Abstract
Arf encodes an important tumor suppressor, p19Arf, which also plays a critical role to control hyperplasia in the primary vitreous during mouse eye development. In the absence of Arf, mice are born blind and display a phenotype closely mimicking severe forms of the human eye disease, persistent hyperplastic primary vitreous (PHPV). In this report, we characterize p19Arf expression in perivascular cells that normally populate the primary vitreous and express the Arf promoter. Using a new ex vivo model, we show that these cells respond to exogenous Tgfβ, despite being isolated at a time when Tgfβ has already turned on the Arf promoter. Treatment of the cells with PDGF-B ligand doubles the population of cells in S-phase and ectopic expression of Arf blunts that effect. We show this effect is mediated through Pdgfrβ as expression of Arf represses expression of Pdgfrβ mRNA and protein to approximately 60%. p53 is not required for Arf-dependent blockade of PDGF-B driven proliferation and repression of Pdgfrβ protein as ectopic expression of Arf is still able to inhibit the 2-fold increase in the S-phase fraction of cells upon treatment with PDGF-B. Finally, induction of mature miR-34a, a microRNA previously identified to be regulated by p19Arf does not depend on p53 while the expression of the primary transcript does require p53. These data corroborate that, as in vivo, p19Arf functions to inhibit PDGF-B driven proliferation ex vivo.
Keywords: p19Arf, Primary vitreous, PHPV, Pdgfrβ, Perivascular cells, p53, miR-34a
1. Introduction
During mammalian eye development, the hyaloid vascular system (HVS) in the primary vitreous provides nutrients to foster lens and retina development. This vasculature consists of the hyaloid artery, which feeds the vasa hyaloidea propria (VHP) and tunica vasculosa lentis (TVL) (Goldberg, 1997; Ito and Yoshioka, 1999). To achieve optimal vision, this fully formed vasculature regresses in later stages of eye development, resulting in the avascular and largely acellular secondary vitreous. Defects in HVS regression and primary vitreous maturation lead to ocular disease in children. This disease, variously known as either persistent hyperplastic primary vitreous (PHPV) or persistent fetal vasculature (PFV) (Goldberg, 1997; Haddad et al., 1978) covers a wide spectrum of severity from mere remnants of the hyaloid artery stalk to cellular hyperplasia leading to erosion of the lens capsule posteriorly and dysplasia and tractional detachment of the retina, leading to blindness (Goldberg, 1997; Haddad et al., 1978). Currently, therapeutic interventions largely hinge on surgical interventions to try to preserve or restore vision or remove a severely diseased eye (Hunt et al., 2005; Pollard, 1997).
The underlying pathogenetic mechanisms are likely to vary with disease severity, and this notion is supported in pre-clinical mouse models of the disease. These mechanisms can largely be divided into fundamental defects in pro-apoptotic processes needed to clear the cells from the vitreous and defects in processes that check hyperplastic expansion of primary vitreous cells – quite literally paralleling the two descriptors for the disease: PFV and PHPV. The former is reflected in mouse models with germ-line destruction of the p53 tumor suppressor gene (Ikeda et al., 1999) or angiopoietin-2 (Gale et al., 2002; Hackett et al., 2002) or in mice lacking macrophage-like hyalocytes (Lang and Bishop, 1993) or the WNT7B signals needed for them to exert cytotoxic effects on endothelial cells in the HVS (Lobov et al., 2005). In contrast, deregulated vascular endothelial growth factor (VEGF) (Mitchell et al., 1998; Rutland et al., 2007) or platelet-derived growth factor-B (PDGF-B) (Lei et al., 2010; Niklasson et al., 2010) support the hyperplastic expansion of primary vitreous cells and PHPV-like phenotype. Anti-proliferative factors have also been implicated. In particular, loss of the Arf tumor suppressor gene leads to hyperplasia of Pdgfrβ-expressing perivascular cells that normally flank the hyaloid artery mice mimics many aspects of PHPV, including microphthalmia; retrolental, fibrovascular mass that erodes the lens capsule and distorts the retina; and dense lens opacity resulting in blindness (Martin et al., 2004; McKeller et al., 2002). Although Arf−/− mice develop bilateral, severe eye disease, contrasting the typical clinical presentation, loss of Arf in just a subset of primary vitreous cells through somatic mosaic deletion leads to a more variable phenotype with incomplete disease penetrance – better mimicking the clinical scenario (Mary-Sinclair et al., 2014).
Because Arf is normally expressed in perivascular cells of the primary vitreous, it represents an essential component of normal mouse eye development (Martin et al., 2004; McKeller et al., 2002; Silva et al., 2005). Arf encodes p19Arf, a nuclear protein best known for its capacity to negatively regulate Mdm2, thereby stabilizing (and activating) p53 (Honda and Yasuda, 1999; Weber et al., 1999). A linear pathway from p19Arf to p53 cannot fully account for the developmental effects, though, because p53 deficient mice usually have normal eyes, whereas Arf loss leads to PHPV in a variety of pure and mixed mouse strains (Ikeda et al., 1999; McKeller et al., 2002; Reichel et al., 1998). Clues to the biochemical effects of p19Arf first came from genetic studies showing that the hyperplastic phenotype is ameliorated in Arf−/−, Pdgfrβ−/− embryos (Widau et al., 2012), and p19Arf expression correlates with lower expression of Pdgfrβ in the developing eye, and in cultured mouse embryonic fibroblasts (MEFs) (Silva et al., 2005; Thornton et al., 2007; Widau et al., 2012). More detailed mechanistic studies – also conducted in MEFs and 10T1/2 fibroblasts – demonstrated that p19Arf expression can block Pdgfrβ mRNA transcription in a p53-dependent way and Pdgfrβ protein translation independently of p53 (Widau et al., 2012). Finally, miR34a, a microRNA known to be controlled by p53, is also induced by p19Arf and is required for p53-indendent translational repression of Pdgfrβ (Iqbal et al., 2014a). Though this detailed molecular mechanism explains the ocular phenotype in the absence of Arf, it is important to recognize that the experiments were conducted in mouse fibroblasts, whereas the cells that normally express p19Arf are pericyte-like cells derived from the neural crest (Silva et al., 2005; Zheng et al., 2010). In this report, we functionally characterize and confirm aspects of Arf biology previously defined in other cell types and in vivo in an ex vivo cell culture model that represents the first model in which the Arf promoter is normally active.
