Abstract
Background
Esophagogastric junction contractile integral (EGJ-CI) assesses EGJ barrier function on esophageal high resolution manometry (HRM). We assessed EGJ-CI values in achalasia and gastroesophageal reflux disease (GERD) to determine if postoperative EGJ-CI changes reflected surgical intervention.
Methods
21 achalasia patients (42.8±3.2 yrs, 62% F) with HRM before and after Heller myotomy (HM) and 68 GERD patients (53.9±1.8 yrs, 66% F) undergoing anti-reflux surgery (ARS) were compared to 21 healthy controls (27.6±0.6 yrs, 52% F). EGJ-CI (mmHg.cm) was calculated using the distal contractile integral (DCI) measurement across the EGJ, measured above the gastric baseline and corrected for respiration. Pre- and post-surgical EGJ-CI and conventional lower esophageal sphincter pressure (LESP) metrics were compared within and between these groups using non-parametric tests. Correlation between EGJ-CI and conventional LESP metrics was assessed.
Results
Baseline EGJ-CI was higher in achalasia compared to GERD (p<0.001) or controls (p=0.03). EGJ-CI declined by 59.2% after HM in achalasia (p=0.001), and increased by 26.3% after ARS in GERD (p=0.005). End-expiratory and basal LESP decreased by 74.5% and 64.5% with HM, but increased by only 17.8% and 4.3% with ARS. Differences were noted between Dor vs. Toupet fundoplication in achalasia (p=0.007), and partial vs. complete ARS in GERD (p=0.03). EGJ-CI correlated modestly with both end-expiratory and basal LESP (Pearson’s r of 0.8 for all), but was less robust in GERD (0.7).
Conclusions
EGJ-CI has clinical utility in assessing EGJ barrier function at baseline and after surgical intervention to the EGJ, and could complement conventional EGJ metrics.
Keywords: high-resolution manometry, esophagogastric junction, antireflux surgery, myotomy
INTRODUCTION
The introduction of esophageal high-resolution manometry (HRM) over the past decade has advanced the diagnosis and classification of esophageal motor disorders, mainly through the use of software tools and intuitive HRM-specific metrics1, 2. However, similar software tools have not been adopted for assessing esophagogastric junction (EGJ) barrier function. Physiologic or structural deficiencies at the EGJ barrier facilitate GERD3, 4, and evaluation of EGJ barrier function may be relevant in the evaluation of patients referred for anti-reflux surgery (ARS)5. The single HRM EGJ metric in common use, integrated relaxation pressure (IRP), assesses the EGJ only in terms of post swallow residual pressures, and adequacy of transit through the EGJ with swallows, but not anti-reflux barrier function1. Indeed, metrics generated with conventional esophageal manometry (including mean basal and end-expiratory lower esophageal sphincter (LES) pressures) continue to be reported and utilized. Although these measures generate descriptive and meaningful EGJ metrics, EGJ function as an anti-reflux barrier is not comprehensively addressed.
The esophagogastric junction contractile integral (EGJ-CI) is a novel esophageal high-resolution manometry (HRM) metric that evaluates EGJ barrier function6–9. Designed similar to the distal contractile integral (DCI), the EGJ-CI takes both inspiratory and expiratory LES pressures, and EGJ length into account, as all are fundamental to the assessment of the EGJ barrier10, 11. The current version of the EGJ-CI is measured above the gastric baseline, and is corrected for respiratory cycle duration6, 8, 9. While the EGJ-CI makes physiologic sense, the impact of surgical interventions to the EGJ on the EGJ-CI metric have not been reported. Documentation of re-establishment of EGJ barrier function with ARS may be useful in investigating recurrent or persistent post-operative symptoms, while description of adequate LES disruption following Heller myotomy (HM) may help determine adequacy of this therapeutic measure.
In this study, we evaluated baseline and post-operative EGJ-CI values in patients with achalasia and GERD, and compared these to measurements from healthy controls. We assessed whether post-operative EGJ-CI changes reflected surgical intervention in achalasia (HM) and GERD (ARS). Specifically, we sought to verify the value of EGJ-CI as representative of EGJ barrier function by comparing it to conventional EGJ metrics of EGJ barrier integrity.
METHODS
Adult patients (≥18 years) with both pre-and post-operative HRM following surgical intervention for achalasia (HM) and GERD (ARS), were identified from our institutional database over an 8-year-period from 2007 to 2014. Demographic data (age, gender) and clinical characteristics (achalasia subtype, fundoplication type, presenting symptom prompting postoperative evaluation) were collected. Exclusion criteria included incomplete esophageal HRM or lack of clinical and symptom data. HRM and clinical data from these study subjects were extracted from the institutional HRM database and electronic medical records. The control group was composed of healthy asymptomatic patients who had no history of gastrointestinal symptoms, no upper gastrointestinal tract surgery, no major medical conditions, and were not on any regular medications; these control subjects underwent HRM as part of our institutional normative data assessment. The study protocol was approved by the Human Research Protection Office (Institutional Review Board) at Washington University in Saint Louis.
HRM studies were performed after an overnight fast using a 36-channel solid-state catheter system with high-fidelity circumferential sensors 1-cm apart along the catheter (Covidien/Given Imaging, Duluth, GA). After calibration, the catheter was passed through an anesthetized nasal canal. A 20-second swallow-free period was obtained while the subject remained still, resting quietly in the recumbent position (landmark period), from which basal lower esophageal sphincter pressures (LESP) were obtained12. Ten swallows were recorded using 4–5 mL of ambient temperature water spaced >20 s apart. Studies were acquired and analyzed using dedicated computerized HRM acquisition, display, and analysis systems (ManoView; Covidien/Given Imaging, Duluth, GA).
