Abstract
Animal models are crucial for the study of fibrosis. Keloids represent a unique type of fibrotic scarring that occurs only in humans, thus presenting a challenge for those studying the pathogenesis of this disease and its therapeutic options. Here, several animal models of fibrosis currently in use are described, emphasizing recent progress and highlighting encouraging challenges.
Keywords: animal models, tissue fibrosis, keloids, hypertrophic scar
Introduction
Many of the challenges associated with the study of keloid scarring arise from our incomplete understanding of the disease and its pathobiology. Keloids have been proven to involve multi-factorial and complex processes with many pathologic etiologies identified. Accumulating evidence indicates certain genetic susceptibilities and traits, however, the underlying pathogenesis and the principal signaling pathways remain to be defined in further precision.
Keloid scarring has been known to occur only in homo sapiens. No other animal species, including non-human primates, have been found to naturally develop scar tissue comparable to that of human keloids. Thus, keloids represent a disease process that is uniquely human, and establishes a challenging precedent to overcome.
In any disease process, studying etiology and progression in the host organism would be optimal. However, given the ethical and medical risks this would pose to patients, animal model equivalents have been sought as a surrogate to allow for in-depth analysis to take place. Generally, these models have been developed in one of two ways: (a) Engineer a comparable degree of fibrosis in an animal that does not normally develop it, or (b) Transplant human-derived tissue and/or cells into a host animal for in vivo analysis (Table 1). Both avenues have the same goal of giving researchers a reliable way of measuring and studying fibrosis, and each has its own set of specific challenges to overcome. Despite these challenges, animal models hold value in approximating events that occur in humans and can help lead to better understanding of the disease, with therapeutic implications.
Table 1.
Characteristics of animal models of keloids and hypertrophic scarring
| Animal Model |
Trau ma- indu ced |
Model Fully Devel oped |
Systemi c Involve ment |
Model Duratio n |
Gross Observat ions |
Histolog ic Findings |
Therap eutic Viabilit y |
Key Finding s |
Reference s |
|---|---|---|---|---|---|---|---|---|---|
| Tsk 1/2+mouse | − | 2–3 weeks | + | Indefinite | Tightness in skin of interscapular region | Thickened dermis, abnormal dermal componenets | Known genetic defect, reproducible fibrosis; no specific lesion site to inject | Genetic basis for fibrosis, without true keloid formation | [1] – [6] |
| Duroc Pig | + | 3 weeks | − | 4–5 months | Raised, firm hyperpigmented “thick” scars | Thickened dermis, presence of myofibroblasts, mast cells, increased collagen fibers in whorls and nodules | Specific lesion to treat; reproducible | Thick scar with features approaching HS; not representative of human HS or keloid. | [7 – 10] |
| Rabbit Ear | + | 3–4 weeks | − | 60+ days | Raised, firm papules | Thickened dermis with horizontally arranged collagen fibers, increased vascular tissue, mild chronic inflammation | Specific lesion to treat; reproducible | Thick scar with features approaching HS; not representative of human HS or keloid. | [11 – 13] |
| Horse “Proud Flesh” | + | several weeks | − | weeks to months | Exuberant granulation tissue | Increased fibroblast s, increased collagen | Specific lesion to treat | Limited degree of scar tissue development; not representative of human HS or keloid development | [17], [18] |
| Bleomycin | − | 2–4 weeks | + | persist days to weeks following cessation of drug administration | Difficult to identify lesional skin vs. non-lesional | Thickened dermis with bundles of collagen, mixed inflammatory-cell infiltrate, elevated hydroxyproline content | No specific lesion to treat; useful for systemic anti-fibrotic therapy | Reproducible model of fibrosis, with skip lesions; not representative of HS or keloid development. | [19], [21], [22], [26] |
| ROS model | − | 2–4 weeks | + | persist days to weeks following cessation of drug administration | Difficult to identify lesional skin vs. non-lesional | Increased dermal thickness and collagen content, with features similar to bleomycin model | No specific lesion to treat, useful for systemic anti-fibrotic therapy | Reproducible model of fibrosis, with skip lesions; not representative of HS or keloid development. | [31 – 33] |
| Xenograft | − | 1 week | − | weeks to months | Subcutaneous implantation, visually observable | Dense collagenous whorls with evidence of angiogenesis | Specific human-derived lesion to treat | Reproducible; graft viability and host rejection limits use for in-depth analysis. | [34 – 40] |
| Bio-Scaffold | − | 1–2 weeks | − | 2–4 weeks | Subcutaneous implantation, visually observable | Scaffolds show collagen and GAG deposition, evidence of angiogenesis from host | Specific human-derived scaffold to treat | Reproducible; cell viability, phenotypic change and host rejection limit use for in-depth analysis. | [42 – 45] |
Animal models
Genetic Models of Fibrosis: Tight skin mouse 1 and 2 (Tsk)
