Abstract
While seizures ultimately result from aberrant firing of neuronal networks, several laboratories have embraced a non-neurocentric view of epilepsy to show that other cells in the brain also bear an etiologic impact in epilepsy. Astrocytes and brain endothelial cells are examples of controllers of neuronal homeostasis; failure of proper function of either cell type has been shown to have profound consequences on neurophysiology. Recently, an even more holistic view of the cellular and molecular mechanisms of epilepsy has emerged to include white blood cells, immunological synapses, the extracellular matrix and the neurovascular unit. This review will briefly summarize these findings and propose mechanisms and targets for future research efforts on non-neuronal features of neurological disorders including epilepsy.
Keywords: Anti-epileptic drugs, Cerebrovasculature, Drug resistance, Brain endothelium, Glia-neuronal interactions, Extracellular matrix
This book is devoted to one of the all-time leaders in epilepsy research, Philip Alan Schwartzkroin. Phil has not only changed the traditional understanding of mammalian neurophysiology but he also revolutionized the tools we employ to study the brain as one of the people to perfect the brain slice preparation [70,71,74–76]. Last but not least, Phil has edited many seminal books and papers, and incessantly contributed to the recruitment and scientific development of scores of young scientists. Under the shadow of this giant (and former mentor for one of us (DJ)) writing this review is a humbling experience; one way to start the process is to refer the reader to Phil’s recent introduction to the field of epilepsy research [72].
20.1 Why Study Non-neuronal Mechanisms in Neurology or Neuroscience?
We study the brain for many reasons, not least of which is for its intrinsic interest (see Schwartzkroin’s recent introduction to the field [72]). The fascination with neurons and neuronal circuitries is not surprising since neurons are the collectors and effectors of our daily experiences and actions. In the specific case of epilepsy research (clinical or basic/translational), the quest for “epileptic neurons” or “epileptic circuits” has produced remarkable results, leading to the discovery of viable anti-epileptic drug targets and to the multimodal definition of the “epileptic focus”, an invaluable clinical tool for the neurosurgeon. However, as in other neurological disorders, a neurocentric approach has left certain questions unanswered and experimental opportunities remain. The most striking example of why neuroscience should become more “holistic” is embolic stroke, a disease stemming from cerebrovascular disease that has devastating consequences on brain function. After the NIH convened a Stroke Progress Review Group in 2001, stroke research shifted from a purely neurocentric focus to a more integrated view wherein dynamic interactions between all cell types contribute to function and dysfunction in the brain. In the field of epilepsy research and treatment, there is no pressing need for such a sharp re-direction, since the field is already characterized by the study of many cell types, and non-neuronal processes. For example:
Many neuronal molecular, morphological defects or functional abnormalities described in human epileptic brain are present throughout the cycle of interictal-to-ictal states that characterize the epileptic brain. The persistence of these neuronal abnormalities does not fully explain why at a given time point an interictal cortex develops a seizure. Other mechanisms, such as changes in cerebral blood flow or blood-brain barrier permeability have been proposed to mediate the interictal to ictal transition.
It has been proposed that the process of epileptogenesis is distinct from the process of ictogenesis. According to this hypothesis, what makes a brain epileptic (e.g., genetic mutations, acquired or inherited; malformations of brain development) does not directly cause seizures. In fact, seizures can occur in “non-epileptic” brain and people with epilepsy spend most of the time not having seizures, indeed many experience only a few seizures per year. Again, as in (1), non-neuronal mechanisms spanning from altered cerebral blood flow to glial dysfunction have been used to explain how an asymptomatic neurologic condition can suddenly develop into a seizure state or the fact that seizures can occur in non-epileptic brain (e.g., stroke).
Multiple drug resistance to anti-epileptic drugs affects over 20 % of patients with epilepsy. Multiple drug resistance cannot be fully explained in pharmacodynamic or neuronal terms, and great emphasis has been put on pharmacokinetic mechanisms that include the blood-brain barrier.
Analysis of resected or post-mortem epileptic brain reveals a number of pathophysiological changes in astrocytes and microglia. MRI studies show, in addition to persistent structural changes such as malformations of brain development, an array of transient changes that reflect post-ictal or interictal functional fluctuations in the extracellular space (increased FLAIR signal, perfusion changes etc.).
The analysis of molecular transcripts and changes in gene expression in patients with epilepsy reveal a surprising number of genes and proteins that are involved in astrocytic function, blood-brain barrier maintenance and transport, as well as immune signaling and extracellular matrix proteins.
The following paragraphs detail the rationale for new or corroborative experiments that will help understand the extent and nature of non-neuronal mechanisms of seizure disorders.
