Abstract
Membrane wrapped nanoparticles represent a versatile platform for utilizing specific lipid-receptor interactions, such as siallyllactose-mediated binding of the ganglioside GM3 to Siglec1 (CD169), for targeting purposes. The membrane wrap around the nanoparticles does not only serve as a matrix to incorporate GM3 as targeting moiety for antigen presenting cells but also offers unique opportunities for constructing a biomimetic surface from lipids with potentially protein repellent properties. We characterize non-specific protein adsorption (corona formation) to membrane wrapped nanoparticles with core diameters of approx. 35 nm and 80 nm and its effect on the GM3-mediated targeting efficacy as function of surface charge through combined in vitro and in vivo studies. The stability and fate of the membrane wrap around the nanoparticles in a simulated biological fluid and after uptake in CD169 expressing antigen presenting cells is experimentally tested. Finally, we demonstrate in hock immunization studies in mice that GM3 decorated membrane wrapped nanoparticles achieve a selective enrichment in the peripheral regions of popliteal lymph nodes that contain high concentrations of CD169 expressing antigen presenting cells.
Keywords: zwitterion, GM3, Siglec1, stealth nanoparticle, hyperspectral imaging, antigen presenting cells, gangliosides
Active targeting of nanoparticles (NPs) to selected cell populations through specific ligand – receptor interactions has become a leading theme in drug delivery and NP-based imaging. Nanoconjugated antibodies,1 peptides,2 aptamers3 and small molecules4 that recognize specific surface groups have been used to guide NP binding to a particular subset of cells that (over)express these functionalities. Recent findings that some gangliosides, i.e. glycosphingolipids with at least one sialic acid, enhance the targeting of specific host cells by the enveloped human immunodeficiency virus (HIV-1) have generated significant interest in these glycolipids as alternative targeting functionalities for NPs.5–8 A series of recent studies have shown that the sialyllactose group of gangliosides, such as the monosialoldihexosyl-ganglioside GM3, facilitates a selective binding of GM3-presenting NPs to Siglec1 (CD169)-expressing myeloid dendritic cells and macrophages.6,8–12 This particular set of antigen presenting cells (APCs) plays a key role in priming and activating B cells,13–16 iNKT,7,17 and CD8+ T18–20 cells, and the selective targeting of the above-mentioned cells provides new opportunities for enhancing cell-mediated immunity and for improving vaccination efficacy.
Lipid membrane wrapped NPs represent a tailorable platform for endowing GM3-presenting NPs with targeting functionality for CD169+ APCs. Due to their conceptual similarity with enveloped virus particles, we refer to these hybrid NPs as artificial virus NPs (AVNs).5,6 Although the GM3-CD169 binding affinity is relatively low (10−3 M),18 multivalent presentation of the ligand in the NP supported membrane can achieve a significant enhancement of the binding avidity.12 Since GM3 is an endogenous ganglioside that plays a ubiquitous role in exosome-mediated intercellular communication,21,22 it has an intrinsically low immunogenicity.23 Consequently, unlike many of the conventional targeting moieties that utilize antibodies or recombinant receptor-binding domains,24 multivalent presentation of GM3 does not risk eliciting an unintentional innate immune response.25
One general challenge for all active NP targeting methods, including ganglioside-based approaches, is that in biological fluids a broad variety of different proteins rapidly adsorb to the charged NP surface.26–29 The resulting formation of a “corona” around a NP impacts the fate and distribution of NPs in vivo due to non-specific opsonization and scavenging.27,30–33 Furthermore, non-specific protein adsorption can cover NP-bound surface ligands and, thus, result in a reduction of their bio-availability and trigger ligand unrelated biological responses.34 The conventional strategy for suppressing corona effects is based on PEGylation. PEGs have demonstrated to suppress corona formation around NPs,35 and they are also widely used in NP vaccines.36 Several studies have, however, indicated that PEGs are immunogenic37–39 and reduce intracellular uptake40–42 and transfection efficiency.43 Furthermore, especially for lipids with relatively small headgroups as targeting functionality, loss of activity through bulky PEGs that bury the active sites in a membrane is a potential concern. All of these points together prompted our interest in enhancing the efficacy of NP targeting strategies through AVNs with appropriate targeting moieties. Common lipids, such as phosphatidylcholine, are zwitterionic and self-assembled monolayers of zwitterionic ligands have shown great promise for effectively suppressing corona formation around a wide variety of NPs.44–48 The lipid membrane wrapping approach underlying the AVN strategy does, therefore, not only provide a matrix for the decoration of NPs with gangliosides but also improves the biocompatibility of the NP core49–55 and provides a platform for engineering protein repellent surface properties.
In the following, we will 1.) characterize the non-specific protein adsorption to GM3 containing AVNs (GM3-AVN) with a 35 and 80 nm core, 2.) evaluate the effect of NP core size and membrane charge on corona formation and CD169 targeting selectivity in vitro, 3.) test the stability of AVN membrane in complex biological environments, and 4.) validate the ability of optimized GM3-AVN particles to target CD169+ APCs in popliteal lymph nodes after hock injection in a murine immunization model.
RESULTS AND DISCUSSION
NP Design and Assembly
AVNs were generated by integrating lipids via their hydrophobic tail into an octadecanethiol monolayer self-assembled around a 34.6 ± 0.3 nm or 82.0 ± 1.1 nm diameter gold NP core. We refer to these two AVNs with different Au NPs core sizes as AVN35 and AVN80 throughout this manuscript. We applied a “one-pot” assembly6,56 procedure in which citrate stabilized gold colloid was incubated with octadecanethiol and liposomes as lipid reservoir to generate AVNs (see Figure 1 & Methods). Our design of a membrane wrapped NP that combines protein repellent surface properties and targeting efficacy is inspired by evolutionarily optimized enveloped virus particles, such as the human immunodeficiency virus (HIV). The composition of the liposomes (≥55mol% 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), ~40 mol% cholesterol) was, consequently, chosen to imitate the basic membrane properties of enveloped HIV particles.57 3 mol% GM3 was included for targeting purposes. DPPC was selected as major component due to its zwitterionic nature and small headgroup size (Figure 1), which minimizes any potential interference with the CD169-binding sialyllactose group of GM3. We found that a small concentration of negatively charged lipid was necessary to ensure the colloidal stability of the AVNs over several days. We, consequently, added 1–5 mol% 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (DOPS) to vary the surface charge of the AVNs in a rational fashion. One particular appeal of the AVN approach is that it promises a NP passivation and stabilization without the need of bulky surface ligands. An alternative ligand of similar size as the lipids that can stabilize 35 – 80 nm diameter NPs against agglomeration in the salt concentrations of typical biological buffers is the alkyl-PEG-carboxylic acid HS-(CH2)11-EG6-OCH2-COOH.58,59 We included NPs passivated with these PEGs as benchmark for non-specific protein adsorption as the resulting NPs have similar zeta potentials as AVNs dosed with 5% DOPS (Table 1). We refer to these NPs as PEG-NP throughout this manuscript. The zeta potentials of all NPs considered in this work are summarized in Table 1. The data confirm that variation of the DOPS concentration in the AVN membrane allows a systematic variation of the AVN surface potential between approximately −10 and −30 mV. Importantly, this range covers the zeta potential of HIV particles at neutral pH, which has been determined as −15–20 mV.60 The AVN membrane differs from the lipid bilayer membranes of conventional liposomes as it does not contain two leaflets but, instead, contains lipids inserted into an octadecanethiol brush (Figure 1). To further characterize this unique membrane we recorded differential scanning calorimetry (DSC) thermograms for AVNs (80 nm gold core, 1 mol% DOPS and 40 mol% cholesterol) and the respective liposomes used for their assembly. Thermograms for representative preparations are shown in Figure 2. While the unilamellar liposomes exhibit an endothermic peak at 49.2°C corresponding to a gel-liquid phase transition, the thermogram of the AVN does not contain a well-defined peak. Instead, a broad feature with a minimum at 46.0°C is observed. A broadening of phase transitions in membranes has been interpreted before as a sign of reduced cooperativity and reduced lateral structural correlation.61 The broad features observed for AVNs indicate, thus, a less ordered membrane than in the liposome. A decrease in structural order could result in increased lateral lipid mobility in AVN membranes. The latter would increase the efficacy of multivalent binding interactions between lipid ligands and their cellular targets and have important biological implications.
Figure 1.