2. Results
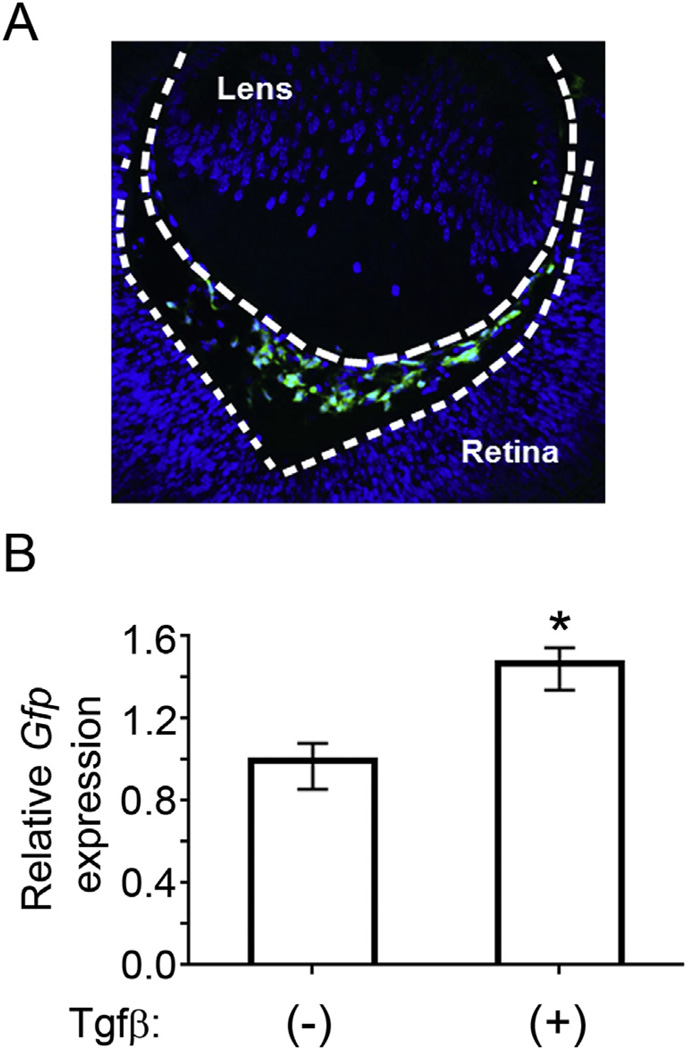
To verify that the aforementioned molecular and genetic pathway is relevant to the cells normally expressing Arf, we took advantage of primary cultures of primary vitreous cells (PVCs) that we purified by flow cytometry based on expression of a Gfp reporter in ArfGfp/Gfp (functionally, Arf−/−) animals (Iqbal et al., 2014b). We previously characterized the PVCs by global gene expression analysis that highlighted, among other things, their pericyte-like nature (Iqbal et al., 2014b). These cells are readily apparent as early as embryonic day (E) 12.5 (Martin et al., 2004; McKeller et al., 2002; Silva et al., 2005) and dramatically increase in number in the primary vitreous space by E13.5 (Silva et al., 2005) and Fig. 1A. Several pieces of data indicate that Tgfβ2 is required for Arf induction in the primary vitreous. First, Tgfβ2−/− mice have a variety of developmental defects, including primary vitreous hyperplasia (Saika et al., 2001; Sanford et al., 1997), and this correlates with decreased expression of p19Arf (Freeman-Anderson et al., 2009). Second, transgenic expression of Tgfβ1 driven from the α-crystallin promoter can correct the primary vitreous hyperplasia in Tgfβ2−/− mouse embryos, but not in Arf−/− embryos, which indicates that p19Arf is needed for the anti-proliferative effects of Tgfβ in the eye (Zheng et al., 2010). Dual immunofluorescence staining shows p19Arf and the Tgfβ receptor TbrII to be coexpressed, suggesting that this protein signals to induce Arf directly (Freeman-Anderson et al., 2009). This was confirmed to be true in MEFs and 10T1/2 cells: exogenous Tgfβ1, 2, or 3 increases Smad2/3 binding to the Arf gene, recruits RNA polymerase II, and then increases Arf mRNA and protein expression (Freeman-Anderson et al., 2009; Zheng et al., 2010). Interestingly, even though we isolated the PVCs by virtue of Tgfβ2-driven Arf promoter activation and Gfp expression, addition of Tgfβ1 (5 ng/ml) further increased Gfp mRNA expression in these cells ex vivo (Fig. 1B). Hence, the developmental signaling pathway that is critical for eye development is maintained in cultured PVCs. This finding is also consistent with our previous global gene expression analysis showing that components of the Tgfβ pathway defined as being important for Arf induction, such as Smad2/3, Sp1 and Cebpβ, are expressed in PVCs (Iqbal et al., 2014b).
