Abstract
lntravitreal injection of substances dissolved in a vehicle solution is a common tool used to assess retinal function. We examined the effect of injection procedures (three groups) and vehicle solutions (four groups) on the development of form deprivation myopia (FDM) in juvenile tree shrews, mammals closely related to primates, starting at 24 days of visual experience (about 45 days of age). In seven groups (n = 7 per group), the myopia produced by monocular form deprivation (FD) was measured daily for 12 days during an 11-day treatment period. The FD eye was randomly selected; the contralateral eye served as an untreated control. The refractive state of both eyes was measured daily, starting just before FD began (day 1); axial component dimensions were measured on day 1 and after eleven days of treatment (day 12). Procedure groups: the myopia (treated eye refraction – control eye) in the FD group was the reference. The sham group only underwent brief daily anesthesia and opening of the conjunctiva to expose the sclera. The puncture group, in addition, had a pipette inserted daily into the vitreous. In four vehicle groups, 5 μL of vehicle was injected daily. The NaCl group received 0.85% NaCl. In the NaCl+ascorbic acid group, 1mg/mL of ascorbic acid was added. The water group received sterile water. The water+ascorbic acid group received water with ascorbic acid (1mg/mL). We found that the procedures associated with intravitreal injections (anesthesia, opening of the conjunctiva, and puncture of the sclera) did not significantly affect the development of FDM. However, injecting 5 μL of any of the four vehicle solutions slowed the development of FDM. NaCl had a small effect; myopia development in the last 6 days (−0.15 ± 0.08 D/day) was significantly less than in the FD group (−0.53 ± 0.06 D/day). NaCl+ Ascorbic acid further slowed the development of FDM on several treatment days. H2O (−0.09 ± 0.05 D/day) and H2O+ascorbic acid (−0.08 ± 0.05 D/day) both almost completely blocked myopia development. The treated eye vitreous chamber elongation, compared with the control eye, in all groups was consistent with the amount of myopia. When FD continued (days 12–16) without injections in the water and water+ascorbic acid groups, the rate of myopia development quickly increased. Thus, it appears the vehicles affected retinal signaling rather than causing damage. The effect of H2O and H2O+ascorbic acid may be due to reduced osmolality or ionic concentration near the tip of the injection pipette. The effect of ascorbic acid, compared to NaCl alone, may be due to its reported dopaminergic activity.
Keywords: emmetropization, myopia, animal models, vitreous, axial elongation, retinal signaling
Graphical Abstract
1. INTRODUCTION
Intravitreal injection, in which a substance, dissolved in a vehicle solution, is placed into the vitreous chamber, is a frequently used tool in both clinical and basic research studies (Avery, Pieramici, Rabena, Castellarin, Nasir and Giust, 2006; Brown, Kaiser, Michels, Soubrane, Heier, Kim, Sy and Schneider, 2006; Feldkaemper, Neacsu and Schaeffel, 2009; Ganesan and Wildsoet, 2010; Haritoglou, Kook, Neubauer, Wolf, Priglinger, Strauss, Gandorfer, Ulbig and Kampik, 2006; Iturralde, Spaide, Meyerle, Klancnik, Yannuzzi, Fisher, Sorenson, Slakter, Freund, Cooney and Fine, 2006; Norton, Essinger and McBrien, 1994; Pickett-Seltner and Stell, 1995; Rohrer, Iuvone and Stell, 1995; Rohrer, Spira and Stell, 1993; Stone, Lin, Laties and Iuvone, 1989; Zhu and Wallman, 2009). This approach is often used to deliver neurotransmitter agonists and antagonists to the vicinity of the retina so as to observe their impact on retinal function. From the vitreous, these substances, typically small molecules, are presumably moved by diffusion across the inner limiting membrane into the retina (Araie and Maurice, 1991; Park, Bungay, Lutz, Augsburger, Millard, Sinha and Banerjee, 2005) where they come into contact with the target receptors.
In isolated retinal preparations, known concentrations of neurotransmitter analogs can be maintained in the fluid bath and at the retinal surface. However, connections to central brain structures are disrupted in these preparations and visual behaviors cannot occur. When intravitreal injections into intact eyes are used, there is less control over the precise initial concentration and dissipation over time of the injected substances. Nonetheless, intravitreal injection is a useful approach because administration is simple and because the substances that disperse in the vitreous are localized near the retina and are carried through it. In addition, except for anesthesia during the intravitreal injection, animals can be awake with potentially normal retinal signaling and visual behaviors. Alternative approaches, such as sub-conjunctival or peri-bulbar injections are more indirect than intravitreal administration; the substances must pass through the sclera, choroid, and retinal pigment epithelium to reach the retina. In addition, an unknown proportion of the administered substance remains outside the eye and diffuses away, making it more difficult to know the dose that actually reaches the retina; these substances may also affect the choroid, the sclera, and/or extraocular structures.
The choice of the vehicle solution into which the substances of interest are dissolved is a potentially important factor because the vehicle itself might affect retinal signaling. Previous studies (when the vehicle has been specified) have used a variety of solutions, including normal saline, phosphate buffered saline (PBS), and water. Sometimes ascorbic acid (typically 1mg/mL) has been added to these vehicles as an anti-oxidant (Rohrer, Spira and Stell, 1993; Schaeffel, Bartmann, Hagel and Zrenner, 1995; Schaeffel, Hagel, Bartmann, Kohler and Zrenner, 1994; Schmid and Wildsoet, 2004). The vehicle solution used has been reported to affect the response to form deprivation in some (Rohrer, Spira and Stell, 1993; Schmid, Strasberg, Rayner and Hartfield, 2013), but not all cases (F. Schaeffel, personal communication, 2013). We examined two vehicles, 0.85% NaCl and water, both alone and supplemented with 1 mg/mL ascorbic acid. We avoided phosphate buffered saline (PBS) because some substances we planned to investigate in subsequent studies would not dissolve at the needed concentrations in this vehicle.
The impact of these vehicle solutions was assessed in tree shrews, a well-established model of myopia development that, like most mammals, lack the inner cartilaginous scleral layer present in chicks and many other vertebrates. The dependent variables in the present study were the amount of monocular form-deprivation myopia (FDM) and the rate of myopia development (slope in diopters [D]/day) that occurred over an 11-day treatment period, compared with the untreated fellow eye. When a translucent diffuser is held in front of an eye early in postnatal development, the retina detects the form deprivation (FD) and generates what have been described as “GO” signals (He, Frost, Siegwart, Jr. and Norton, 2014; Norton, 1999; Rohrer and Stell, 1994; Schaeffel and Howland, 1991). Retinally-generated signals are not only sent to central visual targets, but also pass through a direct retino-scleral pathway comprised of the retinal pigment epithelium (RPE) and choroid to reach the sclera where they produce scleral remodeling (Gao, Frost, Siegwart, Jr. and Norton, 2011; Guo, Frost, Siegwart, Jr. and Norton, 2014; McBrien, Cornell and Gentle, 2001; Moring, Baker and Norton, 2007; Norton, Essinger and McBrien, 1994; Norton and Rada, 1995; Siegwart, Jr. and Norton, 1999) that results in ocular (vitreous chamber) elongation (Marsh-Tootle and Norton, 1989). As measured in choroid and sclera, nearly identical GO signals are produced by FD and another myopiagenic stimulus, a negative-power lens, and these GO signals are distinct from the STOP signals that occur during recovery from induced myopia (Guo, Frost, Siegwart, Jr. and Norton, 2014; He, Frost, Siegwart, Jr. and Norton, 2014). Thus, the GO signals produced by FD are not merely the absence of STOP signals. As FD continues, the elongation of the deprived eye moves the retina behind the focal plane so that the eye becomes myopic (McFadden, Howlett and Mertz, 2004; Shen and Sivak, 2007; Sherman, Norton and Casagrande, 1977; Wallman, Turkel and Trachtman, 1978; Wiesel and Raviola, 1977). In the present study, FD was selected rather than negative-lens wear because the myopia produced by a negative lens is limited by the dioptric power of the lens. With FD there is no limitation since it is an open loop condition; eyes continue to elongate for many days as long as the diffuser remains in place (McBrien and Norton, 1992; Smith, III, Bradley, Fernandes and Boothe, 1999; Troilo and Judge, 1993; Wallman and Adams, 1987).
