Abstract
Fibrosis and scarring are the end stage of many disease processes. In effect, the collagen fibers that initially provide a necessary strength during the repair of injured tissues are frequently synthesized in excessive amounts and become irreversible fibrotic deposits that limit regeneration of the endogenous cells of a tissue. This review will focus on the potential of mesenchymal stem/stromal cells for treatment of fibrotic diseases, with emphasis on the role of TSG-6 as a mediator of anti-inflammatory effects.
Keywords: Tissue fibrosis, regenerative medicine, Mesenchymal stem/stromal cells, TSG-6
Development of Novel Therapies for Tissue Fibrosis
Collagen biosynthesis – a historical perspective
Early in its development, my laboratory took an interest in fibrosis. At the time, I was privileged to work with Joel Rosenblum and a cadre of other outstanding scientists, including two who are contributors to this mini-review, Jouni Uitto and Sergio Jimenez. Our efforts produced 12 publications with Joel, 20 with Jouni, and 10 with Sergio, most in the most prestigious scientific journals (for examples, see refs 1, 2 and 3 [1-3]). With technologies that now seem pre-historic, we were able to discover the complex and fascinating steps involved in the biosynthesis of collagen [4]. Our discoveries began with the demonstration that hydroxyproline is a unique among amino acids since it is synthesized post-translationally by the hydroxylation of prolyl residues in peptide precursors. We also demonstrated the remarkable importance of this post-translational step in that the hydroxylation of about 100 prolyl residues in each of the three chains of the protein is essential for its correct folding, and that the triple-helical conformation, a characteristic of collagen, is required for its normal secretion. In the process, we provided some of the first evidence that collagen was synthesized as a precursor protein called procollagen with additional amino acid sequences on both the NH2- and COOH-ends of the three chains of the protein. In addition, we made the unexpected observation that several analogues of proline were readily incorporated into procollagen in place of proline and that the unusual conformation of the proline analogues prevented the correct folding of the protein. The unfolded procollagen was poorly secreted and most of it underwent intracellular degradation, probably by a process now known as autophagy. The observations with proline analogues suggested that they might be useful as drugs for inhibiting or modulating fibrosis and scarring, however, for a variety of reasons, their therapeutic prospects were not developed further. In subsequent work with Joel, Jouni, Sergio and many others, we isolated and defined the structure of genes for human collagens and used the information to identify mutations in the genes that caused skeletal disorders [5]. Most of the disorders were relatively rare genetic diseases of children such as osteogenesis imperfect (brittle bone disease of children) and dwarfism. However, in a few instances we found mutations of collagen genes in families that had been diagnosed with osteoporosis and osteoarthritis [3,5].
New perspectives on prolyl hydroxylation
Potential new therapies are now being developed largely on the basis of our work in defining the structure and properties of prolyl hydroxylase, the unusual enzyme responsible for synthesizing the hydroxyproline in collagen. An inhibitor of the enzyme is now in Phase 3 clinical trials for therapy of anemias. The inhibitor is effective because of the serendipitous observation [6] that although the major forms of prolyl hydroxylase are involved in collagen biosynthesis, a minor form of the enzyme is required for hydroxylation of a single prolyl residue in hypoxia induced factor (HIF). Hydroxylation of the residue is essential to initiate degradation of HIF by proteasomes. The inhibitor increases the levels of HIF and thereby increases responses to ischemia, including increases in the hormone erythropoietin that stimulates erythropoiesis. Erythropoietin is widely used in the treatment of anemias but therapy with the inhibitor of prolyl hydroxylase may have an advantage since it can be administered by mouth instead of by injection.
Mesenchymal Stem/Stromal Cells (MSCs)
As reflected in the accompanying articles in this Mini-Review Cluster, Joel, Jouni and Sergio have continued the important research on fibrosis. For better or worse, the interest in my own laboratory has turned to trying to develop new therapies first for osteogenesis imperfecta and then for a broad range of more common diseases. Our primary focus has been on the potential of the special class of cells from bone marrow referred to as mesenchymal stem/stromal cells or MSCs [7].
