Abstract
The imidazoline I2 receptor is an emerging drug target for analgesics. This study extended previous studies by examining the antinociceptive effects of three I2 receptor agonists (2-BFI, BU224 and CR4056) in the formalin test. The receptor mechanisms and anatomical mediation of I2 receptor agonist-induced antinociception were also examined. Formalin-induced flinching responses (2%, 50µl) were quantified after treatment with I2 receptor agonists alone or in combination with the I2 receptor antagonist idazoxan. Anatomical mediation was studied by locally administering 2-BFI into the plantar surface or into the right lateral ventricle via cannulae (i.c.v). The locomotor activity was also examined after central (i.c.v.) administration of 2-BFI. 2-BFI (1–10 mg/kg, i.p.) and BU224 (1–10 mg/kg, i.p.) attenuated the spontaneous flinching response observed during 10 min (phase 1) and 20–60 min (phase 2) following formalin treatment, while CR4056 (1–32 mg/kg, i.p.) only decreased phase 2 flinching response. The I2 receptor antagonist idazoxan attenuated the antinociceptive effects of 2-BFI and BU224 during phase 1, but not phase 2. Peripheral administration of 2-BFI (1–10 mg/kg, i.pl) to the hindpaw of rats had no antinociceptive effects. In contrast, centrally delivered 2-BFI (10–100 µg, i.c.v.) dose-dependently attenuated phase 1 and phase 2 flinching at doses that did not reduce the locomotor activity. Together, these data revealed the differential antinociceptive effects of I2 receptor agonists and the differential antagonism profiles by idazoxan, suggesting the involvement of different I2 receptor subtypes in reducing different phases of formalin-induced pain-like behaviors. In addition, the results also suggest the central mediation of I2 receptor agonist-induced antinociceptive actions.
Keywords: formalin test, imidazoline I2 receptor ligands, Intracerebroventricular, idazoxan, rat
INTRODUCTION
Chronic pain reduces the quality of life and imparts high health costs and economic loss to society. Unfortunately, currently available analgesics are not adequate to meet the clinical needs, leaving a large population with undertreated pain (Institute of Medicine, 2011). Opioids and non-steroidal anti-inflammatory drugs (NSAIDs) are the most widely used analgesics; however, their use is constrained by limited effectiveness to certain painful conditions and adverse side effects. Although the understanding of pain mechanisms has greatly improved over the past few decades, this has not translated into adequate new clinically effective analgesics (Mogil, 2009). Thus, there is still a dire need for the development of novel pharmacotherapy to treat pain.
The imidazoline I2 receptor is an emerging drug target for the development of analgesics as I2 receptor agonists demonstrate antinociceptive effects in rodent models of acute and chronic pain (Ferrari et al., 2011; Li et al., 2011,2014; Meregalli et al., 2012; Sampson et al., 2012). For example, the I2 receptor agonist CR4056 reduces mechanical hyperalgesia in rat models of complete Freund’s adjuvant (CFA)-induced inflammatory pain and chemotherapy-induced painful neuropathy in animals (Ferrari et al., 2011; Meregalli et al., 2012). We also found that I2 receptor agonists reduce CFA-induced inflammatory pain and nerve injury-induced neuropathic pain (Li et al., 2014). Te formalin test is one of the most commonly used pain assay. Introduced in 1977, this test allows for the study of nocifensive behaviors without restraint, and with a continuous source of stimulation (Dubuisson and Dennis, 1977). After an injection of formalin into the skin of the rodent hind paw, an intense early phase (phase 1) of hindpaw guarding, licking and flinching behaviors emerges and subsides about 10 min after formalin injection, which is followed by reappearing nocifensive behaviors that last as long as 1 hour (phase 2). Phase 1 of this test is thought to be a direct effect of formalin on nociceptive fibers (neurogenic pain), while phase 2 has long been ascribed to formalin-induced inflammation (inflammatory pain) (Hunskaar and Hole, 1987). Thus, one assay can examine the antinociceptive effects of potential analgesics on two types of pain. However, the effects of I2 receptor agonists on formalin pain are unknown. The purpose of the current study is two-fold. Firstly, this study examined the antinociceptive effects of three I2 receptor agonists, [2-BFI (2-(2-benzofuranyl)-2-imidazoline) hydrochloride, BU224 (2-(4, 5-dihydroimidazol-2-yl) quinolone) hydrochloride and CR4056 (2-phenyl-6-(1H imidazol-1yl) quinazoline)], which reportedly all have anti-hyperalgesic effects in animal models of inflammatory and neuropathic pain (Ferrari et al., 2011; Li et al., 2014) in the formalin test, to examine the generality of the antinociceptive actions of I2 receptor agonists. Given the reported antinociceptive profiles of the three I2 receptor agonists, it was expected that all would produce similar and dose-dependent antinociceptive effects in both phases of the formalin test. Because the anti-hyperalgesic effects of all the three I2 receptor agonists were antagonized by the commonly used non-selective I2 receptor antagonist idazoxan (Ferrari et al., 2011; Li et al., 2014), it was also anticipated that idazoxan would be able to attenuate the antinociceptive effects of these same I2 receptor agonists in the formalin test in the current study. Secondly, because little is known of the anatomical (i.e., central versus peripheral) mediation of I2 receptor agonist-induced antinociception, this study also examined whether I2 receptor agonists are acting through a peripherally- or centrally-mediated mechanism to produce their antinociceptive effects.