20.2 Identification of Important Problems
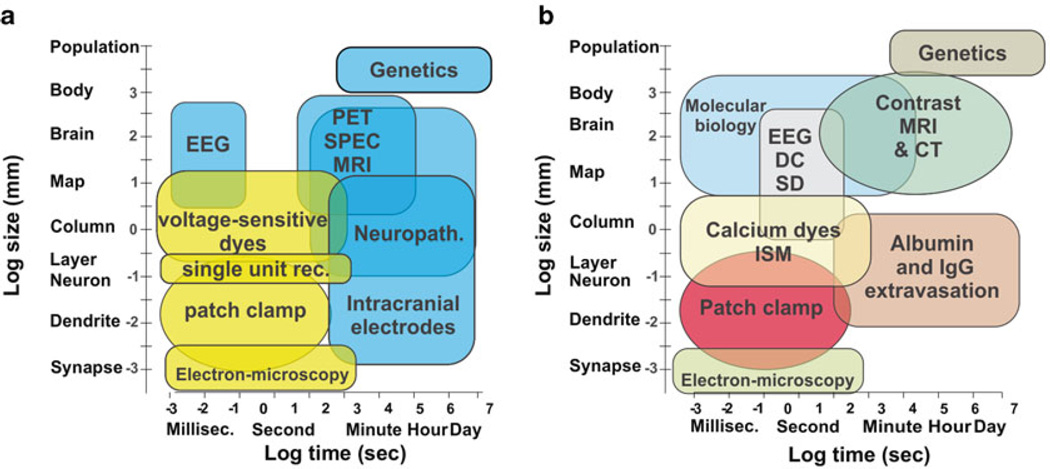
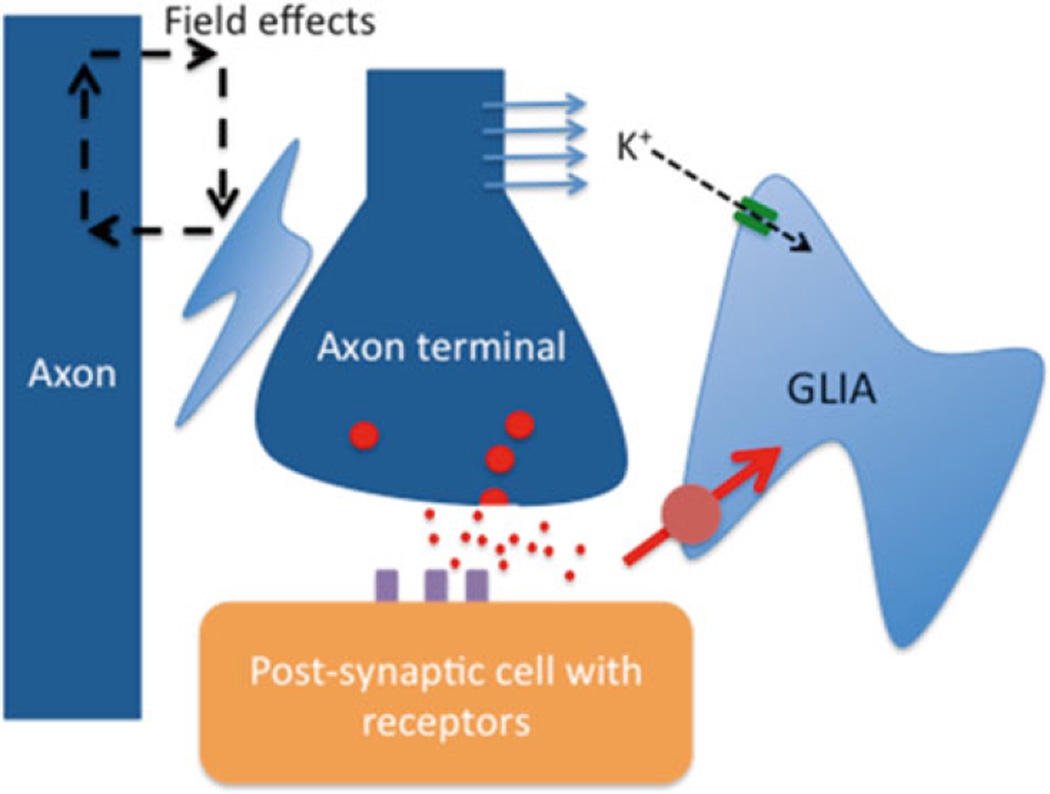
The translational nature of modern research affords the unique opportunity to use real life clinical problems and “translate” these into meaningful laboratory efforts. As beautifully illustrated by Phil in his summary of basic mechanisms [72], the tools used for research are not always the same used in clinical practice. In fact, a substantial discrepancy in size and temporal resolution becomes evident when comparing clinical and laboratory-based approaches (Fig. 20.1). For this mini review, we will focus on three fundamental yet often neglected aspects of ictogenesis and epileptogenesis: the blood-brain barrier, glia (Fig. 20.2) and the extracellular matrix. The following paragraphs will summarize current understanding and knowledge gaps related to these cellular and molecular mechanisms of neuronal pathophysiology.
Fig. 20.1.
Comparison of methods used in basic (a) or clinical (b) neuroscience. Note the partial overlap and significant differences
Fig. 20.2.
Some mechanisms by which glia can affect seizure activity. Glia regulate the concentration of extracellular potassium, the size of extracellular space and so electrical field effects (ephaptic communication), and uptake of neurotransmitter
20.3 The Blood-Brain Barrier (BBB)
The BBB is the most important vascular barrier of the CNS. The BBB protects the brain from harmful substances of the blood stream, while supplying the brain with the nutrients required for proper function. The BBB strictly regulates the trafficking of cells of the immune system and pro-inflammatory cytokines from the blood into the brain. Recent findings indicate that neurovascular dysfunction is an integral part of many neurological disorders [35, 88]. In diseases with a compromised BBB, the microenvironment of neurons is altered; infiltration into the brain of cells, ions, or molecules may initiate a CNS response. Failure of the BBB is observed in association with a variety of pathological events, occurring as consequence of either systemic pathologies such as stroke, systemic inflammation and CNS disease such as multiple sclerosis (MS) and epilepsy. Increasing evidence has shown that BBB damage causes abnormal neuronal activity. For example, seizures are observed in MS patients, as consequence of stroke, or during systemic or local inflammation. As a proof-of-principle, we (DJ et al.) and others have demonstrated that failure of the BBB induced by “mechanical” means (such as osmotic shock) can play a key role in the onset of seizures [45].
In vitro and in vivo experiments on various models of neurological diseases have shown that blood-brain barrier damage accompanies the development of neurological symptoms; in contrast, managing BBB failure promotes recovery and affords neuroprotection. BBB disruption (BBBD) causes seizures in animal models and human subjects [19, 45, 46, 48, 50, 51, 85]. In particular, a model of temporal lobe epilepsy (pilocarpine, PILO) also depends on BBBD [19, 51, 84]. The currently accepted mechanism of BBBD-induced seizures predicts activation of adhesion molecules on endothelial cells and leukocytes [19]. According to this hypothesis, and in analogy to what is observed in multiple sclerosis, leukocyte adhesion to or interaction with BBB endothelial cells is an essential step leading to BBBD. Published results have shown that anti-inflammatory therapy (e.g., glucocorticosteroids) effectively reduce BBBD and associated symptoms [48]. The specific cell types involved in inflammation-promoted blood-brain barrier dysfunction are poorly understood but many leukocyte families have been shown to be involved, including natural killer cells and cytotoxic lymphocytes [4,46, 50]. Attempts to curb the immune response, such as the extreme case of splenectomy, have been shown to decrease experimental seizures [50]. While BBBD-induced seizures were independent from the means used to obtain disruption (osmotic, pilocarpine, albumin), a specific molecular effector of pilocarpine-induced seizures, perforin, was only recently identified [50]. Perforin released by T cells may explain how activation of T lymphocytes leads to increased BBB permeability; in fact, this molecule can effectively “perforate” the cell membrane causing a rapid loss of function and eventually cell death. In many ways, perforin actions mimic those of membrane-permeating antibiotics, nystatin or gramicidin.