Characteristics of MSCs
MSCs were initially discovered by Friedenstein and others in the 1950s as the small fraction of cells from bone marrow that are readily isolated as spindle-shaped cells that adhere to tissue culture surfaces and rapidly expand in culture. The cells were of special interest because Friedenstein and others demonstrated that they readily differentiated in culture and in implants in vivo into mineralizing cells, adipocytes, and chondrocytes. Some of the research on MSCs was sparked by their apparent role in forming the stroma of bone marrow and their ability to serve as feeder layers for cultures of hematopoietic cells. Beginning about 25 years ago, interest developed in their potential therapeutic benefits [7,8]. On the basis of promising results obtained in a murine model for the disease in my own laboratory [9], the first clinical trial with MSCs was carried out in patients with severe osteogenesis imperfecta [10]. Four of five patients had a favorable response with improvements in growth rate and mobility in that several could sit up and walk with support for the first time. While the improvements persisted for only a few months, most importantly, however, there were no adverse effects in these patients. The results in part prompted a subsequent trial in a patient with life-threatening graft-versus-host-disease [11]. The results of these two initial trials opened a floodgate of interest in MSCs [12-14]. Surprisingly, these studies have led us back to re-examining the basic processes by which the body responds to injury, including the inflammatory and immune responses that produce either normal repair or fibrosis.
Therapeutic potential of MSCs
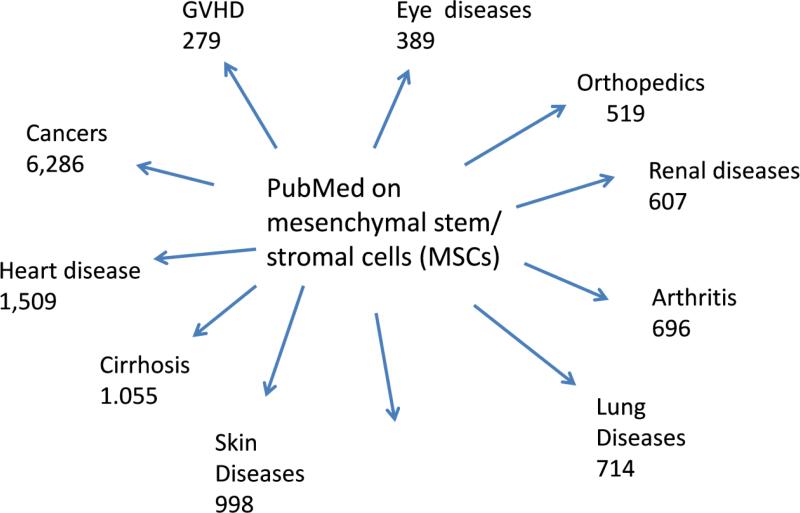
Exploring the therapeutic potentials of MSCs has proceeded on two levels. One level has been to test the cells in animal models for human diseases. Here there have been over 10,000 citations on publications in which MSCs were administered in models for diseases of essentially every organ (see Fig. 1). In essentially all the reports, improvements were observed. The only striking exception is some cancer models in which MSCs accelerated growth of the primary tumor or enhanced metastases. A few reports indicated that the MSCs became tumorigenic but on closer examination these appeared to reflect contamination of MSC cultures by neoplastic cells or to reflect the spontaneous transformation of murine cells in culture [15]. The majority of the experiments have been performed with MSCs derived from bone marrow, but use of the same or similar cells from other tissues, including fat, synovial membranes, and umbilical cord, have generated similar data. The embryonic origin and the normal function of endogenous MSCs is still being investigated, but they appear to be reticular cells associated with most small blood vessels that serve as niche for hematopoietic cells in bone marrow [16] and that proliferate locally after tissue injury [17].