The development of both FDM and lens-induced myopia (LIM) is dependent on the presence of nearly continuous FD or lens wear. If FD or lens wear is discontinued for short daily periods (30 minutes to 1 hour), the amount of myopia that develops is greatly reduced (Kee, Hung, Qiao-Grider, Ramamirtham, Winawer, Wallman and Smith, III, 2007; Napper, Brennan, Barrington, Squires, Vessey and Vingrys, 1995; Schmid and Wildsoet, 1996; Shaikh, Siegwart and Norton, 1999). Thus, FDM is a sensitive system in which to evaluate the possible impact of injected vehicles.
Ideally, the injection procedures (daily anesthesia and scleral puncture) also should not affect retinal signaling so the development of FDM should be unaffected. However, repeated anesthesia and re-opening of a scleral puncture potentially could affect myopia development by reducing intraocular pressure (IOP), by producing tissue responses, or by interrupting retinal GO signaling. To assess this issue, two relevant procedure groups were assessed in addition to four groups that received vehicle solutions.
2. MATERIALS AND METHODS
2.1 Subjects and experimental groups
As in previous studies from this laboratory (Guo, Frost, He, Siegwart, Jr. and Norton, 2013; He, Frost, Siegwart, Jr. and Norton, 2014; McBrien and Norton, 1992), juvenile tree shrews (Tupaia glis belangeri) were raised in our breeding colony by their mothers on a 14 h light/10 h dark cycle. Tree shrews are small mammals (dichromats) that are closely related to primates with excellent vision for their size (2–4 cyc/deg) (Norton, Wu and Siegwart, Jr., 2003; Petry, Fox and Casagrande, 1984). All procedures complied with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. The first day both eyes are open, which occurs about three weeks after birth, is considered to be the first day of visual experience. Experimental groups were balanced to include both males and females and avoided multiple pups from the same parents. Which eye was treated with FD (and injections) was balanced between left and right eyes in each group.
The seven groups in this study (n=7 per group) are summarized in Table 1. Animals in all groups received monocular FD for 11 days with a translucent diffuser held in a goggle frame. The groups are divided into two classes: three procedure groups and four vehicle groups. Procedure groups included the FD, sham, and puncture groups. The FD group was our reference group; these animals received only monocular form deprivation with daily measures of refractive state. Sham group: on day 1 of treatment, after pre-treatment refractive and axial component measures were made, the animals were anesthetized with 5% isoflurane. After instilling a drop of topical anesthetic in the to-be treated eye, an opening was made in the temporal conjunctiva that was re-opened on subsequent days. After application of a drop of artificial tears to both eyes, the goggle containing the diffuser was attached; the animal was weighed and allowed to recover from anesthesia in a nesting box in the lab for at least 5–10 min before it was returned to its cage in the animal colony. This procedure, including brief (approximately 5 minutes) removal of the diffuser, was repeated for 10 more days. The removal of the diffuser mimicked the amount of time taken to perform the intravitreal injections. The final refractive and axial measures were made on day 12, which was 24 hours after the 11th treatment.
Table 1.
Experimental groups.
| Procedure | Vehicle | ||||||
|---|---|---|---|---|---|---|---|
| Treatment: | FD | Sham | Puncture | NaCl | NaCl+ Ascorbic Acid | Water | Water+ Ascorbic Acid |
| Form deprivation | X | X | X | X | X | X | X |
| Daily anesthesia, ~5 min diffuser removal, conjunctival opening | X | X | X | X | X | X | |
| Scleral puncture, daily pipette insertion | X | X | X | X | X | ||
| 5μL 0.85% NaCl | X | ||||||
| 5μL 1mg/mL ascorbic acid in 0.85% NaCl | X | ||||||
| 5μL water | X | ||||||
| 5μL 1mg/mL ascorbic acid in water | X | ||||||
The puncture group underwent the same procedures as the sham group but, in addition, the sclera was punctured temporally at the ora serrata, under the conjunctival opening, with a sterile 33-gauge needle on day 1. A sterile glass pipette, pulled to a diameter smaller than the scleral opening and filled with sterile water was inserted to a depth of approximately 3–4 mm, taking care to keep the tip behind the lens and in front of the retina. The pipette was held in place for approximately 20 seconds and then removed (no fluid was ejected into the vitreous). The scleral opening was located on days 2–11 and a fresh pipette was inserted as before.
The four vehicle groups underwent the same procedures as the puncture group and, in addition, received a daily 5 μL intravitreal injection of the solution listed in Table 1. Sterile water was obtained from an Ultrapure system with a 0.22μm final filter (Millipore Corporation, Billerica, MA). NaCl (0.85%) and ascorbic acid (1mg/mL; L-Ascorbic acid, Fisher Scientific, Pittsburgh, PA) solutions were both prepared using this sterile water. The vehicle solutions were made in advance, divided into 50μL aliquots and stored at −20°C until use. Delivery was controlled by a 25 μL Hamilton micro syringe (Hamilton Company, Reno, NV) connected to the pipette through PE-90 tubing.
2.2 Form Deprivation
To produce form-deprivation myopia, all animals wore a goggle frame that contained a monocular translucent diffuser that covered the treated eye (Norton and Rada, 1995). The other (control) eye had unobstructed vision through an open goggle frame. The goggle was clipped to a dental acrylic pedestal that was attached to the skull at 21 ± 1 days of visual experience (Siegwart and Norton, 1994). For pedestal installation, each animal was anesthetized with ketamine (100 mg/kg) with xylazine (7.5 mg/kg) supplemented with 0.5 – 2.0% isoflurane as needed. After pedestal installation and recovery from anesthesia, the animals were placed in individual cages with fluorescent lighting (GE, F34CW-RS-WM-ECO lamps), 100 – 300 lux measured on the floor of the cage. Three days later, the goggle frame holding the monocular diffuser was clipped to the pedestal, firmly holding the diffuser in front of the treated eye. Goggles were removed daily for cleaning. The eyes were examined periodically with an indirect ophthalmoscope for signs of inflammation and lenticular or retinal damage.
