Abstract
Objective. To evaluate the relationship between TGFβ signaling and endothelial lncRNA expression. Methods. Human umbilical vein endothelial cell (HUVECs) lncRNAs and mRNAs were profiled with the Arraystar Human lncRNA Expression Microarray V3.0 after 24 hours of exposure to TGFβ1 (10 ng/mL). Results. Of the 30,584 lncRNAs screened, 2,051 were significantly upregulated and 2,393 were appreciably downregulated (P < 0.05) in response to TGFβ. In the same HUVEC samples, 2,148 of the 26,106 mRNAs screened were upregulated and 1,290 were downregulated. Of these 2,051 differentially expressed upregulated lncRNAs, MALAT1, which is known to be induced by TGFβ in endothelial cells, was the most (~220-fold) upregulated lncRNA. Bioinformatics analyses indicated that the differentially expressed upregulated mRNAs are primarily enriched in hippo signaling, Wnt signaling, focal adhesion, neuroactive ligand-receptor interaction, and pathways in cancer. The most downregulated are notably involved in olfactory transduction, PI3-Akt signaling, Ras signaling, neuroactive ligand-receptor interaction, and apoptosis. Conclusions. This is the first lncRNA and mRNA transcriptome profile of TGFβ-mediated changes in human endothelial cells. These observations may reveal potential new targets of TGFβ in endothelial cells and novel therapeutic avenues for cardiovascular disease-associated endothelial dysfunction.
1. Introduction
Transforming growth factor-β (TGFβ) belongs to a large superfamily of linked proteins, comprising activins, bone morphogenetic proteins (BMPs), growth/differentiation factors, and anti-Müllerian hormone [1] that regulates proliferation, differentiation, migration, and survival in diverse cell populations depending on the cell type [2]. TGFβ1, TGFβ2, and TGFβ3 are the most common of the isoforms that are involved in these functions [3]. Prior to binding to its specific type I and type II serine/threonine kinase receptors, the latent form of TGFβ is activated by proteases or thrombospondin. It is well documented that TGFβ signaling involves one TGFβ type II receptor and two distinct TGFβ type I receptors, that is, the endothelium limited activin receptor-like kinase (ALK1) and the largely expressed ALK5. Activated ALK5 after ligand binding transduces signals from the membrane to the nucleus via phosphorylation of a specific subset of intracellular effectors termed Smads [3, 4]. While ALK1 activation phosphorylates Smad1, Smad5, and Smad8, ALK5 mediates Smad2 and Smad3 phosphorylation. The heteromeric complex of phosphorylated Smad2/Smad3 with Smad4 then translocates to the nucleus, where, together with various transcriptional regulators, it leads to the transcription of a wide array of target genes [5, 6].
Several in vivo studies have shown that interfering with the components of the TGFβ1 signaling pathway, including TGFβ1 [7], TGFβR-II [8], ALK5 [9], endoglin [10], ALK1 [11], or Smad5 [12], through gene targeting results in extreme vascular anomalies in mice as illustrated by enlarged vessels and defective differentiation of smooth muscle cells. Depending on the experimental conditions and animal models, TGFβ1 has been also shown to function as an inhibitor or a promoter of angiogenesis [13, 14]. Given its multifunctional role in cellular processes, disturbed TGFβ1 signaling is notably evident in various human disorders [15, 16]. Evidence for how TGFβ1 contributes to the advancement of tumors is conflicting and appears to be dependent on the developmental stage of the tumor. TGFβ1 acts as an inhibitor of proliferation during the initial stages of tumor development. However, upon attenuation of this antiproliferative signal, tumor cells often secrete great amounts of TGFβ1 which promote cell invasion, epithelial-to-mesenchymal transition (EMT) metastasis, and angiogenesis which collectively establish a growth-supportive tumor microenvironment [4, 17, 18].
In recent years, the long noncoding RNAs (lncRNAs) have emerged as regulators and potential therapeutic targets for a wide variety of physiological and pathological processes [19, 20]. Typically, lncRNAs are transcripts greater than 200 nucleotides that lack an open reading frame and protein-coding ability. Although the lncRNAs are not as well conserved as protein-coding genes and microRNA, increasing evidence suggests that lncRNAs are involved in a variety of cellular functions like proliferation, survival, migration, invasion, angiogenesis, and differentiation and could serve as alternative therapeutic targets [21–26]. MALAT1 (metastasis associated lung adenocarcinoma transcript 1), which is amongst the most abundant and highly conserved lncRNAs, exhibits specific nuclear localization, developmental regulation, and dysregulation in cancer, all of which are indicative of its critical role in multiple biological processes [27]. MALAT1 is an important mediator of TGFβ signaling and may represent a promising therapeutic option for suppressing bladder cancer progression [28]. MALAT1 is highly expressed in endothelial cells and loss of MALAT1 tips the balance from a proliferative to a migratory endothelial cell phenotype in vitro and reduces vascular growth in vivo [29].
To date, the nuances underlying the transcriptional regulation of lncRNAs by TGFβ1 in endothelial cells remain unexplored. The goal of the current study was to profile the changes in lncRNA expression in association with TGFβ1 signaling in endothelial cells that may provide insights into regulation of endothelial function by TGFβ1-associated lncRNAs. This approach also allowed us to identify novel lncRNA targets and associated pathways of TGFβ1 in endothelial cells.
2. Materials and Methods
2.1. Cell Culture
Human umbilical vein endothelial cells (HUVECs, Lonza) were cultured in endothelial cell growth medium-2 (EGM™-2 Bulletkit™; Lonza) supplemented with growth factors, serum, and antibiotics at 37°C in humidified 5% CO2. Confluent HUVECs were split into 6 and maintained in 6-well plates for 24 hours in the absence (3 plates) or presence (3 plates) of recombinant TGFβ1 (10 ng/mL; R&D Systems).
2.2. Microarray Profiling
Total RNA was isolated using TRIzol™ (Invitrogen) reagent and quantified with the NanoDrop ND-1000 spectrophotometer. RNA integrity was confirmed by standard denaturing agarose gel electrophoresis. The expression profile of 30,584 human lncRNAs and 26,106 protein-coding transcripts was conducted with the Arraystar Human LncRNA Microarray V3.0. Sample labeling and array hybridization were performed on the Agilent Array platform. Briefly, total RNA from each sample was amplified and transcribed into fluorescent cRNA (Arraystar Flash RNA Labeling Kit, Arraystar) before 1 μg of each labeled cRNA was hybridized onto the microarray slide. The hybridized arrays were washed, fixed, and scanned with the Agilent DNA Microarray Scanner (Product# G2505C). The acquired array images were analyzed with the Agilent Feature Extraction software (version 11.0.1.1). Quantile normalization and subsequent data processing were performed with the GeneSpring GX v11.5.1 software package (Agilent Technologies). P values for the differentially expressed genes were determined with the t-test and adjusted for multiple testing with the Benjamini Hochberg method to minimize the false discovery rate. Volcano plot filtering, set at a threshold of ≥2.0-fold, was used to screen for lncRNAs and mRNAs that exhibited significantly different (P < 0.05; unpaired t-test) expression levels in the two study groups. Pathway analysis was based on the current Kyoto Encyclopedia of Genes and Genomes (KEGG) database. Gene ontology (GO) analysis was performed with the topGO package of bioconductor system.