Conventional HRM metrics
LES pressures were extracted from the landmark phase using the electronic sleeve function (eSleeve, Manoview, Covidien/Given Imaging, Duluth, GA) during quiet rest. This included mean basal LES pressure and end expiratory LES pressure, from both pre- and postoperative HRM studies. Control HRM studies were similarly evaluated. Augmentation with respiration was measured as the difference between mean basal LES pressure and end expiratory LES pressure in mmHg, which describes the contribution of diaphragmatic crural contraction in mid respiration to the EGJ barrier. Achalasia was subtyped as per Chicago Classification 3.01 on the pre-operative HRM study; median IRP of >15 mmHg was required for achalasia diagnosis. EGJ morphology was characterized and recorded as follows: type 1: no separation between LES and crural diaphragm; type 2: ≤2 cm separation between LES and crural diaphragm, type 3: >2 cm separation between LES and crural diaphragm1.
Calculation of EGJ-CI
In addition to standard analysis of the motor pattern using the Chicago Classification 3.01, particular attention was focused on EGJ metrics (mean basal LES pressure, end-expiratory LES pressure). The EGJ-CI was calculated as previously reported6 from the same landmark phase where conventional LES metrics were extracted. Briefly, the landmark period was used to identify 3 respiratory cycles, and the duration of the cycles was recorded. The gastric baseline was determined during the landmark phase, and set as the threshold for EGJ-CI calculation. The EGJ-CI was determined by forcing the distal contractile integral (DCI) measurement box across the EGJ, for exactly three respiratory cycles and recorded above the gastric baseline; by convention, three respiratory cycles were chosen instead of one to make the metric more representative given propensity for variation in respiratory cyclic duration6. Next, this value was corrected for time by dividing it by the duration of the three respiratory cycles, to yield the corrected EGJ-CI in mmHg.cm (Figure 1).
Figure 1.
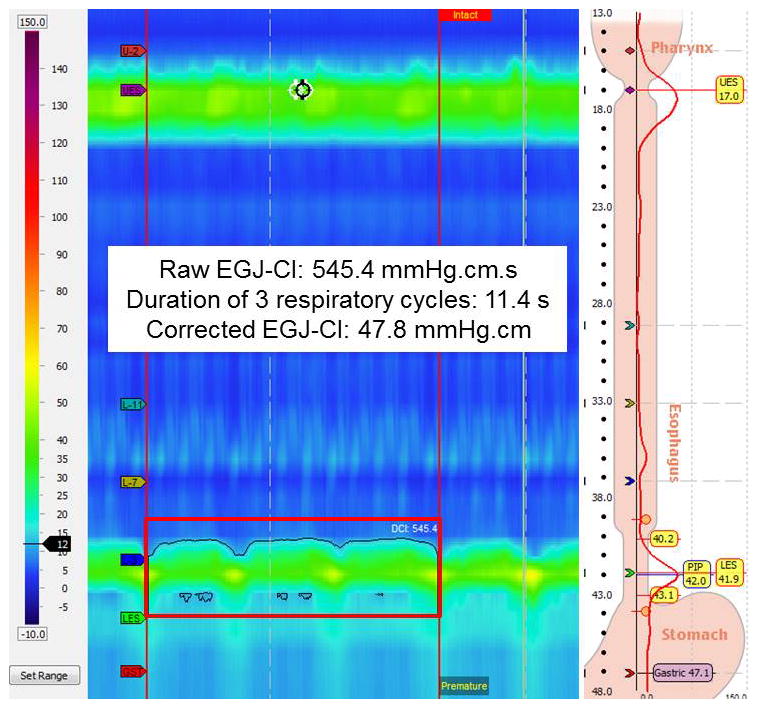
Measurement of the esophagogastric junction contractile integral (EGJ-CI). The distal contractile integral (DCI) box is placed over the esophagogastric junction covering exactly 3 respiratory cycles, during the initial landmark phase when basal LES metrics are obtained. The recorded value (raw EGJ-CI, in mmHg.cm.sec) is divided by the duration of three respiratory cycles to yield the corrected EGJ-CI in mmHg.cm.
Data Analysis
Continuous variables are reported as mean ± standard error of the mean (SEM) or median and interquartile range (IQR), as appropriate. Categorical data are reported using frequencies and proportions. Normative values for EGJ-CI were determined from analysis of data from normal controls. Age and gender were compared between the three groups with ANOVA and chi-square tests, respectively. Pre- and post-surgical EGJ-CI values and conventional EGJ metrics were compared between groups with non-parametric independent-samples Kruskal-Wallis or Mann-Whitney U tests, and within groups with the related-samples Wilcoxon signed-rank test. The degree of correlation between EGJ-CI and LESP metrics was assessed with Pearson’s r and Spearman’s rho correlations. In all cases, p<0.05 was required for statistical significance. All statistical analyses were performed using IBM SPSS Statistics V.22.0 (Armonk, NY).