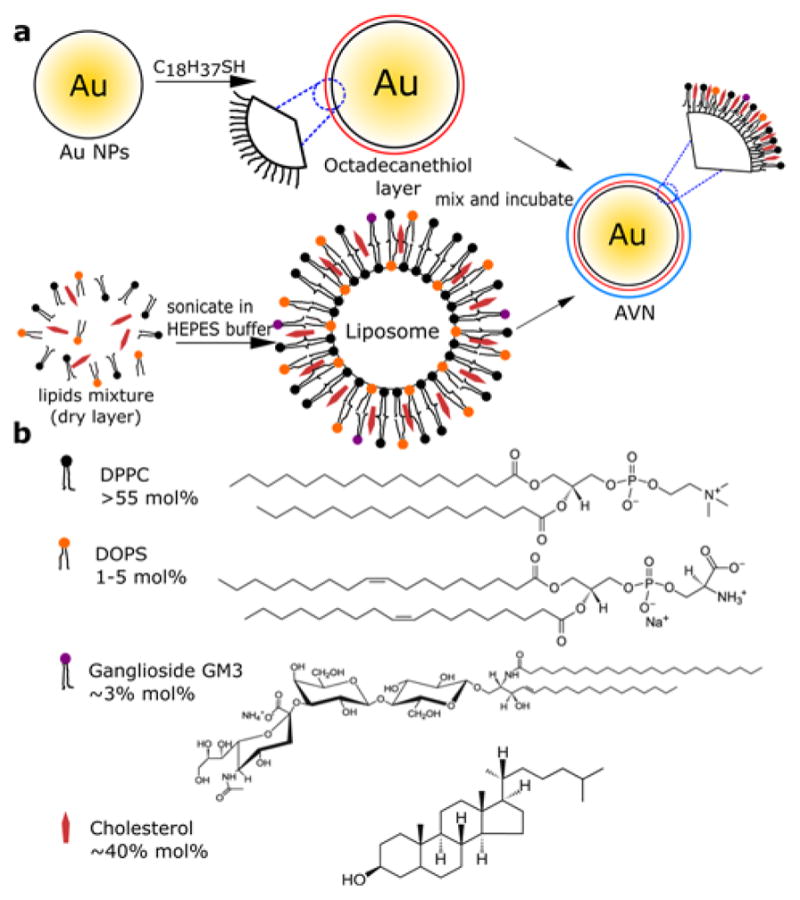
a) Schematic overview of AVN preparation. b) Molecular structures of DPPC, DOPS, GM3, cholesterol and their respective concentrations in the liposomes.
Table 1.
Zeta potentials (ζ) for all investigated NPs in DDI water
| Nanoparticle | ζ (mV) |
|---|---|
| 1mol% DOPS AVN35 | −12.9±2.0 |
| 2mol% DOPS AVN35 | −21.9±1.5 |
| 5mol% DOPS AVN35 | −30.1±3.0 |
| 1mol% DOPS AVN80 | −14.9±1.9 |
| 2mol% DOPS AVN80 | −26.0±0.3 |
| 5mol% DOPS AVN80 | −30.6±1.1 |
| PEG-NP (35nm) | −30.7±3.0 |
| PEG-NP (80nm) | −35.0±0.9 |
Figure 2.

DSC thermograms of a) liposome precursors (see text) and b) AVNs.
Corona Formation around Membrane Wrapped NPs
The chemical composition of the surface as well as the surface charge are key factors that effect corona formation around NPs.27,62 Furthermore, the NP size can modulate the corona formation due to curvature-related changes in the membrane composition63–68 and NP-protein interactions.26,69 The National Institutes of Health defines nanomaterials as materials with characteristic length scales < 100 nm.70 For the sake of simplicity we limited our investigation to two representative NP core sizes (AVN35 and AVN80) in the characteristic size range of most virus particles. Corona formation around NPs is frequently studied in vitro under standardized cell growth conditions (10% FBS in DMEM at 37°C).71–74 To facilitate comparability with previous studies we applied the same conditions and quantified corona formation by measuring hydrodynamic radii and the zeta potentials for AVN35 and AVN80 containing three different DOPS concentrations (1, 2, and 5 mol%).
Non-specific NP-protein interactions lead to protein deposits classified as hard (non-reversible binding) or soft (reversible binding) corona.27,75 We differentiated these coronas for the investigated AVNs by measuring Dhyd of the NPs under three different conditions: i) before addition to FBS containing medium (no corona), ii) after incubation in FBS containing medium for 24h (hard + soft corona, without any washing procedures), and iii) after removal of the soft corona through centrifugation and re-suspension in water (hard corona).76 For all conditions we included PEG-NPs as benchmark.
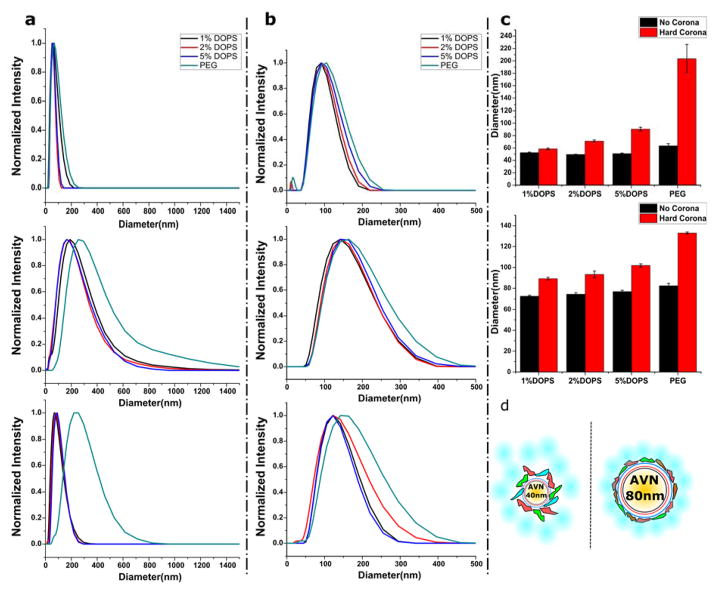
Representative distributions of the hydrodynamic diameter as determined by dynamic light scattering (DLS) for the investigated AVN35 and AVN80 are summarized in Figure 3a and b, respectively. For all AVN35, a large (ΔDhyd ≈100 nm) shift of the average hydrodynamic diameter is obtained upon incubation in FBS containing medium (Figure 3a, middle, hard + soft corona). While the differences in ΔDhyd between the three AVN35 preparations lie within the range of typical experimental fluctuations associated with the preparations of AVNs, the shift for the PEG-NPs is (ΔDhyd ≈ 200 nm) significantly larger than for all AVN35 conditions, including AVN35 with 5 mol% DOPS, whose zeta potentials are comparable to those of PEG-NPs (Table 1). Even more distinct differences between AVN35 and PEG-NPs become apparent when the NPs are washed to remove the soft corona (Figure 3a, bottom, hard corona).76 AVNs with 1–5 mol% PS containing membranes show a substantial decrease of Dhyd by ~70–100 nm and an overall sharpening of the Dhyd distributions after removal of the soft corona. In contrast, the Dhyd distribution of the 35 nm core PEG-NPs before and after washing remains nearly unchanged. Only the width of the distribution decreased somewhat after removal of the soft corona.
Figure 3.
Hydrodynamic diameter distribution (based on DLS, intensity statistics) for AVN35 (a) or AVN80 (b) for three different conditions: i) no corona (top). ii) hard + soft corona (middle) and iii) hard corona (bottom). All DLS histograms are normalized. c) Bar plots of average hydrodynamic diameters (Z-average size) for AVN35 (top) and AVN80 (bottom) without corona (black) and with hard corona (red). The error bars are standard deviations from two independent measurements d) Structural models for corona formation around AVN35 and AVN80. The inner hard corona is strongly tethered to the AVN surface and attracts and structures an outer, more weakly bound soft corona.
Interestingly, the behavior of AVN80 differs from that of AVN35. The average shift of the Dhyd distribution after corona (hard + soft) formation (Figure 3b, middle) is with ΔDhyd ≈ 50 nm noticeably smaller than for AVN35 (ΔDhyd ≈ 100 nm). Also, the difference in the corona thickness between PEG-NP and the three types of AVN80 is less drastic. The PEG-NP distribution shows only a slight broadening on the right side of the distribution. After removal of the soft corona through washing (Figure 3b, bottom) the average ΔDhyd values for AVN80 reduce by ~25 nm, independent of the DOPS concentration. The smaller change in ΔDhyd associated with the removal of the soft corona for AVN80 when compared with AVN35 indicates a thinner soft corona for the larger NP core. As in the case of the 35 nm NP core the effect of washing is the smallest for 80nm PEG-NP. The Dhyd distribution remains almost unchanged in this case.
For a closer systematic comparison of the hard corona formation for the different experimental conditions, we summarized in Figure 3c the average hydrodynamic diameters for AVN35 and AVN80 without corona and with hard corona, i.e. experimental conditions i.) and iii.) from multiple independent experiments. For both NP core sizes Dhyd increases with increasing DOPS concentration (= increasing negative surface potential) in the assembled AVN membrane. The effect of the increasing negative charge on the hard corona of AVN35 and AVN80 is, however, smaller compared with the increase in size obtained for the corresponding PEG-NPs.