METHODS
Subjects
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed individually on a 12/12-h light/dark cycle (behavioral experiments were conducted during the light period) with free access to water and food except during experimental sessions. Animals were maintained and experiments were conducted in accordance with guidelines of the International Association for the Study of Pain (Zimmermann, 1983) and were approved by the Institutional Animal Care and Use Committee, University at Buffalo, the State University of New York, and with the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington DC).
Formalin test
Groups of 6–7 rats were assigned to each dose/drug combination and each rat was tested once. Formalin (2%, 50 µl) was injected using a 28 G needle into the dorsal surface of the right hindpaw while the rat was restrained manually. Immediately after formalin injection, the rat was placed into the observation arena (acrylic clear chamber 40 cm × 40 cm × 30 cm) and began the scoring. A mirror was placed behind the chamber and opposite to the experimenter to facilitate visual observation. Flinching is characterized as a rapid and brief withdrawal or flexion of the injected paw. This pain-related behavior was quantified by counting the number of flinches for 5 min periods at 0 – 80 min following formalin administration. Two phases of spontaneous flinching behavior were observed: an initial acute phase (phase 1: during the first 10 min after the formalin injection) and a prolonged tonic phase (phase 2: beginning about 20 min after the formalin injection). For drug studies, formalin was injected 10 min following pretreatment with I2 receptor agonists (intraperitoneally [i.p.] or subcutaneously [s.c]), or immediately following intracerebroventricular (i.c.v.) administration. When drugs were studied in combination, the first drug was administered 10 min prior to the second drug, which was followed by formalin injection 10 min later. The pretreatment time was based on previous studies showing that this time was sufficient to achieve maximal behavioral effects (Qiu et al., 2014,2015). For peripheral drug administration, the study drug was administered into the plantar surface skin of the right hindpaw (intraplantar, i.pl). The experienced observers were blind to drug treatments. For the drug combination studies, the doses of agonists that produced the maximal antinociceptive effects in the single acute dose studies were used and the doses of idazoxan were chosen according to previous studies which show significant blockade of antinociceptive effects induced by I2 receptor agonists (Meregalli et al., 2012; Li et al., 2014).
Stereotaxic surgery
Rats were anesthetized with ketamine/xylazine (60/5 mg/kg, i.p.) and placed into a stereotaxic frame (Model # 962; David Kopf Instruments, Tujunga, CA, USA). Stainless steel guide cannulae (26 gauge; Plastics One, Roanoke, VA, USA) were inserted into the right lateral ventricle. The stereotaxic coordinates for intracranial cannulae were: A/P = −0.9; M/L = +1.4; D/V = −1.8. Following implantation, guide cannulae were secured to the skull with cranioplastic cement and cranial screws, and stylets were placed into the guide cannulae to prevent blockage. Cannula placement was verified by administering 10 ng of angiotensin II in TBS using injector cannulae that extended 1.5 mm past the guide cannulae and observing drinking behavior for 30 min (Vento et al., 2012).
Locomotor activity
Locomotor activity was monitored by an infrared motor-sensor system (AccuScan Instruments, Columbus, OH) fitted outside clear acrylic chambers (40 × 40 × 30 cm) that were cleaned between test sessions. Locomotor activity (distance travelled) was analyzed for 1 hr immediately after i.c.v drug administration with the Versa Max animal activity monitoring software (AccuScan Instruments, Columbus, OH) (Thorn et al., 2014).
Drugs
2-BFI, BU224 and CR4056 were synthesized according to standard procedures (Jarry et al., 1997; Ishihara and Togo, 2007). Idazoxan hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO, USA). Unless otherwise noted, all drugs were dissolved in physiological saline and administered either i.p. or i.pl (peripheral), or dissolved in DPBS and administered i.c.v. (central). CR4056 was dissolved in 20% DMSO with saline and a drop of HCl and administered i.p. Doses are expressed as mg of the form indicated earlier per kg body weight. Injection volumes were 1 ml/kg for i.p., 50 µl for i.pl, and 4 µl for i.c.v.
Data analyses
The total number of flinches observed during 10 min (phase 1) and 20–60 min (phase 2) following formalin treatment were collected and analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc analysis. P < 0.05 was considered statistically significant. The analysis of phase 2 only included the data between 20–60 min after formalin injection because it was found that data after 60 min tended to converge among groups and did not represent the drug effects.