Another reason to focus on the BBB when studying epilepsy is the failure to generate new brain therapeutics owing to insufficient knowledge of the mechanisms involved in brain drug distribution under pathological conditions. Drug resistance affects a significant number of people with epilepsy; it is estimated that approximately 20–30 % of people with epilepsy fail to respond to available anti-epileptic drugs (AEDs) [4, 26, 30, 37, 48, 61, 67]. In the past decade the over-expression of multidrug transporter proteins (e.g., MDR1) at the blood-brain barrier (BBB) has been proposed as a mechanism that contributes to the failure of AEDs to penetrate into epileptic brain [1,9,16,41–43,47,49, 59,77]. In addition to multidrug transporters, it was shown that transcripts of P450 enzymes are elevated in primary endothelial cells (EC) isolated from drug resistant epileptic (DRE) patients; these enzymes include AED-metabolizers such as CYP3A4, CYP2C19, etc. [21]. In addition, transcripts for PHASE II metabolic enzymes are present in DRE EC; these enzymes are responsible for the metabolism of 1st and 2nd generation AEDs; CYP3A4 and MDR1 co-localize at the BBB (and neurons) in human DRE brain [22] and overexpression of CYP3A4 in DRE EC is associated with exaggerated carbamazepine (CBZ) metabolism. This new metabolic pathway produces the toxic CBZ metabolite quinolic acid (QA) leading to the paradoxical situation of an anti-epileptic drug being metabolized in the proximity of the epileptic focus to a seizure-promoting agent.
In summary, therapeutic considerations (use of anti-inflammatory therapy to treat seizures, BBB transporters in multiple drug resistance to anti-epileptic drugs) and etiologic factors (loss of BBB in seizures) suggest that the BBB is a viable and important target for studies aimed at the understanding and treatment of epilepsy. In addition to the role of the blood brain barrier, two other non-neuronal elements need to be considered – glia and brain extracellular matrix – both of which have been shown to have an increasing repertoire of roles in regulating network and brain excitability.
20.4 Neuroglia
“Glia” comes from the Greek meaning glue, and Virchow in his search for connective tissue in the brain, first coined the term neuroglia, considering them a sort of putty that supported the neurons [79]. Later, Golgi distinguished glia from neurons by the lack of an axon and ascribed to them a nutritive as well as supportive role. Ramon y Cajal determined that they were involved in the insulation of nerve cells and axons, a role later confirmed for oligodendroglia by a young Penfield who also established a role of glia in phagocytosis [24]. The repertoire of glia has, however, expanded in recent years from supportive tissue to playing an active role in determining network excitability, both modulating and responding to neuronal activity (Table 20.1).
Table 20.1.
Role of glia in the central nervous system
| Roles of Glia |
| A supportive and protective role for neurons |
| A role in inflammation |
| Regulation of the size of the extracellular space |
| Maintenance of ion homeostasis in the extracellular space |
| Neurotransmitter uptake and synthesis |
| Providing neurons with energy |
| Detecting glutamate release from neurons and other glia |
| Release of neurotransmitters, and regulatory proteins |
| Synapse formation and regulation |
| Communication between neuronal activity and cerebral blood flow |
20.4.1 Glia, Extracellular Space and Potassium Buffering
Glia play a critical part in the regulation of the size of the extracellular space, and extracellular ion homeostasis. In particular, they play a crucial role in the regulation of the concentration of potassium [73]. Glia express both aquaporins and potassium channels (inward rectifying and delayed rectifying) that play a role in this glial function through maintaining potassium and water homeostasis [5, 11, 18]. In addition, the connection of glia through gap junctions results in a glial syncytium, which facilitates not only water and potassium buffering but also glial communication [23]. Abnormalities of glial buffering of potassium result in potassium accumulation during neuronal activity. Such an increase in extracellular potassium will result in the depolarization of neurons and may therefore play a role in seizure initiation and spread [44, 73]. Reductions in the size of the extracellular space can affect neuronal communication through enhancement of ephaptic transmission (electrical interactions occurring though juxtaposed neuronal elements, which are lessened by increasing the conductive space between these elements), alterations in neurotransmitter “spill-over” and clearance, and changes in the regulation of extracellular ion concentrations. It is noteworthy that decreasing the extracellular space can promote seizure activity, whilst strategies aimed at increasing the extracellular space and decreasing glial and neuronal swelling can terminate seizure activity [31].
20.4.2 Glia and Neurotransmitter Concentrations
Glia also regulate the extracellular concentration of glutamate and GABA. They express the glutamate transporters GLAST (EAAT1) and GLT1 (EAAT2), which are responsible for most glutamate clearance [12]. These transporters determine the extracellular glutamate concentration, thus shaping the NMDA receptor response and the “spill-over” of glutamate following synaptic release onto other synapses (heterosynaptic activation) and extra-synaptic receptors [36]. Through this means, glial glutamate clearance plays a role in long-term synaptic plasticity. The expression of these transporters is regulated by an interaction between neurons and glia mediated by ephrins [55], which are extracellular proteins involved in neuronal development but which may be altered in injury and have been proposed to be involved in synaptic reorganisation following status epilepticus. Thus mechanisms that may play a part in synaptic reorganisation during epileptogenesis could also be involved in alterations in the expression of glutamate transporters. These possible roles of ephrins in epileptogenesis (see also below) have yet to be fully investigated.