RESULTS
Demographic and Clinical Characteristics
During the study period, 89 patients with pre- and post-operative HRM after surgical intervention to the EGJ were identified. 21 achalasia patients underwent HM (42.8±3.2 years, 62% female), and 68 GERD patients underwent ARS (53.9±1.8 years, 66% female). Of the 21 patients with achalasia, 23.8% had achalasia subtype 1, 52.4% had subtype 2, and the remaining 23.8% had subtype 3. Partial fundoplication was performed along with HM in 95.2% (Dor fundoplication =16, Toupet fundoplication =4); only one patient underwent Nissen fundoplication. In contrast, of the 68 ARS patients, 60 (88.2%) underwent Nissen fundoplication (p<0.0001 compared to HM); the remainder underwent either Toupet (7 patients) or Dor fundoplication (1 patient). Indications for postoperative HRM among HM patients included reflux symptoms (5 patients), dysphagia (12 patients), and both reflux symptoms and dysphagia (3 patients); one patient was asymptomatic. Of the ARS patients, 29 presented post-operatively with reflux symptoms, 27 with dysphagia, and 12 with both reflux symptoms and dysphagia. The control group consisted of 21 healthy volunteers (27.6 ±0.6 years, 52% female). Baseline clinical characteristics are described in Table 1.
Table 1.
Demographic and Clinical Characteristics
| HM n=21 | ARS n=68 | Controls n=21 | p values* | |
|---|---|---|---|---|
| Mean age (yr) | 42.8±3.2 | 53.9±1.8 | 27.6±0.6 | <0.001 |
| Sex (F) | 13 (62%) | 45 (66%) | 11 (52%) | 0.519 |
| Duration between HRM studies (yr) | 2.2±0.3 | 2.0±0.2 | - | 0.741 |
| Post-operative reflux symptoms | 8 (40%) | 41 (60%) | 0.177 | |
| Post-operative dysphagia | 15 (75%) | 39 (57%) | 0.245 | |
| Nissen fundoplication | 1 (4.8%) | 60 (88.2%) | <0.0001 | |
| Dor fundoplication | 16 (76.2%) | 1 (1.5%) | ||
| Toupet fundoplication | 4 (19.0%) | 7 (10.3%) |
Values reported as mean ± standard error of mean.
ANOVA for age; 2-tailed Student’s t-test for duration; Chi-square tests for other comparisons.
Baseline EGJ Characteristics
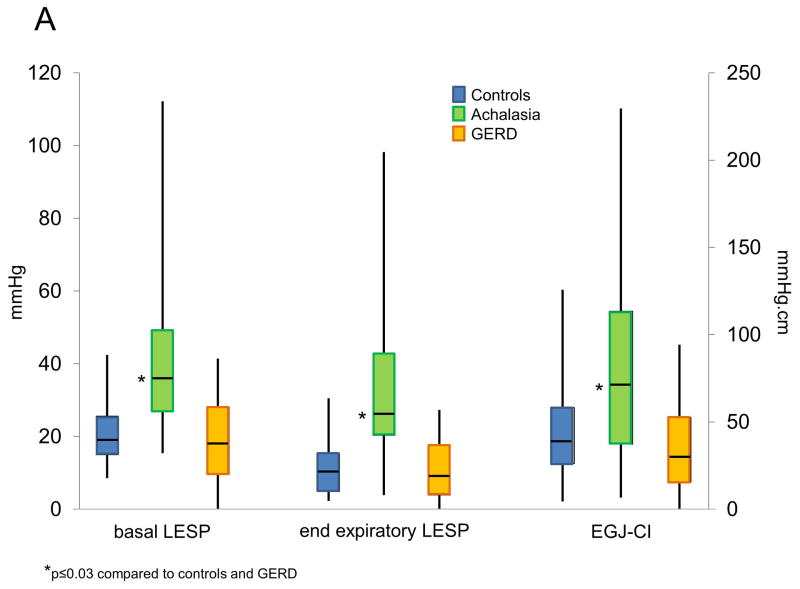
Baseline conventional EGJ metrics (basal LESP and end expiratory LESP) were calculated for each group of patients pre- and post-operatively, and compared to controls (Table 2. Figure 2). Pre-operative end expiratory and basal LESP were significantly higher among achalasia patients compared to GERD patients or controls (p≤0.001 for each comparison, Figure 2A). However, these LESP metrics did not differ between GERD patients and controls (p≥0.29 for both comparisons). Augmentation of LESP with breathing was lower in GERD compared to controls (p=0.03), but did not differ between achalasia and the other two groups (p≥0.18 for both comparisons, Table 2). Within the GERD cohort, 31 subjects had type 1 EGJ morphology, 11 type 2, and 26 had type 3 morphology, with pre-operative EGJ-CI values of 44.0±5.2, 28.3±6.6, and 29.1±5.5 mmHg.cm, respectively. While these trended towards significance across the three subtypes (p=0.06), EGJ-CI was significantly higher in type 1 compared to type 3 EGJ morphology (p=0.03); other comparisons were non-significant.
Table 2.