Figure 1.
Citations in PubMed as of April, 2015 to publications in which MSCs were administered to animal models for the diseases indicated.
The second level for exploring the therapeutic potentials of MSCs has been to test them directly in patients. Several hundred clinical trials with MSCs or similar cells have been registered (www.clinicaltrials.gov), and large numbers of patients, perhaps thousands, have been entered in these trials. Most reports indicate promising results but, as discussed below, the data have several limitations.
Molecular mechanisms of MSCs
At the same time as the clinical trials were progressing, we and others were trying to explain the molecular mechanism by which MSCs produced their therapeutic effects. This led to a re-examination of the basic processes by which tissues respond to injury, e.g., the inflammatory and immune responses, the regeneration of injured cells, and, apparently as a default mechanism, fibrosis. An initial hypothesis was that MSCs engrafted at sites of injury and differentiation to replace injured cells [7]. This hypothesis was supported by some early observations involving extreme tissue injury or injecting cells into rapidly growing embryos. Subsequently, however, it was found that MSCs produce beneficial effects in many animal models even though the cells engraft poorly and are degraded rapidly [12,13]. Among the most perplexing observations was that intravenous (IV) infusions of MSCs in mice improved induced injuries to distal organs like the heart, brain or kidney even though most of the cells were trapped in the lung. The conclusion from multiple experiments was that MSCs produced long-term effects in vivo by responding to signals from injured tissues in multiple ways. Among the primary effects was modulating the inflammatory and immune responses to tissue injury that are frequently excessive and contribute to the pathologic changes that follow. In effect MSCs served as “guardians” of these systems ([17]; Figure 2). However, in some circumstances the cells enhanced proliferation and differentiation of tissue endogenous stem cells [18]. In other circumstances, they enhanced regeneration of injured cells by transfer of vesicles containing proteins, microRNAs, mRNAs and even mitochondria [19-22].
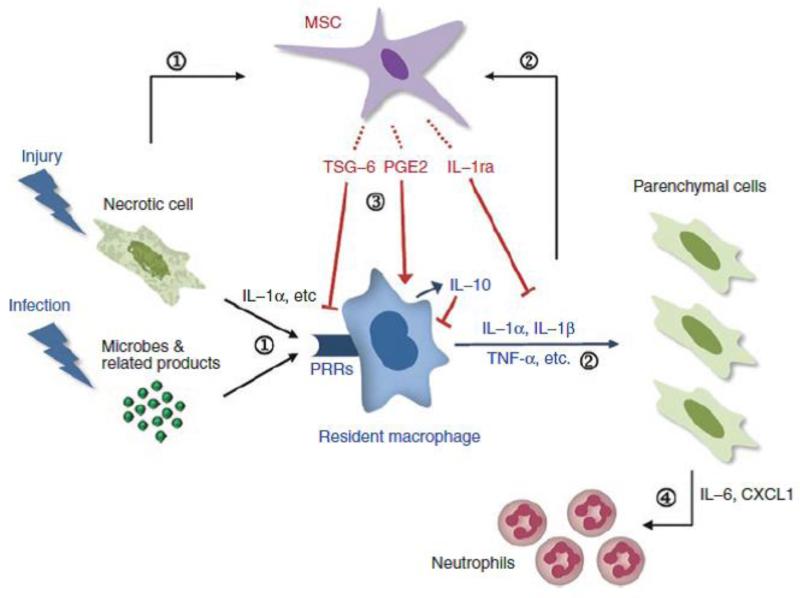
Figure 2.