Excessive collagen deposition is the hallmark of many fibrotic conditions. Systemic sclerosis (SSc) is a devastating disorder in which collagen deposition affects the skin and internal organs, leading to poor survival rate (< 65% at 10 years) in extreme cases with no identifiable cause [1]. The tight skin 1 and 2 (Tsk1/2) mutant mouse strains were identified as animal models to study scleroderma and SSc and have been used extensively to test various therapeutic modalities [2,3]. The Tsk1/+ mouse spontaneously occurred following partial in-frame duplication of the fibrillin-1 gene, located on mouse chromosome 2 [2]. In a similar fashion, the Tsk2/+ mouse was identified from the offspring of a male 101/H mouse following administration of ethylnitrosourea, a mutagenic agent, developing gain-of-function missense mutations in Col3a1 on chromosome 1 [3–5]. Both mouse strains develop tightness in the skin of the interscapular region, thickened dermis, and abnormal dermal structural components. Similarly, the mutations that occur in both strains are inherited in an autosomal dominant fashion, with homozygous mutations in both strains resulting in death in utero [2,3]. As a model for scleroderma, both the Tsk1/2+ mouse strains demonstrate appreciable cutaneous fibrosis, as early as 2–3 weeks postnatal. However, as a model for trauma-induced fibrosis, such as keloids, neither model adequately recapitulates the disease. Wound healing in both Tsk models is delayed, occurring 5–6 weeks post-injury compared with 2–3 weeks in wild-type (WT) mice [4,6]. This is thought to be due, in part, to the structural and tensile forces the surrounding skin has on the forming granulation tissue. Once sufficient granulation tissue has formed, the tensile force it exerts over the surrounding wound edge exceeds that of the surrounding wound edge and wound closure slowly occurs [4,6]. Both Tsk strains offer a unique vantage point into systemic cutaneous fibrosis in the mouse, and have proven useful for studying other forms of fibrosis. More study is needed, however, to assess the wound healing process in these animal models and their applicability in the study of keloids.
Trauma-induced animal models
Duroc Pig
Wound healing in the Duroc pig has been described as a close analogue to human hypertrophic scarring (HS). Following dermatomal injury, deep wounds are permitted to heal, and as early as 3 weeks post-injury they begin to elicit signs of thickened scarring: raised, firm, hyperpigmented scars are noted [7–9]. The degree of fibrosis achieved, while not fully comparable to human HS, does demonstrate excess collagen production. Histologic appearance reveals an increase in dermal thickness following deep wounding, increased numbers of myofibroblasts, mast cells and increased collagen fibers in whorls and nodules [8,9]. Transforming growth factor-β (TGF-β) gene expression is increased at early timepoints following injury, slowly declining to levels similar to uninjured skin by 5 months post-injury. Expression of decorin, a proteoglycan involved in the formation of collagen fibrils, is reduced while collagen types I and III, insulin-like growth factor-1 (IGF-1), versican, and nitric oxide are elevated in the early post-wound timeframe with decline in the subsequent weeks to months as the wound matures [9,10]. These changes in protein expression coupled with the gross and histological examination have led to the proposal that this model is an appropriate analogue for studying fibroproliferation [10]. However, scarring in this model occurs most often following deep dermal injury and is not considered hypertrophic by nature, rather it is simply a “thick” scar with features that approach HS development [9]. Severe effects of keloids and HS, including disfigurement and contractures, are limited in this animal model, and the scars that do develop are not invasive in the surround tissue, a hallmark of keloids [8,9]. While this model does not fully recapitulate human scarring, it is a close approximation to human HS development and could prove useful for testing anti-fibrotic treatment modalities.
Rabbit ear model
The rabbit ear model, which was first developed in the late 1960s, involves the creation of a sizeable wound, either through electro-cautery, incisional shaving or excision on the ventral surface of the ear of a rabbit. The extent of scarring in the rabbit ear has been compared to that of hypertrophic scar formation in humans, and is readily visible as early as 3–4 weeks post-trauma [11–13]. Grossly, the lesions appear as raised, firm papules and have been known to persist, in some cases in excess of 60 days [12,13]. Histopathologic features can include horizontally arranged collagen fibers, increased vascular tissue and mild chronic inflammation. Finally, corticosteroid therapy has been shown to reduce the dermal thickness and fibrosis observed in the rabbit ear model, akin to the effects observed in the treatment of hypertrophic scars and keloids in humans. This model has been well documented and used to study the efficacy of many proposed anti-fibrotic treatments, however, it does not recapitulate the findings observed in keloid development [7,13–16]. The scar tissue present is limited in its development, does not extend beyond the confines of the original wound, and the degree of collagen-matrix production observed does not approach the thickened, haphazardly arranged collagenous bundles seen in keloids. This is due to the rabbit’s ability to heal as the initial wound created must be of sufficient size to yield adequate scar formation [13,14]. Ultimately, the scar that develops in the rabbit ear model has been shown to be useful in testing anti-fibrotic therapies, however, it is unsuitable for elucidating the pathomechanisms underlying keloid development in humans.