One caveat of our interpretation of the DLS data so far is that the hydrodynamic diameter of the NPs measured via DLS depends both on the size of the NPs as well as their agglomeration state. To exclude that the observed increases in the hydrodynamic diameter result from agglomeration rather than corona formation we monitored the plasmon resonances of the NPs, which are sensitive to NP clustering.77–81 We recorded UV-Vis spectra of the investigated NPs to determine the association state of the NPs for all three conditions i.) – iii.). The spectra (Figures S1 & S2) of all investigated AVNs show red-shifts in the range between 0–5 nm after corona formation; for the PEG-NPs the peak wavelength shifts between 4–7 nm. The absence of an asymmetric broadening of the plasmon resonance or the appearance of a new shoulder on the long wavelength side in all cases except for the 80 nm PEG-NPs argues against a systematic agglomeration of the AVNs under the chosen experimental conditions. Instead, the magnitude of the observed systematic spectral shifts is consistent with an increase in the local refractive index around AVNs due to non-specific protein adsorption.82,83 Even in the case of the 80 nm PEG-NPs the broadening of the spectrum is relatively small, indicating only moderate levels of agglomeration.
The polydispersity index (PDI) in DLS can provide additional information about the level of agglomeration in solution. The PDI values (Figure S3) for AVN80 are < 0.25 for all three experimental conditions i.)-iii.), confirming relatively sharp size distributions.84 For AVN35 the PDI values for NPs without corona and after hard corona formation are also < 0.25, only for the soft corona condition the PDI value increases to 0.39. We attribute this increase in PDI to the presence of 10% FBS in DMEM in the soft corona case. The serum contains undefined particles with an average particle size of 21 nm and a PDI of 0.56. As the scattering signal is much weaker for AVN35 than for AVN80, this background will particularly affect the measured PDI value of the former.
Based on the UV-Vis data and the measured PDI values, we attribute changes in Dhyd to the (de-) assembly of a corona for all three experimental conditions. Given the fact that the same DOPS concentration yields comparable zeta potential values for AVN35 and AVN80, the pronounced differences in the thickness of the soft and hard corona around these particles observed in Figure 3 demonstrate that even for NP core sizes in the tens of nanometer range the core diameter still has a profound impact on corona formation. The differences between AVN35 and AVN80 imply curvature-induced changes in the interactions between AVNs and the proteins in their environment. The trends observed for the AVNs are consistent with previous studies34 that demonstrated a larger increase in size after corona formation for smaller NPs. One possible model to account for the observed soft corona behaviors is outlined in Figure 3d. Size or curvature-dependent binding interactions between the proteins and the NP induce different structural configurations of the proteins in the inner coordination shell (= hard corona) of AVN35 and AVN80. The size-dependent interactions result in the presentation of different groups or surfaces to the proteins (and other components) of the ambient medium. According to this model AVN80 with an intrinsically lower curvature contains a well-ordered inner-protein shell that saturates the binding sites of the attached proteins so that only a relatively well organized thin soft-corona is formed. In contrast, for the smaller AVN35 the packing of the proteins in the inner layers is less perfect, creating a broad-range of available binding sites for additional proteins from the immediate environment, resulting in an extensive and relatively random soft corona protein network85 associated with the NP core.
Our DLS data also shows that the chemical nature of the NP surface (compare PEG-NPs with AVNs) influences the binding and structure of the inner protein layer and, thus, further modulates the corona properties. PEG-NPs and AVNs with identical core size and comparable zeta potential values (Table 1) have different hydrodynamic diameters (compare PEG-NPs with 5 mol% DOPS containing AVN35 and AVN80 in Figure 3a and b). In fact, the absence of a systematic decrease in hydrodynamic diameter after washing the PEG passivated 35 and 80 nm NPs questions the differentiation between hard and soft corona for these particles.
Corona Formation and Electrophoretic Mobility
To further characterize the effect of corona formation around AVNs, we investigated the electrophoretic mobility of AVN35 and AVN80 (1, 2, or 5 mol% DOPS) before and after incubation in 10 mol% FBS containing medium for 24 h at 37°C (Figure S4). The AVNs were loaded in a 1% agarose gel after washing by centrifugation and resuspension in gel running buffer (0.5× tris/borate/edta (TBE)), so that the gel experiments tested primarily the effect of the hard corona. In the absence of corona, the mobility of AVN35 and AVN80 increases continuously as function of DOPS concentration. Corona formation removes, however, all differences between the AVNs. After hard corona formation all AVNs with a given NP core show the same mobility, independent of the DOPS concentration, and move overall slightly slower than corona-free AVNs with the lowest DOPS concentration (1 mol%). Importantly, corona formation eliminates the diffuse spread and focuses the bands in the gel, indicating that corona formation stabilizes the particles against agglomeration in the gel running buffer.86
Corona Formation and GM3-Mediated Binding Selectivity
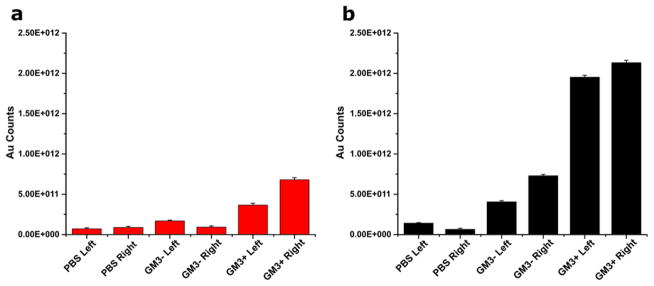
The corona formation characterized in the previous sections raises the question whether the protein adsorption onto the NPs interferes with the applicability of AVNs as a platform for lipid-guided targeting due to a loss in binding affinity and/or selectivity. We tested this important question for GM3-mediated targeting of CD169. To that end, we pre-incubated GM3 presenting AVN35 in 10% FBS containing DMEM at 37°C for 24 h. After that, the NPs were collected by centrifugation, washed and then added to CD169 expressing HeLa cells in FBS containing medium. After 90min of co-incubation with an AVN35 preparation containing 1 or 5 mol% DOPS, we quantified the gold content in the cells by inductively coupled mass spectrometry (ICP-MS). As control we used AVN35 with 1 mol% DOPS but no GM3. Figure 4 summarizes the measured relative gold contents. The GM3-AVN preparation (3 mol% GM3+) with 1 mol% DOPS shows an approximately 6-fold higher binding than the control without GM3 (GM3−). Interestingly, for the 5 mol% DOPS containing GM3-AVN sample the measured gold concentration drops and nearly approaches that of the GM3− control. The 5 mol% DOPS containing AVN35 particles have a higher negative surface charge, which results in a thicker hard corona (Figure 3c). The decreased binding observed in this case indicates an increased perturbation of GM3-CD169 binding interactions due to a more efficient masking of the GM3 ligand by the thicker protein layer.
Figure 4.
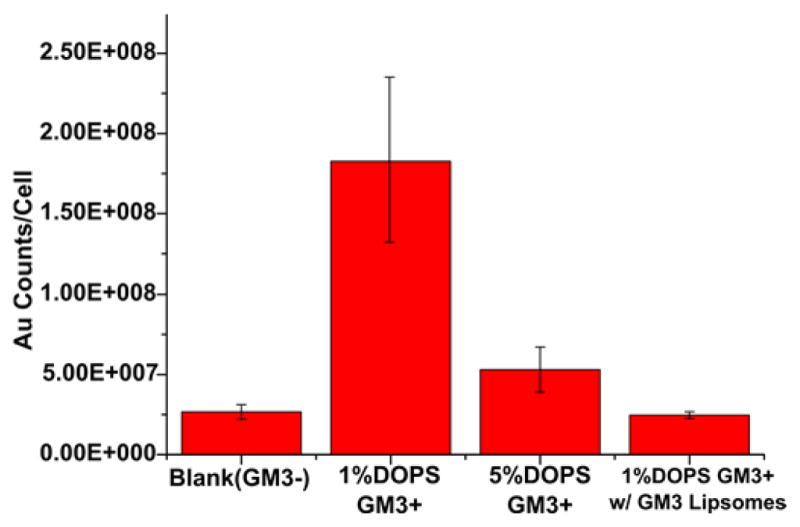
AVN35 binding to CD169 transfected HeLa quantified by ICP-MS for three different AVN configurations (from left to right): blank (GM3− / 1 mol% DOPS); GM3+ / 1 mol% DOPS; GM3+ / 5 mol% DOPS; GM3+ / 1 mol% DOPS in the presence of an excess of GM3 containing liposomes. Error bars are standard deviations from three experiments.
To further validate that the observed binding is GM3-mediated, we incubated GM3 containing AVNs (1 mol% DOPS) with cells in the presence of an excess of GM3 containing liposomes. The GM3-containing liposomes outcompete AVN binding and achieve a large reduction in the measured gold ion concentration (Figure 4). The successful competitive inhibition of AVN binding by GM3 presenting liposomes further confirms that the AVN binding is GM3-mediated.