The role of glia in the regulation of extracellular GABA is less clear since the glial GABA transporter (GAT3) seems to be mainly effective when the neuronal GABA transporters (predominantly GAT1) are blocked [34]. However, it is likely that GAT3 regulates a different pool of GABA that derives from non-vesicular sources. Further, GAT3 seems to play a greater part in regulating the extracellular GABA detected by interneurons than that detected by principal cells [80]. It has been proposed that GAT3 can reverse during periods of excessive activity, thus increasing extracellular GABA concentrations [28]. Finally, glia also are involved in the synthesis of neurotransmitters and in the glutamate-GABA shunt by which glutamate is converted to GABA [10]. Glutamate taken up by glia is converted to glutamine, which is then released into the extracellular space. Glutamine is taken up by neurons and converted to GABA. Inhibition of any of these processes results in a decrease in vesicular GABA content, GABA release and consequently GABAergic transmission [39]. Decreases in glutamate uptake that have been observed during epileptogenesis could therefore not only increase extracellular glutamate but also decrease GABAergic transmission.
20.4.3 Glia and Metabolism
The uptake of glutamate by glia may have a further important role in neuronal energetics. Glutamate enters the Krebs cycle and therefore acts as an energy substrate. Glial glutamate uptake also activates the sodium-potassium ATPase, increasing glucose uptake and glycolysis [63]. Thus increases in extracellular glutamate during seizure activity can increase glial metabolism. Consequently, glia release lactate, which is taken up by neurons and used as an energy substrate, particularly during periods of excessive neuronal activity [6]. The role of glia in neuronal metabolism is probably even more extensive than this. Neurons lack pyruvate carboxylase [68], an enzyme that is crucial for replenishment of oxalo-acetate in the Krebs cycle. As a result of this, the synthesis of GABA and glutamate can rapidly deplete Krebs cycle intermediaries in neurons. Replenishment of these intermediaries in neurons can, however, occur from direct transport of these intermediaries from glia to neurons. Glia are also a major producer of glutathione from glutamate, cysteine and glycine; glial glutathione production is necessary for protection of neurons from free radicals, which are produced during excessive neuronal activity [17]. Failure of glia to provide energy substrates for neurons could therefore promote neuronal death and disorders of neurotransmitter production and neuronal function. Indeed, glia play a crucial role in neurometabolism but how this is altered during and to what extent it plays a part in epileptogenesis are still unclear.
20.4.4 Glia, the Tripartite Synapse and Synaptic Plasticity
One of the main recent advances in our understanding of glia in modulating network activity has been the concept of the tripartite synapse in which glia in close proximity to synapses play a part in synaptic transmission, along with the presynaptic terminal and postsynaptic cell [2]. Vesicles and vesicle-associated proteins have been detected in astrocytes, often in close association to nerve terminals. Glia can detect glutamate via metabotropic glutamate receptors, which mediate a focal rise in astrocyte calcium, which has been proposed to mediate vesicular transmitter release. Calcium rises in one astrocyte can trigger a calcium wave through the glial syncytium, suggesting a mechanism by which focal activity can spread. Glia can also release neurotransmitter through reverse transport and membrane channels. Most of the studies in this area support glial release of glutamate, d-serine and ATP (which is converted to adenosine by extracellular ectonucleotidases) [27]. Glutamate released from glia can act at post-synaptic NMDA receptors and has been proposed to contribute to paroxysmal depolarizing shifts underlying epileptiform activity [82]. D-serine is a co-agonist at NMDA receptors and d-serine release from glia seems to be necessary for NMDA receptor mediated long term potentiation [29]. Lastly, increased adenosine levels through glial ATP release modulates presynaptic release of glutamate in a bimodal fashion through A1 (decreasing release probability) and A2 (increasing release probability) receptors [81]. Thus glia can alter network excitability over short time periods, and could play a role in both seizure initiation (glutamate/D-serine release) and termination (adenosine).
Glia can also play a longer term role in modulating synaptic transmission through the interaction of ephrins, specifically ephrin-A3 on astrocytes with the EphA4 receptor on dendrites [55]. This is a bidirectional interaction, which regulates the expression of glutamate transporters in glia and modulates spine and synapse formation in neurons. Such interactions are important in synaptic plasticity. In addition, glial ephrin signaling is important for neurogenesis, indicating a role for glia in modulating neuronal development and connectivity [55]. The role that this plays in epileptogenesis has yet to be explored.
20.4.5 Glia and Neurovascular Coupling
When neuronal activity increases in an area of the brain, there is a concomitant increase in cerebral blood flow to that area – a phenomenon termed “neurovascular coupling.” There appear to be multiple mechanisms mediating this effect, but there is evidence that glutamate acting via metabotropic glutamate receptors and glutamate uptake by glia can affect the release of vasoactive compounds that directly affect cerebral vasculature [64]. One important consequence of this scenario is that neurovascular coupling may depend upon the release of glutamate rather than local neuronal firing. Indeed, there is accumulating evidence that, although neurovascular coupling correlates both with neuronal firing and local field potentials (i.e. post-synaptic receptor activation through glutamate release), the coupling with field potentials is stronger [40]. This increased blood flow is a critical component of seizure activity that can be detected with ictal SPECT or as an increase in the MRI blood oxygen level dependent (BOLD) signal.
20.4.6 Changes in Glia with Epileptogenesis
Brain injury and neuronal loss invariably leads to a reactive gliosis in which there is not only a proliferation of astrocytes but also changes in astrocytic morphology and gene expression [78]. Moreover, a reactive gliosis is observed in multiple pathologies associated with epileptogenesis, including traumatic brain injury, stroke, tumors, vascular lesions and hippocampal sclerosis. Abnormal glia are also found in tuberous sclerosis; specific knockout of the Tsc1 gene in glia results in seizures [83].
Reactive gliosis may alter regulation of the extracellular space and promote ephaptic transmission. Aquaporin expression in astrocytes changes from astrocyte end feet (i.e., their perivascular location) to a more diffuse expression [5]. This has been proposed to lead to abnormal water regulation, with perivascular water accumulation and increased water uptake by astrocytes resulting in astrocyte swelling and a decrease in the extracellular space. Breakdown of the blood brain barrier and accumulation of albumin within glia also leads to a reduction in glial inward rectifying potassium channel expression and so decreased buffering of potassium rises [13]. Moreover there is evidence in human epileptic tissue of a change in glial glutamate transporter expression and, from rodent studies of epileptogenesis, decreased efficacy of glutamate uptake [13, 65].