Summary of some of the anti-inflammatory effects of MSCs. (1) Damage-associated molecular patterns (DAMPs) and interleukin (IL)-1α released by sterile injury or pathogen-associated molecular patterns (PAMPs) released by infectious injury to tissues activate resident macrophages through receptors involving pattern recognition receptors (PRRs). (2) The activated macrophages produce proinflammatory cytokines such as IL-1α, IL-1β, or tumor necrosis factor-α (TNF-α) to initiate the inflammatory cascade. (3) Simultaneously, the proinflammatory cytokines and probably other signals from injured cells activate MSCs to secrete anti-inflammatory factors, including TNF-α stimulated gene/protein 6 (TSG-6), PGE, and IL-1ra, that either modulate the activation of the resident macrophages or decrease the downstream effects of the proinflammatory cytokines. (4) The net effect is to decrease the amplification of the proinflammatory signals by parenchymal cells though the secretion of IL-6, CXCL1, and related factors and as a result to decrease the recruitment of neutrophils. (Reproduced from Prockop and Oh [17], with permission).
The role of TSG-6 as a mediator of anti-inflammatory effects
The interest in my own laboratory returned to fibrosis and its triggering by inflammation largely as a result of experiments with a brilliant young associate, Ryang Hwa Lee. Previous reports by others demonstrated that IV infusions of human MSCs greatly reduced the size of the myocardial infarcts in animal models of myocardial infarction [23]. We confirmed the observations but the results were perplexing because it seemed apparent that most of the MSCs were trapped in the lung after IV infusion [24]. We developed quantitative PCR and RT-PCR assays to track human MSCs in mice and demonstrated that over 80% of the cells were trapped in the lung of mice after IV infusion; only 0.1 % reached the heart and they were there for no more than a day [25]. The explanation for the therapeutic effect came from microarray and RT-PCR assays for human genes 10 hours after the human MSCs were infused. The results demonstrated an over 50-fold increase in the level of mRNA for TSG-6 (TNF-α stimulated gene/protein 6), a protein previously shown to be a natural modulator of inflammatory responses [26,27]. The role of TSG-6 was confirmed by the observations that MSCs with a knock down of TSG-6 expression were ineffective in the model and the observation that infusion of recombinant TSG-6 reproduced most of the beneficial effects of MSCs [25]. The results linked the beneficial effects directly to inflammation in that previous reports had demonstrated that myocardial infarction can be regarded as a form of sterile inflammation that releases a cascade of proteases and the proteases enhance the damage to the heart. MSCs and TSG-6 limited this sequence of events. In effect, by limiting the inflammatory response to sterile inflammation they decreased the fibrosis. We subsequently observed similar effects with MSCs and recombinant TSG-6 in two other models: induced inflammation of the cornea [28] and peritonitis induced with the yeast extract zymosan [29]. Also, in a murine model for bleomycin-induced lung injury, we found that suppressing the early inflammatory phase improved arterial oxygen saturation and survival, apparently by decreasing fibrosis of the lung [30]. In addition, both MSCs and TSG-6 decreased rejection of allogeneic grafts of the cornea in mice, in part by decreasing the early inflammatory response to the transplantations [31].
MSCs as a regulator of inflammation
Interpretation of our results was greatly facilitated by the progress that was being made at about the same time on the molecular and cellular events involved in inflammatory responses [32,33]. As with most biological processes, inflammation and the subsequent fibrosis are controlled by both positive and negative regulators. The positive regulators include a host of factors released by injured cells or invading micro-organisms referred to as damage associate molecular pattern molecules (DAMPs) or pathology associated molecular pattern molecules (PAMPs). The DAMPs include many nuclear and cytoplasmic components of cells. The PAMPs are small molecular motifs found in microbes and include LPS, flagellin and nucleic acids. The DAMPs and PAMPs become what Medzhitov [32] has referred to as “inducers” that act on “sensor” cells that consist of the macrophages, monocytes and dendritic cells that are endogenous residents in all tissues. The sensor cells respond by synthesizing and secreting pro-inflammatory signals that are amplified by adjacent cells to generate a pro-inflammatory storm that ushers in the cardinal signs of inflammation: infusions of fluid, neutrophils, and macrophages that generate redness, swelling, pain, and heat.