Intracelluar Stability of AVNs
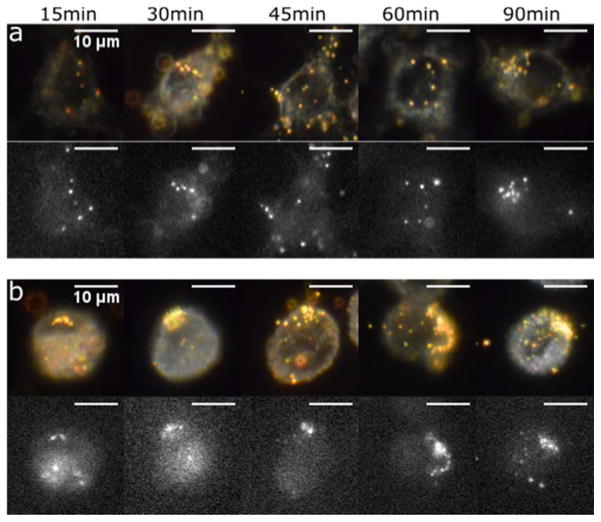
AVNs are intended for use in biological matrices where other lipids derived from cellular membranes are abundant. The possibility of lipid exchange and fusion in such an environment raises concerns about the stability of the AVN membrane. Since GM3 presenting AVNs are intended for targeting CD169+ APCs, we evaluated the intracellular stability with CD169+ macrophages and mature human dendritic cells (mDCs). We used AVN80 for these experiments as the bright 80 nm gold NP core allows for an unambiguous detection of even individual AVNs. We included a fluorescent lipid marker (~0.1mol%, Liss-Rho-PE) in the AVN membrane (1 mol% DOPS), which facilitated a spatial mapping of the lipids in the APCs as a function of time through epi-fluorescence microscopy. The position of the gold NP cores was monitored in parallel by darkfield microscopy. In both CD169+ macrophages (Figure 5a) and mDCs (Figure 5b) we observed a strong correlation of lipid fluorescence and darkfield scattering signals from cell-associated AVNs for over 90 min, which is a characteristic time scale for NP uptake and processing in APCs. Based on these observations, we conclude that for the chosen membrane composition, which is rich in cholesterol, the AVNs retain a high degree of lipid membrane integrity in both CD169+ macrophages and mDCs. APCs are well-known for their “harsh” endocytotic conditions (acidic pH and high concentration of proteolytic enzymes) that facilitate efficient antigen presentation. The observation of a stable AVN membrane under these conditions highlights the robust nature of the AVNs.
Figure 5.
Optical colocalization of NP core darkfield signal and lipid label fluorescence signal confirms membrane stability of uptaken AVNs. a) Correlated color darkfield (top) and fluorescence (bottom) images of GM3-AVN80 in CD169+ macrophages recorded at the specified time points. b) Correlated color darkfield (top) and fluorescence (bottom) images of GM3-AVN80 in mDCs as function of time. Scale bars are 10μm.
Interestingly, even though both CD169+ macrophages and mDCs express the CD169 receptor (Figure S5) that is responsible for an efficient binding of GM3-AVNs, the fate of the AVNs spatial distribution pattern in these two APCs is very different. In CD169+ macrophages, AVNs are seemingly randomly distributed in a scattered pattern, while in mDCs the AVNs are segregated in large clusters. This GM3-specific segregation in mDCs has been observed before and was interpreted as a successful mimicry of GM3-mediated compartmentalization of the enveloped virus particles in deep peripheral membrane invaginations.5,6 These differences can be exploited in the future for developing smart cell-specific delivery and drug release strategies.
Targeting of CD169+ Cells in vivo
After demonstrating that – despite corona formation – GM3-AVNs retain their binding selectivity for CD169 expressing APCs in vitro, we tested their applicability to target CD169 expressing APCs in secondary lymphoid tissues in mice. CD169+ macrophages and DCs are enriched in the subcapsular sinus and perifollicular sinus,14,87 respectively, where they act as sentinels for invading pathogens. To assess whether GM3-AVNs facilitate a selective targeting of these cells in the peripheral regions of lymph nodes even in the presence of a protein corona on the NP surface, we injected AVN80 (1 mol% DOPS) with(+) and without(-) 3 mol% GM3 in the right and left hock, respectively, of living mice. We chose AVN80 with 1 mol% DOPS as these particles minimized corona formation in our in vitro studies. In each experiment 10 μL (2×1010 AVNs/mL in 1× PBS) of GM3-AVN80 colloid was injected. PBS injections (no AVNs) served as additional negative controls. We excised the popliteal lymph nodes and analyzed their gold content via ICP-MS 1 h and 4 h after injection. The measured gold levels for GM3+ and GM3− AVNs (2 repeats per condition) and controls are summarized in Figure 6. Both time points show a significant GM3-dependent enrichment in the lymph nodes, but the total GM3-AVN concentration in the lymph nodes increased by a factor of approximately 4 between 1 h and 4 h. CD169+ APCs are known to show a strong preferential localization at the periphery of the lymph nodes.14,87 To further validate that the observed enrichment of GM3 containing AVN80 in lymph nodes is due to GM3-CD169 specific interactions, we investigated the spatial distribution of GM3-AVNs (80 nm core, 1 mol% DOPS, +/− 3 mol% GM3) in lymph node sections prepared 1 h after AVN injection through darkfield microscopy. The areas of increased scattering intensity with a metallic appearance in Figure 7a indicate gold NP enriched areas. To unambiguously identify the position of the AVNs we augmented this digital color image with a quantitative analysis based on hyperspectral widefield imaging. As the elastic scattering spectrum of resonantly scattering plasmonic metal NPs differs from that of the tissue, the intensity distribution recorded for each individual pixel in the field of view at different wavelengths provides a characteristic fingerprint for the identification of the AVNs.88 Figure 7b contains a series of monochromatic widefield images of the same tissue slice as in Figure 7a collected at selected wavelength between 500 and 640 nm in 20 nm (with a ±10 nm FWHM bandwidth) intervals. Figure 7c plots the wavelength-dependent scattering intensities for selected pixels, which belong either to metal NPs or tissue background. Whereas the scattering intensity of the gold NP clusters exhibits an almost linear increase as a function of wavelength in the investigated spectral range, the tissue background is flat. The distinct differences in the spectral response between metal NPs and tissue background make it possible to identify the locations of all gold NPs in the field of view using uncomplicated image processing algorithms. We generated the binary image shown in Figure 7d applying a linear model based differentiation strategy, which discerns gold NP signal from background by applying a threshold in the slope of the intensity vs. wavelength relationship for each individual pixel. The resulting AVN distribution map shows that the GM3 containing AVNs accumulate preferentially in the periphery of the lymph node where the CD169+ APCs are located. The peripheral localization of CD169+ macrophages is illustrated by fluorescence immunostaining in an independent lymph node section in Figure 7e. Importantly, the corresponding image for the AVN control without GM3 (Figure 7f and g) contains no detectable gold NP signal and lacks any enrichment in the periphery of the lymph node.
Figure 6.
Gold concentration in excised popliteal lymph nodes 1 h (a) and 4 h (b) after hock injection of GM3-AVNs (GM3+) determined by ICP-MS. The data from two independent experiments is shown. Controls include AVNs without GM3 (GM3−) and PBS. Left/Right refers to the flank of the animal from which the samples were taken.
Figure 7.
Optical mapping of GM3-AVN80 in a lymph node section prepared 1 h after hock injection. a) Darkfield image of tissue section obtained for GM3-AVN80 (bottom). b) Monochromatic images from same field of view as in (a) but recorded every 20 nm between 500 nm to 640 nm. c) Intensity as function of wavelength for four marked positions in (a) of which two are located in putative gold NP containing regions and two are located in tissue regions. d) Binary image generated through spectral filtering using a linear model (see text) shows the enrichment of AVNs in the peripheral region of the lymph node section. e) Immunostaining of CD169 from an independent lymph node shows that CD169 expressing APCs mainly accumulate around the peripheral area of a lymph node. f) Darkfield image of a lymph node section after hock injection of AVN controls (no GM3) acquired with digital color camera. g) Binary image for (f) with the same algorithms applied in (d). Scale bars are 100 μm.
Together, the GM3-specific enrichment of AVNs in lymph nodes and the spatial enrichment of the GM3-AVNs in the CD169+ APC containing peripheral region of lymph nodes provides strong experimental evidence for a successful GM3-mediated targeting of AVNs to CD169+ APCs in vivo.
CONCLUSION
AVNs, which contain a membrane wrapped around a central NP core, represent a unique platform for utilizing low-immunogenic lipids in a multivalent presentation as NP targeting moiety. In this manuscript we have demonstrated that GM3 presenting AVNs achieve a selective homing to the peripheral regions of lymph nodes that are enriched in CD169+ APCs in vivo. Like any NP targeting approach, the intrinsically large surface area of GM3 presenting NPs invites non-specific interactions with proteins and other small molecules in biological fluids. Although the AVN membrane cannot prevent non-specific protein adsorption, corona formation was significantly reduced compared to PEG-NPs. AVNs (35 and 80 nm gold NP core) with identical zeta potentials as PEG-NPs showed less non-specific protein adsorption as indicated by a thinner hard corona, and in the case of AVN35, also soft corona. Corona structure and stability were significantly affected by the NP core radius. AVN35 showed a much more prominent soft corona formation than AVN80, highlighting a strong dependence of the corona characteristics on the NP size. Despite corona formation, GM3 embedded in the AVN membrane remained accessible to CD169 receptor binding. In serum containing medium AVNs whose zeta potential is only slightly higher than that of typical enveloped virus particles achieved the highest binding to CD169+ APCs, but the binding efficiency deteriorated with increasing negative membrane charge. Importantly, AVNs were found to retain their membrane cloak for extended periods of time even under the relatively harsh conditions after cellular uptake.