Glial metabolism also changes during epileptogenesis. There is an increase in the expression of adenosine kinase and along with astrocytosis, this leads to decreased adenosine levels with epileptogenesis [7]. There are decreased levels of glutamine synthetase, and a consequent decrease in the glutamate-GABA shunt, resulting in decreased inhibitory transmission [10]. Indeed, a specific reactive gliosis mediated by transfection with a viral vector had no effect on the intrinsic excitability of neighboring neurons, but selectively decreased inhibitory transmission, leading to an inhibitory deficit and increased propagation of excitatory transmission [60]. This is a clear demonstration that reactive gliosis alone is sufficient to promote hyperexcitability. Glial metabolism may also be affected by a reactive gliosis due to decreased glutamate uptake, although the role that changes in glial metabolism have on the development of epilepsy are unclear.
Although it is uncertain to what extent reactive gliosis affects the tripartite synapse, astrocyte calcium rises mediated by activation of metabotropic glutamate and purinergic receptors can promote the generation of seizure activity in vitro and in vivo [25]. Also glial metabotropic receptors are upregulated in epilepsy [3].
The critical role that glia play in the inflammatory process underlying epileptogenesis is discussed elsewhere in this book.
There has thus been growing evidence that glia can alter network excitability through multiple mechanisms. The possible roles of reactive gliosis and the part that it plays both in the development of epilepsy and the generation of seizures need to be further modeled and studied. The extensive role that glia play in many critical functions will need to be carefully dissected in order to target specific glia mediated processes during epileptogenesis (Fig. 20.2).
20.5 The Extracellular Matrix (ECM)
20.5.1 Physiological Role of the Extracellular Matrix
The extracellular matrix (ECM) consists of molecules that are secreted both by neurons and glia, and that aggregate in the extracellular space. About 20 % of the volume of the adult brain consists of extracellular matrix, and the extracellular matrix plays an essential role in determining the diffusion of small molecules [57]. In contrast to ECM elsewhere in the body, the brain ECM predominantly consists of proteoglycans, glycos-aminoglycans (in particular hyaluronic acid), and glycoproteins of the tenascin family. There are also proteins that link the ECM to ECM and to molecules on neurons and glia [15].
The vast majority of the ECM is present in the extra-synaptic space. The ECM also makes up the basal lamina, which contributes to the blood-brain barrier. It has also been increasingly recognized that brain ECM consists of other well-defined components including peri-neuronal nets (mesh-like structures which surround cell bodies and proximal dendrites particularly of parvalbumin-expressing interneurons as a meshlike structure), and specific components present at synapses which are linked to proteins at the post-synaptic and pre-synaptic membrane [15].
Peri-neuronal nets consist of proteoglycans of the lectican family which link with hyaluronic acid and tenascin-R [86]. Peri-neuronal nets are critical in development, closing critical periods and stabilizing synapses and neuronal plasticity. Digestion of proteoglycans associated with peri-neuronal nets or knockout of tenascin-R affect both synaptic plasticity and the excitability of interneurons. Peri-neuronal nets therefore play a crucial role in regulating network excitability and plasticity.
The extracellular matrix can undergo remodeling, which is dependent upon a series of serine proteases, such as plasminogen activators (in particular urokinase-type plasminogen activator), thrombin, metalloproteinase’s, and reelin. All of these have been implicated in neuronal and network plasticity [14]. Alterations and remodeling of peri-neuronal nets permit neuronal reorganization following brain damage and seizures, and during development.
The interaction of the extracellular matrix with neurons can occur via specific receptors, integrins, which are transmembrane heterodimeric transmembrane glycoproteins composed of two of 26 subunits. Integrins bind to intracellular cytoskeleton and secondary messenger systems and extracellularly to other cells and the ECM [32]. They are closely associated with glutamate receptors and various ion channels. Integrins regulate multiple processes including synaptic plasticity, neuronal migration and development, axonal growth and synaptogenesis. They are also involved in angiogenesis.
20.5.2 Changes in the ECM in Epilepsy
There are persistent changes in multiple components of the ECM during the development of epilepsy. Peri-neuronal net components, including aggrecan, neurocan, hyaluronan, tenascin-R and some of the linking proteins, decrease during epileptogenesis; a progressive decrease in perinenuronal nets is associated with a progressive decrease in inhibition and the occurrence of seizures (months after traumatic brain injury) [53, 62]. In addition, degradation of the ECM may permit aberrant neuronal and synaptic reorganisation. ECM remodelling and the increased secretion of proteases may also contribute to this process. There is robust evidence that expression of MMP-9 is increased during epileptogenesis, and that this increase may promote kindling [54]. Other serine proteases are also up-regulated in epilepsy including urokinase-type plasminogen activator (uPA) and its receptor (uPAR) [38]. Intriguingly, uPAR up-regulation may be protective as uPAR knockouts develop a more severe epilepsy phenotype following status epilepticus [56]. This indicates that some of the changes of ECM during epileptogenesis may be adaptive rather than pathogenic.
In addition, mutations in the gene encoding SRPX2 (Sushi-repeat Protein, X-linked 2), one of the ligands of uPAR, results in bilateral peri-sylvian polymicrogyria and epilepsy in humans [66]. Integrin expression is also increased during epileptogenesis and in pathologies associated with the development of epilepsy [87].
Lastly, an extracellularly secreted molecule, leucine rich, glioma-inactivated 1 (LGI1) has been strongly associated with epilepsy [8, 20, 33, 58, 69]. LGI1 interconnects presynaptic disintegrin and metalloproteinase domain-containing protein 23 (ADAM23) to postsynaptic ADAM22 at the synaptic cleft. LGI1 is important for trafficking and kinetics of a presynaptic potassium channel, Kv1.1, and also for trafficking of post-synaptic AMPA receptors. In humans, mutations in LGI1 cause autosomal dominant lateral temporal epilepsy or autosomal dominant partial epilepsy with auditory features with onset in childhood/adolescence [58]. In addition, autoantibodies directed against LGI1 have been shown to underlie limbic encephalitis and temporal lobe seizures in humans [69].