Fig. 1.
p19Arf is expressed in the primary vitreous and responds to exogenous Tgfβ. A) Representative photomicrograph image of developing vitreous at E13.5 from ArfGfp/Gfp mouse. Gfp expression, as a marker for Arf promoter activation, is robustly detected in cells of the primary vitreous space. B) Relative expression of Gfp as a surrogate marker for exon1β measured by qRT-PCR. PVCs were treated with 5 ng/mL Tgfβ1 or 4 mM HCl vehicle control. RNA was extracted 48 h after treatment. Gfp expression is normalized to Gapdh control (* = p < 0.02).
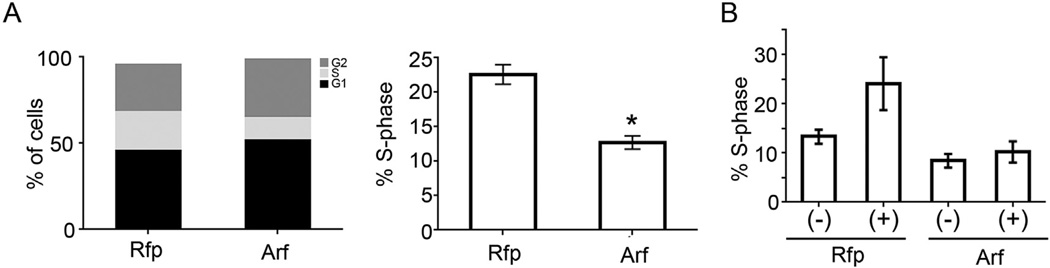
As highlighted above, p19Arf inhibits cell proliferation and, in the context of eye development, it specifically blunts mitogenic effects of PDGF-B by down-regulating the expression of Pdgfrβ (Silva et al., 2005). To test whether p19Arf similarly arrests cultured PVCs, we utilized propidium iodide staining followed by flow cytometry and quantification using the Watson Pragmatic Model Watson 1987 (Watson et al., 1987). We generated MSCV-based retroviral vectors containing Arf cDNA upstream of an IRES element driving expression of Rfp (Iqbal et al., 2014b). Transducing PVCs with the Arf-IRES-RFP vector significantly decreased the fraction of cells in S-phase with an accumulation of cells in G1 and G2 phases as compared to the Rfp control (Fig. 2A). Of note, it is not currently possible to study the effect of endogenous Arf expression in PVCs for several reasons. First, p19Arf expression from the wild type allele in an ArfGfp/+ mouse severely restricts cell accumulation in vivo, and would likely do so ex vivo. Indeed, earlier work with Arf+/− MEFs demonstrated that serial expansion (which would be required if attempting to propagate PVCs from the very small numbers in a phenotypically normal ArfGfp/+ eye) usually results in cells that have lost the remaining wild type allele (Zindy et al., 1998). Second, it is not yet possible to purify ArfGfp/+ PVCs before Arf is expressed as we depend on Gfp expression for the flow cytometry sorting, and the Arf promoter drives the Gfp reporter. Nonetheless, the capacity for ectopic expression of p19Arf to mimic the in vivo arrest provides a new opportunity for structure-function analyses of p19Arf and identification of downstream effectors in one of the very few cell types known to normally express this protein in the developing mouse.
Fig. 2.
p19Arf inhibits PDGF-B driven proliferation in the PVCs. A) Quantification of DNA content of PVCs as detected by propidium iodide and flow cytometry. PVCs were transduced with MSCV-Rfp control or MSCV-Arf-Rfp retrovirus and harvested 48 h later. Cell cycle phases were defined using the Watson Pragmatic Model. Quantification of S-phase fraction is shown on right. B) PVCs were transduced with MSCV-Rfp control or MSCV-Arf-Rfp. Cells were stimulated with 50 ng/mL PDGF-B for 16 h and cell cycle was analyzed as above (* = p < 0.02).
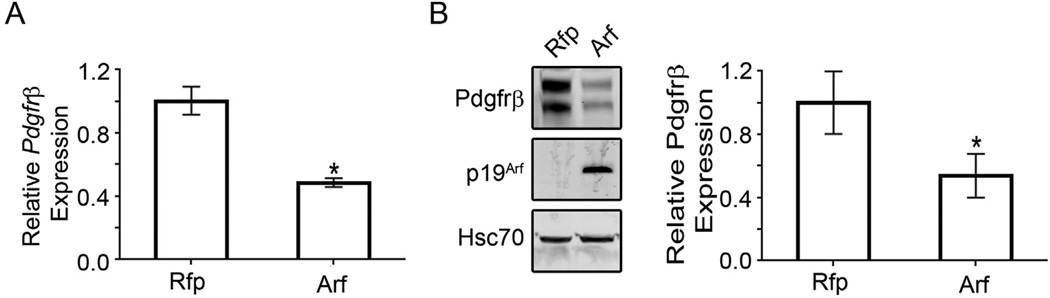
As mentioned above, we know that primary vitreous hyperplasia in the Arf−/− mouse is driven by Pdgfrβ (Silva et al., 2005; Widau et al., 2012). Hence, we evaluated how PDGF-B, the ligand for this receptor, influenced PVC proliferation and the capacity for p19Arf to block the effects using the explanted cells. First, exposure of serum-starved PVCs to PDGF-B (50 ng/ml) for 16 h increased the S-phase fraction by over two fold, but ectopic expression of p19Arf completely abrogated this effect (Fig. 2B). We correlated this blockade with the ability for p19Arf to significantly repress Pdgfrβ mRNA and protein (Fig. 3A and B).We conclude that ex vivo studies of PVCs faithfully reflect the in vivo biology: PDGF-B drives PVC proliferation and Arf expression blocks it by a mechanism that leads to decreased Pdgfrβ expression.