RESULTS
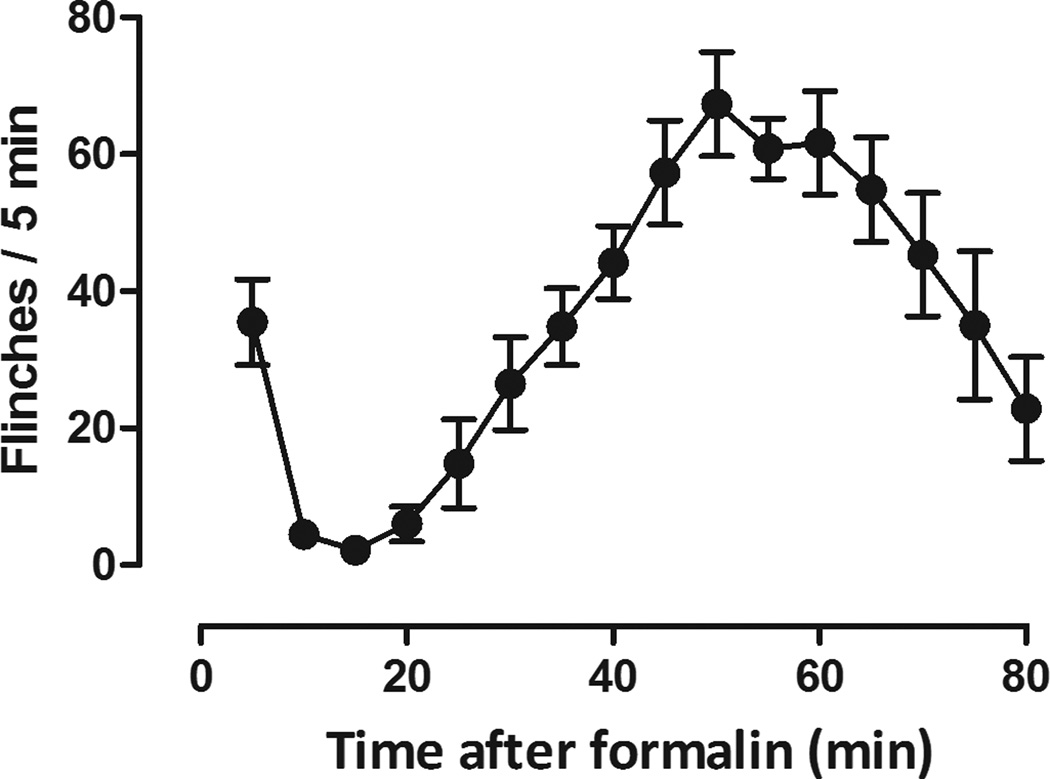
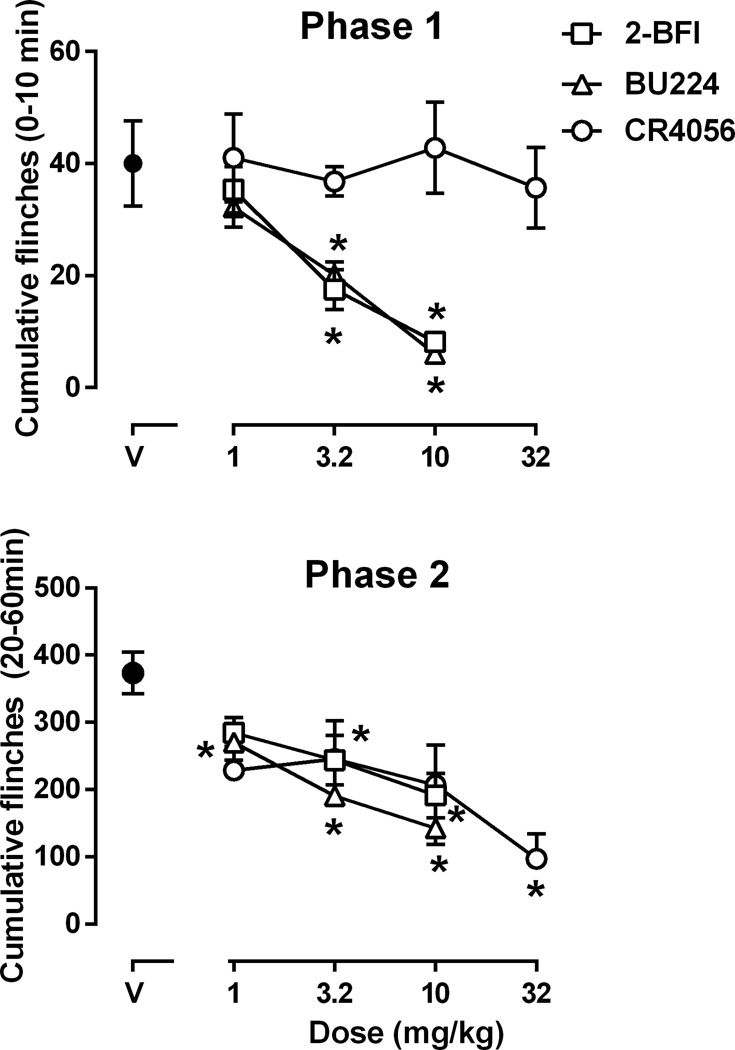
The flinching response is not a natural behavior and rats without formalin treatment did not display flinching (data not shown). Under control conditions, rats displayed flinching behavior in two distinct phases following injection of formalin to their hindpaw (Fig. 1). Formalin produced flinching immediately following injection which lasted 10 min and corresponded to phase 1 (Fig. 1). Phase 2 flinching occurred 20 min following formalin treatment and lasted 60 min (Fig 1). The total flinches observed during phase 1 (0–10 min) and phase 2 (20–60 min) following formalin were plotted as a function of pretreatment drug dose (Fig. 2). Both 2-BFI (left, Fig. 2) and BU224 (middle, Fig. 2) decreased flinching behavior in both phases. One-way ANOVA revealed a significant main effect of 2-BFI on phase 1 (F [3, 23] = 10.06, P < 0.001) and phase 2 (F [3, 23] = 6.09, P < 0.01) flinching. Post hoc analyses indicated that the effects induced by 3.2 and 10 mg/kg 2-BFI was significantly different from that induced by vehicle control for both phases. Similarly, one-way ANOVA revealed that BU224 produced a significant main effect on phase 1 (F [3, 23] = 11.58, P < 0.001) and phase 2 (F [3, 23] = 17.77, P < 0.001) flinching. Post hoc analyses indicated that the effects induced by 3.2 and 10 mg/kg BU224 were significantly different from control during phase 1, while effects induced by all doses of BU224 were significantly different from control during phase 2. In contrast, CR4056 failed to produce a significant effect on flinching behavior during phase 1 (top right, Fig. 2). One-way ANOVA revealed that CR4056 produced a significant effect in phase 2 (F [4, 29] = 4.68, P < 0.01). Post hoc analysis indicated that the effect induced by 32 mg/kg CR4056 was significantly different from that of control group during phase 2 flinching (bottom right, Fig. 2).