The appeal of the enveloped virus inspired AVN approach is that it combines the versatility of the self-assembled membrane to act as matrix for bio-active lipids and to generate a protein-repellent coating with the superior materials properties of a central NP core. The successful demonstration of GM3-mediated APC targeting in vivo, together with the obtained insight in corona formation around membrane wrapped NPs and its dependence on NP size and charge, paves the path to a broader utilization of AVNs in immuno-targeting approaches and use of lipids as NP targeting moiety in general.
METHODS
Liposome and AVN Preparation
A total amount of 1 μmole of lipid mix containing 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), cholesterol, 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (DOPS), GM3, and the fluorescence marker 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (ammonium salt) (liss-RHO-PE) was dissolved in around 100uL chloroform in a 25ml round-bottom flask. The contributions from the individual lipids varied for different preparations as specified in the text. The solvent was then evaporated and the samples dried over night in a vacuum rotary evaporator. 1 mL of 20 mM HEPES buffer (pH = 7.2) was then added to the lipid dry layer, forming a cloudy solution after vigorous agitation. The mixture was then probe-sonicated for 3–5 min under the argon protection until the solution became clear. The resulting liposome solution was stored at 4°C until further use. 1 mL of 80 nm citrate stabilized gold colloid solution (~1.0 × 1010 particles/mL), synthesized following the Turkevich method 89,90 was pelleted via centrifugation at 2.4 krpm for 10 min. A volume of 0.5 mL of the prepared liposome solution was added to the gold NP pellet. The volume was increased to 1 mL with the same 20 mM HEPES buffer. A volume of 20 μL of 1 mg/mL 1-octadecanethiol solution in ethanol was then added to the mixture. The mixed solution was incubated overnight on a rocker. After that, the AVNs were washed three times through centrifugation (2.4 krpm, 10 min) and resuspended in Milli-Q water. Finally, the AVN pellet was resuspended in 200 μL 20 mM HEPES buffer and ready to use.
DSC Measurements
Liposome and AVN solutions were concentrated through increasing start-up amount of lipids by a factor of 10. 25 uL of the concentrated solution was loaded into an aluminum pan and sealed. Thermograms were recorded between 30–80 °C using a Mettler Toledo Polymer DSC R at a heating rate of 10 °C/min.
Dynamic light scattering and zeta potential measurements
Measurements were performed on a Zetasizer Nano ZS90 (Malvern, Worcestershire, UK). For size measurements, AVNs were diluted with Milli-Q water to a final concentration of 1× 108 particles/mL. For zeta measurements, the AVN samples were further diluted to 1×107 particles/mL.
UV-vis spectroscopy
Spectra of diluted AVN solutions (~1 × 108 particles/mL) of a total volume of around ~70 μL in Milli-Q water (pH=7.0) were acquired in a quartz cuvette on an Agilent Cary 5000 UV/VIS spectrometer. Milli-Q water was used for baseline correction. All spectra were normalized by dividing through the peak intensities.
Gel Electrophoresis
After AVNs were incubated in 10% fetal bovine serum in Advanced DMEM (Gibco® Cell Culture, Thermo Fisher Scientific, USA) for overnight, particles were washed with DI water through 3 times centrifugation at a speed of 2.4 krpm for AVN80 and 4.8 krpm for AVN35 both for 10mins to get rid of the soft corona. The pellets was collected after the final centrifugation and 25 uL of 10% in mass Ficoll 400 (Alfa Aesar, USA) aqueous solution was added into the pellet. The resulting solution was then loaded into a 1% agarose gel and run in an electrophoresis box at a voltage of 125 V for 30 min with 0.5× TBE buffer added as the electrophoresis buffer.
Cell-culture
Primary monocyte-derived DCs were differentiated from CD14+ peripheral blood monocytes, and matured with LPS (100 ng mL−1) for 2 days prior to use, as described previously.10 HeLa cells stably expressing CD169-mCherry fusion protein have been described previously.6
Macrophages (RAW 264.7, ATCC) were obtained directly from the vendor and cultured in 10% fetal bovine serum, 1% penicillin–streptomycin in Advanced DMEM (Gibco Cell Culture, Thermo Fisher Scientific, USA). CD169 expression on both CD169-mCherry HeLa cells and RAW264.7 macrophages were validated through Flow Cytometry (Figure S5).
AVN Administration
In vitro experiments
For experiments with CD169-HeLa, CD169+ macrophages, and mDCs, AVNs were incubated with cells as specified for 15 – 90 min before the cells were washed by sequential centrifugation and resuspension with 1× PBS buffer three times. The cells were subsequently fixed with 4% paraformaldehyde and cytospun onto poly-L-lysine-treated glass coverslips.
Hock vaccinations
A hock vaccination model was used to examine recruitment to draining lymph nodes.91 Briefly, mice were gently restrained and 10 μl of AVNs into the lateral aspect of the ankle, avoiding all major blood vessels. Mice were injected with 2×108 particles in a total volume of 10 μL with or without GM3 in both ankles. 10 μL of PBS solution served as negative control. All animal procedures were carried out under BU IACUC protocol #14155 approved to Lee Wetzler.
Lymph node preparation and sectioning
One or four hours after hock vaccination, mice were euthanized using CO2. The draining popliteal lymph nodes (LNs) were removed and either directly frozen at −80°C for ICP-MS analysis or embedded in molds using Optimal Cutting Temperature (OCT) medium (Richard Allan Scientific, USA). Lymph nodes were flash frozen using dry ice and stored at −80°C. Sectioning was performed on a Microm HM 550 (Microm International GmbH, Germany). 8 μm sections were obtained and placed on Colorfrost Plus slides (ThermoFisher, USA). Sections were stored at −80°C until further processing. Sections were air dried for 15 min at room temperature, then fixed in acetone at −20°C for 10 min and afterwards dried for additional 10 min. Sections were re-hydrated in TBS buffer with 0.05% Tween-20 (TBS-T) then blocked for 20 min at room temperature with TBS-T with 5% BSA. Sections were washed with TBS three times for 5 min and mounted in SlowFade® Gold Antifade mounting medium containing DAPI (life technologies, USA).
ICP-MS
In vitro experiments
Binding studies were performed with the CD169 transfected HeLa cell line mentioned in the cell-culture section. After incubating the cell with GM3-presenting AVNs(~1.0×108 particles/mL) for 90 min, the cells were washed, harvested and then transferred into centrifuge tubes. Excess particles and cells were separated by 3 times centrifugation and subsequent resuspension at 1.5 krpm for 5 min. The cell concentration was then measured with a hemacytometer. Cell samples of known concentration were transferred into a 6-well dish and aqua regia was added to dissolve cells and contained gold NPs. The dish was then placed on top of a hot plate pre-set to 55 °C for overnight to evaporate the aqua regia. The dried sample was re-dispersed in 2% HCl solution and measured, together with defined calibration standards in VG Plasma Quad ExCell ICP-MS to determine the gold concentration in the sample.
In vivo experiments
Popliteal lymph nodes were excised from mice and placed into separate centrifuge tubes and stored at −80 °C until further use. Before ICP-MS analysis, each lymph node in the centrifuge tube was first dissolved in aqua regia at 80 °C for 1h and the resultant solution was then transferred into a 6-well dish. The dish was then placed on top of a hot plate pre-set to 55 °C for overnight to evaporate the aqua regia, On the next day, 2% HCl solution was used to re-disperse the dried samples before the samples were measured together with calibration standards of known concentration in a VG Plasma Quad ExCell ICP-MS to determine the gold concentration in the sample.
Image acquisition and data processing
All optical imaging experiments were performed with an Olympus IX71 inverted microscope. Images were taken with a 10× or 60× oil objective with variable NA (NA=0.65–1.25). For darkfield imaging, the samples were illuminated with a 100W tungsten lamp through a high NA oil darkfield condenser (NA=1.2–1.4). Darkfield images were recorded with a Nikon D3100 SLR digital camera connected to the microscope through an eyepiece adaptor. Fluorescence imaging was performed under epi-illumination using appropriate filter sets. Images were recorded with an Andor Ixon+ electron multiplying charge coupled device detector. The NA of the collecting objective was adjusted for darkfield and fluorescence imaging to optimize signal to noise. The recorded images were further processed by ImageJ to correct for image drifts. Hyperspectral darkfield imaging was performed with a VariSpec Liquid Crystal Tunable Filter placed into the darkfield system to tune the excitation wavelength. The filter had a FWHM bandwidth of 10 nm and was scanned from 500nm to 640nm in steps of 20nm. Prior to a sample measurement a calibration based on the Averaged Optical Density (AOD) equalization method92 was performed on a reference sample (empty glass substrate) to compensate for optical density variations stemming from the excitation light source and the optical system. An image from a lymph node sample was then acquired at each wavelength. The images were imported into Matlab where background corrections were performed and binary images were computed (see main text).
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NCI) through grant 5R01CA138509 to BMR.