Fig. 3.
Pdgfrβ expression in PVCs depends on p19Arf. A) Relative expression of Pdgfrβ mRNA as measured by qRT-PCR upon transduction with MSCV-Rfp control or MSCV-Arf-Rfp. B) Representative western blot showing Pdgfrβ, p19Arf and HSC-70 protein expression in lysates prepared from PVCs transduced with MSCV-Arf-Rfp retrovirus or MSCV-Rfp control. Western signal (on right) is quantified using the Odyssey Image Studio Lite system and normalized to Hsc-70 (* = p < 0.05).
Although p19Arf is most well-known for its capacity to sequester Mdm2 and thereby stabilize p53 (Honda and Yasuda, 1999; Weber et al., 1999), the protein also acts independently of p53 to inhibit ribosomal RNA processing (Sugimoto et al., 2003), associate with E2F1 and c-Myc to inhibit their trans-activating potential (Datta et al., 2004; Eymin et al., 2001; Qi et al., 2004) and promote sumoylation of Mdm2, nucleophosmin and other proteins (Rizos et al., 2005; Tago et al., 2005). Genetic evidence from mouse studies suggests that p19Arf likely controls primary vitreous expansion in a manner that does not strictly depend on p53. Ocular development is normal in most strains of mice lacking p53; however, certain pure BALB/c and pure C57Bl/6 lines of p53−/− mice have a PHPV/PFV-like phenotype with variable penetrance (Ikeda et al., 1999). Further, the developmental defect in C57Bl/6 mice is abrogated when the animals are bred into a mixed C57Bl/6 × 129/Sv background (Reichel et al., 1998). In contrast, PHPV consistently develops in Arf−/− mice in pure C57Bl/6 and pure 129/Sv lines as well as mixed C57Bl/6 × 129/Sv animals (McKeller et al., 2002).We have taken this to mean that while p53 may play a role in transcriptional repression of Pdgfrβ mRNA, Arf expression represses the protein through p53 independent mechanisms (Widau et al., 2012).
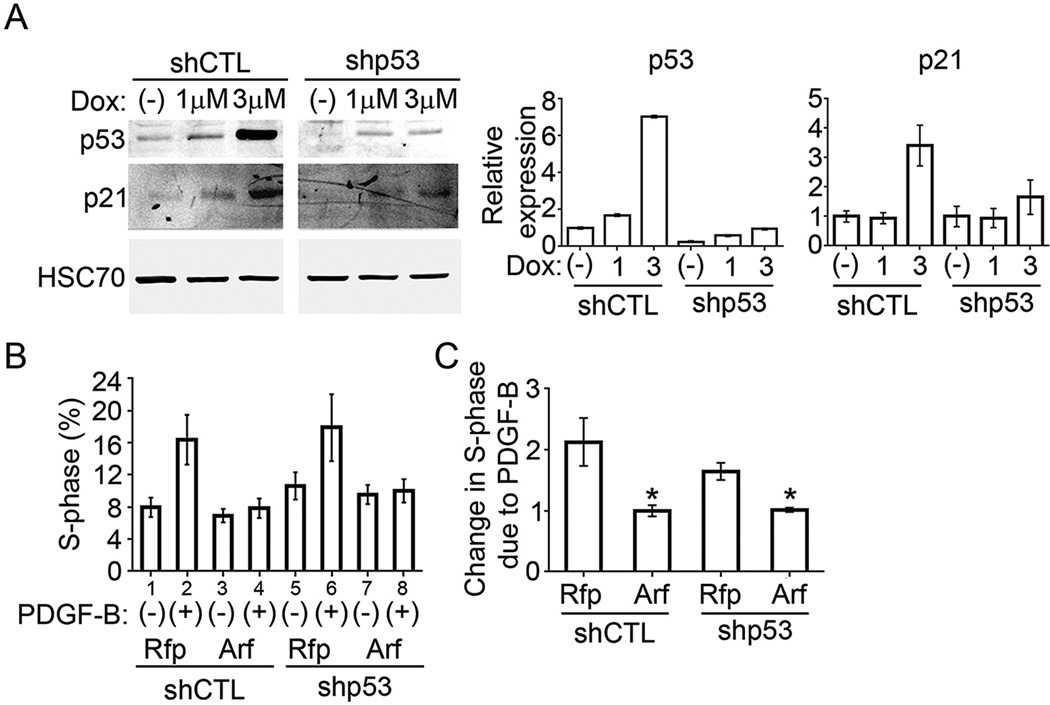
We utilized the PVC model to evaluate the role that p53 plays in Arf-dependent repression of Pdgfrβ mRNA and protein. Because our PVCs retain the p53 gene, we developed PVC sub-lines in which LMP-based retroviral vectors (obtained from S. Lowe, Memorial Sloan Kettering Cancer Center) delivered either control or p53-specific shRNA, and puromycin was used to select pools of PVCs retaining p53 (shCTL) and those with p53 knockdown (shp53). Although not complete, the knockdown is functionally significant: ectopic expression of Arf in the shCTL cells robustly increases p53 (Fig. 5B, lanes 1 and 2), but similar transduction of Arf into shp53 cells only increases p53 to a level that is still lower than baseline in the control cells (Fig. 5B, compare lanes 2 and 4). As another functional measure, doxorubicin (Dox) augments the expression of p21Cip1, a well-known p53 target (Harper et al., 1993), in a dose-dependent manner; this effect is also dramatically decreased in the p53 knockdown PVCs (Fig. 4A). We also observed that the baseline S phase fraction was slightly increased in the shp53 PVCs at baseline (Fig. 4B, lanes 1 vs 5), but in both cases exogenous PDGF-B drove additional cells into S phase (Fig. 4B, lanes 1 vs 2 and 5 vs 6). Importantly, ectopic Arf expression blunted the mitogenic effects of PDGF-B in the presence and in the absence of p53 knockdown (Fig. 4B, lanes 2 vs 4 and 6 vs 8, and Fig. 4C).