Figure 1.
Formalin-induced flinching in rats (n=6 per group). Vertical axis, total flinches every 5 min; horixontal axis, time following formalin treatment (min).
Figure 2.
Effects of 2-BFI, BU224 and CR4056 on formalin-induced flinching (n=6 per group). Vertical axis, total flinches during 0–10 min (phase 1, upper panel) and 20–60 min (phase 2, lower panel) following formalin treatment; horixontal axis, drug dose (mg/kg). * P < 0.05 as compared to vehicle control.
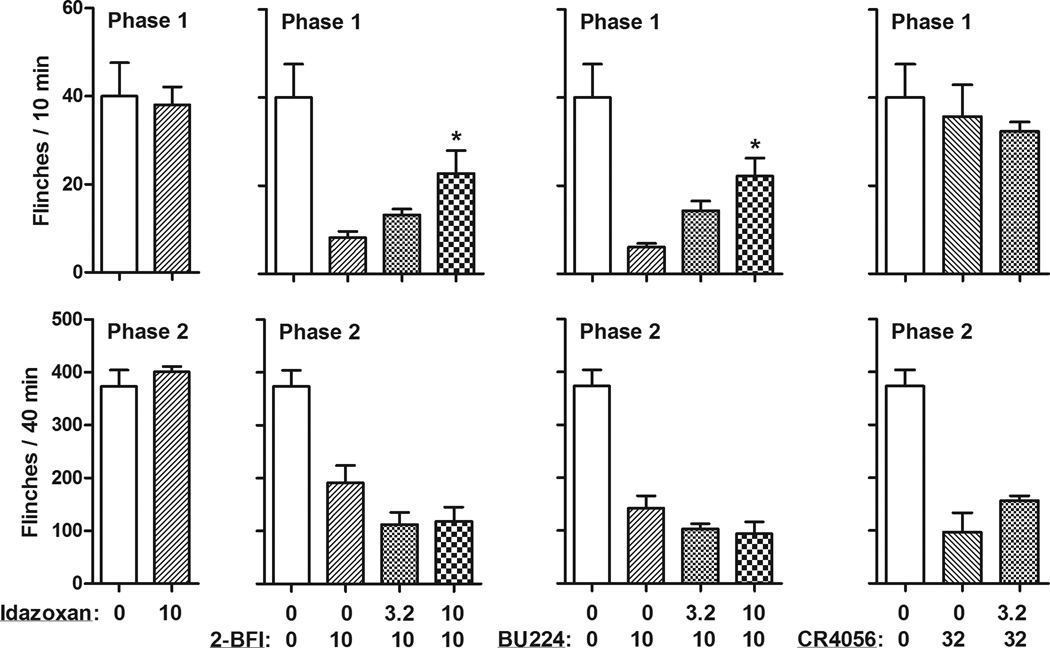
In order to examine the pharmacological mechanism of the antinociceptive effects induced by I2 receptor agonists, the commonly used imidazoline I2 receptor antagonist idazoxan was studied in combination with all the I2 receptor agonists. Idazoxan alone up to a dose of 10 mg/kg had no significant effect on formalin-induced flinching behavior (left, Fig. 3). When studied as a pretreatment, idazoxan significantly attenuated the antinociceptive effects of 2-BFI (F [2, 16] = 6.39, P < 0.05) and BU224 (F [2, 17] = 8.99, P < 0.01) during phase 1. Post hoc analyses indicated that 10 mg/kg idazoxan significantly prevented the antinociceptive effects of 2-BFI and BU224. However, idazoxan failed to alter the antinociceptive effects of 2-BFI or BU224 during phase 2. Furthermore, although CR4056 only produced antinociceptive effects during phase 2 flinching, 3.2 mg/kg idazoxan failed to attenuate this effect. The larger dose of idazoxan (10 mg/kg) was studied as a pretreatment in one rat receiving 32 mg/kg CR4056 to examine whether the effect of CR4056 could be antagonized. A marked sedation was observed in this rat which likely precluded the expression of flinching behavior. Thus, testing with this dose combination was discontinued.
Figure 3.
Effects of idazoxan pretreatment on the antinociceptive effects of 2-BFI, BU224 and CR4056 (n=6 per group). Vertical axis, total flinches during 0–10 min (phase 1, top) and 20–60 min (phase 2, bottom) following formalin treatment; horixontal axis, drug dose (mg/kg).* P < 0.05 as compared to 2-BFI, BU224, or CR4056 alone. The control group data were re-plotted from Figure 2.
