit.
Participants taking aspirin were asked to abstain from its use for at least two weeks prior to Clinic Visit 1. All other medications, vitamins, and supplements were discontinued one week prior to Clinic Visit 1. After an overnight fast, subjects were brought to the Amish Research Clinic in Lancaster, PA. A nurse or physician performed a physical examination, administered a urine pregnancy test (if applicable), measured vital signs, and collected a fasting blood sample. This blood sample was used in analyses of platelet aggregation and fasting lipids, whole blood was obtained for DNA isolation, and plasma, serum, and urine were stored for future use.
After platelet aggregation measurements were recorded, subjects were given a 300 mg oral loading dose of clopidogrel at the clinic and were instructed to take 75 mg daily for the following 6 days from pre-loaded pill boxes (Fig. 1). Subjects were informed of the potential side effects of study drugs such as gastrointestinal bleeding, intracranial hemorrhage, and other GI events (e.g. nausea, indigestion, heartburn and vomiting). In addition, participants were advised to call the clinic immediately if any of the side effects occurred. A pill count was carried out by a field team at least once during that week to verify adherence to the intervention and to check for adverse events. Subjects returned to the clinic 1 week later (Clinic Visit 2) after an overnight fast. At the clinic, they took the final 75 mg dose of clopidogrel, and 1 h later blood was drawn for measures of platelet aggregation. After follow-up platelet aggregation measurements were recorded, participants were given 325 mg of chewable aspirin at the clinic. Two hours after the aspirin was taken, platelet aggregation studies were repeated to assess the combined response of clopidogrel and aspirin (DAPT).
Platelet Aggregation
Platelet aggregometry was completed at all 3 time-points: (1) at baseline before clopidogrel administration (Clinic Visit 1), (2) post-clopidogrel alone (Clinic Visit 2) and (3) post-DAPT (Clinic Visit 2). Platelet rich plasma (PRP) was prepared from whole blood and adjusted to a platelet count of 200,000 platelets/μL using a Coulter 3-part differential analyzer (Sysmex, Lancaster PA) [8]. Platelet function was measured by optical aggregometry using a PAP8E aggregometer (Bio/Data Corporation, Horsham, PA) after stimulation of PRP samples with adenosine diphosphate (ADP; 2, 5, 10, and 20 μM), collagen (1, 2, 5, and 10 μg/ml), epinephrine (10 μM), and arachidonic acid (AA; 1.6 mM) using platelet-poor plasma as a referent (Chrono-Log, Lancaster PA). Repeat measures were calculated on 15 subjects and intra-assay coefficients of variation ranged from 3–16%.
Other Study Measures
PAPI study participants were examined at the first clinic visit and administered questionnaires on health history and lifestyle factors including smoking history. Body weight and height were measured using a stadiometer and calibrated scale without shoes during the first clinic visit. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in duplicate with a standard sphygmomanometer. Serum lipid and high density lipoprotein cholesterol (HDL-C) were measured by Quest Diagnostics (Horsham, PA) and LDL-C was calculated using the Friedewald formula [9].
Statistical Analysis
The primary outcome of this analysis is maximum ex vivo platelet aggregation. Summary statistics (i.e. means and standard deviations) and frequencies of participant characteristics and agonists at each time-point were calculated using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). Changes (Δ) in platelet aggregation between time-points were calculated as:
Δ between baseline and post-clopidogrel = clopidogrel response
Δ between post-clopidogrel and post-DAPT = aspirin response
Δ between baseline and post-DAPT = DAPT response
We used general linear models to compare platelet aggregation at baseline, after clopidogrel or post-DAPT between men and women and to assess the effect of age on platelet aggregation at all 3 time-points. Additionally, we evaluated the relationship between platelet aggregation measures and other participant characteristics (i.e. BMI, blood pressure, smoking status, and lipid measures) while simultaneously adjusting for age and sex. Statistical analyses were performed using SOLAR (The Southwest Foundation for Biomedical Research, San Antonio, TX) which is a robust software package used to perform various statistical analyses in complex family studies [10]. SOLAR has been described extensively elsewhere, but in brief, we conducted analysis using a variance components analytical framework which allows us to account for non-independence of study participants due to family structure through the inclusion of a random effect which is a function of the kinship coefficient. Heritability, or the proportion of trait variation between individuals that is due to genetic factors, was also estimated using SOLAR. We estimated the residual heritability for change in platelet aggregation between time-points for each agonist while accounting for age and sex. Finally, we calculated the partial phenotypic correlations, after adjusting for age and sex, using this variance components framework.