3. Results
3.1. Quality Assessment of LncRNAs and mRNAs Data
The 6 samples evaluated had a 2 : 1 intensity ratio for their 28S : 18S rRNA bands and OD260/OD280 ratios of >1.8 thereby verifying RNA integrity, purity, and concentration (Supplementary Figure 1 in Supplementary Material available online at http://dx.doi.org/10.1155/2016/2459687). Box-and-Whisker plots constructed to visualize the distribution of the fluorescent intensities revealed very similar normalized log 2 ratios for both lncRNA and mRNA and accordingly comparable quality of the array data, across the board (Supplementary Figure 1).
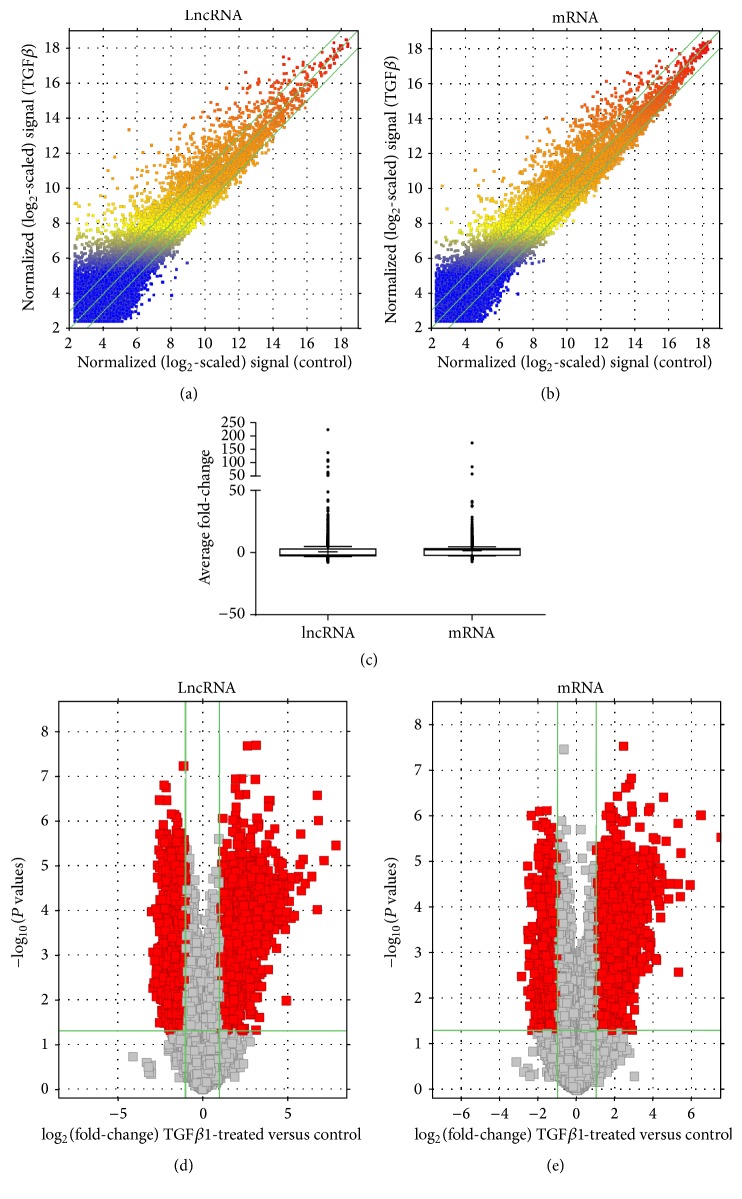
Scatter plots provided a profile of HUVEC lncRNAs (Figure 1(a)) and mRNAs (Figure 1(b)) that were upregulated, downregulated, or unaffected by exposure to TGFβ1 treatment. Overall, the average fold-changes of lncRNAs and mRNAs differentially expressed under the study conditions were similar (Figure 1(c)). Subsequent volcano plot filtering uncovered 2,051 significantly upregulated and 2,393 significantly downregulated lncRNAs in HUVECs cultured with TGFβ1 relative to control samples (Figure 1(d); P < 0.05; Supplement Tables A and B). LncRNAs that demonstrated the greatest differences in expression ranged from 177 bp to 17.85 kb. Specifically, MALAT1 (RNA length: 8,708 bp, chromosome 15) was the most upregulated lncRNA and AC144521.1 (RNA length: 919 bp, chromosome 3) was the most downregulated in HUVECs subjected to TGFβ1 treatment. Table 1 lists the 10 most up-/downregulated lncRNAs depending on the fold-change expression. TGFβ1 treatment associated changes at the transcript level were also noted amongst 3,436 mRNAs with 2,148 upregulated and 1,290 downregulated (Figure 1(e); P < 0.05; Supplement Tables C and D).
Figure 1.
LncRNA and mRNA expression profiles in HUVECs exposed to TGFβ1 (10 ng/mL) versus control. (a and b) Scatter plots comparing the variation in lncRNA and mRNA expression. The values plotted are the averaged normalized signal values (log 2 scaled) for the control (x-axis) and the TGFβ1 treatment (y-axis) groups. The green lines indicate fold-change. LncRNAs and mRNAs above the top green line and below the bottom green line exhibit at least a 2.0-fold difference between the two study groups. (c) Box-and-Whisker plots (10th and 90th percentiles) showing average fold-change of lncRNAs and mRNAs. Median intensity is denoted with a “−” sign and mean intensity is denoted with a “+” sign. (d and e) Volcano plots detailing magnitude of expression difference. The vertical green lines correspond to 2.0-fold upregulation and 2.0-fold downregulation of expression. The horizontal green line indicates a P value of ≤0.05. Red points represent lncRNAs and mRNAs with statistically significant differential expression (fold-change ≥ 2.0, P ≤ 0.05).
Table 1.