Comparison of EGJ metrics between study groups
| Achalasia-HM* n=21 | GERD-ARS* n=68 | Controls n=21 | p values* | |
|---|---|---|---|---|
| Baseline parameters | ||||
| End expiratory LESP (mmHg) | 25.6 (20.3–42.8) | 8.6 (3.9–15.8) | 10.3 (4.9–15.2) | <0.001 |
| Mean basal LESP (mmHg) | 36.2 (26.8–49.3) | 17.9 (9.6–28.1) | 19.0 (15.0–25.6) | <0.001 |
| Inspiratory augmentation (mmHg) | 7.5 (5.4–13.3) | 7.0 (4.2–9.4) | 8.7 (6.4–14.2) | 0.065 |
| EGJ CI (mmHg.cm) | 67.1 (37.3–113.5) | 29.5 (15.4–52.7) | 34.7 (26.2–58.3) | 0.001 |
| Postoperative parameters | ||||
| End expiratory LESP (mmHg) | 6.0 (3.1–10.1) | 11.3 (4.6–18.5) | - | 0.077 |
| Mean basal LESP (mmHg) | 12.0 (8.4–19.5) | 19.9 (10.0–26.8) | - | 0.096 |
| Inspiratory augmentation (mmHg) | 5.2 (3.4–10.6) | 5.9 (3.6–9.9) | - | 0.873 |
| EGJ CI (mmHg.cm) | 27.0 (11.3–43.0) | 43.8 (20.9–61.9) | - | 0.051 |
Values reported as median (interquartile range)
Independent-samples Kruskal-Wallis test or Mann-Whitney U test
Figure 2.
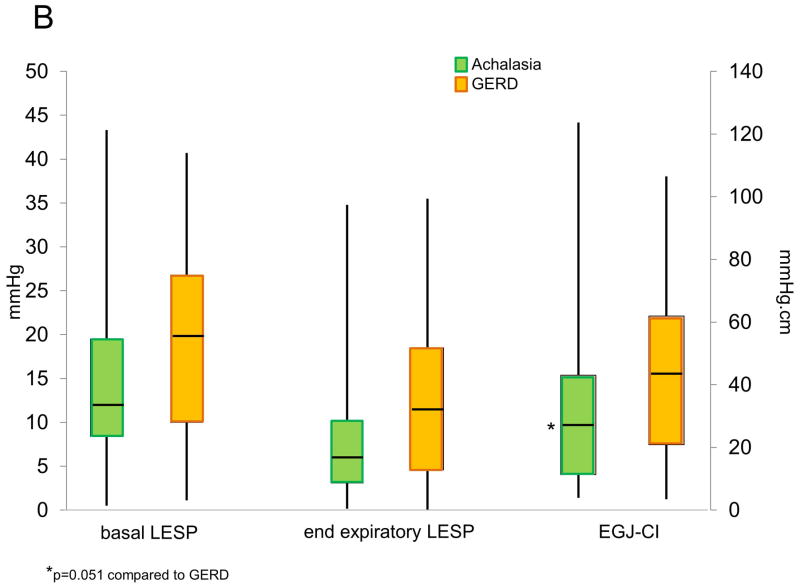
Comparison of lower esophageal sphincter pressure (LESP) metrics and EGJ-CI between achalasia, GERD and controls at baseline (A) and between achalasia and GERD postoperatively (B). Baseline conventional metrics (end expiratory LESP, basal LESP) as well as EGJ-CI were higher in achalasia compared to the other two groups (p≤0.03 for each comparison). Post-operative EGJ-CI strongly trended to be lower in achalasia compared to GERD (p=0.051) but conventional LESP metrics were not different between the two groups.
Achalasia patients had significantly higher pre-operative EGJ-CI values compared to those with GERD or controls (p≤0.03 for both comparisons; Figure 2A), Baseline EGJ-CI values were numerically lower in GERD patients compared to controls, but did not reach statistical significance (p=0.18).
Changes with Surgical Intervention
With HM, basal LESP, and end-expiratory LESP significantly decreased from pre-operative values (74.5 and 64.5% decline respectively, p≤0.001 for all comparisons, Figure 3). Mean end-expiratory and basal LESP decreased by 74.5% and 64.5% with HM. With ARS, although end-expiratory LESP increased 17.7% from pre-operative values (p=0.057 and p=0.005, respectively), basal LESP remained similar (4.3% increase, p=0.262). On the other hand, EGJ-CI declined by 59.2% after HM in achalasia (p<0.001), and increased by 26.3% after ARS in GERD (p=0.005, Figure 3). Augmentation with respiration significantly decreased with HM (p=0.025) but remained similar after ARS (p=0.164). Only 3 (14%) achalasia patients augmented EGJ-CI following HM, compared to 44 (65%) of GERD patients following ARS (p<0.001). Among the post-ARS cohort, the 27 patients who presented with dysphagia trended towards higher post-ARS EGJ-CI values compared to the 29 patients with reflux symptoms (48.7±5.3 vs 37.0±5.3 mmHg.cm respectively, p=0.06); 12 patients with both dysphagia and reflux were excluded from this comparison. There was no difference in post-ARS EGJ-CI across the three EGJ morphology subtypes (p=0.27 across groups).
Figure 3.