RESULTS
Participant Characteristics and Platelet Aggregation
Characteristics of male and female PAPI participants were generally similar though, in accordance with Amish cultural traditions, tobacco use is restricted to men (Table 1). Females also had slightly higher SBP (p=0.0007), LDL-C (p = 0.0019), BMI (p < 0.0001) and platelet count (p = 0.0172) compared with males. As expected, mean levels of agonist-induced platelet aggregation were lower for each agonist compared with baseline after both clopidogrel and DAPT intervention (p < 0.05 for all) (Fig. 2, Suppl Table 2). Consistent with the known mechanism of action of clopidogrel, significant decreases in ADP-stimulated platelet aggregation were observed after intervention with clopidogrel alone and after DAPT. For example, platelet aggregation decreased by 53% with ADP (20 μM) stimulation, and by another 8% with addition of aspirin. In contrast, larger changes were observed for AA- and epinephrine-stimulated platelet aggregation after the DAPT intervention as compared with the clopidogrel only intervention. For example, the reduction in AA-stimulated platelet aggregation was 12% after clopidogrel and 96% following the DAPT intervention. Collagen-stimulated platelet aggregation showed moderate reductions after both clopidogrel and DAPT; platelet aggregation decreased by 18% post-clopidogrel and 67% post-DAPT.
Table 1.
PAPI participant characteristics.
| Female | Male | Combined | |
|---|---|---|---|
| N = 346 | N = 341 | N = 687 | |
| Age (years) | 46.5 (13.8) | 43.6 (12.9) | 45.0 (13.4) |
| BMI (kg/m2) | 28.2 (5.4) | 26.0 (3.6) | 27.1 (4.7)* |
| Systolic BP (mmHg) | 118 (14) | 117 (12) | 117 (13)* |
| Diastolic BP (mmHg) | 70 (7) | 71 (7) | 70 (7) |
| Hypertension (%) | 7.2% | 6.2% | 6.7% |
| Ever Used Tobacco (%) | N/A** | 45.2% | N/A |
| Current Smoker (%) | N/A** | 20.2% | N/A |
| Post-Menopausal Status (%) | 37.1% | N/A | N/A |
| Platelet Count (thous/mcl) | 245 (52) | 235 (46) | 240 (49)* |
| RBC Count (mill/mcl) | 4.2 (0.3) | 4.61 (0.3) | 4.4 (0.4) |
| WBC Count (thous/mcl) | 6.1 (1.4) | 6.1 (1.5) | 6.1 (1.5) |
| Hemoglobin (g/dL) | 12.9 (0.8) | 14.4 (0.8) | 13.6 (1.1) |
| Hematocrit (%) | 37.7 (2.3) | 41.7 (2.4) | 39.7 (3.1) |
| LDL (mg/dl) | 138 (48) | 137 (40) | 138 (44)* |
| HDL (mg/dl) | 62 (15) | 55 (15) | 59 (15) |
| Triglycerides (mg/dl) | 74.8 (41.9) | 68.4 (39.8) | 71.6 (41.0) |
Means (standard deviations) or percentages are presented
p-value <0.05 for difference between males and females
Due to Amish cultural traditions, women do not use tobacco products.
N/A – Not applicable
BMI:Body Mass Index; BP:Blood pressure; RBC:Red Blood Cell; WBC:White Blood Cell; HDL: high-density cholesterol; LDL: low-density cholesterol;
Fig. 2. Platelet Aggregation Before or After Clopidogrel or After DAPT.