10 Most differentially expressed (up- and downregulated) lncRNAs in HUVECs upon TGFβ1 (10 ng/mL) stimulation.
| Sequence name | RNA length | Chr. | Fold | P value | |
|---|---|---|---|---|---|
| Upregulated lncRNAs | MALAT1 | 8708 | 15 | 223.69 | 3.72601E − 06 |
| RP11-327I22.8 | 1761 | 6 | 137.52 | 8.04408E − 06 | |
| PSMD6-AS2 | 2555 | 11 | 110.50 | 1.03209E − 06 | |
| BC016035 | 1170 | 18 | 105.83 | 9.96754E − 05 | |
| CRNDE | 659 | 3 | 105.66 | 2.85351E − 07 | |
| RP5-1103G7.4 | 750 | 2 | 84.49 | 1.8145E − 05 | |
| DA315543 | 538 | 16 | 64.10 | 2.62297E − 05 | |
| TM4SF19-TCTEX1D2 | 1022 | 9 | 59.10 | 7.56202E − 06 | |
| DMD-AS3 | 293 | 1 | 54.68 | 3.75391E − 05 | |
| CTD-2026G22.1 | 1035 | 7 | 53.39 | 2.06995E − 06 | |
|
| |||||
| Downregulated lncRNAs | AC144521.1 | 919 | 3 | 7.90 | 0.000111558 |
| BX114362 | 693 | 5 | 7.47 | 0.000885235 | |
| D16471 | 2448 | X | 7.43 | 0.005627670 | |
| uc.117 | 251 | 3 | 7.17 | 0.004263809 | |
| LOC729678 | 2874 | 5 | 7.01 | 0.001343734 | |
| LINC00593 | 1330 | 15 | 6.81 | 9.68611E − 05 | |
| RP11-594C13.2 | 369 | 14 | 6.71 | 0.002673799 | |
| LINC00494 | 508 | 20 | 6.64 | 0.008476439 | |
| RP11-574O16.1 | 585 | 2 | 6.57 | 0.001869003 | |
| LOC643401 | 490 | 5 | 6.54 | 9.53526E − 05 | |
3.2. LncRNA Chromosomal Distribution and Subtype Analysis
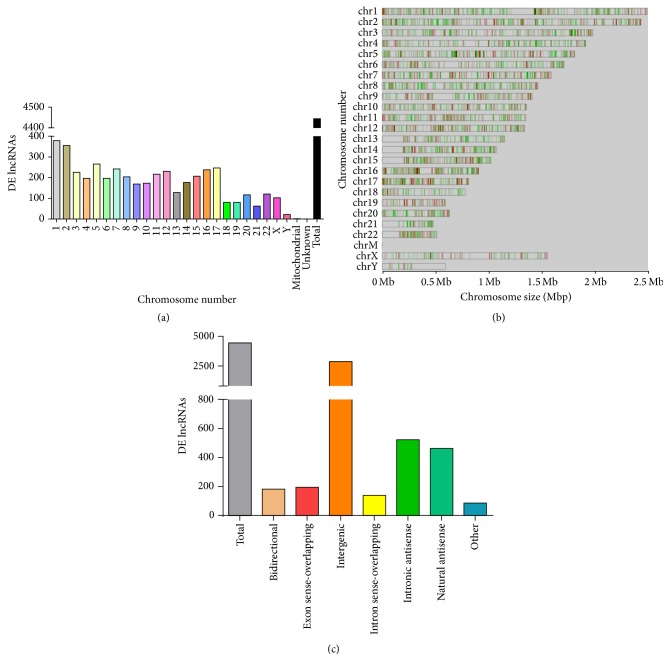
Supplementary Figure 2 shows the dendrograms generated for hierarchical analysis of clustered lncRNAs and mRNAs that were differentially expressed in HUVECs cultured in media with TGFβ1 in comparison to controls. Although lncRNAs modulated by TGFβ1 treatment were abundant and found on every human chromosome, most were located on chromosomes 1, 2, and 17 (Figure 2(a)). Further probing revealed that while these differentially expressed lncRNAs are expressed along the entire length of the chromosomes, there is a notable clustering of lncRNAs (Figure 2(b)). LncRNA subgroup analysis, which helps identify the functional relationship between lncRNAs and their associated protein-coding genes, demonstrated that the majority (~50%) of lncRNAs were intergenic in origin followed by intron and natural antisense lncRNAs (Figure 2(c)). We also identified bidirectional, exon sense-overlapping, and intron sense-overlapping lncRNAs (Figure 2(c)).
Figure 2.
Distribution, location, and classification of differentially expressed lncRNAs in HUVECs exposed to TGFβ1 (10 ng/mL) versus control. Demonstration of (a) numbers and (b) chromosomal location of differentially expressed (DE) lncRNAs on different chromosomes. (c) Bar graph representing types of differently expressed lncRNAs, depending on their genomic location.
3.3. LncRNAs and Associated Protein-Coding Transcripts
We conducted additional profiling to gather insight into differentially expressed lncRNAs and associated protein-coding transcripts. The fold-change calculated for the top 10 highly up-/downregulated lncRNA with known associated protein-coding genes is summarized in Figure 3. Interestingly, MALAT1, which is highly expressed in endothelial cells [29] and is an important mediator of TGFβ signaling [28], was the most upregulated lncRNA after TGFβ-stimulation (Figure 3). The protein-coding genes LTBP3, KCNK7, and TGD3, which are adjacent to MALAT1 on chromosome 15 [27], were also significantly upregulated (Figure 3). Of note, 9 of the 20 lncRNAs demonstrated a direct correlation in fold-change with its associated mRNA, whereas the remaining 11 displayed an inverse correlation. Inverse relation was mainly observed for the downregulated (9 out of 10) lncRNAs (Figure 3).
Figure 3.
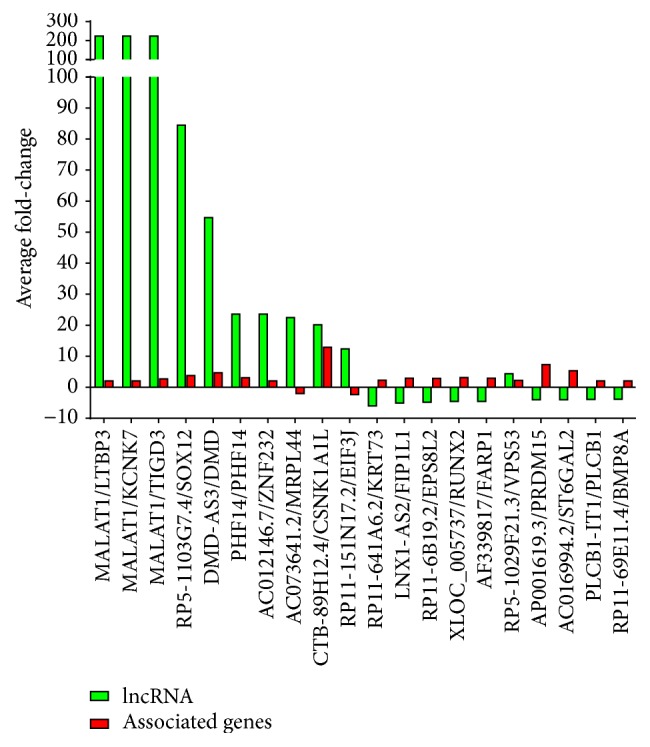
Network coexpression and bioinformatics analyses of samples from HUVECs exposed to TGFβ1 (10 ng/mL) versus control. Representation of differentially expressed lncRNAs and associated genes with respect to fold-change. Eight significantly upregulated and 10 downregulated lncRNAs with known target genes were selected for presentation in the figure.