2.3 Refractive and Axial Measures
Immediately before the start of form deprivation (day 1) and every morning for the next 11 days, awake, non-cycloplegic refractive measures were taken on the animals with a Nidek ARK-700A infrared auto-refractor (Marco Ophthalmic, Jacksonville, FL)(Norton, Wu and Siegwart, Jr., 2003). Since atropine may interfere with the development of FDM, cycloplegic refractive measures were not performed (McKanna and Casagrande, 1981). However, previous studies have shown that non-cycloplegic measures provide a valid estimate of the refractive state, and of induced myopia, in tree shrews (Norton, Siegwart, Jr. and Amedo, 2006; Norton, Siegwart, German, Robertson and Wu, 2000). All refractive values were measured at the corneal plane and were corrected for the small eye artifact (Glickstein and Millodot, 1970) previously shown to be approximately +4 D in tree shrews (Norton, Wu and Siegwart, Jr., 2003). The final refractive measure, on day 12, occurred 24 hours after the final anesthesia and injection.
On day 1 and day 12, a Lenstar LS-900 optical biometer (Haag-Streit USA, Mason, OH) was used to measure corneal thickness, anterior chamber depth, lens thickness, vitreous chamber depth, retinal thickness, and choroidal thickness while the animals were awake. Because the Lenstar software does not provide a direct measure of the vitreous chamber, this measure was obtained during waveform analysis using the retinal cursors (international software version). The anterior retinal cursor was placed on the peak that denoted the posterior surface of the crystalline lens while the posterior retinal cursor was located at the front of the retina. The same cursors were relocated to measure the retinal and choroidal thicknesses. At the start of form deprivation, none of the ocular component dimensions differed significantly across groups (1-way analysis of variance, P > 0.05).
2.4 Post-injection Measures
To assess if the vehicle solutions might have interfered with the development of FDM because they damaged the retina (in which case FDM would continue to be retarded), or disrupted retinal GO signaling (in which case the rate of FDM development should increase), form deprivation was continued in some animals for at least 4 days beyond day 12 (along with daily refractive measures). This additional FD occurred in animals from the NaCl (n=1), NaCl+ascorbic acid (n=2), water (n=6), and water+ascorbic acid (n=5) groups.
We also recorded binocular photopic electroretinograms (ERGs) from animals in the FD (n=1), sham (n=1), puncture (n=1), NaCl (n=1), and water+ascorbic acid (n=2) groups with a KC UTAS-3000 Diagnostic System (Gaithersburg, MD). The photopic ERGs were elicited with 10-msec flashes of “white” light with a 1-min delay at intensities of: 0 dB (2.5 cd*s/ m2), 5 dB (7.91 cd*s/m2), 10 dB (25 cd*s/m2), and 15 dB (79.1 cd*s/m2) with a “white” background (2.5 cd/ m2). Scans produced values that were averaged from 15 total scans at each light intensity. These measures were made on day 12 after the final refractive and axial component measures had been taken. For animals in the vehicle groups, an additional (12th) vehicle injection was then made under anesthesia as on previous days. After the animals awakened, the pupils were dilated with 1% atropine sulfate ophthalmic solution (Bausch & Lomb, Tampa, FL). The animals were then transported to the nearby ERG facility where they were anesthetized with ketamine/xylazine and measured within one hour of the intravitreal injection.
2.5 Data Analysis
Refractive measures from each animal for each day were examined in Excel spreadsheets. To determine the rate at which the groups developed FDM, slope values (D/day of increased myopia) were calculated for each animal by fitting a linear regression to the refractive differences (treated – control eye values). Slopes were analyzed for the entire 11-day period (days 1–12), the first 5 days of FD (days 1–6), the last 7 days (days 6–12), and, for animals in which FD continued after injections stopped, the 4-day post-injection period (days 12–16). The mean squared error of the linear regression fits for all these time periods did not differ significantly across groups (1-way analysis of variance, P > 0.05). One-way analysis of variance with the Fisher LSD post-hoc test (ANOVA; IBM SPSS Statistics 22) was used to assess group differences in the amount of FD, the slope values, and the axial component dimensions; P < 0.05 was considered to be significant. Paired t-tests were performed on the a- and b-wave amplitudes of the injected (treated) and non-injected (control) eyes for each of the 4 light intensities.
3. RESULTS
3.1 Animal Development
One concern related to administering daily intravitreal injections under anesthesia is that the procedures involved and substances injected might, in themselves, affect the overall development of the juvenile animals (24 days of visual experience). We thus examined the weight of all animals when they were anesthetized for the first (day 1) and last time (day 11). The weights did not differ significantly across groups at either time point (ANOVA, P > 0.05).
3.2 Treated-Control Eye Data
The myopia values are reported as the form-deprived treated eye minus the fellow control eye because, normally, the refractions of the two eyes of an individual animal are highly correlated; differences between the eyes can be attributed to the treated eyes (Norton and McBrien, 1992). In addition, the refractions of the control eyes across groups did not differ significantly during the 11 days of treatment, except on treatment day 9 when the water and water+ascorbic acid group control eye refractions were slightly hyperopic compared with the other groups. However, these differences did not importantly affect the treated vs. control eye results. The vitreous chamber depths also are reported as the treated eye - control eye; the control eye values on day 12 did not differ significantly across groups (ANOVA, P > 0.05).
3.3 Procedure Groups
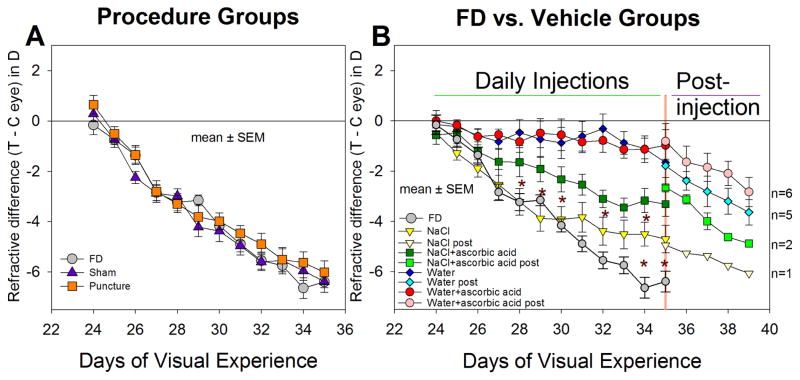
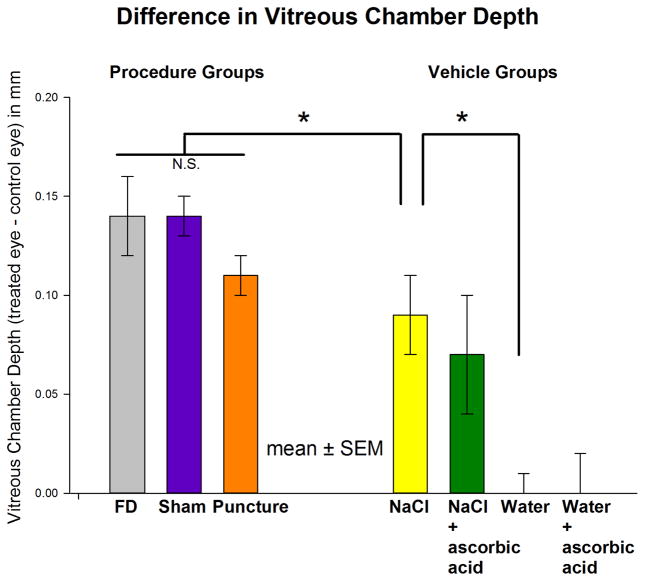
Figure 1A shows the refractions of the three procedure groups. In the FD group, myopia developed rapidly over the first 6 days (mean ± SEM, slope, −0.68 ± 0.05 D/day, Table 2) and slightly less rapidly during the last 6 days (−0.55 ± 0.06 D/day). As is evident in the figure, the myopia development in the sham and the puncture groups was nearly identical to that in the FD group; the amount of myopia in these two groups and the slope of myopia development did not differ from those in the FD group on any day during treatment (ANOVA, P > 0.05). Thus, daily anesthesia, sham surgery, and re-inserting a pipette did not affect the development of FDM. As shown in Figure 2, the vitreous chamber depths in these three groups at the end of treatment also were not significantly different (ANOVA, P > 0.05). Interestingly, across the 21 animals of the FD, sham, and puncture groups, the thickness of the retina (0.178 ± 0.005 mm) and choroid (0.058 ± 0.003 mm) in the FD eyes was significantly thinner (t-test, P < 0.05) than the control eye retina (0.184 ± 0.004 mm) and choroid (0.065 ± 0.003 mm). The corneal thickness, anterior chamber depth, and lens thickness did not differ significantly between treated and control eyes (t-test, P > 0.05).