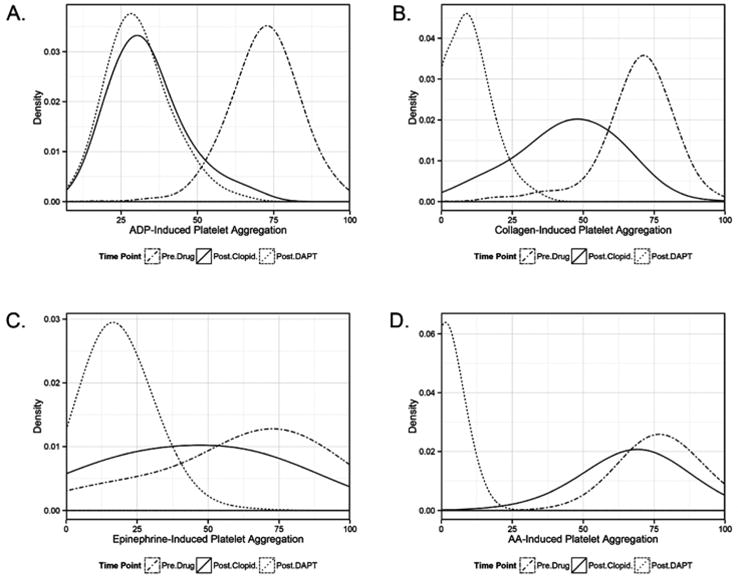
Baseline is denoted by a solid black line, post-clopidogrel by dashed line, and post-DAPT by a dotted-hyphen line. The variation of platelet aggregation response by clopidogrel or DAPT due to A) adenosine diphosphate (ADP) at 20 μM, B) collagen at 2 μg/ml C) epinephrine at 10 μg/ml and D) arachidonic acid (AA) at 1.6 mM.
Association of Participant Characteristics with Platelet-Related Traits
Baseline subject characteristics, including age, sex, BMI, systolic and DBP, HDL-C, LDL-C, triglycerides, and current smoking status, were tested for association with each platelet-related trait (Table 2 and Suppl Table 3). Regression analyses were performed to determine if the traits showed a positive or negative association with agonist-stimulated maximal platelet aggregation. Female sex and older age were associated with higher maximum aggregation for the majority of traits at all 3 time-points. Consequently, other subject characteristics were simultaneously adjusted for age and sex in all analyses. An inverse relationship between triglycerides and collagen (1, 2 and 5 μg/mL) or epinephrine-stimulated aggregation was observed at baseline. While this inverse relationship persisted for epinephrine-stimulated aggregation post-clopidogrel and post-DAPT, it did not for collagen-stimulated aggregation. In fact, a positive association between triglycerides and ADP-stimulated aggregation was observed post-clopidogrel and post-DAPT. Lower blood pressure (both SBP and DBP) was associated with higher platelet aggregation for ADP-, collagen- and epinephrine-stimulated aggregation at baseline, but was, in general, not associated after drug interventions.
Table 2.
Platelet Aggregation Predictors at Baseline, Post-Clopidogrel and Post-DAPT.