Footnotes
UV-VIS spectra, PDI statistics from DLS measurements, electrophoresis images, and flow cytometer expression profiles of CD169 are provided in Figure S1–5. The Supporting Information is available free of charge on the ACS Publications website.
References
- 1.Fay F, Scott CJ. Antibody-Targeted Nanoparticles for Cancer Therapy. Immunotherapy. 2011;3:381–394. doi: 10.2217/imt.11.5. [DOI] [PubMed] [Google Scholar]
- 2.Delehanty JB, Boeneman K, Bradburne CE, Robertson K, Bongard JE, Medintz IL. Peptides for Specific Intracellular Delivery and Targeting of Nanoparticles: Implications for Developing Nanoparticle-Mediated Drug Delivery. Ther Deliv. 2010;1:411–433. doi: 10.4155/tde.10.27. [DOI] [PubMed] [Google Scholar]
- 3.Farokhzad OC, JC, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Targeted Nanoparticle-Aptamer Bioconjugates for Cancer Chemotherapy In Vivo. Proc Natl Acad Sci USA. 2006;103:6315. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weissleder R, Kelly K, Sun EY, Shtaland T, Josephson L. Cell-Specific Targeting of Nanoparticles by Multivalent Attachment of Small Molecules. Nat Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 5.Yu X, Xu F, Ramirez NGP, Kijewski SDG, Akiyama H, Gummuluru S, Reinhard BM. Dressing up Nanoparticles: A Membrane Wrap to Induce Formation of the Virological Synapse. ACS Nano. 2015;9:4182–4192. doi: 10.1021/acsnano.5b00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu X, Feizpour A, Ramirez NGP, Wu L, Akiyama H, Yu X, Gummuluru S, Reinhard BM. Glycosphingolipid-Functionalized Nanoparticles Recapitulate CD169-Dependent HIV-1 Uptake and Trafficking in Dendritic Cells. Nat Commun. 2014;5:4136. doi: 10.1038/ncomms5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawasaki N, Vela JL, Nycholat CM, Rademacher C, Khurana A, van Rooijen N, Crocker PR, Kronenberg M, Paulson JC. Targeted Delivery of Lipid Antigen to Macrophages via the CD169/Sialoadhesin Endocytic Pathway Induces Robust Invariant Natural Killer T Cell Activation. Proc Natl Acad Sci USA. 2013;110:7826–7831. doi: 10.1073/pnas.1219888110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen WC, Kawasaki N, Nycholat CM, Han S, Pilotte J, Crocker PR, Paulson JC. Antigen Delivery to Macrophages Using Liposomal Nanoparticles Targeting Sialoadhesin / CD169. PloS ONE. 2012;7:e39039. doi: 10.1371/journal.pone.0039039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puryear WB, Yu X, Ramirez NP, Reinhard BM, Gummuluru S. HIV-1 Incorporation of Host-Cell-Derived Glycosphingolipid GM3 Allows for Capture by Mature Dendritic Cells. Proc Natl Acad Sci USA. 2012;109:7475–7480. doi: 10.1073/pnas.1201104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puryear WB, Akiyama H, Geer SD, Ramirez NP, Yu X, Reinhard BM, Gummuluru S. Interferon-Inducible Mechanism of Dendritic Cell-Mediated HIV-1 Dissemination is Dependent on the Siglec, CD169. PloS Pathog. 2013;9:e1003291. doi: 10.1371/journal.ppat.1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson E, Pino M, Erkizia I, Glass B, Clotet B, Keppler OT, Telenti, Kräusslich H-G, Martinez-Picado J. Siglec-1 Is a Novel Dendritic Cell Receptor That Mediates HIV-1 Trans-Infection Through Recognition of Viral Membrane Gangliosides. PLoS Biol. 2012;10:e1001448. doi: 10.1371/journal.pbio.1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nycholat CM, Rademacher C, Kawasaki N, Paulson JC. In Silico-Aided Design of a Glycan Ligand of Sialoadhesin for In Vivo Targeting of Macrophages. J Am Chem Soc. 2012;134:15696–15699. doi: 10.1021/ja307501e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrasco YR, Batista FD. B Cells Acquire Particulate Antigen in a Macrophage-Rich Area at the Boundary Between the Follicle and the Subcapsular Sinus of the Lymph Node. Immunity. 2007;27:160– 171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen NV, Mempel TR, Whelan SP, von Andrian UH. Subcapsular Sinus Macrophages in Lymph Nodes Clear Lymph-Borne Viruses and Present Them to Antiviral B Cells. Nature. 2007;450:110– 114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Pomares L, Gordon S. Antigen Presentation the Macrophage Way. Cell. 2007;131:641–643. doi: 10.1016/j.cell.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 16.Veninga H, Borg EG, Vreeman K, Taylor PR, Kalay H, van Kooyk Y, Martinez-Promares L, den Haan JM. Antigen Targeting Reveals Splenic CD169+ Macrophages as Promotors of Germinal Center B-cell Responses. Eur J Immunol. 2015;45:747–757. doi: 10.1002/eji.201444983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barral P, Polzella P, Bruckbauer A, van Rooijen N, Besra CG, Cerundolo V, Batista FD. CD169(+) Macrophages Present Lipid Antigens to Mediate Early Activation of Inkt Cells in Lymph Nodes. Nature Immunol. 2010;11:303– 3012. doi: 10.1038/ni.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Pomares L, Gordon S. CD169+ Macrophages at the Crossroads of Antigen Presentation. Trends Immunol. 2012;33:66– 70. doi: 10.1016/j.it.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Chtanova T, Han SJ, Schaeffler M, van Dooren GG, Herzmark P, Striepen B, Robey EA. Dynamics of T Cell, Antigen-Presenting Cell, and Pathogen Interactions During Recall Responses in the Lymph Node. Immunity. 2009;31:342–355. doi: 10.1016/j.immuni.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, Kanagawa O, Fujii SI, Tanaka M. CD169-Positive Macrophages Dominate Antitumor Immunity by Crosspresenting Dead Cell-Associated Antigen. Immunity. 2011;34:85–95. doi: 10.1016/j.immuni.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Yoon YJ, Kim OY, Gho YS. Extracellular Vesicles as Emerging Intercellular Communicasomes. BMB Rep. 2014;47:531–539. doi: 10.5483/BMBRep.2014.47.10.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaput N, Flament C, Viaud S, Taieb J, Roux S, Spatz A, Andre F, LePecq JB, Boussac M, Garin J, Amigorena S, Thery C, Zitvogel L. Dendritic Cell Derived-Exosomes: Biology and Clinical Implementations. J Leukoc Biol. 2006;80:471–478. doi: 10.1189/jlb.0206094. [DOI] [PubMed] [Google Scholar]
- 23.Nores GA, Dohi T, Taniguchi M, Hakomori S. Density-Dependent Recognition of Cell Surface GM3 by a Certain Anti-Melanoma Antibody, and GM3 Lactone as Possible Immunogen: Requirements for Tumor-Associated Antigen and Immunogen. J Immunol. 1987;139:3171–3176. [PubMed] [Google Scholar]
- 24.Montenegro JM, Grazu V, Sukhanova A, Agarwal S, de la Fuente JM, Nabiev I, Greiner A, Parak WJ. Controlled Antibody/(Bio-) Conjugation of Inorganic Nanoparticles for Targeted Delivery. Adv Drug Deliv Rev. 2013;65:677–688. doi: 10.1016/j.addr.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Pan Y, Chefalo P, Nagy N, Harding C, Guo Z. Synthesis and Immunological Properties of N-Modified GM3 Antigens as Therapeutic Cancer Vaccines. J Med Chem. 2005;48:875–883. doi: 10.1021/jm0494422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the Nanoparticle-Protein Corona Using Methods to Quantify Exchange Rates and Afinities of Proteins to Nanoparticles. Proc Natl Acad Sci USA. 2007;104:2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle Size and Surface Properties Determine the Protein Corona with Possible Implications for Biological Impacts. Proc Natl Acad Sci USA. 2008;105:14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Docter D, Westmeier D, Markiewicz M, Stolte S, Knauer SK, Stauber RH. The Nanoparticle Biomolecule Corona: Lessons Learned - Challenge Accepted? Chem Soc Rev. 2015;44:6094–6121. doi: 10.1039/c5cs00217f. [DOI] [PubMed] [Google Scholar]
- 29.Rocker C, Potzl M, Zhang F, Parak WJ, Nienhaus GU. A Quantitative Fluorescence Study of Protein Monolayer Formation on Colloidal Nanoparticles. Nat Nanotechnol. 2009;4:577–580. doi: 10.1038/nnano.2009.195. [DOI] [PubMed] [Google Scholar]