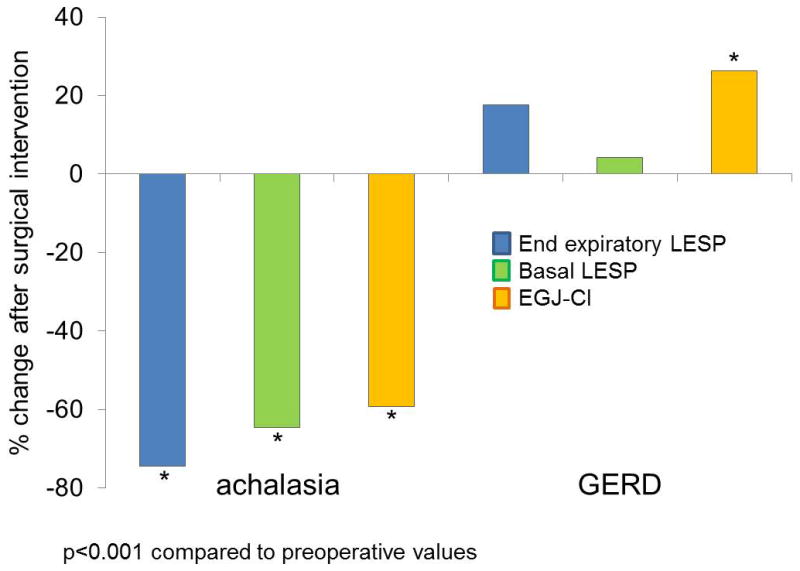
Change in esophagogastric junction contractile integral (EGJ-CI) following surgical intervention in achalasia and GERD. All LES metrics declined significantly following Heller myotomy for achalasia (p<0.001 for each comparison). Only EGJ CI augmented significantly following antireflux surgery for GERD (p<0.001).
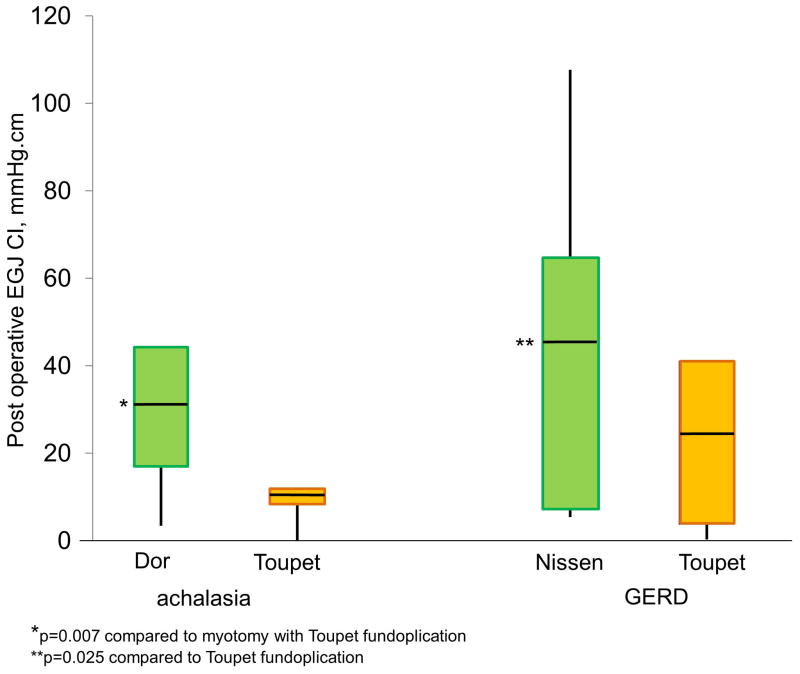
When differences between types of fundoplication with HM were evaluated, the 16 patients with Dor fundoplication had significantly higher post-HM EGJ-CI values (median 29.8 mmHg.cm, IQR 16.8–44.3 mmHg.cm) compared to the 4 patients who underwent Toupet fundoplication (median 9.8 mmHg.cm, IQR 8.5–11.4 mmHg.cm; Figure 4, p=0.007), despite similar pre-operative conventional and HRM LES metrics (p≥0.4 for all comparisons). With ARS, the 60 patients who underwent Nissen fundoplication had significantly higher postoperative EGJ-CI values (median 46.0 mmHg.cm, IQR 22.0–64.9 mmHg.cm) compared to the 7 patients who had a Toupet fundoplication (median 25.4 mmHg.cm, IQR 3.8–41.1 mmHg.cm; Figure 4, p=0.025), again despite similar LESP metrics (p≥0.1 for all comparisons).
Figure 4.
Differences in esophagogastric junction contractile integral (EGJ-CI) between types of antireflux procedures performed with Heller myotomy (HM) in achalasia, and in GERD. Augmentation of EGJ-CI was more robust following Dor fundoplication with HM (p=0.007 compared with Toupet fundoplication in this setting. Nissen fundoplication in GERD resulted in the highest augmentation of EGJ-CI (p=0.025 compared to Toupet fundoplication in this setting).