| Sex* | Age* | BMI | DBP | SBP | Current Smoker** | HDL | LDL | TG | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | ADP 2 μM | + | + | − | − | − | ||||
| ADP 5 μM | + | + | − | |||||||
| ADP 10 μM | + | + | − | − | ||||||
| ADP 20 μM | + | + | − | − | − | |||||
| Coll. 1 μg/ml | + | + | − | − | − | |||||
| Coll. 2 μg/ml | + | − | − | − | ||||||
| Coll. 5 μg/ml | − | − | − | |||||||
| Coll. 10 μg/ml | − | |||||||||
| Epinephrine | + | + | − | − | − | − | ||||
| AA | ||||||||||
| Post-Clopidogrel | ADP 2 μM | + | + | + | − | + | + | |||
| ADP 5 μM | + | + | + | − | + | |||||
| ADP 10 μM | + | + | + | − | + | |||||
| ADP 20 μM | + | + | + | − | + | |||||
| Coll. 1 μg/ml | + | + | ||||||||
| Coll. 2 μg/ml | + | + | ||||||||
| Coll. 5 μg/ml | + | − | − | |||||||
| Coll. 10 μg/ml | + | |||||||||
| Epinephrine | + | + | − | |||||||
| AA | + | + | + | + | − | + | ||||
| Post-DAPT | ADP 2 μM | + | + | − | + | |||||
| ADP 5 μM | + | + | + | − | + | |||||
| ADP 10 μM | + | + | + | − | + | |||||
| ADP 20 μM | + | + | + | − | + | |||||
| Coll. 1 μg/ml | + | + | − | |||||||
| Coll. 2 μg/ml | + | + | − | |||||||
| Coll. 5 μg/ml | + | + | − | − | ||||||
| Coll. 10 μg/ml | + | + | − | − | ||||||
| Epinephrine | + | + | − | − | − | + | − | |||
| AA |
SBP: systemic blood pressure; DBP: diastolic blood pressure; HDL: high-density cholesterol; LDL: low-density cholesterol; TG: triglycerides; ADP: adenosine diphosphate; Coll.: collagen; AA: Arachidonic Acid
Results for age and sex are unadjusted, results for all other predictors are simultaneously adjusted for age and sex.
Association with current smoking was calculated in males only since females are non-smokers.
Correlations between Changes in Aggregation Across Different Agonists
Age and sex adjusted partial correlations were calculated for change in aggregation between all agonists for each time-point (Fig. 3). In general, different concentrations of the same agonist were highly correlated for clopidogrel response (ADP: 0.29–0.89; collagen: 0.40–0.77), DAPT response (ADP: 0.28–0.88; collagen: −0.01–0.79) and aspirin response (ADP: 0.56–0.88; collagen: −0.05–0.82). Also, more similar concentrations of the same agonist were generally more highly correlated. For example, DAPT response aggregation was more similar between 5 and 10 μg/mL of collagen agonist (r = 0.79) compared with 1 and 10 μg/mL of collagen agonist (r = − 0.01). At all time-points, there was some correlation between change in aggregation across different agonists (r = − 0.03–0.64), but generally to a lower extent than the correlation between different concentrations of the same agonists.
Fig. 3. Correlations between Agonists for Change in Aggregation between Interventions.
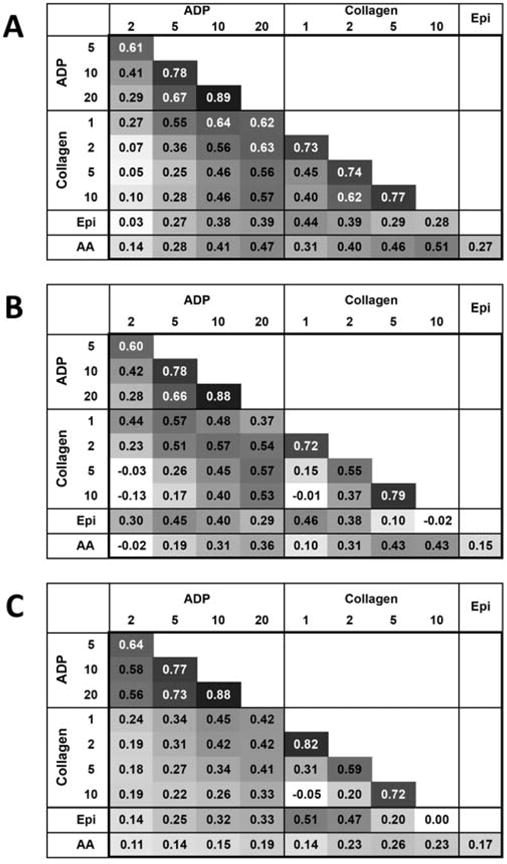
Pair-wise correlations between agonists for change in aggregation between A) baseline and post-clopidogrel (clopidogrel response) B) baseline and post-DAPT (DAPT response) C) Post-clopidogrel and post-DAPT (aspirin response). Darker shading indicates higher correlation.