Figure 1.
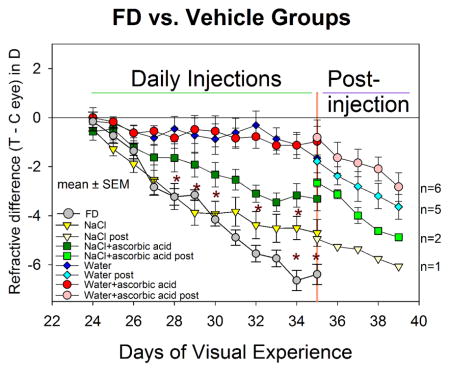
Development of form-deprivation myopia in the (A) procedure groups, and (B) vehicle groups, compared with the FD group. The refractive difference (treated eyes - control eyes) is shown for all groups. Error bars indicate standard error of the mean (SEM). Asterisks indicate days on which there were significant differences in the amount of FDM between the FD and the NaCl groups, and between the NaCl and NaCl+ascorbic acid groups. The water and the water+ascorbic acid groups had significantly less myopia than the NaCl group starting on day 2 and less than the FD group starting on day 4. Starting on day 12 (35 days of visual experience) fewer animals were measured during the post-injection period as indicated by the different symbols. n = number of animals in which myopia development was followed post-injection for a least four days.
Table 2.
Rate (D/day) of myopia development (treated - control eyes) during FD.
| Progression Days | FD | NaCl | NaCl+ Ascorbic Acid | Water | Water+ Ascorbic Acid |
|---|---|---|---|---|---|
| 1–6 | −0.68 ± 0.05 | −0.67 ± 0.13 | −0.30 ± 0.15*† | −0.11 ± 0.15*† | −0.12 ± 0.08*† |
| 6–12 | −0.55 ± 0.06 | −0.15 ± 0.08* | −0.24 ± 0.15* | −0.12 ± 0.04* | −0.10 ± 0.05* |
| 1–12 | −0.60 ± 0.03 | −0.37 ± 0.05* | −0.28 ± 0.07* | −0.09 ± 0.05*† | −0.08 ± 0.05*† |
| 12–16 (n=) |
−0.27 1 |
−0.60 ± 0.01 2 |
−0.39 ± 0.12╪ 6 |
−0.44 ± 0.12╪ 5 |
Values expressed as mean ± SEM D/day.
significantly different from FD;
significantly different from NaCl;
days 12–16 significantly different from days 1–12.
Figure 2.
Vitreous Chamber Differences. The elongation of the vitreous chamber (mean ± SEM) in the treated eyes, compared with their control eyes, measured on day 12 (at the end of 11 days of FD and 24 hours after the last intravitreal injection in the vehicle groups). “N.S” represents differences that were not statistically significant between groups. Asterisks indicate that the vitreous elongation in the NaCl group was significantly less than in the FD group and that the (negligible) elongation in the water group was significantly less than in the NaCl group (ANOVA, P < 0.05).
3.4 Vehicle Groups
Figure 1B shows that the vehicle solution can have dramatic effects on the amount of FDM. In the NaCl group, myopia during the first five days, developed at nearly the same rate (−0.67 ± 0.13 D/day) as in the FD group. During days 6–12, the rate in the NaCl group slowed to −0.15 ± 0.08 D/day, significantly slower than in the FD group (ANOVA, P < 0.05); the amount of myopia was significantly less than the FD group on the last two days of injections (asterisks). As indicated in Figure 2, the vitreous chamber elongation of the NaCl group on day 12 (0.09 ± 0.02 mm) was significantly less than in the FD group (0.14 ± 0.02 mm). Compared with the NaCl group, the myopia in the NaCl+ascorbic acid group developed at a slower rate during the first 5 days (−0.30 ± 0.15 D/day, Table 2), and the amount of myopia present in the NaCl+ascorbic acid group was significantly less than in the NaCl group on several days during the injection series (indicated by asterisks in Figure 1B). However, on the final treatment day, the myopia in the two groups did not differ significantly nor did the vitreous chamber depths (Figure 2).
The use of water (with or without ascorbic acid) dramatically reduced the amount of myopia that developed during 11 days of form deprivation. The amount of myopia in both groups was significantly less than in the NaCl group as soon as 24 hours after the first injection and on all subsequent injection days. The rate of myopia development across the 11 days of treatment in both groups (−0.10 ± 0.10 D/day) was significantly less than for the NaCl group and the FD group (ANOVA, P < 0.05). As shown in Figure 2, there was essentially no elongation of the vitreous chamber in the water or water+ascorbic acid groups. As shown in Figure 1B and Table 2, the rate of myopia development increased significantly in these two groups after injections ceased but FD continued, suggesting that it was the presence of injected vehicle solution, rather than retinal damage, that had blocked FDM development,.
3.5 Ocular Components
We assessed whether ocular components other than vitreous chamber size were significantly affected in the treated eyes of the vehicle groups. There were no significant treated vs. control eye differences amongst the groups in anterior chamber depth, lens thickness, retinal thickness, or choroidal thickness (ANOVA, P > 0.05). The auto-refractor also provided a measure of the amount of astigmatism; the amount of astigmatism treated and control eyes did not differ significantly across groups (ANOVA, P > 0.05).
3.6 Post-Injection Values
The dramatic slowing in the development of FDM in the water and water+ascorbic acid groups raised concerns that the solutions might have damaged the retina. We thus followed most of the animals in these two groups for at least four days after the injection series ended but while FD continued. As may be seen in Figure 1B, the rate of myopia development seemed to rebound immediately after injections ceased; the effect of these vehicles was transient. ERGs in animals from the FD (n=1), sham (n=1), puncture (n=1), NaCl (n=1), and water+ascorbic acid (n=2) groups, taken within an hour after vehicle injection. The a- and b-wave amplitudes were averaged for each eye separately and compared at each intensity. The treated vs. control eye amplitudes were not significantly different (paired t-test, P > 0.05) for the a- or b-waves at any of the 4 light intensities.