Comparison of EGJ-CI with Conventional
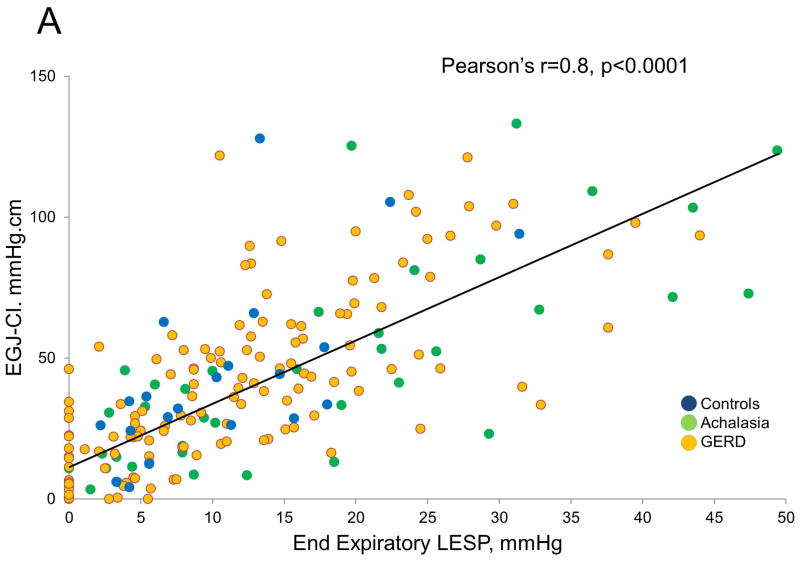
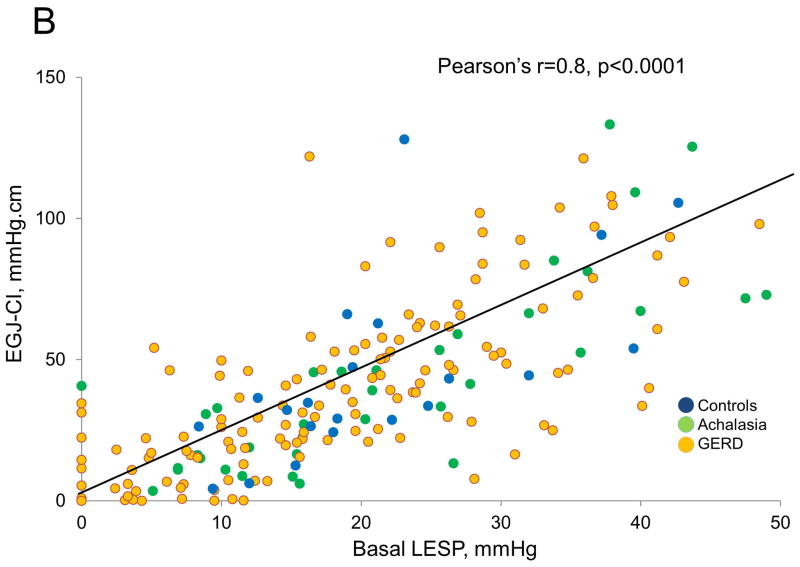
EGJ Metrics Overall, for all subjects, the EGJ-CI correlated with both end-expiratory and basal LESP (Pearson’s r and Spearman coefficients of 0.8 for each comparison, p<0.001 for all correlations, Figure 5). Although all correlations were significant (p<0.001), those between EGJ-CI and basal LESP were higher in achalasia (Pearson’s r and Spearman coefficients of 0.9, 0.8 respectively) and controls (0.8, 0.8 respectively), but less robust in GERD (0.7, 0.7 respectively). Similarly, although all relationships were significant (p<0.001), correlations between EGJ-CI and end-expiratory LESP were higher in achalasia (0.9, 0.8 respectively) and controls (0.9, 0.8 respectively), but less robust in GERD (0.7, 0.8 respectively). Similar degrees of correlation were seen pre-operatively and post-operatively.
Figure 5.
Comparison of conventional lower esophageal sphincter pressure (LESP) metrics with esophagogastric junction contractile integral (EGJ-CI). There was excellent correlation between individual LESP metrics (end-expiratory LESP, A; basal LESP, B) and EGJ-CI (Pearson’s r=0.8, p<0.001 for each comparison). Correlation was similar for controls and achalasia (r=0.8–0.9), but less robust for GERD (r=0.7).
DISCUSSION
In this report, we show that the esophagogastric junction contractile integral (EGJ-CI) functions well as a metric for EGJ barrier function when comparing pre- and post-operative EGJ-CI values in achalasia and GERD patients. Baseline EGJ-CI is higher among patients with achalasia compared to those with GERD or institutional controls, and decreases appropriately with HM disruption of the EGJ barrier and increases with ARS augmentation of the EGJ barrier. Post-operative EGJ-CI mirrors expectations following 360-degree fundoplication vs. partial fundoplication in GERD, and following variants of partial fundoplication after HM. EGJ-CI correlates well with conventional EGJ metrics (end-expiratory and basal LESP), although in a less robust fashion in GERD. Therefore, our results demonstrate that the EGJ-CI metric has clinical utility in assessing EGJ barrier function at baseline and after surgical intervention to the EGJ, and combines elements of the EGJ barrier into a single convenient metric that correlates with individual conventional EGJ metrics.
The EGJ barrier is composed of two elements: the intrinsic lower esophageal sphincter (LES) component which has a constant resting tone, and a superimposed crural diaphragmatic component which augments the barrier during inspiration to prevent reflux when intrathoracic pressures are negative8, 11, 13. Conventional esophageal manometry utilizes the stationary pull-through maneuver to localize the LES, and to assess basal tone, using either a single unidirectional or circumferential sensor, or a sleeve. This technique is limited in its ability to assess all aspects of the barrier, especially inspiratory augmentation from an asymmetric EGJ barrier11, 13. Esophageal HRM has advanced assessment of the EGJ barrier, and morphologic descriptions of superimposition of separation of the LES and diaphragmatic crural pressure signatures are now utilized to describe the barrier1, 10. While categorization of the components of the barrier with HRM is undoubtedly useful, pressure metrics are frequently utilized in describing EGJ resting tone, especially in assessing a disrupted barrier prior to ARS. 3-dimensional HRM has demonstrated profound asymmetry of the crural diaphragmatic component of the EGJ barrier14, making point pressure assessments, especially at mid respiration or at peak expiration not fully representative. In other words, changes in the EGJ barrier with respiration, especially with inspiratory augmentation, are particularly significant, but these dynamics are, at the present time, not always assessed or reported even with HRM. EGJ-CI has the potential to overcome some of the limitations of existing reporting of EGJ barrier function, and combines important elements, especially augmentation of barrier function with respiration and esophageal length into a single metric.