Heritability of Change in Platelet Aggregation between Different Interventions
In general, change in platelet aggregation between interventions was heritable (Table 3). Change in aggregation was heritable for clopidogrel response (h2: 0.16–0.33) as was DAPT response (h2: 0.17–0.26) for all concentrations of ADP agonist, but aspirin response was only heritable for the two highest agonist ADP concentrations (h2: 0.21–0.25). Change in aggregation stimulated by collagen was heritable for clopidogrel response at the 2, 5 and 10 μg/mL concentrations (h2: 0.14–0.24) as was DAPT response for the two highest concentrations of collagen (h2: 0.38–0.57). Change in aggregation for aspirin response was heritable for all collagen agonist concentrations (h2: 0.24–0.48). Change in aggregation stimulated by epinephrine agonist was not heritable for clopidogrel response but was among DAPT and aspirin responses (h2: 0.21 and 0.52, respectively). Finally, change in AA-stimulated aggregation was only heritable between clopidogrel and aspirin responses (h2: 0.35 and 0.37, respectively). Heritability of aggregation at the 3 time points (as opposed to change) is provided in Suppl Table 4.
Table 3.
Heritability of change in platelet aggregation between interventions.
| Agonist | Clopidogrel Response | DAPT Response | Aspirin Response | |||
|---|---|---|---|---|---|---|
| h2 ± SE | p-value | h2 ± SE | p-value | h2 ± SE | p-value | |
| ADP | ||||||
| 2 μM | 0.23 ± 0.07 | 3.6×10−4 | 0.26 ± 0.08 | 1.5×10−4 | 0.08 ± 0.09 | 0.251 |
| 5 μM | 0.16 ± 0.08 | 0.019 | 0.25 ± 0.10 | 0.003 | 0.06 ± 0.08 | 0.249 |
| 10 μM | 0.24 ± 0.09 | 9.7×10−8 | 0.17 ± 0.09 | 0.023 | 0.21 ± 0.09 | 0.006 |
| 20 μM | 0.33 ± 0.09 | 1.7×10−5 | 0.22 ± 0.09 | 0.006 | 0.25 ± 0.09 | 0.001 |
| Collagen | ||||||
| 1 μg/mL | 0.12 ± 0.08 | 0.078 | 0.04 ± 0.13 | 0.500 | 0.48 ± 0.10 | 6.0×10−7 |
| 2 μg/mL | 0.24 ± 0.09 | 0.003 | 0.11 ± 0.09 | 0.134 | 0.37 ± 0.09 | 2.5×10−5 |
| 5 μg/mL | 0.19 ± 0.08 | 0.009 | 0.38 ± 0.09 | 7.0×10−7 | 0.24 ± 0.08 | 4.6×10−4 |
| 10 μg/mL | 0.14 ± 0.08 | 0.050 | 0.57 ± 0.09 | 5.0×10−13 | 0.32 ± 0.07 | 1.0×10−7 |
| Epinephrine | 0.10 ± 0.10 | 0.220 | 0.21 ± 0.10 | 0.015 | 0.52 ± 0.09 | 5.9×10−10 |
| Arachidonic Acid | 0.35 ± 0.08 | 1.7×10−6 | 0 | 0.5 | 0.37 ± 0.08 | 1.0×10−7 |
Clopid. = Clopidogrel; h2 = heritability; SE = Standard Error; Heritability estimates are adjusted for age and sex.