- 30.Morghimi SM, Hunter AC, Andresen TL. Factors Controlling Nanoparticle Pharmacokinetics: An Integrated Analysis and Perspective. Annu Rev Pharmacol Toxicol. 2012;52:481–503. doi: 10.1146/annurev-pharmtox-010611-134623. [DOI] [PubMed] [Google Scholar]
- 31.Almeida JP, Chen AL, Foster A, Drezek R. In Vivo Biodistribution of Nanoparticles. Nanomedicine. 2011;6:815– 835. doi: 10.2217/nnm.11.79. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Byrne JD, Napier ME, DeSimone JM. More effective Nanomedicines through Particle Design. Small. 2011;7:1919– 1931. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dell’Orco D, Lundqvist M, Linse S, Cedervall T. Mathematical Modeling of the Protein Corona: Implications for Nanoparticulate Delivery Systems. Nanomedicine. 2014;9:851–858. doi: 10.2217/nnm.14.39. [DOI] [PubMed] [Google Scholar]
- 34.Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, Schlenk F, Fischer D, Kiouptsi K, Reinhardt C, Landfester K, Schild H, Maskos M, Knauer SK, Stauber RH. Rapid Formation of Plasma Protein Corona Critically Affects Nanoparticle Pathophysiology. Nat Nanotechnol. 2013;8:772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 35.Pelaz B, del Pino P, Maffre P, Hartmann R, Gallego M, Rivera-Fernández S, de la Fuente JM, Nienhaus GU, Parak WJ. Surface Functionalization of Nanoparticles with Polyethylene Glycol: Effects on Protein Adsorption and Cellular Uptake. ACS Nano. 2015;9:6996–7008. doi: 10.1021/acsnano.5b01326. [DOI] [PubMed] [Google Scholar]
- 36.Irvine DJ, Hanson MC, Rakrha K, Tokatlian T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem Soc Rev. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garay RP, El-Gewely R, Armstrong JK, Garraty G, Richette P. Antibodies Against Polyethylene Glycol in Healthy Subjects and in Patients Treated with PEG-Conjugated Agents. Expert Opin Drug Deliv. 2012;9:1319–1323. doi: 10.1517/17425247.2012.720969. [DOI] [PubMed] [Google Scholar]
- 38.Ishida T, Kiwada H. Anti-Polyethyleneglycol Antibody Response to Pegylated Substances. Biol Pharm Bull. 2013;36:889–891. doi: 10.1248/bpb.b13-00107. [DOI] [PubMed] [Google Scholar]
- 39.Ishida T, Atobe K, Wang X, Kiwada H. The Contribution of Phagocytotic Activity of Liver Macrophages to the Accelerated Blood Clearance (ABC) Phenomenon of Pegylated Liposomes in Rats. J Control Release. 2008;126:162–165. doi: 10.1016/j.jconrel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Mishra S, Webster P, Davis ME. Pegylation Significantly Affects Cellular Uptake and Inctracellular Traficking of Non-Viral Gene Delivery Particles. Eur J Cell Biol. 2004;83:97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- 41.Bao Y, Jin Y, Chivukula P, Zhang J, Liu YP, Liu J, Clamme JP, Mahato RI, Ng D, Ying W, Wang Y, Yu L. Effect of Pegylation on Biodistribution and Gene Silencing of Sirna/Lipid Nanoparticle Complexes. Pharm Res. 2013;30:342–351. doi: 10.1007/s11095-012-0874-6. [DOI] [PubMed] [Google Scholar]
- 42.Xu L, Wempe MF, Anchordoquy TJ. The Effect of Cholesterol Domains on Pegylated Liposomal Gene Delivery In Vitro. Ther Del. 2011;2:451–460. doi: 10.4155/tde.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harvie P, Wong FMP, Bally MB. Use of Poly(Ethylene Glycol)-Lipid Conjugates to Regulate the Surface Attributes and Transfection Activity of Lipid-DNA Particles. J Pharm Sci. 2000;89:652–663. doi: 10.1002/(SICI)1520-6017(200005)89:5<652::AID-JPS11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 44.Keefe AJ, Jiang S. Poly(Zwitterionic)Protein Conjugates Offer Increased Stability Without Sacrificing Binding Affinity or Bioactivity. Nature Chemistry. 2012;4:59– 63. doi: 10.1038/nchem.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Susumu K, Oh E, Delehanty JB, Blanko-Canosa JB, Johnson BJ, Jain V, Hervey WJ, IV, Russ Algar W, Boeneman K, Dawson PE, Medintz IL. Multifunctional Compact Zwitterionic Ligands for Preparing Robust Biocompatible Semiconductor Quantum Dots and Gold Nanoparticles. J Am Chem Soc. 133:9480–9496. doi: 10.1021/ja201919s. [DOI] [PubMed] [Google Scholar]
- 46.Rouhana LL, Jaber JA, Schlenoff JB. Aggregation-Resistant Water-Soluble Gold Nanoparticles. Langmuir. 2007;23:12799– 12801. doi: 10.1021/la702151q. [DOI] [PubMed] [Google Scholar]
- 47.Muro E, Pons T, Lequeux N, Fragola A, Sanson N, Lenkei Z, Dubertret B. Small and Stable Sulfobetaine Zwitterionic Quantum Dots for Functional Live-Cell Imaging. J Am Chem Soc. 132:4566–4567. doi: 10.1021/ja1005493. [DOI] [PubMed] [Google Scholar]
- 48.Muro E, Fragola A, Pons T, Lequeux N, Ioannou A, Skourides P, Dubertret B. Comparing Intracellular Stability and Targeting of Sulfobetaine Quantum Dots with Other Surface Chemistries in Live Cells. Small. 2012;8:1029– 1037. doi: 10.1002/smll.201101787. [DOI] [PubMed] [Google Scholar]
- 49.Allijin IE, Leong W, Tang J, Gianella A, Mieszawska AJ, Fay FS, Ma G, Russel S, Callo CB, Gordon RE, Korkmaz E, Post JA, Zhao Y, Gerritsen HC, Thran A, Proksa R, Daerr H, Storm G, Fuster V, Fisher EA, et al. Gold Nanocrystal Labeling Allows Low-Density Lipoprotein Imaging from the SubCellular to Macroscopic Level. ACS Nano. 2013;7:9761–9770. doi: 10.1021/nn403258w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cormode DP, Skajaa T, van Schooneveld MM, Koole R, Jarzyna P, Lobatto ME, Calcagno C, Barazza A, Gordon RE, Zanzonico P, Fisher EA, Fayad ZA, Mulder WJM. Nanocrystal Core High Density Lipoproteins: A Multimodality Contrast Agent Platform. Nano Lett. 2008;8:3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Schooneveld MM, Vucic E, Koole R, Zhou Y, Stocks J, Cormode DP, Tang CY, Gordon RE, Nicolay K, Meijerink A, Fayad ZA, Mulder WJ. Improved Biocompatibility and Pharmacokinetics of Silica Nanoparticles by Means of a Lipid Coating: A Multimodality Investigation. Nano Lett. 2008;8:2517–1525. doi: 10.1021/nl801596a. [DOI] [PubMed] [Google Scholar]
- 52.Tarn D, Ashley CE, Xue M, Carnes EC, Zink JI, Brinker CJ. Mesoporous Silica Nanoparticle Nanocarriers: Biofunctionality and Biocompatibility. Acc Chem Res. 2013;46:792–801. doi: 10.1021/ar3000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ashley CE, Carnes EC, Phillips GK, Padilla D, Durfee PN, Brown PA, Hanna TN, Liu J, Phillips B, Carter MB, Carrol NJ, Jiang X, Dunphy DR, Willman CL, Petsev DN, Evans DG, Parikh AN, Chackerian B, Wharton W, Peabody DS, et al. The Targeted Delivery of Multicomponent Cargos to Cancer Cells by Nanoporous Particle-Supported Lipid Bilayers. Nat Mater. 2011;10:389–397. doi: 10.1038/nmat2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu C-MJ, Fang RH, Wang K-C, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, et al. Nanoparticle Biointerfacing by Platelet Membrane Cloaking. Nature. 2015;526:118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu CMJ, Fang RH, Luk BT, Chen KNH, Carpenter C, Gao W, Zhang K, Zhang L. ‘Marker-Of-Self’ Functionalization of Nanoscale Particles through a Top-Down Cellular Membrane Coating Approach. Nanoscale. 2013;5:2664–2668. doi: 10.1039/c3nr00015j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang JA, Murphy CJ. Evidence for Patchy Lipid Layers on Gold Nanoparticle Surfaces. Langmuir. 2012;28:5404–5416. doi: 10.1021/la300325p. [DOI] [PubMed] [Google Scholar]
- 57.Brugger B, Glass B, Haberkant P, Leibrecht I, Wieland FT, Kräusslich HG. The HIV Lipidome: A Raft with an Unusual Composition. Proc Natl Acad Sci USA. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu C, Bhatt LR, Jun HY, Park SH, Chai KY. Carboxyl-Polyethylene Glycol-Phosphoric Acid: A Ligand for Highly Stabilized Iron Oxide Nanoparticles. J Mater Chem. 2012;22:19806–19811. [Google Scholar]