3.4. Bioinformatics Analyses
Pathway analysis with the current KEGG database yielded several pertinent findings (Tables 2 and 3). In brief, mRNAs upregulated in response to TGFβ1 treatment are involved in hippo signaling, Wnt signaling, focal adhesion, neuroactive ligand-receptor interaction, and cancer-associated pathways (Table 2). The most downregulated mRNAs are notably involved in olfactory transduction, PI3K-Akt signaling, Ras signaling, neuroactive ligand-receptor interaction, and apoptosis (Table 3).
Table 2.
Results of bioinformatics analyses on upregulated pathways in HUVECs after TGFβ1 (10 ng/mL) stimulation.
| Pathway analysis | Upregulated gene count | P value |
|---|---|---|
| Hippo signaling pathway | 153 | 0.0002709 |
| Wnt signaling pathway | 139 | 0.0006834 |
| Basal cell carcinoma | 55 | 0.0017771 |
| Hedgehog signaling pathway | 51 | 0.0027492 |
| Pathways in cancer | 327 | 0.0052783 |
| Osteoclast differentiation | 131 | 0.0067513 |
| Melanogenesis | 101 | 0.0073718 |
| Axon guidance | 127 | 0.0095634 |
| Pertussis | 75 | 0.0114669 |
| Neuroactive ligand-receptor interaction | 321 | 0.0157052 |
| Synaptic vesicle cycle | 63 | 0.0158835 |
| NOD-like receptor signaling pathway | 57 | 0.0186762 |
| Acute myeloid leukemia | 57 | 0.0186762 |
| Neurotrophin signaling pathway | 120 | 0.0205091 |
| Focal adhesion | 206 | 0.0236221 |
| Proteoglycans in cancer | 225 | 0.0259569 |
| Adrenergic signaling in cardiomyocytes | 149 | 0.0279908 |
| Notch signaling pathway | 48 | 0.0376197 |
| Prolactin signaling pathway | 72 | 0.0414023 |
| Jak-STAT signaling pathway | 156 | 0.0438904 |
| Cytokine-cytokine receptor interaction | 271 | 0.0439402 |
| Prostate cancer | 89 | 0.0448626 |
Table 3.
Results of bioinformatics analyses on downregulated pathways in HUVECs after TGFβ1 (10 ng/mL) stimulation.
| Pathway analysis | Downregulated gene count | P value |
|---|---|---|
| Olfactory transduction | 405 | 0.0036241 |
| Apoptosis | 86 | 0.0050919 |
| PI3K-Akt signaling pathway | 346 | 0.006535 |
| mRNA surveillance pathway | 91 | 0.0082309 |
| Ribosome biogenesis in eukaryotes | 85 | 0.0119435 |
| Circadian rhythm | 30 | 0.0140969 |
| Chemical carcinogenesis | 80 | 0.0189201 |
| Ras signaling pathway | 227 | 0.0201366 |
| Melanoma | 71 | 0.0211199 |
| Hypertrophic cardiomyopathy (HCM) | 85 | 0.0284512 |
| Rap1 signaling pathway | 213 | 0.0320182 |
| Estrogen signaling pathway | 100 | 0.0381713 |
| Tight junction | 134 | 0.0382214 |
| Nicotinate and nicotinamide metabolism | 28 | 0.0382726 |
| Drug metabolism-cytochrome P450 | 68 | 0.0398014 |
| Serotonergic synapse | 114 | 0.0453301 |
| Neuroactive ligand-receptor interaction | 321 | 0.0459533 |
| Tyrosine metabolism | 39 | 0.0463610 |
Bioinformatics GO analyses grouped the differentially expressed mRNAs under the following three categories: biological processes, cellular component, and molecular function. GO terms most broadly associated with upregulated mRNAs were biological function, protein binding, and signalling (Table 4). GO terms associated with downregulated mRNA were mainly enriched in cell, response to stimulus, and multicellular organism process (Table 4).
Table 4.
Results of bioinformatics GO (gene ontology) enrichment analyses to determine the roles of differentially expressed mRNAs in GO terms.
| Upregulated | Downregulated | |||||||
|---|---|---|---|---|---|---|---|---|
| GO term | Count | % of total DE genes | P value | GO term | Count | % of total DE genes | P value | |
| Biological process | Cell communication | 686 | 41.7 | 5.77E − 06 | Response to stimulus | 587 | 55.3 | 1E − 06 |
| Biological regulation | 1136 | 69.1 | 8.48E − 06 | Cation transport | 97 | 9.1 | 8E − 06 | |
| Organ development | 356 | 21.7 | 9.07E − 06 | Multicellular organismal process | 494 | 46.6 | 1E − 05 | |
| Anatomical structure development | 540 | 32.8 | 1.27E − 05 | Single-multicellular organism process | 478 | 45.1 | 2E − 05 | |
| Signaling | 674 | 41.0 | 1.6E − 05 | Cell surface receptor signaling pathway | 265 | 25.0 | 5E − 05 | |
|
| ||||||||
| Cellular component | Plasma membrane part | 273 | 15.5 | 5.43E − 05 | Plasma membrane | 380 | 33.9 | 6E − 09 |
| Neuron part | 123 | 7.0 | 0.000426 | Cell periphery | 386 | 34.5 | 8E − 09 | |
| Intrinsic component of plasma membrane | 170 | 9.7 | 0.00069 | Cell part | 1006 | 89.8 | 0.002 | |
| Cell projection | 180 | 10.2 | 0.00236 | Cell | 1006 | 89.8 | 0.002 | |
| Cell periphery | 525 | 29.9 | 0.00265 | Integral component of membrane | 389 | 34.7 | 0.0026 | |
|
| ||||||||
| Molecular function | Channel activity | 68 | 4.2 | 5.66E − 05 | Signaling receptor activity | 124 | 12.0 | 3E − 05 |
| Passive transmembrane transporter activity | 68 | 4.2 | 5.66E − 05 | Receptor activity | 138 | 13.3 | 9E − 05 | |
| Transmembrane transporter activity | 124 | 7.7 | 0.000639 | Signal transducer activity | 144 | 13.9 | 0.0001 | |
| Protein binding | 917 | 57.1 | 0.000751 | Molecular transducer activity | 144 | 13.9 | 0.0001 | |
| Cation transmembrane transporter activity | 85 | 5.3 | 0.000822 | Transmembrane signaling receptor activity | 113 | 10.9 | 0.0001 | |
4. Discussion
The underlying dogma of molecular biology for the last few decades has been that the purpose of RNA is to direct the assembly of proteins from amino acids through translation. A few exceptions to this paradigm are ribosomal RNA and transfer RNA which are functional RNA macromolecules that do not encode protein. A large proportion (>80%) of the human genome is transcribed, but protein-coding transcripts account for only ~2% of whole transcriptome [30]. This suggests that the majority of the genomes are transcribed as non-protein-coding RNAs. Among noncoding RNAs, a novel class of noncoding RNAs, which stretch more than 200 nucleotides and are termed long noncoding RNAs (lncRNAs), has recently emerged [31]. Evidence to date suggests that the mechanisms underlying gene regulation by lncRNAs are highly complex and involve both inhibition and activation of gene expression [32].