What keeps the inflammatory cycle from spinning out of control? The question is not yet fully answered, but a large series of negative regulators have been identified. These include specialized lipid mediators (lipoxins, resolvins, protectins, and maresins), proteins (annexin A1, galectins), peptides, gaseous mediators (hydrogen sulfide), a purine (adenine), and neuromodulators [33]. These negative regulators can limit neutrophil infiltration, clearance of neutrophils by macrophages, and change the phenotype of macrophages and probably other cells from pro-inflammatory (M1) to anti-inflammatory (M2). To this list of negative regulators the data say we must add MSCs. In some circumstances, MSCs may be major negative regulators. A striking feature of both MSCs and TSG-6 is that they act very early in the inflammatory response to injury [28]. TSG-6, either directly or through a complex with hyaluronan, binds to the CD44 receptor on resident macrophages [29]. The binding dissociates co-receptors (MyD 88 and TIRAP) from TLR-2 and decreases NF-ḳB signaling in the resident macrophages and other sensor cells. The result is that the cytokine storm of inflammation is aborted.
Progress and challenges in clinical trials
But let me return to the question of clinical trials with MSCs. Definitive data have been difficult to generate in clinical trials for several reasons. One is that most of the trials have involved small numbers of patients with diseases in which therapeutic effects are difficult to evaluate without large scale, well-controlled and expensive trials. Another problem is that there have not been any adequate biomarkers to define the cells. As a result, there are marked variations in the quality of the tissue-derived samples of MSCs from different donors. Also, the conditions for expanding the cells in culture markedly affect their properties. For example, the characteristics of the MSCs differ if the cells are incubated at low or high density, an observation we originally made [34,35] and has now been extensively confirmed [36-41].Therefore some patients may have received very efficacious MSCs and others not. And most surprising of all, as summarized briefly above, it has proven difficult to define all the multiple molecular mechanisms by which the cells produce their therapeutic benefits.
The limited size of the clinical trials is currently being addressed by several new efforts. One is that the National Heart Lung and Blood Institute has sponsored a multi-center trial to treat patients with severe Acute Respiratory Distress Syndrome (ARDS) with bone marrow MSCs prepared with a standardized protocol developed in our laboratory [42]. Another is that several large multi-center trials in a variety of diseases are either planned or underway in the European Union and elsewhere [43].
Identification of biomarkers for the cells has proven difficult. Our laboratory recently reported that an assay of MSCs for expression of TSG-6 can serve as a biomarker to predict the efficacy of different preparations of human MSCs in suppressing induced inflammation in mice [44]. A critical feature of the data was that the efficacy in vivo of the MSCs was assayed in mice with induced inflammation of the cornea under conditions that provided quantitative measures and dose response data [28]. We encountered an unpleasant surprise when we used the assay to test bone marrow-derived MSCs. Our laboratory has been preparing such cells with a carefully standardized protocol and distributing them to over 250 laboratories for the past 14 years under the auspices of an NIH grant. We were surprised that only one-third of 15 different preparations of our standardized MSCs expressed high levels of TSG-6. The same one-third of preparations were optimally effective in a mouse model for induced inflammation of the cornea. One-third of the preparations expressed very low levels of TSG-6 and were largely ineffective in the same models. The results were therefore consistent with the possibility that some of the variations seen in the responses of patients to therapy with MSCs might be explained by differences in the efficacy of different preparations of the cells. They also suggested that pre-testing the MSCs for expression of a natural anti-inflammatory gene (TSG-6) might provide a method for selecting the most efficacious cells if the MSCs were being used to suppress or modulate inflammatory responses in patients. It is unlikely however that expression of TSG-6 accounts for all the beneficial effects observed with administration of MSCs in disease models or in patients. Therefore it will be important to search for additional biomarkers that are predictive of efficacy. The major barrier appears to be adequate animal models in which the efficacy of MSCs can be assayed quantitatively and dose response data generated. Such models remain a challenge because the major effects of MSCs are to either limit tissue injury or enhance its repair, but mice and rats may have more efficient means of dealing with these issues than humans.