Overall, there is growing evidence for the importance of the ECM in epileptogenesis, plasticity and determining network excitability. Further studies aimed at modeling disruption and reorganization of the ECM will be important for a greater understanding of the epileptogenic process. Moreover, the ECM provides an ideal target for therapies aimed at disrupting epileptogenesis and modifying established epilepsy, as it is extracellular and so easily accessible to drugs and has multiple downstream effects, regulating receptors, channels and synaptic transmission.
20.6 Conclusions
There is burgeoning evidence to support a critical role for non-neuronal mechanisms in epileptogenesis and the generation of seizures. Both animal experiments and experiments of nature (gene mutations) indicate that pathology of non-neuronal elements are sufficient for epileptogenesis. However, most of our present therapies are neurocentric, indicating that there may be enormous undiscovered therapeutic potential in targeting these non-neuronal elements. Moreover, it is a concern that many of the large scale mathematical models of brain function (e.g., the blue brain project [52]) have thus far ignored the role of these non-neuronal constituents.
Acknowledgments
This work was supported by the National Institutes of Health (R01NS078307,R01NS43284, R41MH093302, R21NS077236, R42MH093302, and R21HD057256 to DJ).
Contributor Information
Damir Janigro, Department of Molecular Medicine, Cerebrovascular Research, Cleveland Clinic Lerner College of Medicine, Cleveland, OH, USA, janigrd@ccf.org
Matthew C. Walker, Department of Clinical and Experimental Epilepsy, UCL Institute of Neurology, University College London, London WC1N 3BG, UK, m.walker@ucl.ac.uk.
References
- 1.Abbott NJ, Khan EU, Rollinson CM, Reichel A, Janigro D, Dombrowski SM, et al. Drug resistance in epilepsy: the role of the blood-brain barrier. Novartis Found Symp. 2002;243:38–47. [PubMed] [Google Scholar]
- 2.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 3.Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci. 2000;12:2333–2344. doi: 10.1046/j.1460-9568.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- 4.Bauer S, Koller M, Cepok S, Todorova-Rudolph A, Nowak M, Nockher WA, et al. NK and CD4+ T cell changes in blood after seizures in temporal lobe epilepsy. Exp Neurol. 2008;211:370–377. doi: 10.1016/j.expneurol.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Binder DK, Nagelhus EA, Ottersen OP. Aquaporin-4 and epilepsy. Glia. 2012;60:1203–1214. doi: 10.1002/glia.22317. [DOI] [PubMed] [Google Scholar]
- 6.Bittner CX, Valdebenito R, Ruminot I, Loaiza A, Larenas V, Sotelo-Hitschfeld T, et al. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J Neurosci. 2011;31:4709–4713. doi: 10.1523/JNEUROSCI.5311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boison D. Adenosine dysfunction in epilepsy. Glia. 2012;60:1234–1243. doi: 10.1002/glia.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chabrol E, Navarro V, Provenzano G, Cohen I, Dinocourt C, Rivaud-Pechoux S, et al. Electroclinical characterization of epileptic seizures in leucine-rich, glioma-inactivated 1-deficient mice. Brain. 2010;133:2749–2762. doi: 10.1093/brain/awq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornford EM, Oldendorf WH. Epilepsy and the blood-brain barrier. Adv Neurol. 1986;44:787–812. [PubMed] [Google Scholar]
- 10.Coulter DA, Eid T. Astrocytic regulation of glutamate homeostasis in epilepsy. Glia. 2012;60:1215–1226. doi: 10.1002/glia.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Ambrosio R, Wenzel J, Schwartzkroin PA, McKhann GM, Janigro D. Functional specialization and topographic segregation of hippocampal astrocytes. J Neurosci. 1998;18:4425–4438. doi: 10.1523/JNEUROSCI.18-12-04425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 13.David Y, Cacheaux LP, Ivens S, Lapilover E, Heinemann U, Kaufer D, et al. Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? J Neurosci. 2009;29:10588–10599. doi: 10.1523/JNEUROSCI.2323-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dityatev A. Remodeling of extracellular matrix and epileptogenesis. Epilepsia. 2010;51(Suppl 3):61–65. doi: 10.1111/j.1528-1167.2010.02612.x. [DOI] [PubMed] [Google Scholar]
- 15.Dityatev A, Seidenbecher CI, Schachner M. Compartmentalization from the outside: the extracellular matrix and functional microdomains in the brain. Trends Neurosci. 2010;33:503–512. doi: 10.1016/j.tins.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Dombrowski SM, Desai SY, Marroni M, Cucullo L, Goodrich K, Bingaman W, et al. Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia. 2001;42:1501–1506. doi: 10.1046/j.1528-1157.2001.12301.x. [DOI] [PubMed] [Google Scholar]
- 17.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 18.Emmi A, Wenzel HJ, Schwartzkroin PA, Taglialatela M, Castaldo P, Bianchi L, et al. Do glia have heart? Expression and functional role for ether-a-go-go currents in hippocampal astrocytes. J Neurosci. 2000;20:3915–3925. doi: 10.1523/JNEUROSCI.20-10-03915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabene PF, Navarro MG, Martinello M, Rossi B, Merigo F, Ottoboni L, et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med. 2008;14:1377–1383. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukata Y, Lovero KL, Iwanaga T, Watanabe A, Yokoi N, Tabuchi K, et al. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc Natl Acad Sci USA. 2010;107:3799–3804. doi: 10.1073/pnas.0914537107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh C, Gonzalez-Martinez J, Hossain M, Cucullo L, Fazio V, Janigro D, et al. Pattern of P450 expression at the human blood-brain barrier: Roles of epileptic condition and laminar flow. Epilepsia. 2010;51:1408–1417. doi: 10.1111/j.1528-1167.2009.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh C, Marchi N, Desai NK, Puvenna V, Hossain M, Gonzalez-Martinez J, et al. Cellular localization and functional significance of CYP3A4 in the human epileptic brain. Epilepsia. 2011;52:562–571. doi: 10.1111/j.1528-1167.2010.02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- 24.Gill AS, Binder DK. Wilder Penfield, Pio del Rio-Hortega, and the discovery of oligodendroglia. Neurosurgery. 