Horse “Proud Flesh”
Horses have been known to develop exuberant granulation tissue, also known in the equine community as “proud flesh,” given the excessive degree to which it can progress. Proud flesh develops on the extremities of the horse, where skin tension lines are at a maximal and where healing by secondary intention occurs most often following trauma, as approximation of the wound edges is difficult [17,18]. Immobilization of the area is not practical in the horse, thus repeated damage and trauma to the site of injury can occur, making what began as a localized injury a much more drawn out process, leading to protracted healing. Histopathologic analysis of proud flesh reveals increased numbers of fibroblasts and minimal vascularity in the deep dermis as well as increased amounts of haphazardly arranged collagen, although not to the same extent as occurs in keloids [17]. Importantly, the wound healing process concludes without continued production of dense type I collagen meshwork as is seen in keloid pathology, allowing for better resolution of the wound defect. While there may be some similarities between the wound repair processes in horses and in humans, the practicality of using this model as a viable alternative to study tissue fibrosis poses a new subset of technical challenges many researchers would be unable to overcome.
Chemically-induced Fibrosis
Bleomycin Model
Bleomycin is a glycopeptide antibiotic isolated from the bacterium Streptomyces verticillius, and has subsequently been used as an anti-cancer therapy in the treatment of a variety of cancers, notably Hodgkin’s lymphoma and squamous cell carcinoma [19]. In addition to inducing strand breaks in DNA, bleomycin acts as an inflammatory mediator both systemically and at the site of administration. A major adverse effect of this systemic reaction is pulmonary toxicity which has limited its use in humans [19–21]. Ironically, it is this adverse effect that has been exploited to study fibrosis in a variety of different disorders [21–23]. Injections of bleomycin have been used since the 1980s to induce fibrosis in many organ systems, most notably in the skin and lungs. In a mouse model for idiopathic pulmonary fibrosis, single or repeated intra-tracheal doses of bleomycin yield a readily observable degree of fibrosis in a relatively short timeframe [24,25]. In the skin, bleomycin can be administered to produce a localized area of cutaneous fibrosis, the most common route of administration being direct subcutaneous injection. Given the difficulty and variability repeated daily injections can pose, however, newer models have emerged using sustained release of bleomycin via subcutaneously implanted osmotic pumps, eliminating the need for daily injections [21,22,26]. This administration method reduces injection site variability and provides a more consistent distribution of lesions in affected skin [22,26].
Bleomycin itself acts as an inflammatory mediator and elicits a strong inflammatory reaction at the site of administration. Many pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-4, IL-6 and CXCL2, are found to be upregulated in the skin of mice injected with bleomycin [21,22]. Additionally, many inflammatory cells, including CD45+ leukocytes, F4/80+ macrophages, CD3+ T lymphocytes and mast cells as well as α-smooth muscle actin (αSMA) positive myofibroblasts, are found in higher numbers in the skin of bleomycin-treated animals versus control [21,22,27]. A downstream effect of this intense inflammatory process is tissue fibrosis and increased collagen deposition. Both dermal thickness and hydroxyproline content of bleomycin-injected skin are 2–3 fold higher than control [21,22,26,27].
For studying the early inflammatory and immune component of fibrosis, the bleomycin model has an advantage. Many fibrotic conditions, including keloids, have been shown to have an inflammatory component that, in part, helps to drive fibrosis early on in scar development [28]. However, many other factors, such as hypoxia, genetic susceptibility and trauma, known to influence abnormal wound healing and scarring, are not encountered in this model. Furthermore, the fibrosis observed is self-limiting and dissipates with recovery of organ function less than 6 weeks post-cessation of bleomycin administration [19]. Additionally, the level and degree of fibrosis observed can be difficult to quantify. Many studies utilize dermal thickness as an indicator for fibrosis, and while this does reveal the effects of the drug, use of this metric is limited by sample area selection and observer bias. Dermal thickness is not uniformly increased in most specimens, with some exhibiting patches of increased dermal thickness as the bleomycin is dispersed throughout the skin [19,22]. Newer models utilizing sustained release of the drug via osmotic pump implantation have improved on this limitation, however, constant administration is still required for a long duration to achieve the desired effect [21,22].