The growing appreciation of the multitude of mechanisms, functions, and types of lncRNAs has set off a research tsunami to clarify the involvement of lncRNAs in the etiology of disease states. Although there have been reports demonstrating that lncRNAs are dysregulated in several human diseases, it has yet to be confirmed that these molecules can act independently to drive the progression of said pathologies [33]. At present, the strongest association lies with cancer [34] where altered expression of several lncRNAs has been documented [35, 36]. LncRNA PCAT-1 which is a target of histone-modifying PRC2 complex bearing both oncogenic and tumor-suppressive features was found to promote cell proliferation [37]; antisense noncoding RNA in the INK4 locus (ANRIL; also known as CDKN2BAS) is upregulated in prostate cancer and implicated in tumor suppression [38]; HOTAIR upregulation is associated with poor prognosis in pancreatic [39], colorectal [40], liver [41], gastrointestinal [42], and breast [43] cancers and likely also contributes to increased metastasis [43] of these cancer types. MALAT1 was one of the first lncRNAs to be implicated in cancer and a series of studies have established its potential importance as a biomarker and potential therapeutic target for cancer metastasis [44]. Increased expression of MALAT1 is observed in lung, breast, colon, cervical, colorectal, ovarian, gastric, and other cancer types [44]. Mechanistically, MALAT1 affects the transcriptional and posttranscriptional regulation of cytoskeletal and extracellular matrix genes [45]. A similar function has been postulated for lincRNA-p21 (named for its vicinity to the CDKN1A/p21 locus) in cancer, which functions as a repressor in p53-dependent transcriptional responses particularly on genes regulating apoptosis, possibly by directing the recruitment of hnRNP-K to its genomic targets [36].
Although the biological significance of lncRNAs has perhaps been most extensively investigated in cancers, it is noteworthy that several lines of evidence purport a role for lncRNAs in nonneoplastic conditions such as development [46] and cardiovascular diseases (CVDs). The first evidence suggestive of a lncRNA-CVD association stemmed from genome-wide association studies that independently identified a susceptibility locus of coronary artery disease (CAD) on human chromosome 9p21 [47, 48]. This locus is adjacent to the last exon of ANRIL. That the protein-coding genes cyclin-dependent kinase inhibitors 2A and 2B (CDKN2A and CDKN2B, resp.) lie >100 kb from associated single nucleotide polymorphisms (SNPs) suggested to the investigators that SNPs in ANRIL increases the susceptibility to CAD and other vascular diseases [49–51]. The lncRNAs MALAT1, MEG3, and TUG1 are highly expressed in endothelial cells [29] and are induced under low oxygen conditions in vitro in endothelial cells [29]; MALAT1 expression is similarly affected in vivo in ischemic limbs [29]. Inhibition of MALAT1 promoted RNA degradation in an RNase H-dependent mechanism and promoted migration of tip cells but blocked proliferation of subsequent stalk cells leading to an abnormal tube formation in vitro [29]. Genetic deletion or pharmacological inhibition of MALAT1 impaired vascularization in vivo [29]. Bioinformatics analysis of MALAT1-regulated genes revealed that MALAT1 supports the proliferation of endothelial cells through its cell cycle regulatory effects [29, 52]. Notably, the enhanced levels of MALAT1 observed in patients with ischemia [29] are consistent with the upregulation of MALAT1 previously described in in vitro and in vivo models [29].
Deep sequencing studies have identified lncRNAs in human coronary aortic smooth muscle cells (SMCs) by comparing their expression profiles to those of HUVECs [53]. After screening 31 lncRNAs, 1 lncRNA, namely, smooth muscle and endothelial cell-enriched migration/differentiation-associated long noncoding RNA (SENCR), was studied in detail, which is highly expressed in endothelial cells, SMCs, and aortic tissue [53]. In SMCs, loss of SENCR significantly enhanced SMC migration and reduced expressions of SMC contractile markers [53]. Another study evaluating the regulation and function of lncRNAs in human aortic valve cells demonstrated that cyclic stretch reduced the expression of the lncRNA HOTAIR and also that loss of HOTAIR elevated expressions of calcification-related genes, indicating its role in aortic valve calcification [54]. In the heart, Fendrr (Fetal-lethal noncoding developmental regulatory RNA) is an excellent example for the role of lncRNAs in cardiac development as intraventricular septal heart defects were observed embryonically in Fendrr-deficient mice [55].
Role of other lncRNAs in CVDs is demonstrated by lncRNA MIAT, which is associated with increased risk of myocardial infarction [56]; lncRNA ANRIL is associated with increased risk to coronary heart disease [57]; lncRNA DBE-T localizes to the facioscapulohumeral muscular dystrophy (FSHD) locus [58]; and a novel lncRNA is identified in association with HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) [59]. Furthermore, vascular lincRNA-p21 represses proliferation and induces apoptosis in vitro and in vivo in vascular smooth muscle cells [60]. Loss of endogenous lincRNA-p21 exacerbates neointima formation in injured carotid arteries in the carotid artery injury model [60]. This finding is highly relevant because it implicates lncRNAs to CVDs and indicates that lincRNA-p21 may be a novel therapeutic approach to treat human atherosclerosis and related CVDs [60].
TGFβ belongs to a large superfamily of related polypeptides and is involved in diverse biological processes, such as cell proliferation, migration, differentiation, survival, and cell-cell and cell-matrix interaction [1]. TGFβ plays a crucial role in the development of the cardiovascular system, affecting functions of both endothelial and periendothelial cells [61]. TGFβ-associated signaling is a key player in metazoan biology, and its dysregulation can result in either developmental defects or other pathologies like tumor development [15]. Consequently, the output of a TGFβ-response is known to be highly context-dependent in development, across different tissues, as well as in cancer syndromes [15]. Dysregulated TGFβ-associated signaling is linked to human hereditary hemorrhagic telangiectasia (HHT) type II [62] and HHT type I [63]. HHT patients present with dilated blood vessels with thin walls and exhibit abnormal arteriovenous fusion and shunting. Studies have revealed that the dysregulation of the TGFβ signaling pathway results in severe vascular abnormalities in mice models of vasculogenesis [7–12]. The TGFβ-pathway is also responsible for the endothelial to mesenchymal transition (EndMT), a process by which endothelial cells acquire mesenchymal gene signatures to become more motile and invasive [18, 64]. EndMT plays an important role in the developmental process, as well as in the development of organ fibrosis [18, 64]. TGFβ signaling is thus essential for vascular development and maturation, but the mechanisms of transcriptional regulation of this signaling have not been clearly defined.