- 59.Lee SE, Chen Q, Bhat R, Petkiewicz S, Smith JM, Ferry VE, Correia AL, Alivisatos AP, Bissell MJ. Reversible Aptamer-Au Plasmon Rulers for Secreted Single Molecules. Nano Lett. 2015;15:4564–4570. doi: 10.1021/acs.nanolett.5b01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. Human Immunodeficiency Virus Type 1 Is Trapped by Acidic but Not by Neutralized Human Cervicovaginal Mucus. J Virol. 2009;83:11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Metso AJ, Zhao H, Tuunainen I, Kinnunen PKJ. Observation of the Main Phase Transition of Dinervonoylphosphocholine Giant Liposomes by Fluorescence Microscopy. Biochimica et Biophysica Acta - Biomembranes. 2005;1713:83–91. doi: 10.1016/j.bbamem.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Baldelli Bombelli F, Dawson KA. Physical– Chemical Aspects of Protein Corona: Relevance to In Vitro and In Vivo Biological Impacts of Nanoparticles. J Am Chem Soc. 2011;133:2525–2534. doi: 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- 63.Black JC, Cheney PP, Campbell T, Knowles MK. Membrane Curvature Based Lipid Sorting Using a Nanoparticle Patterned Substrate. Soft Matter. 2014;10:2016– 2023. doi: 10.1039/c3sm52522h. [DOI] [PubMed] [Google Scholar]
- 64.Ogunyankin MO, Huber DL, Sasaki DY, Longo ML. Nanoscale Patterning of Membrane-Bound Proteins Formed through Curvature-Induced Partitioning of Phase-Specific Receptor Lipids. Langmuir. 2013;29:6109–6115. doi: 10.1021/la401011d. [DOI] [PubMed] [Google Scholar]
- 65.Sorre B, Calan-Jones A, Manneville J-B, Nassoy P, Joanny J-F, Prost J, Goud B, Bassereau P. Curvature-Driven Lipid Sorting Needs Proximity to a Demixing Point and Is Aided by Proteins. Proc Natl Acad Sci USA. 2009;106:5622–5626. doi: 10.1073/pnas.0811243106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Risselada HJ, Marrink SJ. Curvature Effects on Lipid Packing and Dynamics in Liposomes Revealed by Coarse Grained Molecular Dynamics Simulations. Phys Chem Chem Phys. 2009;11:2056–2067. doi: 10.1039/b818782g. [DOI] [PubMed] [Google Scholar]
- 67.McMahon HT, Gallop JL. Membrane Curvature and Mechanisms of Dynamic Cell Membrane Remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 68.van Meer G, Vaz WLC. Membrane Curvature Sorts Lipids. EMBO Rep. 2005;6:418–419. doi: 10.1038/sj.embor.7400410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lundquist M, Sethson I, Jonsson BH. Protein Adsorption onto Silica Nanoparticles: Conformational Changes Depend on the Particles’ Curvature and Protein Stability. Langmuir. 2004;20:10639–10647. doi: 10.1021/la0484725. [DOI] [PubMed] [Google Scholar]
- 70. [last accessed 12/18 2015];Nanotechnology at NIH. http://www.nih.gov/research-training/nanotechnology-nih.
- 71.Casals E, Pfaller T, Duschl A, Oostingh GJ, Puntes VF. Hardening of the Nanoparticle–Protein Corona in Metal (Au, Ag) and Oxide (Fe3O4, CoO, and CeO2) Nanoparticles. Small. 2011;7:3479–3486. doi: 10.1002/smll.201101511. [DOI] [PubMed] [Google Scholar]
- 72.Clemments AM, Botella P, Landry CC. Protein Adsorption From Biofluids on Silica Nanoparticles: Corona Analysis as a Function of Particle Diameter and Porosity. ACS Appl Mater Interfaces. 2015;7:21682–21689. doi: 10.1021/acsami.5b07631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Casals E, Pfaller T, Duschl A, Oostingh GJ, Puntes V. Time Evolution of the Nanoparticle Protein Corona. ACS Nano. 2010;4:3623–3632. doi: 10.1021/nn901372t. [DOI] [PubMed] [Google Scholar]
- 74.Shannahan JH, Lai X, Ke PC, Podila R, Brown JM, Witzmann FA. Silver Nanoparticle Protein Corona Composition in Cell Culture Media. PLoS ONE. 2013;8:e74001. doi: 10.1371/journal.pone.0074001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Milani S, Baldelli Bombelli F, Pitek AS, Dawson KA, Rädler J. Reversible versus Irreversible Binding of Transferrin to Polystyrene Nanoparticles: Soft and Hard Corona. ACS Nano. 2012;6:2532–2541. doi: 10.1021/nn204951s. [DOI] [PubMed] [Google Scholar]
- 76.Moyano DF, Saha K, Prakash G, Yan B, Kong H, Yazdani M, Rotello VM. Fabrication of Corona-Free Nanoparticles with Tunable Hydrophobicity. ACS Nano. 2014;8:6748–6755. doi: 10.1021/nn5006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang L, Yan B, Premasiri RW, Ziegler LD, Dal Negro L, Reinhard BM. Engineering Nanoparticle Cluster Arrays for Bacterial Biosensing: The Role of the Building Block in Multiscale SERS Substrates. Adv Funct Mater. 2010;20:2619–2628. [Google Scholar]
- 78.Yan B, Thubagere A, Premasiri R, Ziegler L, Dal Negro L, Reinhard BM. Engineered SERS Substrates with Multiscale Signal Enhancement: Nanoparticle Cluster Arrays. ACS Nano. 2009;3:1190– 1202. doi: 10.1021/nn800836f. [DOI] [PubMed] [Google Scholar]
- 79.Yan B, Boriskina SV, Reinhard BM. Optimizing Gold Nanoparticle Cluster Configurations (n≤7) for Array Applications. J Phys Chem C. 2011;115:4578–4583. doi: 10.1021/jp112146d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA. Selective Colorimetric Detection of Polynucleotides Based on the Distance-Dependent Optical Properties of Gold Nanoparticles. Science. 1997;277:1078–1081. doi: 10.1126/science.277.5329.1078. [DOI] [PubMed] [Google Scholar]
- 81.Hühn D, Kantner K, Geidel C, Brandholt S, De Cock I, Soenen SJH, Rivera_Gil P, Montenegro JM, Braeckmans K, Müllen K, Nienhaus GU, Klapper M, Parak WJ. Polymer-Coated Nanoparticles Interacting with Proteins and Cells: Focusing on the Sign of the Net Charge. ACS Nano. 2013;7:3253–3263. doi: 10.1021/nn3059295. [DOI] [PubMed] [Google Scholar]
- 82.Kelly KL, Coronado E, Zhao LL, Schatz GC. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J Phys Chem B. 2003;107:668–677. [Google Scholar]
- 83.Haes AJ, Hall WP, Chang L, Klein WL, Van Duyne RP. A Localized Surface Plasmon Resonance Biosensor: First Steps toward an Assay for Alzheimer’s Disease. Nano Lett. 2004;4:1029–1034. [Google Scholar]
- 84.Baalousha M, Lead JR. Nanoparticle Dispersity in Toxicology. Nat Nanotechnol. 2013;8:308–309. doi: 10.1038/nnano.2013.78. [DOI] [PubMed] [Google Scholar]
- 85.Maffre P, Brandholt S, Nienhaus K, Shang L, Parak WJ, Nienhaus GU. Effects of Surface Functionalization on the Adsorption of Human Serum Albumin onto Nanoparticles – A Fluorescence Correlation Spectroscopy Study. Beilstein J Nanotechnol. 2014;5:2036–2047. doi: 10.3762/bjnano.5.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang JA, Lohse SE, Murphy CJ. Tuning Cellular Response to Nanoparticles via Surface Chemistry and Aggregation. Small. 2014;10:1642–1651. doi: 10.1002/smll.201302835. [DOI] [PubMed] [Google Scholar]
- 87.Sewald X, Ladinsky MS, Uchil PD, Beloor J, Pi R, Herrmann C, Motamedi N, Murooka TT, Brehm MA, Greiner DL, Shultz LD, Mempel TR, Bjorkman PJ, Kumar P, Mothes W. Retroviruses Use CD169-Mediated Transinfection of Permissive Lymphocytes to Establish Infection. Science. 2015;350:563–567. doi: 10.1126/science.aab2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu L, Reinhard BM. Probing Subdiffraction Limit Separations with Plasmon Coupling Microscopy: Concepts and Applications. Chem Soc Rev. 2014;43:3884–3897. doi: 10.1039/c3cs60340g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grzelczak M, Pérez-Juste J, Mulvaney P, Liz-Marzán LM. Shape Control in Gold Nanoparticle Synthesis. Chem Soc Rev. 2008;37:1783–1791. doi: 10.1039/b711490g. [DOI] [PubMed] [Google Scholar]
- 90.Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J Phys Chem B. 2006;110:15700–15707. doi: 10.1021/jp061667w. [DOI] [PubMed] [Google Scholar]
- 91.Kamala T. Hock Immunization: A Humane Alternative to Mouse Footpad Injections. J Immunol Methods. 2007;328:204–214. doi: 10.1016/j.jim.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thigpen J, Merchant F, Shah S. Photometric Calibration for Quantitative Spectral Microscopy under Transmitted Illumination. J Microsc. 2010;239:200–214. doi: 10.1111/j.1365-2818.2010.03366.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.