The reactive fibrosis generated in this model has been used as an analogue to study scleroderma, idiopathic pulmonary fibrosis, hypertrophic scarring, and keloids, however it does not accurately reflect the pathogenesis of any of these diseases [19,21,26]. While it may not represent one particular model of disease, it has proven useful for testing various anti-fibrotic therapies, some of which are currently being investigated in clinical trials [19,29,30].
Reactive Oxygen Species Model
Reactive oxygen species (ROS) have been implicated in the pathogenesis of SSc [31]. Dermal fibroblasts taken from the skin of SSc patients have been known to produce large amounts of ROS and specific autoantibodies have been shown to induce ROS production [31]. Four specific types of ROS-inducing substances have been used to recapitulate the features found in SSc patients in mouse models of disease: hypochlorous acid (HOCL); hydrogen peroxide (H2O2); peroxynitrites (ONOO−); and superoxide anions (O2−). Each of these agents has been used to induce localized and systemic forms of fibrosis. Mice injected subcutaneously on a daily basis develop increased dermal thickness and collagen content, on par with bleomycin, compared to control [31–33]. Fibroblast proliferation when subjected to HOCL treatment is increased by almost 1.5-fold compared to treatment with PBS or bleomycin [31]. Additionally, the effects observed in the ROS model are not limited to dermal fibrosis; animals treated with ROS agents develop systemic reactions leading to pulmonary fibrosis and renal involvement [31,33]. Circulating concentrations of anti-DNA topoisomerase 1 and anti-CENP-B antibodies are elevated in both the ROS and bleomycin mouse models, signifying that both types of agents signal a systemic immune-mediated reaction, similar to SSc patients. However, TGF-β appears not to play a significant role in the ROS model, as its expression is not detected in the lesional skin of mice injected with ROS agents [31].
This model for fibrosis is similar to but distinct from the bleomycin model. A strong immune-mediated reaction is initiated from the administration of the ROS-generating agent, however the particular fibrotic pathways that are activated are independent from classical fibrotic signaling cascades. This model provides additional insight into tissue fibrosis, however, how it relates to keloid pathology remains to be seen.
Humanized animal model equivalents
Direct xenograft/tissue graft
Initial approaches toward establishing a mouse model for keloids included grafting keloid tissue onto immunodeficient mouse strains, particularly athymic nude mice [34,35].
In theory, the resulting xenografts could then be studied in a variety of ways from discerning specific fibrotic signaling pathways to testing various therapeutic agents in vivo. This approach has several advantages: the model itself is comparable to studying the specific human tissue in an in vivo environment while limiting the impact of environmental factors; not one cell type is studied, preserving the complex human cell-cell interactions in both the epidermis and dermis; cells, specifically fibroblasts, that thrive in a 3-dimensional organotypic environment continue to grow in such an environment.
Keloid xenografts can maintain viability for several weeks to months after transplantation, with evidence of angiogenesis and continued dermal collagen production [35–37]. In the immediate days to weeks following transplantation, xenografts display dense collagenous nodules and whorls similar to human hypertrophic scars with evidence of angiogenesis. In one report, Yang et al. describe full-thickness skin grafts maintaining viability in athymic nude mice up to 6 months following transplantation [37]. However, on average after several months most xenografts lose weight (>50%) and collagen and matrix production decreases [35,37,38].
The athymic nude mouse has been used extensively in transplant studies due to its lack of functioning T-cells thus displaying limited graft rejection [39,40]. However, the immune system is not fully absent in athymic nude mice as they still maintain functioning innate and humoral adaptive immune systems, including functioning natural killer cells. This can affect graft viability, specifically from solid human tissues, and limits the overall effectiveness of this model [40,41]. Additionally, resident immune cells in the graft tissue and/or enhanced antigenicity of the skin may further promote graft rejection [37].
While the xenograft model has demonstrated success in the short term, limited longevity, degree of fibrosis and the potential for rejection have prevented it from universal acceptance as a model for keloid pathology [41].
Tissue-Engineered Model
Attempts at utilizing donor-derived keloid tissue have allowed for the development of dermal tissue-equivalent models to study fibrosis. In these models, fibroblasts isolated and cultured from donor-derived keloid tissue are grown in 3-dimensional scaffolding materials and studied either as in vitro models or implanted in immunocompromised mice and analyzed as in vivo models. Both types of models rely heavily upon cells maintaining their cellular phenotypes once removed from their tissue of origin.