To determine targets of TGFβ in endothelial cells, we performed lncRNA and mRNA microarray analysis on total RNA isolated from TGFβ-stimulated HUVECs. This approach allowed us to identify novel target genes of TGFβ and provided insights into the regulation of different lncRNAs and mRNAs by TGFβ in endothelial cells. Of the 30,584 lncRNAs screened, 2051 were significantly upregulated and 2393 were appreciably downregulated (P < 0.05) in response to TGFβ1. In the same HUVEC samples, 2148 of the 26,106 mRNAs screened were upregulated and 1290 were downregulated. Interestingly, of the 2051 differentially expressed upregulated lncRNAs, MALAT1, which is highly expressed in endothelial cells [29] and is an important mediator of TGFβ signaling [28], was the most (~220-fold) upregulated lncRNA after TGFβ-stimulation in endothelial cells (Figure 3). The protein-coding genes LTBP3, KCNK7 and TGD3, which are adjacent to MALAT1 on chromosome 15 [27], were also significantly upregulated in our mRNA array data (Figure 3). Our data shows that 9 of the 20 lncRNAs demonstrated a direct correlation in fold-change with its associated mRNA, whereas the remaining 11 displayed an inverse correlation, which was mainly observed for the downregulated (9 out of 10) lncRNAs (Figure 3).
Pathway analysis revealed that lncRNAs upregulated in response to TGFβ1 treatment are involved in hippo signaling, Wnt signaling, focal adhesion, neuroactive ligand-receptor interaction, and pathways specific to cancer (Table 2). The most downregulated lncRNAs are notably involved in olfactory transduction, PI3-Akt signaling, Ras signaling, neuroactive ligand-receptor interaction, and apoptosis (Table 3). The proposed common pathophysiological basis between cancer and CVDs [65–68] is strengthened by the role of lncRNAs such as MALAT1 [29, 44], p21 [49, 60], ANRIL [38, 49, 60], and HOTAIR [39, 54] in the development of cancer as well as in CVDs. Accordingly, differentially expressed lncRNA MALAT1 and pathway analysis of our data also demonstrate the common pathways indicating similar pathophysiological basis between cancer and CVDs (Table 2). Results of bioinformatics GO analysis, as described in Table 4, grouped the differentially expressed mRNAs under the following three categories: biological processes, cellular component, and molecular function. GO terms most broadly associated with upregulated mRNAs were biological function, protein binding, and signalling (Table 4). GO terms associated with downregulated mRNA were mainly enriched in cell, response to stimulus, and multicellular organism process (Table 4). This is the first lncRNA and mRNA transcriptome profile of TGFβ-mediated changes in human endothelial cells. These observations may reveal some new targets of TGFβ in endothelial cells and CVD-associated endothelial dysfunction. Further investigations of novel genes identified by this study will provide new clues concerning the mechanisms of vascular development by TGFβ and contribute to therapeutic approaches to vascular diseases as well as treating cancer.
Interest in the contribution of LncRNAs to human health and disease is booming, but much effort is required to determine the full contribution and the mechanisms by which lncRNAs exert their effects. Efforts such as the Encyclopedia of DNA Elements (ENCODE) project aiming to identify all functional elements in the human genome are making major progress [69]; methods based on second-generation RNA sequencing are expected to provide a more detailed picture of the whole human lncRNA transcriptome. The lack of a complete understanding of functional motifs, low expression levels of some lncRNAs, and the need for a better definition of lncRNAs regulatory regions make the characterization of lncRNA challenging. One of the most important challenges is to identify all encoded functional lncRNAs, and emerging genomic, epigenomic, and bioinformatics approaches will be crucial in this context. However, the restricted spatiotemporal expression of many lncRNAs, as well as the binding of transcription factors to noncoding loci, could be used as evidence of functionality. The poor conservation and the fact that most lncRNAs are expressed as various transcript variants challenges the identification of specific biological functions and mechanisms of action. Often, identification of lncRNA sequences from published studies is not trivial and chromosomal localization is not provided. To avoid confusion and to facilitate the use and reproduction of the data, more details should be provided (e.g., chromosomal localization and deposition of the identified transcript into publicly available databases), which we have implemented in our data presentation. Furthermore, the mechanism of action has only been identified for a few lncRNAs.
Despite these challenges, in a short period, lncRNAs have become a major new class of transcripts that potentially comprise a major component of the genome's information content in comparison to the abundance and complexity to the proteome. LncRNAs have already been reported in a wide range of human diseases suggesting their crucial activity in human health and disease [33]. In addition, therapeutic strategies that target endogenous mRNA molecules could also be adapted to target lncRNAs, whose expression is dysregulated in human CVDs. These observations suggest that lncRNAs represent a novel and versatile class of molecules that are centrally important to the modulation of different CVD conditions and could potentially be utilized for developing novel diagnostic and therapeutic approaches to cure CVDs. With respect to the predictive value of the measured lncRNAs in human diseases, the increased MALAT1 expression levels in ischemic patients and the initial levels of ANRIL and KCNQ1OT1 in peripheral blood mononuclear cells in patients with left ventricular dysfunction at 4-month follow-up [70] suggest that lncRNAs might also be useful as indicators for CVDs. These important developments are expected in this area and exciting times lie ahead of us.
Supplementary Material
Quality Assessment of RNA Samples and Data Analysis. RNA integrity and genomic DNA contamination were evaluated by denaturing agarose gel electrophoresis. In all of the samples, the intensity of the upper 28S ribosomal RNA band was about twice that of the lower 18S band thereby confirming the integrity of the RNA studied. The absence of smears above the 28S band attests to the purity of the RNA samples. RNA quantity and purity were also assessed with the NanoDrop ND-1000. The combination of optical density (OD) A260/A280 ratios that were close to 2.0 and A260/A230 ratios that exceeded 1.8 further confirmed the purity of the RNA used. The Box Plot is a well accepted means to quickly visualize the distribution of a dataset. Our Box plots (10th, 90th percentile) showed comparable distributions of expression values after normalization. Hierarchical clustering is a widely used method for analysis of gene expression. Cluster analysis groups samples based on their expression levels with dendrograms summarize the arrangement of said clusters.
The supplementary tables report the fold change, P value, false discovery rate, annotations, and raw and normalized intensities for differentially expressed and up-/down-regulated lncRNAs and mRNAs in TGFβ1-treated HUVECs relative to control HUVECs.
Acknowledgments
This work was supported in part by grants from the Canadian Institutes of Health Research and Heart and Stroke Foundation of Canada to S. Verma.