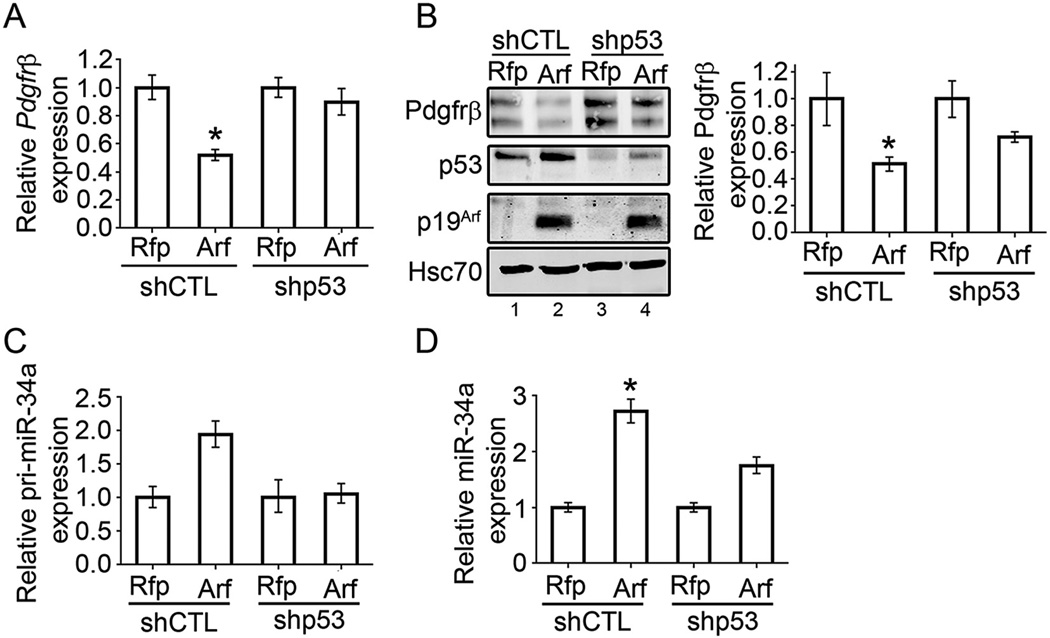
Fig. 5.
p53-dependent effects of p19Arf on Pdgfrβ and miR34a. A) qRT-PCR showing relative expression of Pdgfrβ mRNA upon transduction with MSCV-Rfp control or MSCV-Arf-Rfp retrovirus in shCTL or shp53 cells. B) Representative western blot showing Pdgfrβ, p53, p19Arf and Hsc-70 protein expression in lysates prepared from shCTL or shp53 cells transduced with MSCV-Rfp control or MSCV-Arf-Rfp retrovirus. Western signal (on right) is quantified using the Odyssey Image Studio Lite system and normalized to Hsc-70. C) Relative expression of primary-miR34a transcript in response to ectopic p19Arf expression in shCTL and shp53 cells. D) Relative expression of mature miR34a in response to ectopic p19Arf expression in shCTL and shp53 cells. miR34a is normalized to U6 (* = p < 0.05).
Fig. 4.
p19Arf inhibits PDGF-B driven proliferation independently of p53. A) Representative western blot showing p53 induction in response to doxorubicin (Dox) treatment. p53 and p21 are induced upon treatment in shCTL cells but not shp53 cells. Quantification, normalized to Hsc-70, is on right. B) Quantification of percent of cells in S-phase as measured by propidium iodide and flow cytometry. shCTL or shp53 PVCs were transduced with MSCV-Rfp control or MSCV-Arf-Rfp and stimulated with 50 ng/mL PDGF-B for 16 h. C) Fold change of S-phase fraction of cells in response to PDGF-B (* = p < 0.05).
We used this system to address the importance of p53 in two Arf-dependent responses: Pdgfrβ mRNA and protein repression and miR34a induction. First, as with the parental PVCs (Fig. 3A), ectopic Arf expression significantly decreased Pdgfrβ mRNA but this effect was completely negated when p53 was knocked down (Fig. 5A). In contrast, Arf expression still retained the capacity to repress Pdgfrβ protein in shp53 PVCs, though the effect was somewhat moderated (Fig. 5B). In a similar way, Arf can increase the expression of primary miR34a, but only in the presence of p53 (Fig. 5C), but p53 is at least partly dispensable for induction of mature miR34a – indicating a p53-independent role for p19Arf in processing of this microRNA (Fig. 5D). All of these molecular effects are similar to those that we observed in vivo during primary vitreous development and maturation (Martin et al., 2004; Silva et al., 2005; Widau et al., 2012).
3. Discussion
In the mouse, the mature testis, developing eye, and umbilical arteries are the only sites where numerous Arf expressing cells are normally found (Freeman-Anderson et al., 2009; Silva et al., 2005; Zindy et al., 2003). Arf expressing cells in the eye include those perivascular cells embracing the hyaloid artery and also cells scattered in the cornea (Silva et al., 2005; Thornton et al., 2007; Zindy et al., 2003). Primary vitreous hyperplasia is the only recognized developmental defect due to Arf gene inactivation (Martin et al., 2004; McKeller et al., 2002), and this ocular disease is due to Pdgfrβ-driven proliferation in the aforementioned perivascular cells (Silva et al., 2005; Widau et al., 2012). Because the PVCs are unique as the only cell type known to be altered without Arf, it was important to validate the functional and biochemical effects of p19Arf in these cells. Indeed, using this new ex vivo model, we have established the following: 1) The Arf promoter can be engaged by Tgfβ to induce Arf expression in PVCs ex vivo; 2) Ectopic Arf expression arrests PVC proliferation, including proliferation driven by PDGF-B; 3) Cell proliferation arrest by p19Arf correlates with repression of Pdgfrβ; 4) and Arf expression in PVCs checks proliferation, decreases Pdgfrβ protein, and induces mature miR-34a independently of p53. All of these new findings accurately reflect the in vivo effects of p19Arf during eye development.