When cultured in Matrigel, a commercial mixture of basement membrane and other ECM proteins, keloid fibroblasts maintain their elevated expression of Col1, MMP9 and MMP13. Upon exogenous decorin administration, a GAG involved in collagen fibril organization, expression of Col1, MMP1, MMP13 are downregulated while MMP9 remains unaffected [42]. Wang et al. [43] demonstrated that keloid fibroblasts can maintain their viability and growth when dynamically seeded, i.e. cultured in suspension in specially-designed rotary cell-culture chambers, onto poly(lactic-co-glycolic acid) (PLGA) biomaterials and are implanted subcutaneously in athymic nude mice. Grossly, the fibroblast-containing scaffolds maintain their weight post-implantation and histologically demonstrate increased collagen deposition, mostly type I collagen, within the scaffold matrix, with significant neovascularization from the host animal. In a similar model where a collagen sponge was used as the scaffolding material, keloid fibroblast scaffolds demonstrated increased weight and greater amounts of ground substance and glycosaminoglycan deposition compared to control scaffolds [44].
Use of ex vivo tissue engineering models to study keloid pathogenesis is relatively recent and holds potential. As previously stated, this model is entirely dependent upon fibroblasts maintaining their cellular phenotype in culture once removed from their native environment. This emerging model does show promise for studying particular aspects of keloid pathology, and readily allows for testing different therapies [45].
Conclusions
Animal models aid researchers in elucidating underlying mechanisms of disease and allow for therapeutic interventions to be studied in a controlled environment. Selecting the appropriate model is a crucial first step in the design of a well-thought out study, one that can have a large impact on our understanding of disease and patient outcomes. The number of animal models presented here reflect our incomplete understanding of keloid scarring and abnormal wound healing. While each model can be used for a specific purpose, none are true models recapitulating the complex features of keloid development and progression. Key endpoints need to be defined in order to classify one model as being the “gold standard” by which others should be compared: reliability and reproducibility of results; systematic approach of measuring fibrosis, both histologically, chemically and molecularly; applicability to pharmacologic drug testing. While keloids have only been found to occur in humans, the various models presented herein provide means to study fibrotic diseases in further detail, with pharmacologic implications, the keloid being a paradigm of skin fibrosis.
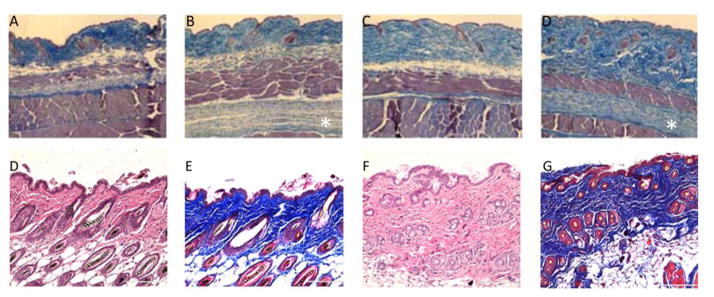
Figure 1. Tsk/+ and Bleomycin Models.
Hematoxylin & Eosin and Masson’s Trichrome staining of wild-type +/+ (A, C), Tsk/+ (B, D), PBS-treated (E,F) and bleomycin-treated (G, H) cutaneous sections. Genetic mutation in fibrillin-1 gene in the Tsk/+ mouse leads to thickened dermis with abundant collagen in both upper and lower dermis (B & D, sub-panniculus carnosus) compared to control (A & C). Daily administration of bleomycin elicits a strong inflammatory reaction in the host animal which, in turn, leads to increased dermal thickness and fibrosis (A & C) compared to control (B & D). Images of Tsk mouse reproduced from: Manne, J., Markova, M., Siracusa, L., Jimenez, S.A. “Collagen content in skin and internal organs of the Tight Skin Mouse: An animal model of scleroderma. Biochemistry Research International. Vol 2013, 1–8.
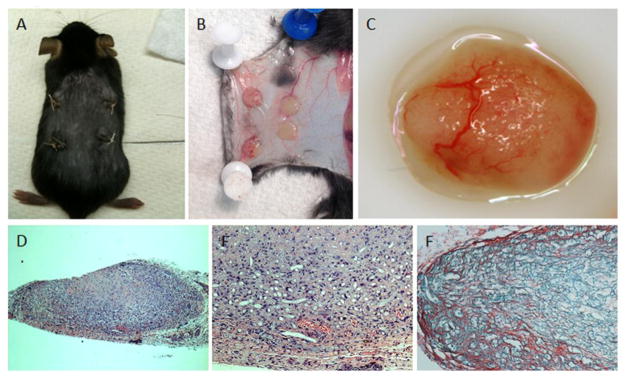
Figure 2. Bio-Scaffold Model.
PLA scaffolds (B & C) are dynamically seeded and subcutaneously implanted into immune-deficient mice (A). Upon retrieval, the scaffolds demonstrate marked neovascularization from the host animal, and continued collagen production (D, E: H&E staining, F: Sirius red staining).
Highlights.