DISCUSSION
There are multiple factors known to confound studies of anti-platelet drug response such as underlying disease burden, medication non-adherence, concurrent use of medications, vitamins or supplements, and diet. To minimize the effects of these potential confounders, we chose to perform a highly controlled, short-term, anti-platelet intervention in healthy drug-naïve research participants from a relatively homogeneous population. As a founder population, the OOA have a unique ancestral history where the population was formed by a limited number of founders. This population bottle neck may result in a loss of genetic variation if it was not carried by the original founder pool. However, the founders were from a European population and likely share with outbred European populations some, if not most, of the common genetic variants that influence drug response [11]. Further, the OOA population has shown outstanding cooperation with our research group resulting in high rates of participation and adherence to study interventions as evidenced here. Further, the family design of the drug intervention study allowed us to estimate heritability of anti-platelet response traits in order to choose the traits most likely to be driven by genetic determinants. An advantage of our study design is that rather than evaluate a clinical endpoint, e.g. CV events, which may be multifactorial in causation, we chose a more proximal quantitative endpoint, agonist-stimulated platelet aggregation, which is known to correlate with on-treatment risk for CV endpoints. While clinical endpoints remain the gold standard for evaluating anti-platelet therapies it is challenging to recruit sufficient numbers of samples to conduct large-scale analysis and these studies are complicated by factors such as polypharmacy and health care delivery differences across sites. While it is critical to validate findings in studies that do have CV event endpoints, we believe this study design is unique and could represent a powerful approach generalizable to other pharmacogenomic studies.
As expected, we observed significant reductions in agonist-stimulated platelet aggregation after both clopidogrel and DAPT exposure. In agreement with the known mechanism of action, ADP-induced platelet aggregation was most significantly influenced upon clopidogrel exposure. Furthermore, the addition of aspirin resulted in only minor changes to ADP-stimulated platelet aggregation, consistent with prior investigations [12, 13]. Collagen-stimulated platelet aggregation was reduced after clopidogrel and even further after DAPT exposure, indicating that aspirin also impacts collagen-induced platelet aggregation [13]. For epinephrine and AA, there was a relatively minor change between baseline and post-clopidogrel aggregation, but a substantial change after aspirin, which is consistent with aspirin’s known mechanism to inhibit cyclooxygenase [12, 13].
In addition to observing variation in both clopidogrel and DAPT response, we sought to determine which baseline clinical characteristics were associated with ex vivo platelet aggregation. We observed a positive association between age and agonist-induced platelet aggregation prior to drug exposure, similar to previous reports [14]. Interestingly, this relationship persisted after both clopidogrel and DAPT intervention. An association between female sex and platelet aggregation was observed at baseline and after intervention. While sex-specific response to aspirin is well established, less is known about sex-specific differences in clopidogrel response, though in other studies the risk of CVD events (death from cardiovascular causes, nonfatal MI, or stroke) in clopidogrel-treated ACS patients was similar in the male and female subgroups [15–17]. In the current analysis, it is unlikely that sex specific differences were observed because of variability in baseline characteristics, as the two sexes were relatively well-matched.
Our results also demonstrated that change in platelet aggregation in response to different agonists is correlated. The highest correlations were observed within ADP- and collagen-induced platelet aggregation for clopidogrel and DAPT responses. As shown in Fig. (3), most of these agonists are moderately correlated with one another, validating that they are dependent on each other but also provide unique information. ADP-stimulated aggregation is likely reflecting clopidogrel response as it interacts with the P2Y12 receptor, while AA-stimulated aggregation is reflective of aspirin’s COX-mediated inhibition. Collagen-stimulate aggregation, on the other hand, may be measuring non-COX mediated platelet aggregation [18]. Taken together, these data may suggest that measurement of multiple agonists is important in order to provide a complete assessment of platelet aggregation and provides further evidence for use of either multiple measures or composite measures in pharmacogenomics studies [19].
Previous reports demonstrate heritability for ADP-, collagen- and epinephrine-stimulated platelet aggregation in population samples not on anti-platelet therapy [20, 21]. A previous short-term intervention of aspirin alone in the OOA reported significant heritability estimates in collagen-, ADP-and AA-stimulated aggregation after aspirin, though post-aspirin heritability was lower than baseline heritability[16]. Our study builds on this previous work by reporting that change in aggregation during clopidogrel or DAPT intervention is also heritable, an observation which has important implications for pharmacogenomics research. We also replicated the heritability for ADP-stimulated aggregation at baseline and post-clopidogrel in the full sample which we previously reported for a subset of samples with genome-wide genotyping from the PAPI study (Suppl Table 4) [22].