4. DISCUSSION
We found that the procedures associated with intravitreal injections (anesthesia, opening of the conjunctiva, and puncture of the sclera) did not significantly affect the development of FDM. However, injecting any of the four vehicle solutions slowed the development of FDM. NaCl had a small effect; H2O and H2O+ascorbic acid both almost completely blocked the development of FDM.
4.1 Procedures: Opening of the Sclera and IOP
It is reassuring that even daily placement of a pipette into the vitreous through a scleral opening, that was re-used each day, did not affect FDM over the course of the 11 sham injections. As was reported by Coulombre (Coulombre, 1956), placing a hollow pipette into the vitreous chamber of embryonic chick eyes greatly slowed the enlargement of the eyes over a four day period, suggesting that intraocular pressure was critically important for embryonic eye enlargement. However, in that study, the pipette was left in place continuously. In addition, the inner diameter of the tube (0.25 mm) was large in proportion to the initial embryonic eye diameter (1.0 mm). Our tree shrews were postnatal, not embryonic, at the age they began treatment. The eyes were much larger (approximately 7.5 mm in axial length) (Norton, Amedo and Siegwart, Jr., 2010) and the scleral opening (0.25 mm) was very small in comparison to the size of the eyes. After withdrawing the pipette, no outflow of vitreous was observed and, except during the pipette insertion, the conjunctival tissues covered the scleral opening, perhaps preserving IOP. Measures of IOP (Tonolab, Tiolat, Helsinki, Finland) in one animal each from the sham and the water groups, 24 hours after the last intravitreal injection, found no indication that the IOP was lower in the injected FD eye, suggesting that the series of injections had no long-term effect on IOP.
4.2 The Effect of Injecting Vehicle Solutions on FDM; Interruption of GO Signals?
Daily injections of all four vehicle solutions slowed the development of FDM to some degree, and the specific substance injected was very important. NaCl (0.85%) had the least impact. Ascorbic acid, added to NaCl, further disrupted FDM. Water, and water+ascorbic acid, strongly disrupted the development of FDM. Something about the specific characteristics of the vehicle solutions disrupted the retinal signals that otherwise would have stimulated the emmetropization mechanism to produce axial ocular elongation and myopia. The factors that may have caused this disruption are considered in the next section. First, however, it is important to consider that the observed reduction in FDM, even the strong effect seen with the water vehicle, could have been produced by a brief daily interruption in retinal GO signaling.
Many details of the retinal signaling that produces vitreous chamber enlargement and refractive myopia are still unknown. However, when the retina is deprived of clear images with a translucent diffuser, the collective response of the retinal neurons may be characterized operationally as GO signals. Some of the retinal signals travel through the central visual pathways to produce visual perception and may affect emmetropization by altering accommodation. In addition, retinally-generated GO (and STOP) signals pass in a signaling cascade through the direct emmetropization pathway (RPE, choroid, sclera) that alters scleral gene expression, producing remodeling and axial elongation and myopia (Gao, Frost, Siegwart, Jr. and Norton, 2011; George, Schmid and Pow, 2005; Guo, Frost, He, Siegwart, Jr. and Norton, 2013; Guo, Frost, Siegwart, Jr. and Norton, 2014; Norton and Rada, 1995). Similar, but probably not identical, GO signals occur in the retina when a negative power lens is held in front of an eye, producing lens-induced myopia (LIM) (Kee, Marzani and Wallman, 2001; Morgan, Ashby and Nickla, 2013; Nickla and Totonelly, 2011; Nickla, Wildsoet and Wallman, 1997; Wildsoet, 1997; Wildsoet and Wallman, 1995). After leaving the retina, FD produces mRNA GO signatures in the tree shrew choroid and sclera that are nearly identical to those produced by a negative lens and are distinct from the STOP signals produced in these tissues during recovery from induced myopia (Guo, Frost, Siegwart, Jr. and Norton, 2014; He, Frost, Siegwart, Jr. and Norton, 2014; Rohrer and Stell, 1994). In these structures, FD causes an active GO signal, rather than a decreased or absent STOP signal.
It has been found that, in order for FDM or LIM to develop, a nearly continuous flow of retinal GO signaling is required. Even short daily disruptions of the signaling, produced by unrestricted vision (removing the diffuser or negative lens) for 30 minutes to 1 hour, significantly reduce the amount of myopia with a very similar temporal dynamic in chicks, tree shrews, and monkeys (Kee, Hung, Qiao-Grider, Ramamirtham, Winawer, Wallman and Smith, III, 2007; Napper, Brennan, Barrington, Squires, Vessey and Vingrys, 1995; Schmid and Wildsoet, 1996; Shaikh, Siegwart and Norton, 1999). For instance, Shaikh et al (1999) found that 1 hr of daily removal of a –5 D lens (which produces retinal STOP signals (Stell, Tao, Karkhanis, Siegwart, Jr. and Norton, 2004)) reduced the myopia produced by 21 days of lens wear by 50%. Two hours of daily lens removal eliminated myopia development. The reduction in the amount of FDM observed in the water and the water+ascorbic acid groups could have been produced if the vehicle reduced or disrupted the retinal GO signaling for between one and two hours each day. Note that the disruption of these very specific retinal GO and STOP signals, that likely occur in a sub-population of amacrine cells (Fischer, Morgan and Stell, 1999; Vessey, Lencses, Rushforth, Hruby and Stell, 2005; Vessey, Rushforth and Stell, 2005), need not involve an overall disruption of retinal function.
As shown in Figure 1B, daily intravitreal injections of water substantially disrupted FDM development. A small amount of FDM developed in this group after two days of FD and water injections. The amount of FDM then remained almost the same for the next six days, suggesting that whatever causes axial elongation and FDM was almost completely blocked day after day. However, the water vehicle did not cause permanent retinal damage. Twenty-four hours after the last injection, normal-appearing a- and b-waves were present in the ERG of the one water vehicle animal studied. After injections stopped, a higher rate of FDM development occurred. The fact that the effect of water was immediate and continued throughout the period of the daily injections, as is the case with brief daily removal of FD, is consistent with an interruption of retinal GO signaling after the injection. The issue then is how could the water (and water+ascorbic acid) mimic the effects of daily diffuser removal?
4.3 Volume, pH, Ionic Concentration and Osmolality as Possible Issues
At least four factors related to the addition of 5 μL of vehicle into the vitreous chamber might have transiently affected retinal functioning without damaging the retina: pressure changes from the injection volume, altered pH, altered concentration of ions and osmolality. The injection volume all of the vehicles was the same, yet the effects were different, suggesting that volume and possible attendant IOP changes, was an unlikely cause of the reduced FDM. Similarly, the pH of the vehicles (water, 6.8; others, 6.9 – 7.1) was similar to the value (7.0 – 7.8) expected in vitreous, which also contains buffers (Bito, 1977) that should minimize pH effects. Lowering of the ionic concentration and the osmolality in the vitreous should have been greatest with the water vehicle, potentially reducing them by 4% (5 μL in an estimated vitreous volume of 125 μL) when the injected water was distributed evenly in the entire vitreous volume. If the osmolality of the vitreous is 290–300 milliosmoles, a 4% overall reduction would produce an osmolality of 270 – 288 milliosmoles. The ionic concentration of each of the many ions in the vitreous (Na+, K+, Cl−, Ca++, PO43−, etc.) would also have been reduced by 4%.