Hoshino et al first evaluated HRM for assessment of EGJ barrier function, and measured the ‘DCI of the LES’ (termed LES pressure integral) over a 10-second period of time. While this was a new concept, intragastric baseline pressure was not used as a reference, and the effect of the duration of respiratory cycles were not factored into the calculation7. Nicodeme et al then refined this metric to be referenced to the gastric baseline and independent of respiration, by measuring the DCI value at the LES using a threshold of 2 mmHg above the gastric baseline, and dividing the recorded value by the duration of the 3 complete respiratory cycles8. This new metric, termed the EGJ-CI, overcomes some of the limitations of the LES pressure integral. Our group recently showed how simplifying the EGJ-CI by referencing to the gastric baseline (instead of correcting to a value above the gastric baseline) yields similar thresholds and normative values as reported by Nicodeme et al 6. Recently, Tolone et al showed that decreased EGJ-CI values correlate with increasing GERD evidence on ambulatory reflux monitoring9. In the current study, the use of the ‘St. Louis method’ of measurement of EGJ-CI1, 6 correlated well with individual conventional LESP metrics. EGJ-CI thus represents a more robust metric than individual LESP metrics because of this ability to capture all contributors to EGJ barrier function, including inspiratory augmentation and barrier length. This simplified technique for assessing the EGJ-CI only requires identification of the gastric baseline and forcing a DCI-like box over the desired frame. While not powered to determine differences between types of antireflux surgical procedures, our findings suggest that the Dor anterior fundoplication, which covers the myotomy site, results in higher EGJ-CI values compared to the posterior Toupet fundoplication. Although further studies, especially prospective evaluations, are needed to better understand the clinical utility of EGJ-CI, we believe that this metric can be adapted into the HRM automated software analysis to guide planning of surgical interventions to the EGJ barrier, and to assess adequacy of such surgical interventions.
Assessment of the EGJ barrier function carries clinical value. Currently utilized methods of assessment of the EGJ barrier – barium imaging studies, upper endoscopy, and LES pressure metrics on esophageal HRM –each add a component to the evaluation, but are each limited in their ability to fully quantify the EGJ barrier. EGJ-CI has the potential to combine elements of the barrier into a single evaluation, and preliminary reports suggest that it has potential to predict abnormal reflux parameters. While individual elements of the EGJ have been described as predictive of abnormal acid exposure time and response to ARS15, there is now evidence to show that an abnormal EGJ-CI barrier is associated with abnormal reflux parameters, and can potentially direct management6, 9. In fact, ARS resolved reflux symptoms better than medical management when the EGJ-CI was abnormally low compared to controls6. However, there are limitations in the clinical use of EGJ-CI at the present time. Foremost, there is no software tool specifically designed to measure EGJ-CI, and the DCI box or smart mouse tool has to be manually employed across the EGJ barrier for measurement. Further, the gastric baseline recorded using the gastric sensor varies, and automated averaging of gastric baseline pressures during the chosen measurement frame will make the EGJ-CI more representative. Both these limitations can be offset by appropriate development of software tools for automated analysis. Another point to consider is uniformity of measurement technique in the presence of sizeable separation between the LES and diaphragmatic components of the barrier. In order to avoid pressure events within such large hiatus hernias, and to maintain a uniform technique when the diaphragm is not traversed by the motility catheter, the ‘St. Louis method’ of measurement isolates the LES in all type 3 hiatus hernias6. With large hiatus hernias, the LES tone provides the ‘final’ component of the EGJ barrier prior to reflux, including reflux of content located within the hiatus hernia, therefore assessing just the LES provides meaningful contribution to EGJ barrier function in our opinion6. Finally, other structures such as distal esophageal strictures or extraesophageal processes have the potential to influence assessments of the EGJ-CI.
Limitations in our study design temper the strength of our conclusions. Our control cohort was younger than the achalasia or GERD cohorts; this difference reflects the difficulty in identifying older normal volunteers meeting our definitions of lack of symptoms, co-morbidities, and prescription medications. However, our institutional normative data are likely representative, as shown by previous comparisons of motor function between these controls and younger cohorts with GERD16. Patients included in this study were not consecutive, since the study design required two HRM studies in order to evaluate the effects of surgical management. The indication for the second study could have affected the degree of change in EGJ-CI after surgical intervention. Despite this, we demonstrate significant changes in EGJ-CI as would be expected after surgical intervention in achalasia and GERD, and there was good correlation with individual conventional LESP metrics in all instances. Finally, the EGJ-CI value was calculated using the DCI tool, which was not originally conceived to evaluate the EGJ. This could have introduced subjectivity to the recorded values; software algorithms specifically designed to measure the EGJ-CI could provide more accurate recordings. Nonetheless, our findings add to the research determining the value of the EGJ-CI as a pathophysiologic tool for assessing EGJ barrier function, and demonstrate its value in the clinical realm.