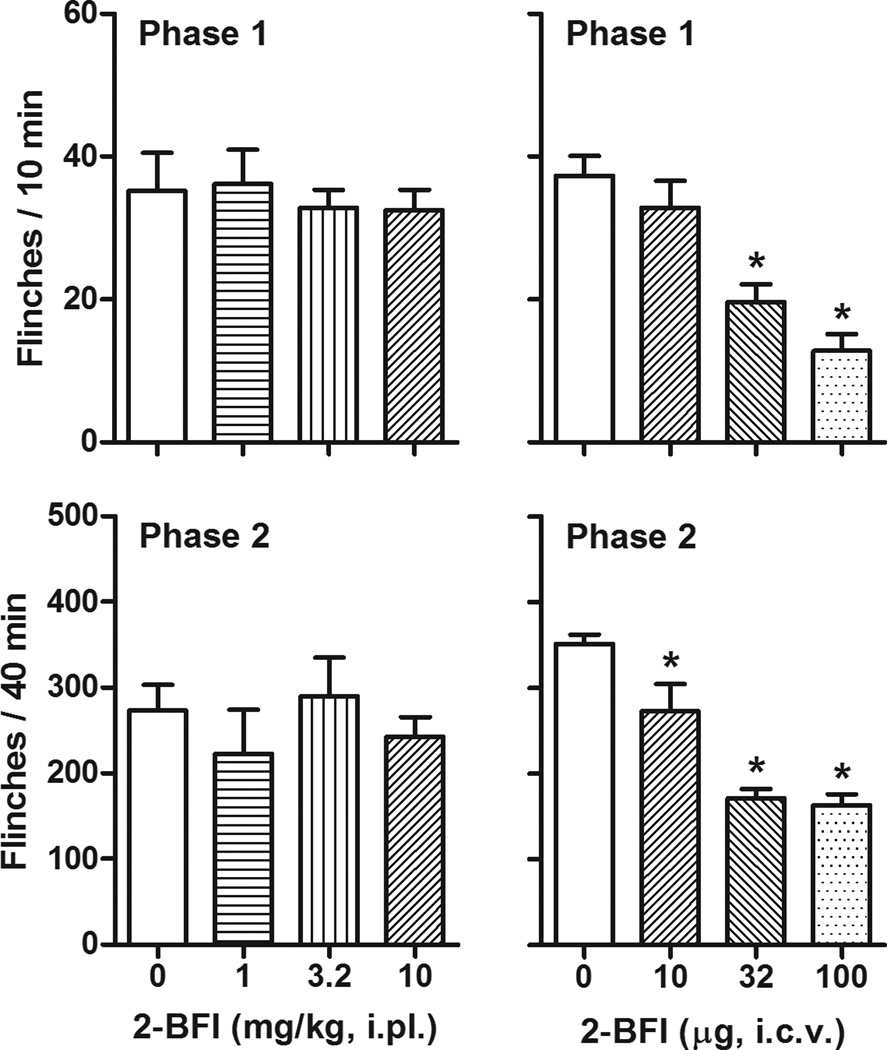
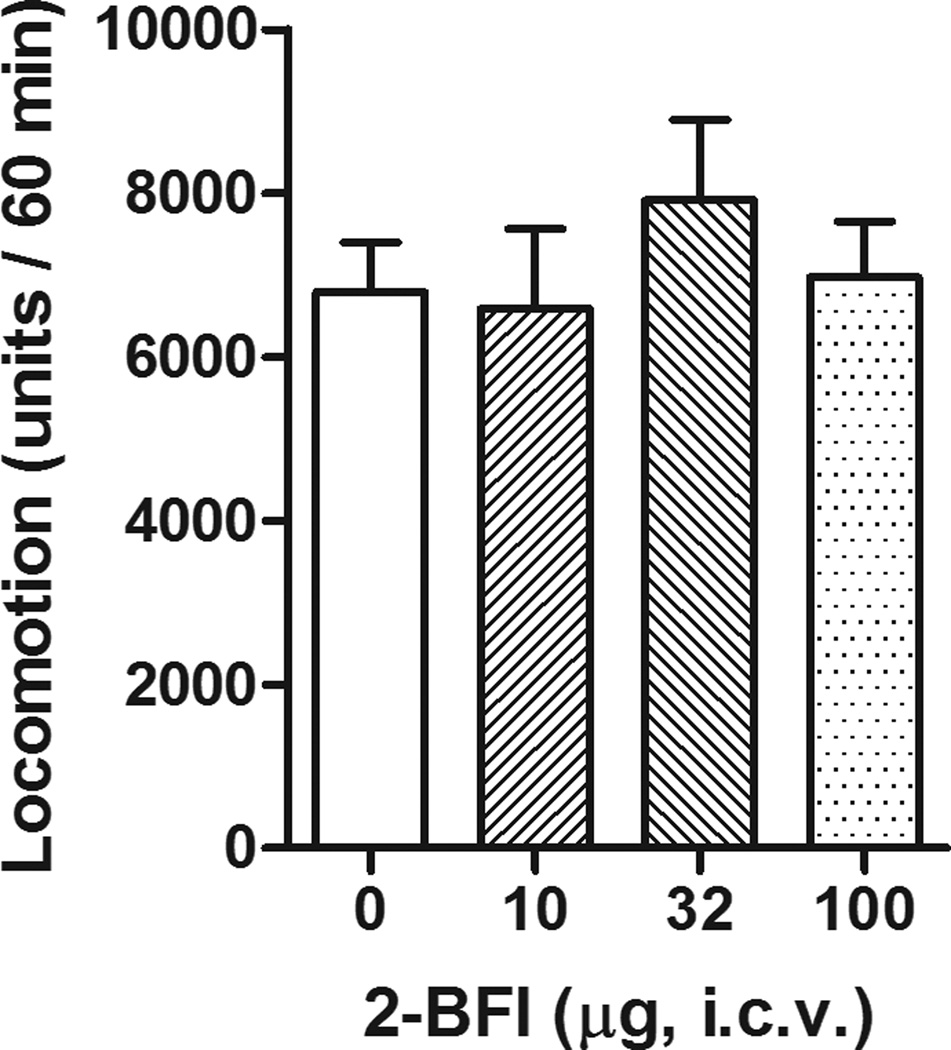
Intraplantar administration of 2-BFI to the rats’ hindpaw failed to produce antinociceptive effects (Fig. 4). In contrast, one-way ANOVA revealed that central 2-BFI administration, by delivering the drug to the right lateral ventricle, produced a significant effect on the flinching behavior during phase 1 (F [3, 23] = 15.55, P < 0.001) and phase 2 (F [3, 23] = 23.07, P < 0.001). Post hoc analyses indicated that 32 and 100 µg 2-BFI produced significant effects compared to those of control group during phase 1, while all doses of 2-BFI produced effects that were significantly different from those of the control group during phase 2 (Fig. 4). Furthermore, centrally administered 2-BFI failed to produce affect the rats’ general locomotor activity (Fig. 5).