Although injection of water would cause an overall 4% reduction in osmolality and ionic content, the decrease it produced at the tip of the pipette would have been much greater. One might speculate that this greater decrease produced a localized disruption of retinal function near the tip of the pipette, perhaps triggering retinal spreading depression (Martins-Ferreira and de Oliveira, 1971) that blocked GO signal generation as observed with atropine in chick by Schwahn et al. (2000) Although spreading depression in retina at body temperature is of short duration (a few minutes)(Weimer and Hanke, 2005), the effects are stronger in amacrine cells (Tomita and Shimoda, 1983). Consistent with the thought that a localized decrease in osmolality and ionic content might trigger a disruption that is proportional to the amount of ionic and osmolality decrease, one additional animal that received 2.5 μL of water showed slowed myopia development compared with the NaCl group, but more myopia than in the 5 μL water group.
Other studies of FDM that have used intravitreal injections of water vehicle have also found that it causes a reduction in FDM. For example, in chick, Schmid et al. (2013) found that water vehicle reduced FDM compared with the amount found previously in animals with only FD (Schmid and Wildsoet, 2004). In the more recent Schmid study (2013), water vehicle had a smaller impact on FDM in chick than we observed in tree shrews, suggesting that other differences exist between these species. One such difference is the presence of a cartilaginous [chick] vs. fibrous [tree shrew] sclera. Another is that the chick has a larger vitreous chamber with a different structural layout than the tree shrew.
Of the vehicle solutions examined, 0.85% NaCl had the smallest impact on the development of FDM in tree shrews and its effect developed slowly over time so that the amount of FDM was less than in the FD group only on the last two days of treatment. This is consistent with the fact that the isotonic 0.85% NaCl (295 milliosmoles) should have had only minimal effects on both the vitreous osmolality and overall ionic concentration, though it would selectively raise the concentrations of Na+ and Cl−. Perhaps this is sufficient to account for the small disruption in FDM the last days of treatment.
Ascorbic acid, added to water (slightly raising the osmolality and ionic content), did not appear to produce an additional slowing of FDM development. However, because little FDM developed with the water vehicle, it would not have been possible to detect a further slowing of FDM when ascorbic acid was added. However, ascorbic acid, which slightly raised the ionic content and osmolality of the vehicle, also did not appear to protect against the effect of the water.
4.4 Could Daily Injections cause Retinal Damage?
A recent study in mouse using NaCl vehicle found localized retinal lesions (Hombrebueno, Luo, Guo, Chen and Xu, 2014). Our ophthalmoscopic observations of the retina in the NaCl group did not detect any damage and the NaCl vehicle produced only a modest reduction in FDM. The volume of NaCl injected in the mice (2 μL) was very large (~33–40%) in comparison with the estimated volume of the vitreous chamber (~5–6 μL), whereas the injected vehicle volume in the present study (5 μL in an estimated 125 μL) was approximately 4% of the vitreous volume. Slightly larger injection volumes (6%) used in chicks by Schmid et al (2013) also did not produce retinal damage. We suggest that the damage produced NaCl in mouse involved the larger relative volume used. In human clinical use, the volumes are a tiny fraction of the vitreous volume (Del Amo and Urtti, 2015) and drugs are administered infrequently, so that the injected vehicle volume is not an important issue.
4.5 Ascorbic Acid and its Role in Dopaminergic Signaling and FDM
It is not surprising that ascorbic acid, added to NaCl, slowed the development of FDM. Dopamine has been implicated as an important neurotransmitter in retinal signaling related to myopia development (Besharse and Iuvone, 1992; Iuvone, Tigges, Stone, Lambert and Laties, 1991; Stone, Lin, Iuvone and Laties, 1990; Stone, Lin, Laties and Iuvone, 1989). Dopamine levels are reduced during the development of FDM and intravitreal injections of dopamine agonists have been found to reduce FDM (Stone, Lin, Iuvone and Laties, 1990). Ascorbic acid is abundant in the retina and could participate in a variety of mechanisms and activities. Ascorbic acid is actively involved in the dopamine pathways as a neuromodulator that reduces the uptake of dopamine by reducing voltage-dependent K+ currents (Fan and Yazulla, 1999). Tolbert et al(1992) found that ascorbic acid inhibited the specific binding of both D1 and D2 receptor agonists. Rohrer and Stell (1993), in a study of dopaminergic influences on FDM, found that a vehicle solution of ascorbic acid in NaCl produced a 50% slowing of FDM in chicks. As suggested by Fan and Yazulla (1999), alternative antioxidants that might be preferable to ascorbic acid include Glutamyl-L-cysteinyl-glycine (GSH, reduced form) and D(−)-isoascorbic acid because they do not cause a reduction of voltage-dependent K+ currents and thus, do not reduce uptake of dopamine or inhibit binding of dopamine agonists. Though these mechanisms are not fully understood and evidence for vehicles affecting eye growth and myopic development is limited, it seems preferable that a chosen vehicle should not interfere with any neurotransmitters’ activity (especially dopamine).
It has been argued that injecting the ascorbic acid vehicle in the control eye, and vehicle plus dopamine analogs in the treated eye, allows the differences in refraction and axial elongation between the treated and control eyes to be a valid indicator of the effects of the dopamine analogs (Rohrer, Spira and Stell, 1993). A concern with this approach, however, is that the treated eye, in such cases, is treated with two agents that affect dopaminergic functioning. The possible interactions of the ascorbate with the dopamine analog make it more difficult to assess what the effect in the treated (form-deprived) eye would be in the absence of ascorbic acid.
4.6 Concluding Thoughts
Although intravitreal injections may be the best way in many instances to deliver pharmacologically active agents near the retina, the present study serves as a cautionary reminder that the choice of the vehicle solution can, in and of itself, affect responses, such as FDM, that depend on continuous retinal signaling. In particular, studies of retinal dopamine signaling might choose alternative antioxidants (if needed) that do not affect dopaminergic or other retinal signaling.
Intravitreal injection procedures had no effect on form-deprivation myopia (FDM).
Daily injections of any vehicle reduced the development of FDM.
NaCl had only a small effect. Ascorbic acid (in NaCl) further reduced FDM.
Water (with or without ascorbic acid) dramatically reduced FDM.
Vehicles could be interfering with biochemical signals driving eye growth.