Genetic animal models with diffuse skin fibrosis have been encountered, including Tight Skin Mutant Mouse (TSK) 1 and 2.
Trauma-induced scarring (rabbit ear model) and chemically-induced fibrosis (bleomycin) can serve as models for fibrotic diseases.
Keloids, the paradigm of fibrotic skin diseases, are uniquely human, and there are no naturally occurring animal models.
Recently, implantation of biodegradable 3-dimensional scaffolds impregnated with human keloid cells, into immuno compromised mice has been developed as an in vivo model of keloids.
Availability of animal models for fibrotic diseases allows testing of novel anti-fibrotic pharmacologic agents
Acknowledgments
Carol Kelly assisted with manuscript preparation. Dr. Marttala was supported by a post-doctoral fellowship from Orion Research Foundation, Finland, and Dr. Andrews was recipient of a NIH/NIAMS Training Grant AR T32060715.
Abbreviations
- HS
hypertrophic scar
- IGF-1
insulin-like growth factor-1
- IL
Interleukin
- ROS
reactive oxygen species
- SSc
systemic sclerosis
- TGF-β
transforming growth factor-β
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubio-Rivas M, Royo C, Simeon CP, Corbella X, Fonollosa V. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44:208–219. doi: 10.1016/j.semarthrit.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Baxter RM, Crowell TP, McCrann ME, Frew EM, Gardner H. Analysis of the tight skin (Tsk1/+) mouse as a model for testing antifibrotic agents. Lab Invest. 2005;85:1199–1209. doi: 10.1038/labinvest.3700331. [DOI] [PubMed] [Google Scholar]
- 3.Christner PJ, Peters J, Hawkins D, Siracusa LD, Jimenez SA. The tight skin 2 mouse. An animal model of scleroderma displaying cutaneous fibrosis and mononuclear cell infiltration. Arthritis Rheum. 1995;38:1791–1798. doi: 10.1002/art.1780381212. [DOI] [PubMed] [Google Scholar]
- 4.Long KB, Burgwin CM, Huneke R, Artlett CM, Blankenhorn EP. Tight Skin 2 Mice Exhibit Delayed Wound Healing Caused by Increased Elastic Fibers in Fibrotic Skin. Adv Wound Care (New Rochelle) 2014;3:573–581. doi: 10.1089/wound.2014.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long KB, Li Z, Burgwin CM, Choe SG, Martyanov V, Sassi-Gaha S, Earl JP, Eutsey RA, Ahmed A, Ehrlich GD, Artlett CM, Whitfield ML, Blankenhorn EP. The Tsk2/+ mouse fibrotic phenotype is due to a gain-of-function mutation in the PIIINP segment of the Col3a1 gene. J Invest Dermatol. 2015;135:718–727. doi: 10.1038/jid.2014.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long KB, Artlett CM, Blankenhorn EP. Tight skin 2 mice exhibit a novel time line of events leading to increased extracellular matrix deposition and dermal fibrosis. Mat Bio. 2014;38:91–100. doi: 10.1016/j.matbio.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Wells A, Nuschke A, Yates C. Skin tissue repair: matrix microenvironmental influences. Mat Bio. 2015 doi: 10.1016/j.matbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harunari N, Zhu KQ, Armendariz RT, Deubner H, Muangman P, Carrougher GJ, Isik FF, Gibran NS, Engrav LH. Histology of the thick scar on the female, red Duroc pig: final similarities to human hypertrophic scar. Burns. 2006;32:669–677. doi: 10.1016/j.burns.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu KQ, Carrougher GJ, Gibran NS, Isik FF, Engrav LH. Review of the female Duroc/Yorkshire pig model of human fibroproliferative scarring. Wound Repair Regen. 2007;15(Suppl 1):S32–39. doi: 10.1111/j.1524-475X.2007.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu KQ, Carrougher GJ, Couture OP, Tuggle CK, Gibran NS, Engrav LH. Expression of collagen genes in the cones of skin in the Duroc/Yorkshire porcine model of fibroproliferative scarring. J Burn Care Res. 2008;29:815–827. doi: 10.1097/BCR.0b013e3181848141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph J, Dyson M. Tissue replacement in the rabbit’s ear. Br J Surg. 1966;53:372–380. doi: 10.1002/bjs.1800530415. [DOI] [PubMed] [Google Scholar]