Figure 4.
Effects of peripheral (i.pl) or central (i.c.v) administration of 2-BFI on formalin-induced flinching (n=6 per group). Vertical axis, total flinches during 0–10 min (phase 1, top) and 20–60 min (phase 2, bottom) following formalin treatment; horixontal axis, drug dose (mg/kg or µg). * P < 0.05 as compared to vehicle control.
Figure 5.
Effects of central (i.c.v) administration of 2-BFI on general locomotor activity (n=6 per group). Vertical axis, total distance traveled (units) during the 60 min session; horixontal axis, drug dose (µg).
DISCUSSION
The primary findings of the current study were that selective imidazoline I2 receptor agonists produced antinociceptive effects in the formalin test, a well-validated rat model of tonic persistent pain. In addition, these effects appear to be acting through a centrally- but not peripherally-mediated mechanism. Interestingly, the data also showed that idazoxan-sensitive I2 receptors primarily participate in the antinociceptive effects during phase 1 but not phase 2 of formalin test. Taken together, these data extend the previous findings that I2 receptor agonists are effective against acute and chronic pain (Ferrari et al., 2011; Li et al., 2011,2014; Meregalli et al., 2012) by showing that these ligands produce antinociception via a central mechanism.
The formalin test is a well-validated procedure and differs from most models of pain in two ways. Firstly, most traditional tests of nociception are based on a phasic stimulus of high intensity and since the pain experience is short-lasting, it is thus not possible to assess modulatory mechanisms that may be triggered by the stimulus itself (Tjolsen et al., 1992). Secondly, formalin induces two distinct phases of nociceptive behavior consisting of an early phase (phase 1) which is predominantly caused by direct chemical stimulation of nociceptors, and a late phase (phase 2) due to an inflammatory reaction (Hunskaar and Hole, 1987). As a result, the formalin test has been suggested to provide a more valid model for clinical pain compared to the traditional tests using a high intensity phasic stimulus (Abbott et al., 1981; Dubuisson and Dennis, 1977). Consistent with the literature, we found that formalin produced spontaneous flinching behavior when injected into the hindpaw of rats and this occurred in two phases (Fig. 1). The I2 receptor agonists 2-BFI and BU224 attenuated flinching behavior during phase 1 and phase 2 following formalin treatment, while CR4056 only decreased phase 2 flinching (Fig. 2). 2-BFI has previously been shown to produce antinociceptive effects in animal models of both nerve-injury induced neuropathic pain and CFA-induced inflammatory pain (Li et al., 2014). In the formalin test, phase 1 is thought to be neurogenic and phase 2 is more related to inflammatory pain (Hunskaar and Hole, 1987). The finding that both 2-BFI and BU224 produced antinociceptive effects for both phases extends previous findings by suggesting that such compounds are efficacious against both neuropathic and inflammatory pain.