2007;60:940–948. doi: 10.1227/01.NEU.0000255448.97730.34. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Gonzalo M, Losi G, Chiavegato A, Zonta M, Cammarota M, Brondi M, et al. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol. 2010;8:el000352. doi: 10.1371/journal.pbio.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granata T, Marchi N, Carlton E, Ghosh C, Gonzalez-Martinez J, Alexopoulos AV, et al. Management of the patient with medically refractory epilepsy. Expert Rev Neurother. 2009;9:1791–1802. doi: 10.1586/ern.09.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heja L, Nyitrai G, Kekesi O, Dobolyi A, Szabo P, Fiath R, et al. Astrocytes convert network excitation to tonic inhibition of neurons. BMC Biol. 2012;10:26. doi: 10.1186/1741-7007-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75:192–196. doi: 10.1016/j.eplepsyres.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Hochman DW, Baraban SC, Owens JW, Schwartzkroin PA. Dissociation of synchronization and excitability in furosemide blockade of epileptiform activity. Science. 1995;270:99–102. doi: 10.1126/science.270.5233.99. [DOI] [PubMed] [Google Scholar]
- 32.Hynes RO. The emergence of integrins: a personal and historical perspective. Matrix Biol. 2004;23:333–340. doi: 10.1016/j.matbio.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, et al. Antibodies to Kvl potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133:2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kersante F, Rowley SC, Pavlov I, Gutierrez-Mecinas M, Semyanov A, Reul JM, et al. A functional role for both -aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus. J Physiol. 2013;591:2429–2441. doi: 10.1113/jphysiol.2012.246298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krizanac-Bengez L, Mayberg MR, Janigro D. The cerebral vasculature as a therapeutic target for neurological disorders and the role of shear stress in vascular homeostatis and pathophysiology. Neurol Res. 2004;26:846–853. doi: 10.1179/016164104X3789. [DOI] [PubMed] [Google Scholar]
- 36.Kullmann DM. Spillover and synaptic cross talk mediated by glutamate and GABA in the mammalian brain. Prog Brain Res. 2000;125:339–351. doi: 10.1016/S0079-6123(00)25023-1. [DOI] [PubMed] [Google Scholar]
- 37.Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen HW, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 38.Lahtinen L, Huusko N, Myohanen H, Lehtivarjo AK, Pellinen R, Turunen MP, et al. Expression of urokinase-type plasminogen activator receptor is increased during epileptogenesis in the rat hippocampus. Neuroscience. 2009;163:316–328. doi: 10.1016/j.neuroscience.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Liang SL, Carlson GC, Coulter DA. Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J Neurosci. 2006;26:8537–8548. doi: 10.1523/JNEUROSCI.0329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 41.Loscher W. Mechanisms of drug resistance in status epilepticus. Epilepsia. 2007;48(Suppl 8):74–77. doi: 10.1111/j.1528-1167.2007.01357.x. [DOI] [PubMed] [Google Scholar]
- 42.Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 43.Loscher W, Sills GJ. Drug resistance in epilepsy: why is a simple explanation not enough? Epilepsia. 2007;48:2370–2372. doi: 10.1111/j.1528-1167.2007.01260_2.x. [DOI] [PubMed] [Google Scholar]
- 44.Lux HD, Heinemann U, Dietzel I. Ionic changes and alterations in the size of the extracellular space during epileptic activity. Adv Neurol. 1986;44:619–639. [PubMed] [Google Scholar]
- 45.Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, Hernandez N, et al. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48(4):732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marchi N, Fan QY, Ghosh C, Fazio V, Bertolini F, Betto G, et al. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis. 2009;33:171–181. doi: 10.1016/j.nbd.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchi N, Gonzalez-Martinez J, Nguyen MT, Granata T, Janigro D. Transporters in drug-refractory epilepsy: clinical significance. Clin Pharmacol Ther. 2010;87:13–15. doi: 10.1038/clpt.2009.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchi N, Granata T, Freri E, Ciusani E, Puvenna V, Teng Q, et al. Efficacy of anti-inflammatory therapy in a model of acute seizures and in a population of pediatric drug resistant epileptics. PLoS One. 2011;6:e18200. doi: 10.1371/journal.pone.0018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marchi N, Hallene KL, Kight KM, Cucullo L, Moddel G, Bingaman W, et al. Significance of MDR1 and multiple drug resistance in refractory human epileptic brain. BMC Med. 2004;2:37. doi: 10.1186/1741-7015-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marchi N, Johnson A, Puvenna V, Tierney W, Ghosh C, Cucullo L, et al. Modulation of peripheral cytotoxic cells and ictogenesis in a model of seizures. Epilepsia. 2011;52:1627–1634. doi: 10.1111/j.1528-1167.2011.03080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marchi N, Oby E, Fernandez N, Uva L, de Curtis M, Batra A, et al. In vivo and in vitro effects of pilocarpine: relevance to epileptogenesis. Epilepsia. 2007;48(10):1934–1946. doi: 10.1111/j.1528-1167.2007.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markram H. The blue brain project. Nat Rev Neurosci. 2006;7:153–160. doi: 10.1038/nrn1848. [DOI] [PubMed] [Google Scholar]
- 53.McRae PA, Baranov E, Rogers SL, Porter BE. Persistent decrease in multiple components of the perineuronal net following status epilepticus. Eur J Neurosci. 2012;36:3471–3482. doi: 10.1111/j.1460-9568.2012.08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizoguchi H, Nakade J, Tachibana M, Ibi D, Someya E, Koike H, et al. Matrix metalloproteinase-9 contributes to kindled seizure development in pentylenetetrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J Neurosci. 2011;31:12963–12971. doi: 10.1523/JNEUROSCI.3118-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murai KK, Pasquale EB. Eph receptors and ephrins in neuron-astrocyte communication at synapses. Glia. 2011;59:1567–1578. doi: 10.1002/glia.21226. [DOI] [PubMed] [Google Scholar]