- 12.Morris DE, Wu L, Zhao LL, Bolton L, Roth SI, Ladin DA, Mustoe TA. Acute and chronic animal models for excessive dermal scarring: quantitative studies. Plast Reconstr Surg. 1997;100:674–681. doi: 10.1097/00006534-199709000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Tollefson TT, Kamangar F, Aminpour S, Lee A, Durbin-Johnson B, Tinling S. Comparison of effectiveness of silicone gel sheeting with microporous paper tape in the prevention of hypertrophic scarring in a rabbit model. Arch Facial Plast Surg. 2012;14:45–51. doi: 10.1001/archfacial.2011.62. [DOI] [PubMed] [Google Scholar]
- 14.Kloeters O, Tandara A, Mustoe TA. Hypertrophic scar model in the rabbit ear: a reproducible model for studying scar tissue behavior with new observations on silicone gel sheeting for scar reduction. Wound Repair Regen. 2007;15(Suppl 1):S40–45. doi: 10.1111/j.1524-475X.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- 15.Uzun H, Bitik O, Hekimoglu R, Atilla P, Kayikcioglu AU. Angiotensin-converting enzyme inhibitor enalapril reduces formation of hypertrophic scars in a rabbit ear wounding model. Plast Reconstr Surg. 2013;132:361e–371e. doi: 10.1097/PRS.0b013e31829acf0a. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Chen Z, Li XJ, Ma L, Tang YL. Anti-inflammatory cytokine TSG-6 inhibits hypertrophic scar formation in a rabbit ear model. Eur J Pharmacol. 2015;751:42–49. doi: 10.1016/j.ejphar.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Theoret CL, Olutoye OO, Parnell LK, Hicks J. Equine exuberant granulation tissue and human keloids: a comparative histopathologic study. Vet Surg. 2013;42:783–789. doi: 10.1111/j.1532-950X.2013.12055.x. [DOI] [PubMed] [Google Scholar]
- 18.Ud-Din S, Volk SW, Bayat A. Regenerative healing, scar-free healing and scar formation across the species: current concepts and future perspectives. Exp Dermatol. 2014;23:615–619. doi: 10.1111/exd.12457. [DOI] [PubMed] [Google Scholar]
- 19.Della Latta V, Cecchettini A, Del Ry S, Morales MA. Bleomycin in the setting of lung fibrosis induction: From biological mechanisms to counteractions. Pharmacol Res. 2015;97:122–130. doi: 10.1016/j.phrs.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Majeski JT, Neidzwiecki JA. Caution in reconstituting bleomycin. J Am Acad Dermatol. 1985;13:517–518. doi: 10.1016/s0190-9622(85)80368-6. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto T, Takagawa S, Katayama I, Yamazaki K, Hamazaki Y, Shinkai H, Nishioka K. Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J Invest Dermatol. 1999;112:456–462. doi: 10.1046/j.1523-1747.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- 22.Liang M, Lv J, Zou L, Yang W, Xiong Y, Chen X, Guan M, He R, Zou H. A modified murine model of systemic sclerosis: bleomycin given by pump infusion induced skin and pulmonary inflammation and fibrosis. Lab Invest. 2015;95:342–350. doi: 10.1038/labinvest.2014.145. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi T, Asano Y, Ichimura Y, Toyama T, Taniguchi T, Noda S, Akamata K, Tada Y, Sugaya M, Kadono T, Sato S. Amelioration of tissue fibrosis by toll-like receptor 4 knockout in murine models of systemic sclerosis. Arthritis Rheumatol. 2015;67:254–265. doi: 10.1002/art.38901. [DOI] [PubMed] [Google Scholar]
- 24.Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299:L442–452. doi: 10.1152/ajplung.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaauboer M, Boeijen F, Emson C, Turner S, Zandieh-Doulabi B, Hanemaaijer R, Smit T, Stoop R, Everts V. Extracellular matrix proteins: a positive feedback loop in lung fibrosis? Mat Bio. 2014;34:170–178. doi: 10.1016/j.matbio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Cameron AM, Adams DH, Greenwood JE, Anderson PJ, Cowin AJ. A novel murine model of hypertrophic scarring using subcutaneous infusion of bleomycin. Plast Reconstr Surg. 2014;133:69–78. doi: 10.1097/01.prs.0000436821.26709.a7. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, Takahashi Y, Takagawa S, Katayama I, Nishioka K. Animal model of sclerotic skin. II Bleomycin induced scleroderma in genetically mast cell deficient WBB6F1-W/W(V) mice. J Rheumatol. 1999;26:2628–2634. [PubMed] [Google Scholar]
- 28.Bagabir R, Byers RJ, Chaudhry IH, Muller W, Paus R, Bayat A. Site-specific immunophenotyping of keloid disease demonstrates immune upregulation and the presence of lymphoid aggregates. Br J Dermatol. 2012;167:1053–1066. doi: 10.1111/j.1365-2133.2012.11190.x. [DOI] [PubMed] [Google Scholar]