Two unexpected findings need to be discussed. Firstly, it was surprising that CR4056 only attenuated the flinching behavior during phase 2 but showed no significant effect during phase 1. In studies that evaluate the antinociceptive effects of CR4056 in models of CFA-induced inflammatory pain and capsaicin-induced mechanical hyperalgesia, the effects of CR4056 were completely blocked by the I2 receptor antagonist idazoxan (Ferrari et al., 2011). Idazoxan also attenuates the antinociceptive effects of 2-BFI in a rat model of CFA-induced pain (Li et al., 2014). Using the drug discrimination assay, a procedure that is widely used to assess and compare the interoceptive effects induced by centrally active compounds, we found that both 2-BFI and CR4056 fully substitute for BU224 in rats discriminating 5.6 mg/kg BU224, which suggests that all the three compounds share similar pharmacological mechanisms (Qiu et al., 2015). In addition, 2-BFI, BU224 and CR4056 all produce I2 receptor-mediated hypothermia in rats (Thorn et al., 2012). Besides the functional studies, receptor binding results also indicate that all the three compounds are highly selective I2 receptor ligands and have little affinity for dozens of other receptors (Ferrari et al., 2011; Thorn et al., 2012). All these results strongly suggest that CR4056 shares similar pharmacological mechanisms with 2-BFI and BU224 and should be expected to produce similar antinociceptive effects in formalin test. The lack of effect of CR4056 on phase 1 is not likely due to inadequate dosing or inadequate pretreatment time because both 2-BFI and BU224 show similar potencies in both phases, the doses of CR4056 studied are well within the dose range that produce many other behavioral effects including antinociception (Ferrari et al., 2011; Thorn et al., 2012; Qiu et al., 2014), and the pretreatment time was sufficient to achieve maximal behavioral effects (Qiu et al., 2014). Despite these similarities between CR4056 and other I2 receptor agonists, emerging evidence suggests that important differences exist. In rats discriminating 10 mg/kg CR4056, 2-BFI only partially substitutes for CR4056 while BU224 neither substitutes for nor blocks the discriminative stimulus effects of CR4056 (Qiu et al., 2014). The asymmetrical substitution between CR4056 and BU224 suggests that the two compounds have critical differences despite the apparent partially overlapping pharmacological effects. Thus, the different effects between BU224 and CR4056 on phase 1 of formalin test may be due to the non-overlapping idazoxan-sensitive I2 receptor subtypes, although the exact mechanism remains to be determined.
Another unexpected finding was that idazoxan was ineffective in blocking the antinociceptive effects of all the three I2 receptor agonists on phase 2 of the formalin test. As discussed above, it should be expected that idazoxan should be able to attenuate the antinociceptive effects of the I2 receptor agonists, as shown in other studies using different models of pain (Ferrari et al., 2011; Li et al., 2011,2014). Although idazoxan blocks some behavioral effects (notably antinociceptive effects) of I2 receptor agonists, it produces similar discriminative stimulus effects with I2 receptor agonists (Jordan et al., 1996; Qiu et al., 2014,2015) and does not affect the behavioral effects of I2 receptor agonists under certain conditions (Min et al, 2013). These apparent discrepancies are interpreted as differential binding and/or functional activities of idazoxan and other I2 receptor agonists on different I2 receptor subtypes (Qiu et al., 2014,2015). I2 receptors are usually identified by [3H] 2-BFI or [3H] idazoxan radio-ligands and such binding sites are heterogeneous and consist of multiple proteins (Escriba et al., 2009; Regunathan and Reis, 1996), including one that was recently identified as brain creatine kinase (Kimura et al., 2009). It is likely that these different I2 receptor subtypes are functionally different and that I2 receptor ligands with differential binding activities on these subtypes have different functional effects (Qiu et al., 2014). In the formalin test, the two phases differentially respond to some analgesics (Ossipov et al., 2000). Thus, the discrepancy that idazoxan only blocked the antinociceptive effects of I2 receptor agonists on phase 1 but not phase 2 might be due to the differential involvement of I2 receptor subtypes in the modulation of formalin pain. It is plausible that 2-BFI and BU224 may exert antinociceptive effects on phase 1 through idazoxan-sensitive subtypes and attenuate the nociceptive behavior during phase 2 through idazoxan-insensitive subtypes of I2 receptors. The lack of antagonism by idazoxan was not due to the low dose used, as much lower doses have been shown to block I2 receptor mediated effects (Li et al., 2014; Thorn et al., 2012). The effects of I2 receptor agonists in the formalin test are behaviorally specific because the doses of the compounds studied do not impair general behavior in rats (Thorn et al., 2012).
I2 receptors are expressed in most tissues and organs that have been studied (Regunathan and Reis, 1996). Almost all studies using selective I2 receptor ligands treat animals systemically. In one study, I2 receptor agonists were found to enhance morphine antinociception when both were administered i.c.v, suggesting that the interaction occurs in the brain (Sanchez-Blazquez et al., 2000). However, it is largely unclear whether the antinociceptive effects produced by I2 receptor agonists are centrally or peripherally mediated. When 2-BFI was delivered directly to the hindpaw prior to formalin treatment, no antinociception was observed (Fig. 4). In contrast, centrally administered 2-BFI into the right lateral ventricle produced significant antinociception during both phases of the formalin test at doses that had no significant effect on the general locomotor behavior of rats (Fig. 5). These results clearly showed that the antinociception of I2 receptor agonists in formalin test is a centrally-mediated event.
In summary, this study found that imidazoline I2 receptor agonists produce antinociceptive effects in the formalin test via both idazoxan-sensitive and -insensitive mechanisms. The observations of differential modulation of formalin pain by I2 receptor agonists and differential antagonism by idazoxan suggest that there are functional differences between I2 receptor subtypes. In addition, the selective I2 receptor agonist 2-BFI produces antinociception via a central, but not peripheral mechanism. Taken together, this study further supports the notion that I2 receptors represent a novel centrally-active drug target to treat pain.
Acknowledgements
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (Awards no. R01DA034806 and R21DA032837) and by a grant from National Natural Science Foundation of China (81373390). The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
There are no conflicts of interest.