In conclusion, the novel EGJ-CI metric facilitates assessment of EGJ barrier function, and demonstrates appropriate change after two different types of surgical intervention to the EGJ with Heller myotomy or fundoplication. Further, EGJ-CI values correlate with individual LESP metrics, and the EGJ-CI therefore could complement evaluation of the EGJ barrier given its ability to assess both LES and crural diaphragmatic contributions. Further prospective studies should systematically assess the value of EGJ-CI in predicting reflux burden and symptomatic outcomes after antireflux therapy, particularly in the selection of patients for surgical interventions to the EGJ.
Key Messages.
The esophagogastric junction contractile integral (EGJ-CI) is a novel high resolution manometry (HRM) tool designed similar to the distal contractile integral (DCI) to assess EGJ barrier function.
EGJ-CI reflects pathophysiology at the EGJ, and correlates with individual LESP metrics.
EGJ-CI changes appropriately with surgical intervention directed at the EGJ, and demonstrates differences between specific interventions.
Acknowledgments
This study was partially funded through NIH/NIDDK (5P30 DK052574-14 –AP), and through the American Neurogastroenterology and Motility Society (ANMS) Clinical Training Program (AS).
Footnotes
Podium Presentation in Preliminary Form at the Annual Meeting of the American College of Gastroenterology Honolulu, Hawaii, October 2015
Author roles: DW, MM & AS: data collection and analysis, manuscript preparation and review; AP: study design, data analysis, manuscript preparation and review; CPG: study concept and design, data analysis, manuscript preparation, critical review and final approval of manuscript
No conflicts of interest exist. No writing assistance was obtained.
References
- 1.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3. 0. Neurogastroenterol Motil. 2015;27:160–74. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gyawali CP, Patel A. Esophageal Motor Function: Technical Aspects of Manometry. Gastrointest Endosc Clin N Am. 2014 doi: 10.1016/j.giec.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Bredenoord AJ, Weusten BL, Timmer R, et al. Intermittent spatial separation of diaphragm and lower esophageal sphincter favors acidic and weakly acidic reflux. Gastroenterology. 2006;130:334–40. doi: 10.1053/j.gastro.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 4.Shaker R, Dodds WJ, Kahrilas PJ, et al. Relationship of intraluminal pH and pressure within the lower esophageal sphincter. Am J Gastroenterol. 1991;86:812–6. [PubMed] [Google Scholar]
- 5.Chan WW, Haroian LR, Gyawali CP. Value of preoperative esophageal function studies before laparoscopic antireflux surgery. Surg Endosc. 2011;25:2943–9. doi: 10.1007/s00464-011-1646-9. [DOI] [PubMed] [Google Scholar]
- 6.Gor P, Li Y, Munigala S, et al. Interrogation of esophagogastric junction barrier function using the esophagogastric junction contractile integral: an observational cohort study. Dis Esophagus. 2015 doi: 10.1111/dote.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoshino M, Sundaram A, Mittal SK. Role of the lower esophageal sphincter on acid exposure revisited with high-resolution manometry. J Am Coll Surg. 2011;213:743–50. doi: 10.1016/j.jamcollsurg.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Nicodeme F, Pipa-Muniz M, Khanna K, et al. Quantifying esophagogastric junction contractility with a novel HRM topographic metric, the EGJ-Contractile Integral: normative values and preliminary evaluation in PPI non-responders. Neurogastroenterol Motil. 2014;26:353–60. doi: 10.1111/nmo.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolone S, De Bortoli N, Marabotto E, et al. Esophagogastric junction contractility for clinical assessment in patients with GERD: a real added value? Neurogastroenterol Motil. 2015 doi: 10.1111/nmo.12638. [DOI] [PubMed] [Google Scholar]
- 10.Pandolfino JE, Kim H, Ghosh SK, et al. High-resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. Am J Gastroenterol. 2007;102:1056–63. doi: 10.1111/j.1572-0241.2007.01138.x. [DOI] [PubMed] [Google Scholar]
- 11.Nicodeme F, Pandolfino JE, Lin Z, et al. Adding a radial dimension to the assessment of esophagogastric junction relaxation: validation studies of the 3D-eSleeve. Am J Physiol Gastrointest Liver Physiol. 2012;303:G275–80. doi: 10.1152/ajpgi.00063.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel A, Ding A, Mirza F, et al. Optimizing the high-resolution manometry (HRM) study protocol. Neurogastroenterol Motil. 2015;27:300–4. doi: 10.1111/nmo.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicodeme F, Lin Z, Pandolfino JE, et al. Esophagogastric Junction pressure morphology: comparison between a station pull-through and real-time 3D-HRM representation. Neurogastroenterol Motil. 2013;25:e591–8. doi: 10.1111/nmo.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwiatek MA, Pandolfino JE, Kahrilas PJ. 3D-high resolution manometry of the esophagogastric junction. Neurogastroenterol Motil. 2011;23:e461–9. doi: 10.1111/j.1365-2982.2011.01733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staehelin A, Zingg U, Devitt PG, et al. Preoperative factors predicting clinical outcome following laparoscopic fundoplication. World J Surg. 2014;38:1431–43. doi: 10.1007/s00268-013-2415-9. [DOI] [PubMed] [Google Scholar]
- 16.Porter RF, Kumar N, Drapekin JE, et al. Fragmented esophageal smooth muscle contraction segments on high resolution manometry: a marker of esophageal hypomotility. Neurogastroenterol Motil. 2012;24:763–8. e353. doi: 10.1111/j.1365-2982.2012.01930.x. [DOI] [PubMed] [Google Scholar]