- 29.Ahluwalia N, Shea BS, Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med. 2014;190:867–878. doi: 10.1164/rccm.201403-0509PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther. 2014;349:209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- 31.Servettaz A, Goulvestre C, Kavian N, Nicco C, Guilpain P, Chereau C, Vuiblet V, Guillevin L, Mouthon L, Weill B, Batteux F. Selective oxidation of DNA topoisomerase 1 induces systemic sclerosis in the mouse. J Immunol. 2009;182:5855–5864. doi: 10.4049/jimmunol.0803705. [DOI] [PubMed] [Google Scholar]
- 32.Bagnato G, Bitto A, Pizzino G, Irrera N, Sangari D, Cinquegrani M, Roberts WN, Matucci Cerinic M, Squadrito F, Altavilla D, Saitta A. Simvastatin attenuates the development of pulmonary and cutaneous fibrosis in a murine model of systemic sclerosis. Rheumatology (Oxford) 2013;52:1377–1386. doi: 10.1093/rheumatology/ket144. [DOI] [PubMed] [Google Scholar]
- 33.Batteux F, Kavian N, Servettaz A. New insights on chemically induced animal models of systemic sclerosis. Curr Opin Rheumatol. 2012;23:511–518. doi: 10.1097/BOR.0b013e32834b1606. [DOI] [PubMed] [Google Scholar]
- 34.Estrem SA, Domayer M, Bardach J, Cram AE. Implantation of human keloid into athymic mice. Laryngoscope. 1987;97:1214–1218. doi: 10.1288/00005537-198710000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Kischer CW, Pindur J, Shetlar MR, Shetlar CL. Implants of hypertrophic scars and keloids into the nude (athymic) mouse: viability and morphology. J Trauma. 1989;29:672–677. doi: 10.1097/00005373-198905000-00023. [DOI] [PubMed] [Google Scholar]
- 36.Kischer CW, Sheridan D, Pindur J. Use of nude (athymic) mice for the study of hypertrophic scars and keloids: vascular continuity between mouse and implants. Anat Rec. 1989;225:189–196. doi: 10.1002/ar.1092250303. [DOI] [PubMed] [Google Scholar]
- 37.Yang D, Li S, Wu J, Chen Y, Li G, Bi S, Dai X. Establishment of a hypertrophic scar model by transplanting full-thickness human skin grafts onto the backs of nude mice. J Plast Reconstr Surg Nurs. 2005;119:104–109. doi: 10.1097/01.prs.0000244828.80490.62. [DOI] [PubMed] [Google Scholar]
- 38.Shetlar MR, Shetlar CL, Kischer CW, Pindur J. Implants of keloid and hypertrophic scars into the athymic nude mouse: changes in the glycosaminoglycans of the implants. Connect Tissue Res. 1991;26:23–36. doi: 10.3109/03008209109152161. [DOI] [PubMed] [Google Scholar]
- 39.Mecklenburg L, Nakamura M, Sundberg JP, Paus R. The nude mouse skin phenotype: the role of Foxn1 in hair follicle development and cycling. Exp Mol Pathol. 2001;71:171–178. doi: 10.1006/exmp.2001.2386. [DOI] [PubMed] [Google Scholar]
- 40.Shultz D, Goodwin N, Ishikawa F, Hosur V, Lyons B, Greiner D. Human cancer growth and therapy in NOD/SCID/IL2Rynull (NSG) mice. Cold spring Harbor Protoc. 2014 doi: 10.1101/pdb.top073585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seo BF, Lee JY, Jung SN. Models of abnormal scarring. Biomed Res Int. 2013;2013:423147. doi: 10.1155/2013/423147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Nahas Z, Feng F, Elisseeff JH, Boahene K. Tissue engineering for in vitro analysis of matrix metalloproteinases in the pathogenesis of keloid lesions. JAMA Facial Plast Surg. 2013;15:448–456. doi: 10.1001/jamafacial.2013.1211. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Luo S. Establishment of an animal model for human keloid scars using tissue engineering method. J Burn Care Res. 2013;34:439–446. doi: 10.1097/BCR.0b013e318269bd64. [DOI] [PubMed] [Google Scholar]
- 44.Yagi Y, Muroga E, Naitoh M, Isogai Z, Matsui S, Ikehara S, Suzuki S, Miyachi Y, Utani A. An ex vivo model employing keloid-derived cell-seeded collagen sponges for therapy development. J Invest Dermatol. 2013;133:386–393. doi: 10.1038/jid.2012.314. [DOI] [PubMed] [Google Scholar]
- 45.Chung HJ, Steplewski A, Chung KY, Uitto J, Fertala A. Collagen fibril formation. A new target to limit fibrosis. J Biol Chem. 2008;283:25879–25886. doi: 10.1074/jbc.M804272200. [DOI] [PMC free article] [PubMed] [Google Scholar]