Acknowledgments
Supported by NIH R01 EY005922 and P30 EY003039 CORE. Alexander Ward was supported by funds from the Department of Vision Sciences. This work was performed in partial fulfillment of the requirements for the degree of Doctor of Philosophy at the University of Alabama at Birmingham (Alexander Ward). Preliminary results were presented in abstract form at the 2014 International Society for Eye Research meeting and 14th International Myopia Conference, 2013. We thank Dr. Karen Gamble for statistical guidance. We also thank Dr. Marina Gorbatyuk and Mr. Vishal Shinde for technical assistance with the ERG recording.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Araie M, Maurice DM. The loss of fluorescein, fluorescein glucuronide and fluorescein isothiocyanate dextran from the vitreous by the anterior and retinal pathways. Exp Eye Res. 1991;52:27–39. doi: 10.1016/0014-4835(91)90125-x. [DOI] [PubMed] [Google Scholar]
- 2.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmol. 2006;113:363–72. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Besharse JC, Iuvone PM. Is dopamine a light-adaptive or a dark-adaptive modulator in retina? Neurochemistry International. 1992;20:193–9. doi: 10.1016/0197-0186(92)90167-p. [DOI] [PubMed] [Google Scholar]
- 4.Bito LZ. The physiology and pathophysiology of intraocular fluids. Exp Eye Res. 1977;25(Suppl):273–89. doi: 10.1016/s0014-4835(77)80024-9. [DOI] [PubMed] [Google Scholar]
- 5.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 6.Coulombre AJ. The role of intraocular pressure in the development of the chick eye - I. Control of eye size. J Exp Zoology. 1956;133:211–25. [Google Scholar]
- 7.Del Amo EM, Urtti A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp Eye Res. 2015;137:111–24. doi: 10.1016/j.exer.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Fan SF, Yazulla S. Suppression of voltage-dependent K+ currents in retinal bipolar cells by ascorbate. Vis Neurosci. 1999;16:141–8. doi: 10.1017/s0952523899161091. [DOI] [PubMed] [Google Scholar]
- 9.Feldkaemper MP, Neacsu I, Schaeffel F. Insulin acts as a powerful stimulator of axial myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50:13–23. doi: 10.1167/iovs.08-1702. [DOI] [PubMed] [Google Scholar]
- 10.Fischer AJ, Morgan IG, Stell WK. Colchicine causes excessive ocular growth and myopia in chicks. Vision Res. 1999;39:685–97. doi: 10.1016/s0042-6989(98)00178-3. [DOI] [PubMed] [Google Scholar]
- 11.Ganesan P, Wildsoet CF. Pharmaceutical intervention for myopia control. Expert Rev Ophthalmol. 2010;5:759–87. doi: 10.1586/eop.10.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao H, Frost MR, Siegwart JT, Jr, Norton TT. Patterns of mRNA and protein expression during minus-lens compensation and recovery in tree shrew sclera. Mol Vis. 2011;17:903–19. [PMC free article] [PubMed] [Google Scholar]
- 13.George A, Schmid KL, Pow DV. Retinal serotonin, eye growth and myopia development in chick. Exp Eye Res. 2005;81:616–25. doi: 10.1016/j.exer.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–6. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- 15.Guo L, Frost MR, He L, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Invest Ophthalmol Vis Sci. 2013;54:6806–19. doi: 10.1167/iovs.13-12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo L, Frost MR, Siegwart JT, Jr, Norton TT. Scleral gene expression during recovery from myopia compared with expression during myopia development in tree shrew. Mol Vis. 2014;20:1643–59. [PMC free article] [PubMed] [Google Scholar]
- 17.Haritoglou C, Kook D, Neubauer A, Wolf A, Priglinger S, Strauss R, Gandorfer A, Ulbig M, Kampik A. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006;26:999–1005. doi: 10.1097/01.iae.0000247165.38655.bf. [DOI] [PubMed] [Google Scholar]
- 18.He L, Frost MR, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Exp Eye Res. 2014;123:56–71. doi: 10.1016/j.exer.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hombrebueno JR, Luo C, Guo L, Chen M, Xu H. Intravitreal Injection of iormal saline induces retinal degeneration in the C57BL/6J mouse. Transl Vis Sci Technol. 2014;3:3. doi: 10.1167/tvst.3.2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iturralde D, Spaide RF, Meyerle CB, Klancnik JM, Yannuzzi LA, Fisher YL, Sorenson J, Slakter JS, Freund KB, Cooney M, Fine HF. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006;26:279–84. doi: 10.1097/00006982-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci. 1991;32:1674–7. [PubMed] [Google Scholar]
- 22.Kee CS, Hung LF, Qiao-Grider Y, Ramamirtham R, Winawer J, Wallman J, Smith EL., III Temporal constraints on experimental emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2007;48:957–62. doi: 10.1167/iovs.06-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kee CS, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001;42:575–83. [PubMed] [Google Scholar]
- 24.Marsh-Tootle WL, Norton TT. Refractive and structural measures of lid-suture myopia in tree shrew. Invest Ophthalmol Vis Sci. 1989;30:2245–57. [PubMed] [Google Scholar]
- 25.Martins-Ferreira H, de Oliveira CG. Spreading depression in isolated chick retina. Vision Res Suppl. 1971;3:171–84. doi: 10.1016/0042-6989(71)90038-1. [DOI] [PubMed] [Google Scholar]
- 26.McBrien NA, Cornell LM, Gentle A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Invest Ophthalmol Vis Sci. 2001;42:2179–87. [PubMed] [Google Scholar]
- 27.McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri) Vision Res. 1992;32:843–52. doi: 10.1016/0042-6989(92)90027-g. [DOI] [PubMed] [Google Scholar]
- 28.McFadden SA, Howlett MH, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004;44:643–53. doi: 10.1016/j.visres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 29.McKanna JA, Casagrande VA. Atropine affects lid-suture myopia development. Doc Ophthalmol Proc Ser. 1981;28:187–92. [Google Scholar]
- 30.Morgan IG, Ashby RS, Nickla DL. Form deprivation and lens-induced myopia: are they different? Ophthalmic Physiol Opt. 2013;33:355–61. doi: 10.1111/opo.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moring AG, Baker JR, Norton TT. Modulation of glycosaminoglycan levels in tree shrew sclera during lens-induced myopia development and recovery. Invest Ophthalmol Vis Sci. 2007;48:2947–56. doi: 10.1167/iovs.06-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Napper GA, Brennan NA, Barrington M, Squires MA, Vessey GA, Vingrys AJ. The duration of normal visual exposure necessary to prevent form deprivation myopia in chicks. Vision Res. 1995;35:1337–44. doi: 10.1016/0042-6989(94)00226-c. [DOI] [PubMed] [Google Scholar]
- 33.Nickla DL, Totonelly K. Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Exp Eye Res. 2011;93:782–5. doi: 10.1016/j.exer.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nickla DL, Wildsoet C, Wallman J. Compensation for spectacle lenses involves changes in proteoglycan synthesis in both the sclera and choroid. Curr Eye Res. 1997;16:320–6. doi: 10.1076/ceyr.16.4.320.10697. [DOI] [PubMed] [Google Scholar]
- 35.Norton TT. Animal models of myopia: Learning how vision controls the size of the eye. ILAR J. 1999;40:59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- 36.Norton TT, Amedo AO, Siegwart JT., Jr The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Vision Res. 2010;50:564–76. doi: 10.1016/j.visres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norton TT, Essinger JA, McBrien NA. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Vis Neurosci. 1994;11:143–53. doi: 10.1017/s0952523800011184. [DOI] [PubMed] [Google Scholar]