Disclosure
S. Verma is the Canada Research Chair in Atherosclerosis at the University of Toronto.
Competing Interests
The authors declare no competing interests.
References
- 1.Heldin C.-H., Miyazono K., ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390(6659):465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 2.Roberts A. B., Sporn M. B. Physiological actions and clinical applications of transforming growth factor-β (TGF-β) Growth Factors. 1993;8(1):1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- 3.Ten Dijke P., Hill C. S. New insights into TGF-β-Smad signalling. Trends in Biochemical Sciences. 2004;29(5):265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Derynck R., Zhang Y. E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 5.Schmierer B., Hill C. S. TGFβ-SMAD signal transduction: molecular specificity and functional flexibility. Nature Reviews Molecular Cell Biology. 2007;8(12):970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 6.Nakao A., Imamura T., Souchelnytskyi S., et al. TGF-β receptor-mediated signalling through Smad2, Smad3 and Smad4. The EMBO Journal. 1997;16(17):5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickson M. C., Martin J. S., Cousins F. M., Kulkarni A. B., Karlsson S., Akhurst R. J. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121(6):1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 8.Oshima M., Oshima H., Taketo M. M. TGF-β receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Developmental Biology. 1996;179(1):297–302. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- 9.Larsson J., Goumans M.-J., Sjöstrand L. J., et al. Abnormal angiogenesis but intact hematopoietic potential in TGF-β type I receptor-deficient mice. The EMBO Journal. 2001;20(7):1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li D. Y., Sorensen L. K., Brooke B. S., et al. Defective angiogenesis in mice lacking endoglin. Science. 1999;284(5419):1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 11.Oh S. P., Seki T., Goss K. A., et al. Activin receptor-like kinase 1 modulates transforming growth factor-β1 signaling in the regulation of angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(6):2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang H., Huylebroeck D., Verschueren K., Guo Q., Matzuk M. M., Zwijsen A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development. 1999;126(8):1631–1642. doi: 10.1242/dev.126.8.1631. [DOI] [PubMed] [Google Scholar]
- 13.Pepper M. S. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine and Growth Factor Reviews. 1997;8(1):21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- 14.Goumans M.-J., Lebrin F., Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-β receptor signaling pathways. Trends in Cardiovascular Medicine. 2003;13(7):301–307. doi: 10.1016/s1050-1738(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 15.Massagué J., Blain S. W., Lo R. S. TGFβ signaling in growth control, cancer, and heritable disorders. Cell. 2000;103(2):295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 16.Blobe G. C., Schiemann W. P., Lodish H. F. Role of transforming growth factor β in human disease. The New England Journal of Medicine. 2000;342(18):1350–1358. doi: 10.1056/nejm200005043421807. [DOI] [PubMed] [Google Scholar]
- 17.Roberts A. B., Wakefield L. M. The two faces of transforming growth factor β in carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(15):8621–8623. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh K. K., Lovren F., Pan Y., et al. The essential autophagy gene ATG7 modulates organ fibrosis via regulation of endothelial-to-mesenchymal transition. The Journal of Biological Chemistry. 2015;290(5):2547–2559. doi: 10.1074/jbc.m114.604603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batista P. J., Chang H. Y. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wapinski O., Chang H. Y. Long noncoding RNAs and human disease. Trends in Cell Biology. 2011;21(6):354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Rinn J. L., Kertesz M., Wang J. K., et al. Functional demarcation of active and silent chromatin domains in human HOX Loci by noncoding RNAs. Cell. 2007;129(7):1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J., Sun B. K., Erwin J. A., Song J.-J., Lee J. T. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322(5902):750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guenther M. G., Levine S. S., Boyer L. A., Jaenisch R., Young R. A. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130(1):77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beltran M., Puig I., Peña C., et al. A natural antisense transcript regulates zeb2/sip1 gene expression during snail1-induced epithelial-mesenchymal transition. Genes and Development. 2008;22(6):756–769. doi: 10.1101/gad.455708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiedler J., Breckwoldt K., Remmele C. W., et al. Development of long noncoding RNA-based strategies to modulate tissue vascularization. Journal of the American College of Cardiology. 2015;66(18):2005–2015. doi: 10.1016/j.jacc.2015.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nature Reviews Drug Discovery. 2013;12(6):433–446. doi: 10.1038/nrd4018. [DOI] [PubMed] [Google Scholar]
- 27.Zhang B., Arun G., Mao Y. S., et al. The lncRNA malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. Cell Reports. 2012;2(1):111–123. doi: 10.1016/j.celrep.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan Y., Shen B., Tan M., et al. TGF-β-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clinical Cancer Research. 2014;20(6):1531–1541. doi: 10.1158/1078-0432.ccr-13-1455. [DOI] [PubMed] [Google Scholar]
- 29.Michalik K. M., You X., Manavski Y., et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circulation Research. 2014;114(9):1389–1397. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 30.Djebali S., Davis C. A., Merkel A., et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapranov P., Cheng J., Dike S., et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316(5830):1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 32.Roberts T. C., Morris K. V., Weinberg M. S. Perspectives on the mechanism of transcriptional regulation by long non-coding RNAs. Epigenetics. 2014;9(1):13–20. doi: 10.4161/epi.26700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esteller M. Non-coding RNAs in human disease. Nature Reviews Genetics. 2011;12(12):861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 34.Tsai M.-C., Spitale R. C., Chang H. Y. Long intergenic noncoding RNAs: new links in cancer progression. Cancer Research. 2011;71(1):3–7. doi: 10.1158/0008-5472.can-10-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hung T., Wang Y., Lin M. F., et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nature Genetics. 2011;43(7):621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huarte M., Guttman M., Feldser D., et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142(3):409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prensner J. R., Chinnaiyan A. M. The emergence of lncRNAs in cancer biology. Cancer Discovery. 2011;1(5):391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotake Y., Nakagawa T., Kitagawa K., et al. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15 INK4B tumor suppressor gene. Oncogene. 2011;30(16):1956–1962. doi: 10.1038/onc.2010.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim K.-J., Moon S.-M., Kim S.-A., Kang K.-W., Yoon J.-H., Ahn S.-G. Transcriptional regulation of MDR-1 by HOXC6 in multidrug-resistant cells. Oncogene. 2013;32(28):3339–3349. doi: 10.1038/onc.2012.354. [DOI] [PubMed] [Google Scholar]
- 40.Kogo R., Shimamura T., Mimori K., et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Research. 2011;71(20):6320–6326. doi: 10.1158/0008-5472.can-11-1021. [DOI] [PubMed] [Google Scholar]