Since the Arf cDNA was first cloned, most of the cell-based functional studies have been carried out in MEFs, a wide range of fibroblast cell lines, and cancer cell lines – none of which reflect a “normal” cellular context. Despite that inherent limitation, much has been gleaned from such studies. Indeed, our own laboratory has defined most of the aforementioned molecular and cell biological effects of p19Arf in MEFs and 10T1/2 fibroblasts (Silva et al., 2005; Weber et al., 2000). However, a more granular understanding of Arf biology is likely to depend on context – expressing the protein in the right cell type. Gaining a better understanding of the nature of the perivascular cells and how Arf influences their biology represents one such area. These particular PVCs are nearly unique for two reasons. First, they embrace blood vessels that undergo dramatic regression in the immediate postnatal period. Second, they are the only cells in the eye that express p19Arf. We state they are “nearly” unique because the umbilical arteries branching from the internal iliac vessels represent another vascular system that becomes superfluous in the immediate postnatal period, and this is the only other vasculature enveloped by Arf expressing perivascular cells (Freeman-Anderson et al., 2009). Of note, we have not yet uncovered a defect in the umbilical vessels when p19Arf is absent. Nearly all work on Arf in the eye has focused on how it controls the number of these PVCs – essentially, how it blocks primary vitreous hyperplasia. Yet, the fact that Arf is expressed in the cells from E11.5, just as the hyaloid vessels are forming (Silva et al., 2005), and the expression is extinguished at P5, just as the vessels begin to regress (Mitchell et al., 1998), suggests another role for p19Arf : it might actually be required to temporarily stabilize the otherwise transient vessels. Perivascular cells, especially pericytes that represent a particular type of vascular mural cell, provide trophic signals to underlying endothelial cells (Hanahan and Folkman, 1996; Yancopoulos et al., 2000). Having developed an ex vivo culture model for those PVCs, we are only now in a position to establish more sophisticated systems to study these potential heterotypic interactions with endothelial cells and the role that p19Arf may play.
Similar comments can be made about the emerging role that p19Arf can play as a microRNA regulator. We previously showed that this protein can increase the expression of miR-34a independently of p53 pathway, and miR34a is needed for post-transcriptional Pdgfrβ repression and p53-independent cell proliferation control (Iqbal et al., 2014a). Others have shown – using MEFs – that p19Arf physically interacts with Drosha, and this interaction both increases and decreases the expression of a wide range of microRNAs (Kuchenreuther and Weber, 2014). We do not yet understand the mechanism by which p19Arf can induce miR-34a independently of p53, nor do we know the full spectrum of microRNA changes that accompany Arf expression in PVCs that normally express the protein. Because both of these are likely to be cell context-dependent, conducting the work in PVCs may provide the correct perspective for such studies. Ultimately, these types of analyses may inform our understanding of PHPV/PFV pathogenesis.
4. Methods
4.1. Cell isolation and culture
PVCs were isolated from early post-natal ArfGfp/Gfp mice. Method for isolation is described in detail in Iqbal et al. (2014b). PVCs were cultured in Pericyte Medium (PM) (ScienCell) and passaged using trypsin/EDTA. Tgfβ1 (R&D Systems), was added to cell culture medium at a dose of 5 ng/ml; an equivalent volume of vehicle (4 mM HCl) was added into the medium as a control. PVCs were transduced with MSCV-RFP or MSCV-Arf-RFP retrovirus. 16 h post transduction, culture media was replaced. 50 ng/mL PDGF-B was added to the cells for 16 h prior to harvesting for cell cycle analysis.
4.2. RNA expression
Total RNA was extracted from PVCs with miRNeasy mini kit (Qiagen). For qRT-PCR, 1 µg of total RNA was reverse transcribed using Superscript III RT kit (Invitrogen) according to the manufacturer's recommendations. Quantitative RT-PCR (qRT-PCR) was performed with KAPA SYBR Green Mastermix (KAPA) on BioRad (CFX96). The PCR program consisted of 20 s at 95 °C, followed by 40 cycles of 95 °C for 15 s and 60 °C for 20 s. Primer quality was analyzed by dissociation curves. The expression of Pdgfrβ and primiR34a was normalized to Gapdh. miR-34a was normalized to U6.
4.3. Cell cycle analysis
Propidium Iodide (PI) (Sigma) staining was performed after cells were harvested by trypsin-EDTA and fixed in 70% ethanol. Fixed cells were washed in PBS and centrifuged at 1200 rpm for 5 min. Cells were resuspended in 0.3 ml PBS and RNaseA (Sigma) was added to the suspension to final concentration of 0.5 mg/ml. After 1 h of incubation at 37 °C, PI was added to the suspension to a final concentration of 10 µg/ml. PI-stained cells were analyzed for DNA content with a BD Calibur flow cytometer and Watson Pragmatic Model to calculate the distribution of cells in G1, S, and G2 cell cycle phases. Results are averaged from 3 biological replicates.