References
- Abbott FV, Franklin KB, Ludwick RJ, Melzack R. Apparent lack of tolerance in the formalin test suggests different mechanisms for morphine analgesia in different types of pain. Pharmacol Biochem Behav. 1981;15:637–640. doi: 10.1016/0091-3057(81)90222-7. [DOI] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Escriba PV, Ozaita A, Garcia-Sevilla JA. Pharmacologic characterization of imidazoline receptor proteins identified by immunologic techniques and other methods. Ann N Y Acad Sci. 1999;881:8–25. doi: 10.1111/j.1749-6632.1999.tb09336.x. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Fiorentino S, Mennuni L, Garofalo P, Letari O, Mandelli S. Analgesic efficacy of CR4056, a novel imidazoline-2 receptor ligand, in rat models of inflammatory and neuropathic pain. J Pain Res. 2011;4:111–125. doi: 10.2147/JPR.S18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (U.S.) Relieving pain in America: A blueprint for transforming prevention, care, education and research. Washington, D.C.: National Academies Press; 2011. [PubMed] [Google Scholar]
- Ishihara M, Togo H. Direct oxidative conversion of aldehydes and alcohols to 2 imidazolines and 2-oxazolines using molecular iodine. Tetrahedron. 2007;63:1474–1480. [Google Scholar]
- Jarry C, Forfar I, Bosc J, Renard P, Scalbert E, Guardiola B. 5-(Arloxymethyl) oxazoline. 5,686,477. Adir e Compagnie; US Patent. 1997
- Jordan S, Jackson HC, Nutt DJ, Handley SL. Discriminative stimulus produced by the imidazoline I2 site ligand, 2 -BFI. J Psychopharmacol. 1996;10:273–278. doi: 10.1177/026988119601000403. [DOI] [PubMed] [Google Scholar]
- Kimura A, Tyacke RJ, Robinson JJ, Husbands SM, Minchin MC, Nutt DJ, Hudson AL. Identification of an imidazoline binding protein: creatine kinase and an imidazoline-2 binding site. Brain Res. 2009;1279:21–28. doi: 10.1016/j.brainres.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Zhang Y, Winter JC. Morphine-induced antinociception in the rat: supra-additive interactions with imidazoline I(2) receptor ligands. Eur J Pharmacol. 2011;669:59–65. doi: 10.1016/j.ejphar.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Li JX, Thorn DA, Qiu Y, Peng BW, Zhang Y. Anti-hyperalgesic effects of imidazoline I2 receptor ligands in rat models of inflammatory and neuropathic pain. Br J Pharmacol. 2014;171:1580–1590. doi: 10.1111/bph.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meregalli C, Ceresa C, Canta A, Carozzi VA, Chiorazzi A, Sala B. CR4056, a new analgesic I2 ligand, is highly effective against bortezomib-induced painful neuropathy in rats. J Pain Res. 2012;5:151–167. doi: 10.2147/JPR.S32122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JW, Peng BW, He X, Zhang Y, Li JX. Gender difference in epileptogenic effects of 2-BFI and BU224 in mice. Eur J Pharmacol. 2013;718:81–86. doi: 10.1016/j.ejphar.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Jerussi TP, Ren K, Sun H, Porreca F. Differential effects of spinal (R)-ketoprofen and (S)-ketoprofen against signs of neuropathic pain and tonic nociception: evidence for a novel mechanism of action of (R)-ketoprofen against tactile allodynia. Pain. 2000;87:193–199. doi: 10.1016/S0304-3959(00)00280-3. [DOI] [PubMed] [Google Scholar]
- Qiu Y, He XH, Zhang Y, Li JX. Discriminative stimulus effects of the novel imidazoline I2 receptor ligand CR4056 in rats. Scientific Reports. 2014;4:6605. doi: 10.1038/srep06605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Zhang Y, Li JX. Discriminative stimulus effects of the imidazoline I2 receptor ligands BU224 and phenyzoline in rats. Eur J Pharmacol. 2015;749:133–141. doi: 10.1016/j.ejphar.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regunathan S, Reis DJ. Imidazoline receptors and their endogenous ligands. Annu Rev Pharmacol Toxicol. 1996;36:511–544. doi: 10.1146/annurev.pa.36.040196.002455. [DOI] [PubMed] [Google Scholar]
- Sampson C, Zhang Y, Del Bello F, Li JX. Effects of imidazoline I2 receptor ligands on acute nociception in rats. Neuroreport. 2012;23:73–77. doi: 10.1097/WNR.0b013e32834e7db3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Blazquez P, Boronat MA, Olmos G, Garcia-Sevilla JA, Garzon J. Activation of I(2)-imidazoline receptors enhances supraspinal morphine analgesia in mice: a model to detect agonist and antagonist activities at these receptors. Br J Pharmacol. 2000;130:146–152. doi: 10.1038/sj.bjp.0703294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, An XF, Zhang Y, Pigini M, Li JX. Characterization of the hypothermic effects of imidazoline I(2) receptor agonists in rats. Br J Pharmacol. 2012;166:1936–1945. doi: 10.1111/j.1476-5381.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Jing L, Qiu Y, Gancarz-Kausch AM, Galuska CM, Dietz DM, Zhang Y, Li JX. Effects of the trace amine-associated receptor 1 agonist RO5263397 on abuse-related effects of cocaine in rats. Neuropsychopharmacology. 2014;39:2309–2316. doi: 10.1038/npp.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Vento PJ, Myers KP, Daniels D. Investigation into the specificity of angiotensin II-induced behavioral desensitization. Physiol Behav. 2012;105:1076–1081. doi: 10.1016/j.physbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]