Conclusions
It is clear that we still have far to go in understanding the processes by which the body responds to tissue injury, processes such as inflammation and fibrosis. Research on MSCs has opened one window on these processes, and may provide a basis for therapeutic interventions in diseases in need of tissue repair and regeneration.
Highlights.
Tissue fibrosis, an end stage of a number of complex processes, is characterized by excessive accumulation of collagen.
Mesenchymal stem/stromal cells (MSCs), a subpopulation of bone marrow derived cells, have the capacity to modulate inflammatory and immune responses to the injury.
TSG-6 is a critical molecule mediating the anti-inflammatory effects of MSCs.
Ongoing clinical trials have suggested that MSC administration may provide a therapeutic intervention in diseases in need of tissue repair and regeneration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenbloom J, Prockop DJ. Incorporation of cis-hydroxyproline into protocollagen and collagen. Collagen containing cis-hydroxyproline in place of proline and trans-hydroxyproline is not extruded at a normal rate. J. Biol. Chem. 1971;246:1549–1555. [PubMed] [Google Scholar]
- 2.Uitto J, Hoffman H, Prockop DJ. Retention of nonhelical procollagen containing cis-hydroxyproline in rough endoplasmic reticulum. Science. 1975;190:1202–1204. doi: 10.1126/science.1198105. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez SA, Ala-Kokko L, Ahmad N, Baldwin C, Dharmavaram R, Reginato A, Knowlton R, Prockop DJ. Type II collagen gene mutations in familial osteoarthritis. In: al., K.K.e., editors. Articular Cartilage and Osteoarthritis. Raven Press Ltd; New York: 1992. pp. 167–178. [Google Scholar]
- 4.Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 5.Prockop DJ, Bateman J. Heritable Disorders of Connective Tissues. In: Jameson L.e.a., editor. Harrison's Principles of Internal Medicine. 19th ed. McGraw Hill Medical; New York: 2014. [Google Scholar]
- 6.Laitala A, Aro E, Walkinshaw G, Maki JM, Rossi M, Heikkila M, Savolainen ER, Arend M, Kivirikko KI, Koivunen P, Myllyharju J. Transmembrane prolyl 4-hydroxylase is a fourth prolyl 4-hydroxylase regulating EPO production and erythropoiesis. Blood. 2012;120:3336–3344. doi: 10.1182/blood-2012-07-441824. [DOI] [PubMed] [Google Scholar]
- 7.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 8.Caplan AI. Mesenchymal stem cells. J. Orthop. Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 9.Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc. Natl. Acad. Sci. U. S. A. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 11.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 12.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs). J Cell Mol Med. 2010;14:2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion (Paris) 2014;54:1418–1437. doi: 10.1111/trf.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prockop DJ, Keating A. Relearning the lessons of genomic stability of human cells during expansion in culture: implications for clinical research. Stem Cells. 2012;30:1051–1052. doi: 10.1002/stem.1103. [DOI] [PubMed] [Google Scholar]
- 16.Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015;16:239–253. doi: 10.1016/j.stem.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penfornis P, Vallabhaneni KC, Whitt J, Pochampally R. Extracellular vesicles as carriers of microRNA, proteins and lipids in tumor microenvironment. Int. J. Cancer. 2015 doi: 10.1002/ijc.29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heldring N, Mager I, Wood MJ, Le Blanc K, Andaloussi SE. Therapeutic Potential of Multipotent Mesenchymal Stromal Cells and Their Extracellular Vesicles. Hum. Gene Ther. 2015 doi: 10.1089/hum.2015.072. [DOI] [PubMed] [Google Scholar]