Although the current analysis does not incorporate any genetic markers, an objective of the PAPI study was to provide a discovery resource for genetic variants influencing the response to anti-platelet interventions. Post-hoc power calculations were conducted using QUANTO, a power and sample size calculator appropriate for a variety of genetic epidemiology study designs [23], and estimate that our sample size of 687 participants provides 80% power at a genome-wide level of significance (p = 5×10−8) to identify variants that explain at least 5.5% of the trait variation. Genome-wide genotyping has been conducted in this study and a genome-wide analysis of the first 429 participants from this study had sufficient power to detect a strong association with the CYP2C19 *2 variant which accounts for 8–9% of the variation in ADP-stimulated platelet aggregation; this association was subsequently followed up in a clinical population of patients undergoing percutaneous coronary intervention [22]. This discovery has led to ongoing pharmacogenomic studies continue to improve our view of anti-platelet therapy [24]. Candidate genes, such as PEAR1 and CES1, have been identified through the PAPI study showing variability in clopidogrel and/or aspirin response [11, 25].
These findings suggest that short-term drug interventions in healthy subjects to minimize non-genetic sources of variation in drug response provide a powerful approach to identify clinically actionable pharmacogenomics variants. While limiting the study to healthy participants may limit generalizability, replication and extension of genetic findings in patient populations has been successfully employed in other studies to help overcome this generalizability limitation. Further, similar pharmacogenomics study designs focusing on short-term interventions with surrogate endpoints could be implemented in earlier stages of drug development to identify potentially actionable genetic variants earlier in the drug development lifecycle.
Supplementary Material
Acknowledgments
We thank the research clinic staff and Amish participants for their dedication to this research. Construction and maintenance of the Anabaptist Genealogy Database (AGDB) is covered under an IRB-approved protocol at the National Institutes of Health (Dr. Leslie Biesecker, Principal Investigator).
This project was supported by research grants from the National Institutes of Health including U01 HL105198 and U01 GM074518. Partial support was also received from the Mid-Atlantic Nutrition and Obesity Center (NORC) (P30 DK072488). LMYA was supported by K01-HL116770 and JPL was supported by K23-GM102678.
Biography

Laura M. Yerges-Armstrong
Footnotes
CONFLICT OF INTEREST
Alan Shuldiner is a consultant for United States Diagnostic Standards, Inc and an employee of Regeneron Pharmaceuticals, Inc. All other authors (LMYA, LMB, JPL, BDM, JRO, KAR, RBH, WH) report no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
References
- 1.Bartholow M. Top 200 Drugs of 2011. Pharmacy Times. 2012 [Google Scholar]
- 2.Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107(23):2908–13. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 3.Wong S, Appleberg M, Ward CM, Lewis DR. Aspirin resistance in cardiovascular disease: a review. Eur J Vasc Endovasc Surg. 2004;27(5):456–65. doi: 10.1016/j.ejvs.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 4.Faraday N, Yanek LR, Mathias R, et al. Heritability of platelet responsiveness to aspirin in activation pathways directly and indirectly related to cyclooxygenase-1. Circulation. 2007;115(19):2490–6. doi: 10.1161/CIRCULATIONAHA.106.667584. [DOI] [PubMed] [Google Scholar]
- 5.Bliden KP, DiChiara J, Tantry US, Bassi AK, Chaganti SK, Gurbel PA. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? J Am Coll Cardiol. 2007;49(6):657–66. doi: 10.1016/j.jacc.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 6.Lee WJ, Pollin TI, O’Connell JR, Agarwala R, Schäffer AA. Ped-Hunter 2.0 and its usage to characterize the founder structure of the Old Order Amish of Lancaster County. BMC Med Genet. 2010;11:68. doi: 10.1186/1471-2350-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwala R, Biesecker LG, Schaffer AA. Anabaptist genealogy database. Am J Med Genet C Semin Med Genet. 2003;121C(1):32–7. doi: 10.1002/ajmg.c.20004. [DOI] [PubMed] [Google Scholar]
- 8.Sysmex. Platelet distribution curves: Interpretation, potentials and limitations. 2011 [cited 2014 March 10]. Available from: http://www.sysmex.ru/files/articles/Xtra_online_Platelet_distribution_curves.pdf.