- 41.Yang X.-J., Huang C.-Q., Suo T., et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Annals of Surgical Oncology. 2011;18(6):1575–1581. doi: 10.1245/s10434-011-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niinuma T., Suzuki H., Nojima M., et al. Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Research. 2012;72(5):1126–1136. doi: 10.1158/0008-5472.CAN-11-1803. [DOI] [PubMed] [Google Scholar]
- 43.Gupta R. A., Shah N., Wang K. C., et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshimoto R., Mayeda A., Yoshida M., Nakagawa S. MALAT1 long non-coding RNA in cancer. Biochimica et Biophysica Acta. 2016;1859(1):192–199. doi: 10.1016/j.bbagrm.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Tano K., Mizuno R., Okada T., et al. MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. FEBS Letters. 2010;584(22):4575–4580. doi: 10.1016/j.febslet.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Fatica A., Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nature Reviews Genetics. 2014;15(1):7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 47.Samani N. J., Erdmann J., Hall A. S., et al. Genomewide association analysis of coronary artery disease. The New England Journal of Medicine. 2007;357(5):443–453. doi: 10.1056/nejmoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McPherson R., Pertsemlidis A., Kavaslar N., et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316(5830):1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holdt L. M., Beutner F., Scholz M., et al. ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(3):620–627. doi: 10.1161/ATVBAHA.109.196832. [DOI] [PubMed] [Google Scholar]
- 50.Congrains A., Kamide K., Oguro R., et al. Genetic variants at the 9p21 locus contribute to atherosclerosis through modulation of ANRIL and CDKN2A/B. Atherosclerosis. 2012;220(2):449–455. doi: 10.1016/j.atherosclerosis.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 51.Tsai P.-C., Liao Y.-C., Lin T.-H., Hsi E., Yang Y.-H., Juo S.-H. H. Additive effect of ANRIL and BRAP polymorphisms on ankle-brachial index in a Taiwanese population. Circulation Journal. 2012;76(2):446–452. doi: 10.1253/circj.CJ-11-0925. [DOI] [PubMed] [Google Scholar]
- 52.Tripathi V., Shen Z., Chakraborty A., et al. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genetics. 2013;9(3) doi: 10.1371/journal.pgen.1003368.e1003368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bell R. D., Long X., Lin M., et al. Identification and initial functional characterization of a human vascular cell-enriched long noncoding RNA. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(6):1249–1259. doi: 10.1161/atvbaha.114.303240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carrion K., Dyo J., Patel V., et al. The long non-coding HOTAIR is modulated by cyclic stretch and WNT/beta-CATENIN in human aortic valve cells and is a novel repressor of calcification genes. PLoS ONE. 2014;9(5) doi: 10.1371/journal.pone.0096577.e96577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grote P., Wittler L., Hendrix D., et al. The tissue-specific lncrna fendrr is an essential regulator of heart and body wall development in the mouse. Developmental Cell. 2013;24(2):206–214. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishii N., Ozaki K., Sato H., et al. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. Journal of Human Genetics. 2006;51(12):1087–1099. doi: 10.1007/s10038-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 57.Broadbent H. M., Peden J. F., Lorkowski S., et al. Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Human Molecular Genetics. 2008;17(6):806–814. doi: 10.1093/hmg/ddm352. [DOI] [PubMed] [Google Scholar]
- 58.Cabianca D. S., Casa V., Bodega B., et al. A long ncRNA links copy number variation to a polycomb/trithorax epigenetic switch in fshd muscular dystrophy. Cell. 2012;149(4):819–831. doi: 10.1016/j.cell.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Dijk M., Thulluru H. K., Mulders J., et al. HELLP babies link a novel lincRNA to the trophoblast cell cycle. The Journal of Clinical Investigation. 2012;122(11):4003–4011. doi: 10.1172/jci65171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu G., Cai J., Han Y., et al. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 2014;130(17):1452–1465. doi: 10.1161/CIRCULATIONAHA.114.011675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nature Medicine. 2000;6(4):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 62.Johnson D. W., Berg J. N., Baldwin M. A., et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type. Nature Genetics. 1996;13(2):189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 63.McAllister K. A., Grogg K. M., Johnson D. W., et al. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nature Genetics. 1994;8(4):345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 64.Zeisberg E. M., Tarnavski O., Zeisberg M., et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nature Medicine. 2007;13(8):952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 65.Shukla P. C., Singh K. K., Yanagawa B., Teoh H., Verma S. DNA damage repair and cardiovascular diseases. Canadian Journal of Cardiology. 2010;26:13A–16A. doi: 10.1016/s0828-282x(10)71055-2. [DOI] [PubMed] [Google Scholar]
- 66.Shukla P. C., Singh K. K., Quan A., et al. BRCA1 is an essential regulator of heart function and survival following myocardial infarction. Nature Communications. 2011;2, article 593 doi: 10.1038/ncomms1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh K. K., Shukla P. C., Quan A., et al. BRCA1 is a novel target to improve endothelial dysfunction and retard atherosclerosis. Journal of Thoracic and Cardiovascular Surgery. 2013;146(4):949–960.e4. doi: 10.1016/j.jtcvs.2012.12.064. [DOI] [PubMed] [Google Scholar]
- 68.Singh K. K., Shukla P. C., Quan A., et al. BRCA2 protein deficiency exaggerates doxorubicin-induced cardiomyocyte apoptosis and cardiac failure. The Journal of Biological Chemistry. 2012;287(9):6604–6614. doi: 10.1074/jbc.m111.292664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Consortium E. P., Birney E., Stamatoyannopoulos J. A., et al. Identification and analysis of functional elements in 1% of the human genome by the encode pilot project. Nature. 2007;447(7146):799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vausort M., Wagner D. R., Devaux Y. Long noncoding RNAs in patients with acute myocardial infarction. Circulation Research. 2014;115(7):668–677. doi: 10.1161/CIRCRESAHA.115.303836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality Assessment of RNA Samples and Data Analysis. RNA integrity and genomic DNA contamination were evaluated by denaturing agarose gel electrophoresis. In all of the samples, the intensity of the upper 28S ribosomal RNA band was about twice that of the lower 18S band thereby confirming the integrity of the RNA studied. The absence of smears above the 28S band attests to the purity of the RNA samples. RNA quantity and purity were also assessed with the NanoDrop ND-1000. The combination of optical density (OD) A260/A280 ratios that were close to 2.0 and A260/A230 ratios that exceeded 1.8 further confirmed the purity of the RNA used. The Box Plot is a well accepted means to quickly visualize the distribution of a dataset. Our Box plots (10th, 90th percentile) showed comparable distributions of expression values after normalization. Hierarchical clustering is a widely used method for analysis of gene expression. Cluster analysis groups samples based on their expression levels with dendrograms summarize the arrangement of said clusters.
The supplementary tables report the fold change, P value, false discovery rate, annotations, and raw and normalized intensities for differentially expressed and up-/down-regulated lncRNAs and mRNAs in TGFβ1-treated HUVECs relative to control HUVECs.