- 38.Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Res. 1992;32:833–42. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- 39.Norton TT, Rada JA. Reduced extracellular matrix accumulation in mammalian sclera with induced myopia. Vision Res. 1995;35:1271–81. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- 40.Norton TT, Siegwart JT, Jr, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006;47:4687–99. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norton TT, Siegwart JT, German AJ, Robertson J, Wu WW. Comparison of cycloplegic streak retinoscopy with autorefractor measures in tree shrew eyes with, and without, induced myopia. Invest Ophthalmol Vis Sci. 2000;41:ARVO Abstract 563. [Google Scholar]
- 42.Norton TT, Wu WW, Siegwart JT., Jr Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optom Vis Sci. 2003;80:623–31. doi: 10.1097/00006324-200309000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park J, Bungay PM, Lutz RJ, Augsburger JJ, Millard RW, Sinha RA, Banerjee RK. Evaluation of coupled convective-diffusive transport of drugs administered by intravitreal injection and controlled release implant. J Control Release. 2005;105:279–95. doi: 10.1016/j.jconrel.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Petry HM, Fox R, Casagrande VA. Spatial contrast sensitivity of the tree shrew. Vision Res. 1984;24:1037–42. doi: 10.1016/0042-6989(84)90080-4. [DOI] [PubMed] [Google Scholar]
- 45.Pickett-Seltner RL, Stell WK. The effect of vasoactive intestinal peptide on development of form deprivation myopia in the chick: a pharmacological and immunocytochemical study. Vision Res. 1995;35:1265–70. doi: 10.1016/0042-6989(94)00244-g. [DOI] [PubMed] [Google Scholar]
- 46.Rohrer B, Iuvone PM, Stell WK. Stimulation of dopaminergic amacrine cells by stroboscopic illumination or fibroblast growth factor (bFGF, FGF-2) injections: possible roles in prevention of form-deprivation myopia in the chick. Brain Res. 1995;686:169–81. doi: 10.1016/0006-8993(95)00370-6. [DOI] [PubMed] [Google Scholar]
- 47.Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D(2)-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci. 1993;10:447–53. doi: 10.1017/s0952523800004673. [DOI] [PubMed] [Google Scholar]
- 48.Rohrer B, Stell WK. Basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-β) act as stop and go signals to modulate postnatal ocular growth in the chick. Exp Eye Res. 1994;58:553–61. doi: 10.1006/exer.1994.1049. [DOI] [PubMed] [Google Scholar]
- 49.Schaeffel F, Bartmann M, Hagel G, Zrenner E. Studies on the role of retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Res. 1995;35:1247–64. doi: 10.1016/0042-6989(94)00221-7. [DOI] [PubMed] [Google Scholar]
- 50.Schaeffel F, Hagel G, Bartmann M, Kohler K, Zrenner E. 6-Hydroxy dopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Res. 1994;34:143–9. doi: 10.1016/0042-6989(94)90327-1. [DOI] [PubMed] [Google Scholar]
- 51.Schaeffel F, Howland HC. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Res. 1991;31:717–34. doi: 10.1016/0042-6989(91)90011-s. [DOI] [PubMed] [Google Scholar]
- 52.Schmid KL, Strasberg G, Rayner CL, Hartfield PJ. The effects and interactions of GABAergic and dopaminergic agents in the prevention of form deprivation myopia by brief periods of normal vision. Exp Eye Res. 2013;110:88–95. doi: 10.1016/j.exer.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996;36:1023–36. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- 54.Schmid KL, Wildsoet CF. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom Vis Sci. 2004;81:137–47. doi: 10.1097/00006324-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 55.Schwahn HN, Kaymak H, Schaeffel F. Effects of atropine on refractive development, dopamine release, and slow retinal potentials in the chick. Vis Neurosci. 2000;17:165–76. doi: 10.1017/s0952523800171184. [DOI] [PubMed] [Google Scholar]
- 56.Shaikh AW, Siegwart JT, Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom Vis Sci. 1999;76:308–15. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- 57.Shen W, Sivak JG. Eyes of a lower vertebrate are susceptible to the visual environment. Invest Ophthalmol Vis Sci. 2007;48:4829–37. doi: 10.1167/iovs.06-1273. [DOI] [PubMed] [Google Scholar]
- 58.Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977;124:154–7. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- 59.Siegwart JT, Norton TT. Goggles for controlling the visual environment of small animals. Lab Animal Sci. 1994;44:292–4. [PubMed] [Google Scholar]
- 60.Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 61.Smith EL, III, Bradley DV, Fernandes A, Boothe RG. Form deprivation myopia in adolescent monkeys. Optom Vis Sci. 1999;76:428–32. doi: 10.1097/00006324-199906000-00023. [DOI] [PubMed] [Google Scholar]
- 62.Stell WK, Tao J, Karkhanis A, Siegwart JT, Jr, Norton TT. Amacrine cells responsive to optical conditions regulating eye growth in the tree shrew, Tupaia glis belangeri. Invest Ophthalmol Vis Sci. 2004;45:ARVO E-abstract 1159. [Google Scholar]
- 63.Stone RA, Lin T, Iuvone PM, Laties AM. Postnatal control of ocular growth: dopaminergic mechanisms. Myopia and the Control of Eye Growth. 1990;155:45–57. doi: 10.1002/9780470514023.ch4. [DOI] [PubMed] [Google Scholar]
- 64.Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci USA. 1989;86:704–6. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tolbert LC, Morris PE, Jr, Spollen JJ, Ashe SC. Stereospecific effects of ascorbic acid and analogues on D1 and D2 agonist binding. Life Sci. 1992;51:921–30. doi: 10.1016/0024-3205(92)90400-j. [DOI] [PubMed] [Google Scholar]
- 66.Tomita T, Shimoda Y. Response to light of various retinal cell types during spreading depression. Vision Res. 1983;23:1309–13. doi: 10.1016/0042-6989(83)90106-2. [DOI] [PubMed] [Google Scholar]
- 67.Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Res. 1993;33:1311–24. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- 68.Vessey KA, Lencses KA, Rushforth DA, Hruby VJ, Stell WK. Glucagon receptor agonists and antagonists affect the growth of the chick eye: a role for glucagonergic regulation of emmetropization? Invest Ophthalmol Vis Sci. 2005;46:3922–31. doi: 10.1167/iovs.04-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vessey KA, Rushforth DA, Stell WK. Glucagon- and secretin-related peptides differentially alter ocular growth and the development of form-deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2005;46:3932–42. doi: 10.1167/iovs.04-1027. [DOI] [PubMed] [Google Scholar]
- 70.Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: Susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–63. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 71.Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201:1249–51. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- 72.Weimer MS, Hanke W. Correlation between the durations of refractory period and intrinsic optical signal of retinal spreading depression during temperature variations. Exp Brain Res. 2005;161:201–8. doi: 10.1007/s00221-004-2060-5. [DOI] [PubMed] [Google Scholar]
- 73.Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–8. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- 74.Wildsoet CF. Active emmetropization - evidence for its existence and ramifications for clinical practice. Ophthal Physiol Opt. 1997;17:279–90. [PubMed] [Google Scholar]
- 75.Wildsoet CF, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–94. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- 76.Zhu X, Wallman J. Opposite effects of glucagon and insulin on compensation for spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009;50:24–36. doi: 10.1167/iovs.08-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]