- 9.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 10.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis JP, Ryan K, O’Connell JR, et al. Genetic variation in PEAR1 is associated with platelet aggregation and cardiovascular outcomes. Circ Cardiovasc Genet. 2013;6(2):184–92. doi: 10.1161/CIRCGENETICS.111.964627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamath S, Blann AD, Chin BS, Lip GY. A prospective randomized trial of aspirin-clopidogrel combination therapy and dose-adjusted warfarin on indices of thrombogenesis and platelet activation in atrial fibrillation. J Am Coll Cardiol. 2002;40(3):484–90. doi: 10.1016/s0735-1097(02)01984-8. [DOI] [PubMed] [Google Scholar]
- 13.Cooke GE, Liu-Stratton Y, Ferketich AK, et al. Effect of platelet antigen polymorphism on platelet inhibition by aspirin, clopidogrel, or their combination. J Am Coll Cardiol. 2006;47(3):541–6. doi: 10.1016/j.jacc.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Terres W, Weber K, Kupper W, Bleifeld W. Age, cardiovascular risk factors and coronary heart disease as determinants of platelet function in men. A multivariate approach. Thromb Res. 1991;62(6):649–61. doi: 10.1016/0049-3848(91)90369-8. [DOI] [PubMed] [Google Scholar]
- 15.Demyanets S, Wojta J. Sex differences in effects and use of anti-inflammatory drugs. Handb Exp Pharmacol. 2012;(214):443–72. doi: 10.1007/978-3-642-30726-3_20. [DOI] [PubMed] [Google Scholar]
- 16.Shen H, Herzog W, Drolet M, et al. Aspirin Resistance in healthy drug-naive men versus women (from the Heredity and Phenotype Intervention Heart Study) Am J Cardiol. 2009;104(4):606–12. doi: 10.1016/j.amjcard.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 18.Sangkuhl K, Shuldiner AR, Klein TE, Altman RB. Platelet aggregation pathway. Pharmacogenet Genomics. 2011;21(8):516–21. doi: 10.1097/FPC.0b013e3283406323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voora D, Ortel TL, Lucas JE, Chi JT, Becker RC, Ginsburg GS. Time-dependent changes in non-COX-1-dependent platelet function with daily aspirin therapy. J Thromb Thrombolysis. 2012;33(3):246–57. doi: 10.1007/s11239-012-0683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Donnell CJ, Larson MG, Feng D, et al. Genetic and environmental contributions to platelet aggregation: the Framingham heart study. Circulation. 2001;103(25):3051–6. doi: 10.1161/01.cir.103.25.3051. [DOI] [PubMed] [Google Scholar]
- 21.Bray PF, Mathias RA, Faraday N, et al. Heritability of platelet function in families with premature coronary artery disease. J Thromb Haemost. 2007;5(8):1617–23. doi: 10.1111/j.1538-7836.2007.02618.x. [DOI] [PubMed] [Google Scholar]
- 22.Shuldiner AR, O’Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–57. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauderman WJ, Morrison JM. QUANTO 1.1: A computer program for power and sample size calculations for genetic-epidemiology studies. 2006 Available from: http://hydra.usc.edu/gxe.
- 24.Horenstein RB, Madabushi R, Zineh I, et al. Effectiveness of clopidogrel dose escalation to normalize active metabolite exposure and antiplatelet effects in CYP2C19 poor metabolizers. J Clin Pharmacol. 2014;54(8):865–73. doi: 10.1002/jcph.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis JP, Horenstein RB, Ryan K, et al. The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenet Genomics. 2013;23(1):1–8. doi: 10.1097/FPC.0b013e32835aa8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