- 21.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 24.Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem. Biophys. Res. Commun. 2007;354:700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milner CM, Higman VA, Day AJ. TSG-6: a pluripotent inflammatory mediator? Biochem. Soc. Trans. 2006;34:446–450. doi: 10.1042/BST0340446. [DOI] [PubMed] [Google Scholar]
- 27.Wisniewski HG, Vilcek J. Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev. 2004;15:129–146. doi: 10.1016/j.cytogfr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Oh JY, Roddy GW, Choi H, Lee RH, Ylostalo JH, Rosa RH, Jr., Prockop DJ. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16875–16880. doi: 10.1073/pnas.1012451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood. 2011;118:330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foskett AM, Bazhanov N, Ti X, Tiblow A, Bartosh TJ, Prockop DJ. Phase-directed therapy: TSG-6 targeted to early inflammation improves bleomycin-injured lungs. Am J Physiol Lung Cell Mol Physiol. 2014;306:L120–131. doi: 10.1152/ajplung.00240.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh JY, Lee RH, Yu JM, Ko JH, Lee HJ, Ko AY, Roddy GW, Prockop DJ. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther. 2012;20:2143–2152. doi: 10.1038/mt.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Headland SE, Norling LV. The resolution of inflammation: Principles and challenges. Semin. Immunol. 2015;27:149–160. doi: 10.1016/j.smim.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc. Natl. Acad. Sci. U. S. A. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Munoz N, Bunnell BA, Logan TM, Ma T. Density-Dependent Metabolic Heterogeneity in Human Mesenchymal Stem Cells. Stem Cells. 2015 doi: 10.1002/stem.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim DS, Lee MW, Yoo KH, Lee TH, Kim HJ, Jang IK, Chun YH, Park SJ, Lee SH, Son MH, Jung HL, Sung KW, Koo HH. Gene expression profiles of human adipose tissue-derived mesenchymal stem cells are modified by cell culture density. PLoS One. 2014;9:e83363. doi: 10.1371/journal.pone.0083363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee MW, Kim DS, Yoo KH, Kim HR, Jang IK, Lee JH, Kim SY, Son MH, Lee SH, Jung HL, Sung KW, Koo HH. Human bone marrow-derived mesenchymal stem cell gene expression patterns vary with culture conditions. Blood Res. 2013;48:107–114. doi: 10.5045/br.2013.48.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mareschi K, Rustichelli D, Calabrese R, Gunetti M, Sanavio F, Castiglia S, Risso A, Ferrero I, Tarella C, Fagioli F. Multipotent mesenchymal stromal stem cell expansion by plating whole bone marrow at a low cellular density: a more advantageous method for clinical use. Stem Cells Int. 2012;2012:920581. doi: 10.1155/2012/920581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon DS, Kim YH, Jung HS, Paik S, Lee JW. Importance of Sox2 in maintenance of cell proliferation and multipotency of mesenchymal stem cells in low-density culture. Cell Prolif. 2011;44:428–440. doi: 10.1111/j.1365-2184.2011.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cholewa D, Stiehl T, Schellenberg A, Bokermann G, Joussen S, Koch C, Walenda T, Pallua N, Marciniak-Czochra A, Suschek CV, Wagner W. Expansion of adipose mesenchymal stromal cells is affected by human platelet lysate and plating density. Cell Transplant. 2011;20:1409–1422. doi: 10.3727/096368910X557218. [DOI] [PubMed] [Google Scholar]
- 42.Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, Levitt J, Wiener-Kronish J, Bajwa EK, Leavitt A, McKenna D, Thompson BT, Matthay MA. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Lee RH, Yu JM, Foskett AM, Peltier G, Reneau JC, Bazhanov N, Oh JY, Prockop DJ. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc. Natl. Acad. Sci. U. S. A. 2014;111:16766–16771. doi: 10.1073/pnas.1416121111. [DOI] [PMC free article] [PubMed] [Google Scholar]