- 56.Ndode-Ekane XE, Pitkanen A. Urokinase-type plasminogen activator receptor modulates epileptogenesis in mouse model of temporal lobe epilepsy. Mol Neurobiol. 2013;47:914–937. doi: 10.1007/s12035-012-8386-2. [DOI] [PubMed] [Google Scholar]
- 57.Nicholson C, Sykova E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21:207–215. doi: 10.1016/s0166-2236(98)01261-2. [DOI] [PubMed] [Google Scholar]
- 58.Nobile C, Michelucci R, Andreazza S, Pasini E, Tosatto SC, Striano P. LGI1 mutations in autosomal dominant and sporadic lateral temporal epilepsy. Hum Mutat. 2009;30:530–536. doi: 10.1002/humu.20925. [DOI] [PubMed] [Google Scholar]
- 59.Oby E, Janigro D. The blood-brain barrier and epilepsy. Epilepsia. 2006;47:1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- 60.Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, et al. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patsalos PN, Berry DJ, Bourgeois BF, Cloyd JC, Glauser TA, Johannessen SI, et al. Antiepileptic drugs – best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring. ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49:1239–1276. doi: 10.1111/j.1528-1167.2008.01561.x. [DOI] [PubMed] [Google Scholar]
- 62.Pavlov I, Huusko N, Drexel M, Kirchmair E, Sperk G, Pitkanen A, et al. Progressive loss of phasic, but not tonic, GABAA receptor-mediated inhibition in dentate granule cells in a model of post-traumatic epilepsy in rats. Neuroscience. 2011;194:208–219. doi: 10.1016/j.neuroscience.2011.07.074. [DOI] [PubMed] [Google Scholar]
- 63.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71:782–797. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 65.Proper EA, Hoogland G, Kappen SM, Jansen GH, Rensen MG, Schrama LH, et al. Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain. 2002;125:32–43. doi: 10.1093/brain/awf001. [DOI] [PubMed] [Google Scholar]
- 66.RoU P, Rudolf G, Pereira S, Royer B, Scheffer IE, Massacrier A, et al. SRPX2 mutations in disorders of language cortex and cognition. Hum Mol Genet. 2006;15:1195–1207. doi: 10.1093/hmg/ddl035. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez Alvarez JC, Serrano Castro PJ, Serratosa Fernandez JM. Clinical implications of mechanisms of resistance to antiepileptic drugs. Neurologist. 2007;13:S38–S46. doi: 10.1097/NRL.0b013e31815bb403. [DOI] [PubMed] [Google Scholar]
- 68.Schousboe A, Bak LK, Waagepetersen HS. Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Front Endocrinol (Lausanne) 2013;4:102. doi: 10.3389/fendo.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schulte U, Thumfart JO, Klocker N, Sailer CA, Bildl W, Biniossek M, et al. The epilepsy-linked Lgil protein assembles into presynaptic Kv1 channels and inhibits inactivation by Kvbetal. Neuron. 2006;49:697–706. doi: 10.1016/j.neuron.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 70.Schwartzkroin PA. Characteristics of CA1 neurons recorded intracellularly in the hippocampal in vitro slice preparation. Brain Res. 1975;85:423–436. doi: 10.1016/0006-8993(75)90817-3. [DOI] [PubMed] [Google Scholar]
- 71.Schwartzkroin PA. Further characteristics of hippocampal CA1 cells in vitro. Brain Res. 1977;128:53–68. doi: 10.1016/0006-8993(77)90235-9. [DOI] [PubMed] [Google Scholar]
- 72.Schwartzkroin PA. Why – and how – do we approach basic epilepsy research? In: Noebels JL, Avoli M, Rogawski MA, et al., editors. Jasper’s basic mechanisms of the epilepsies. 4th. Oxford, New York: 2012. pp. 24–37. [Google Scholar]
- 73.Schwartzkroin PA, Baraban SC, Hochman DW. Osmolality, ionic flux, and changes in brain excitability. Epilepsy Res. 1998;32:275–285. doi: 10.1016/s0920-1211(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 74.Schwartzkroin PA, Mathers LH. Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res. 1978;157:1–10. doi: 10.1016/0006-8993(78)90991-5. [DOI] [PubMed] [Google Scholar]
- 75.Schwartzkroin PA, Prince DA. Cellular and field potential properties of epileptogenic hippocampal slices. Brain Res. 1978;147:117–130. doi: 10.1016/0006-8993(78)90776-x. [DOI] [PubMed] [Google Scholar]
- 76.Schwartzkroin PA, Prince DA. Recordings from presumed glial cells in the hippocampal slice. Brain Res. 1979;161:533–538. doi: 10.1016/0006-8993(79)90683-8. [DOI] [PubMed] [Google Scholar]
- 77.Sisodiya SM, Goldstein DB. Drug resistance in epilepsy: more twists in the tale. Epilepsia. 2007;48:2369–2370. doi: 10.1111/j.1528-1167.2007.01260_1.x. [DOI] [PubMed] [Google Scholar]
- 78.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Somjen GG. Nervenkitt: notes on the history of the concept of neuroglia. Glia. 1988;1:2–9. doi: 10.1002/glia.440010103. [DOI] [PubMed] [Google Scholar]
- 80.Song I, Volynski K, Brenner T, Ushkaryov Y, Walker M, Semyanov A. Different transporter systems regulate extracellular GABA from vesicular and nonvesicular sources. Front Cell Neurosci. 2013;7:23. doi: 10.3389/fncel.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stone TW, Ceruti S, Abbracchio MP. Adenosine receptors and neurological disease: neuroprotection and neurodegeneration. Handb Exp Pharmacol. 2009;193:535–587. doi: 10.1007/978-3-540-89615-9_17. [DOI] [PubMed] [Google Scholar]
- 82.Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, et al. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, et al. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- 84.Uva L, Librizzi L, Marchi N, Noe F, Bongiovanni R, Vezzani A, et al. Acute induction of epileptiform discharges by pilocarpine in the in vitro isolated guinea-pig brain requires enhancement of blood-brain barrier permeability. Neuroscience. 2008;151:303–312. doi: 10.1016/j.neuroscience.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Vliet EA, da Costa AS, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 86.Wang D, Fawcett J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 2012;349:147–160. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- 87.Wu X, Reddy DS. Integrins as receptor targets for neurological disorders. Pharmacol Ther. 2012;134:68–81. doi: 10.1016/j.pharmthera.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]