4.4. Western blot
Protein expression was examined by Western-blotting according to a standard procedure. The following antibodies were used: anti-p19Arf (Ab80, Abcam, 1:1000), anti-Pdgfrβ (AF1042, R&D, 1:1000), anti-Hsc70 (Sc-1059, Santa Cruz, 1:5000), anti-p53 (Sc-6243, Santa Cruz, 1:1000). Band intensity was quantified using the Odyssey Image Studio Lite system and normalized to Hsc-70. Results are averaged from 3 biological replicates.
Acknowledgments
We gratefully acknowledge CJ Sherr (St. Jude Children's Research Hospital) for providing ArfGfp/Gfp mice; SW Lowe (Memorial Sloan Kettering Cancer Center) for LMP-shCTL and shp53 plasmids; the Animal Facility and the Flow Cytometry Core Facility at UT South-western Medical Center. This research is supported by grants to S.X.S. from the National Eye Institute (EY 014368 and EY 019942).
References
- Datta A, Nag A, Pan W, Hay N, Gartel AL, Colamonici O, Mori Y, Raychaudhuri P. Myc-ARF (alternate reading frame) interaction inhibits the functions of Myc. J. Biol. Chem. 2004;279:36698–36707. doi: 10.1074/jbc.M312305200. [DOI] [PubMed] [Google Scholar]
- Eymin B, Karayan L, Seite P, Brambilla C, Brambilla E, Larsen CJ, Gazzeri S. Human ARF binds E2F1 and inhibits its transcriptional activity. Oncogene. 2001;20:1033–1041. doi: 10.1038/sj.onc.1204220. [DOI] [PubMed] [Google Scholar]
- Freeman-Anderson NE, Zheng Y, Calla-Martin AC, Treanor LM, Zhao YD, Garfin PM, He TC, Mary MN, Thornton JD, Anderson C, Gibbons M, Saab R, Baumer SH, Cunningham JM, Skapek SX. Expression of the Arf tumor suppressor gene is controlled by Tgf{beta}2 during development. Development. 2009;136:2081–2089. doi: 10.1242/dev.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campohiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev. Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- Goldberg MF. Persistent fetal vasculature (PFV): an integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV) LIV Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 1997;124:587–626. doi: 10.1016/s0002-9394(14)70899-2. [DOI] [PubMed] [Google Scholar]
- Hackett SF, Wiegand S, Yancopoulos G, Campohiaro PA. Angiopoietin-2 plays an important role in retinal angiogenesis. J. Cell. Physiol. 2002;192:182–187. doi: 10.1002/jcp.10128. [DOI] [PubMed] [Google Scholar]
- Haddad R, Font RL, Reeser F. Persistent hyperplastic primary vitreous. A clinicopathologic study of 62 cases and review of the literature. Surv. Ophthalmol. 1978;23:123–134. doi: 10.1016/0039-6257(78)90091-7. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 CDK-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18:22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt A, Rowe N, Lam A, Martin F. Outcomes in persistent hyperplastic primary vitreous. Br. J. Ophthalmol. 2005;89:859–863. doi: 10.1136/bjo.2004.053595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Hawes NL, Chang B, Avery CS, Smith RS, Nishina PM. Severe ocular abnormalities in C57BL/6 but not in 129/Sv p53-deficient mice. Investig. Ophthalmol. Vis. Sci. 1999;40:1874–1878. [PubMed] [Google Scholar]
- Iqbal N, Mei J, Liu J, Skapek SX. miR-34a is essential for p19-driven cell cycle arrest. Cell Cycle. 2014a;13 doi: 10.4161/cc.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal NS, Xu L, Devitt CC, Skapek SX. Isolation and characterization of mammalian cells expressing the Arf promoter during eye development. Bio-Techniques. 2014b;56:239–249. doi: 10.2144/000114166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yoshioka M. Regression of the hyaloid vessels and pupillary membrane of the mouse. Anat. Embryol. 1999;200:403–411. doi: 10.1007/s004290050289. [DOI] [PubMed] [Google Scholar]
- Kuchenreuther MJ, Weber JD. The ARF tumor-suppressor controls Drosha translation to prevent Ras-driven transformation. Oncogene. 2014;33:300–307. doi: 10.1038/onc.2012.601. PMID: 23318441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RA, Bishop JM. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462. doi: 10.1016/0092-8674(93)80047-i. [DOI] [PubMed] [Google Scholar]
- Lei H, Rheaume MA, Kazlauskas A. Recent developments in our understanding of how platelet-derived growth factor (PDGF) and its receptors contribute to proliferative vitreoretinopathy. Exp. Eye Res. 2010;90:376–381. doi: 10.1016/j.exer.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA. Wnt7b mediates macrophage-induced programmed cell death in patterning the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Thornton JD, Liu J, Wang X, Zuo J, Jablonski MM, Chaum E, Zindy F, Skapek SX. Pathogenesis of persistent hyperplastic primary vitreous in mice lacking the arf tumor suppressor gene. Investig. Ophthalmol. Vis. Sci. 2004;45:3387–3396. doi: 10.1167/iovs.04-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary-Sinclair MN, Wang X, Swanson DJ, Sung CY, Mendonca EA, Wroblewski K, Baumer SH, Goldowitz D, Jablonski MM, Skapek SX. Varied manifestations of persistent hyperplastic primary vitreous with graded somatic mosaic deletion of a single gene. Mol. Vis. 2014;20:215–230. [PMC free article] [PubMed] [Google Scholar]
- McKeller RN, Fowler JL, Cunningham JJ, Warner N, Smeyne RJ, Zindy F, Skapek SX. The Arf tumor suppressor gene promotes hyaloid vascular regression during mouse eye development. Proc. Natl. Acad. Sci. U.S.A. 2002;99:3848–3853. doi: 10.1073/pnas.052484199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CA, Risau W, Drexler HC. Regression of vessels in the tunica vasculosa lentis is initiated by coordinated endothelial apoptosis: a role for vascular endothelial growth factor as a survival factor for endothelium. Dev. Dyn. 1998;213:322–333. doi: 10.1002/(SICI)1097-0177(199811)213:3<322::AID-AJA8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Niklasson M, Bergstrom T, Zhang XQ, Gustafsdottir SM, Sjogren M, Edqvist PH, Vennstrom B, Forsberg M, Forsberg-Nilsson K. Enlarged lateral ventricles and aberrant behavior in mice overexpressing PDGF-B in embryonic neural stem cells. Exp. Cell Res. 2010;316:2779–2789. doi: 10.1016/j.yexcr.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Pollard ZF. Persistent hyperplastic primary vitreous: diagnosis, treatment and results. Trans. Am. Ophthalmol. Soc. 1997:487–549. [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Gregory MA, Li Z, Brousal JP, West K, Hann SR. p19Arf directly and differentially controls the functions of c-Myc independently of p53. Nature. 2004;431:712–717. doi: 10.1038/nature02958. [DOI] [PubMed] [Google Scholar]
- Reichel MB, Ali RR, D'Esposito F, Clarke AR, Luther PJ, Bhattacharya SS, Hunt DM. High frequency of persistent hyperplastic primary vitreous and cataracts in p53-deficient mice. Cell Death Differ. 1998;5:156–162. doi: 10.1038/sj.cdd.4400326. [DOI] [PubMed] [Google Scholar]
- Rizos H, Woodruff S, Kefford RF. p14ARF interacts with the SUMO-conjugating enzyme Ubc9 and promotes the sumoylation of its binding partners. Cell Cycle. 2005;4:597–603. doi: 10.4161/cc.4.4.1597. [DOI] [PubMed] [Google Scholar]
- Rutland CS, Mitchell CA, Nasir M, Konerding MA, Drexler HCA. Microphthalmia, persistent hyperplastic hyaloid vasculature and lens anomalies following overexpression of VEGF-A 188 from the zA-crystallin promoter. Mol. Vis. 2007;13:47–56. [PMC free article] [PubMed] [Google Scholar]
- Saika S, Liu CY, Azhar M, Sanford LP, Doetschman T, Gendron RL, Kao CW, Kao WW. TGFbeta2 in corneal morphogenesis during mouse embryonic development. Dev. Biol. 2001;240:419–432. doi: 10.1006/dbio.2001.0480. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittengerger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFb2 knockout mice have multiple developmental defects that are non-overlapping with other TGFb knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RL, Thornton JD, Martin AC, Rehg JE, Bertwistle D, Zindy F, Skapek SX. Arf-dependent regulation of Pdgf signaling in perivascular cells in the developing mouse eye. EMBO J. 2005;24:2803–2814. doi: 10.1038/sj.emboj.7600751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto M, Kuo ML, Roussel MF, Sherr CJ. Nucleolar Arf tumor suppressor inhibits ribosomal RNA processing. Mol. Cell. 2003;11:415–424. doi: 10.1016/s1097-2765(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Tago K, Chiocca S, Sherr CJ. Sumoylation induced by the Arf tumor suppressor: a p53-independent function. PNAS. 2005;102:7689–7694. doi: 10.1073/pnas.0502978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JD, Swanson DJ, Mary MN, Pei D, Martin AC, Pounds S, Goldowitz D, Skapek SX. Persistent hyperplastic primary vitreous due to somatic mosaic deletion of the arf tumor suppressor. Investig. Ophthalmol. Vis. Sci. 2007;48:491–499. doi: 10.1167/iovs.06-0765. [DOI] [PubMed] [Google Scholar]
- Watson JV, Chambers SH, Smith PJ. A pragmatic approach to the analysis of DNA histograms with a definable G1 peak. Cytometry. 1987;8:1–8. doi: 10.1002/cyto.990080101. [DOI] [PubMed] [Google Scholar]
- Weber JD, Jeffers JR, Rehg JE, Randle DH, Lozano G, Roussel MF, Sherr CJ, Zambetti GP. p53-independent functions of the p19Arf tumor suppressor. Genes Dev. 2000;14:2358–2365. doi: 10.1101/gad.827300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JD, Taylor LJ, Roussel MF, Sherr CJ, Bar-Sagi D. Nucleolar Arf sequesters Mdm2 and activates p53. Nat. Cell Biol. 1999;1:20–26. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- Widau RC, Zheng Y, Sung CY, Zelivianskaia A, Roach LE, Bachmeyer KM, Abramova T, Desgardin A, Rosner A, Cunningham JM, Skapek SX. p19Arf represses platelet-derived growth factor receptor beta by transcriptional and posttranscriptional mechanisms. Mol. Cell. Biol. 2012;32:4270–4282. doi: 10.1128/MCB.06424-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Zhao YD, Gibbons M, Abramova T, Chu PY, Ash JD, Cunningham JM, Skapek SX. Tgfbeta signaling directly induces Arf promoter remodeling by a mechanism involving Smads 2/3 and p38 MAPK. J. Biol. Chem. 2010;285:35654–35664. doi: 10.1074/jbc.M110.128959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, Sherr CJ, Roussel MF. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F, Williams RT, Baudino TA, Rehg JE, Skapek SX, Cleveland JL, Roussel MF, Sherr CJ. Arf tumor suppressor promoter monitors latent oncogenic signals in vivo. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15930–15935. doi: 10.1073/pnas.2536808100. [DOI] [PMC free article] [PubMed] [Google Scholar]