Abstract
The earliest events following mucosal HIV-1 infection, prior to measurable viremia, remain poorly understood. Here we show by detailed necropsy studies that the virus can disseminate rapidly following mucosal SIV infection of rhesus monkeys and trigger components of the inflammasome, both at the site of inoculation and at early sites of distal virus spread. By 24 hours following inoculation, a proinflammatory signature that lacked antiviral restriction factors was observed in viral RNA positive tissues. The early innate response included expression of NLRX1, which inhibits antiviral responses, and activation of the TGF-β pathway, which negatively regulates adaptive immune responses. These data suggest a model in which the virus triggers specific host mechanisms that suppress the generation of antiviral innate and adaptive immune responses in the first few days of infection, thus facilitating its own replication. These findings have important implications for the development of vaccines and other strategies to prevent infection.
INTRODUCTION
The earliest events that occur following mucosal HIV-1 exposure remain poorly understood, largely because the eclipse period of infection prior to detectable viremia is very difficult to study in humans. Studies of the initial events of acute infection have therefore largely utilized the model of SIV infection in rhesus monkeys. Intravaginal SIV infection of rhesus monkeys has been reported to involve expanding foci of virus replicating in CD4+ T cells at the mucosal portal of entry during the first week of infection (Haase, 2005, 2010; Li et al., 2009a; Li et al., 2009b; Miller et al., 2005; Zhang et al., 1999), but little is known about the biology of early systemic virus dissemination. Innate cytokines and chemokines such as MIP-3α have also been reported to play a key role in recruiting CD4+ T cells to the site of inoculation to amplify the local expansion of virus prior to distal spread (Li et al., 2009a).
Viral engagement of cellular pattern recognition receptors (PRRs) trigger the production of type I interferons, which typically represent the first line of innate antiviral defense. Interferons have multiple downstream effects, including upregulation of antiviral restriction factors, such as MX2, TRIM-5, and APOBECs, which inhibit HIV-1 and SIV replication (Schoggins et al., 2011). Nucleotide oligomerization and binding domain (NOD)-like receptors (NLRs) represent another family of genes that sense pathogens and regulate cellular functions including antiviral defense (Kanneganti et al., 2007; Kofoed and Vance, 2011). NLRs include both inflammasome activators (NLRP1, NLRP3), which stimulate the production of proinflammatory cytokines such as IL-1 and IL-18, and triggers of NFκB (NLRC-1, NLRC-2), which augment inflammation (Thaiss and Elinav, 2015). These proinflammatory NLRs are counterbalanced by other NLRs and other molecules that regulate inflammasome activity. These include anti-inflammatory NLRs include NLRC3, NLRP6, NLRP12, NLRX1 and transactivators of MHC II molecules such as CIITA (Lukens et al., 2015; Moore et al., 2008; Parvatiyar and Cheng, 2011). The complexity of the inflammasome highlights the importance of negative feedback loops, in which some members of the inflammasome promote inflammation and others reduce inflammation.
Anecdotal evidence has suggested that the virus can disseminate rapidly to distal sites (Hu et al., 2000; Miller et al., 2005; Ribeiro Dos Santos et al., 2011), but the kinetics and extent of virus dissemination from the site of inoculation remains unknown, and the initial innate response to virus in the first few days of infection is largely unexplored, both at the site of inoculation and at early sites of distal virus spread. Previous studies have reported type I interferon responses, IL-1 expression, and induction of cell death pathways (Hirao et al., 2014; Jakobsen et al., 2015). However, prior studies have been limited by challenges in identifying the earliest sites of virus spread, which has largely precluded a detailed evaluation of the earliest host innate responses in viral RNA positive distal tissues. A detailed characterization of the earliest host response to virus is critical for a comprehensive understanding of initial viral pathogenesis and for the development of vaccines, microbicides, and therapeutics aimed at blocking HIV-1 infection.
In this study, we evaluated the kinetics of virus dissemination to distal tissues and the initial innate and adaptive host immune responses following intravaginal SIV infection of rhesus monkeys. Scheduled necropsies on days 0, 1, 3, 7, and 10 following intravaginal SIV inoculation allowed comprehensive assessments of virology, immunology, histopathology, and gene expression profiles across multiple tissues in each animal. By day 1, virus was occasionally detectable in distal tissues such as the gastrointestinal tract, in addition to the site of inoculation, and triggered proinflammatory transcriptomic responses including nucleotide sensor components of the inflammasome. At both the site of inoculation and in viral RNA positive distal tissues, increased expression of antiviral genes, including interferon-stimulated restriction factors, was notably absent on day 1 and was not consistently observed until peak viral replication on day 10. These data suggest a model for early SIV pathogenesis in which the virus triggers initial proinflammatory host responses that downregulate innate antiviral immunity, inhibit adaptive cellular immunity, and facilitate viral replication at sites of early distal dissemination.
RESULTS
Rapid Virus Dissemination from the Site of Inoculation to Distal Sites
We performed comprehensive serial necropsies in 44 rhesus monkeys (M. mulatta) on day 0 (N=4), day 1 (N=6), day 3 (N=9), day 7 (N=17), and day 10 (N=8) following intravaginal inoculation of 5×104 TCID50 SIVmac251 (Barouch et al., 2012; Liu et al., 2010; Liu et al., 2009). These animals did not express the protective MHC class I alleles Mamu-A*01, Mamu-B*08, or Mamu-B*17. All animals were cycling adult females with the exception of 5 juvenile animals in the day 7 group and 3 juvenile animals in the day 10 group, and inoculations were timed to avoid the menstrual phase. To establish a uniformly infectious but not overwhelming challenge dose, we performed a preliminary titration study and selected the dose that led to infection in 100% (8 of 8) of animals while restricting the number of transmitted/founder viruses in plasma to a median of 5 (range 1-9), as assessed by single genome amplification sequencing (data not shown).
In both the preliminary titration and serial necropsy studies, peak plasma viremia occurred in the majority of animals on day 10-14 following SIVmac251 infection, and the pre-viremic eclipse period was a median of 7 days (Supplementary Figure S1). Tissue viral RNA levels were quantitated using an ultrasensitive nested RT-PCR assay (Hansen et al., 2013) using 23-31 tissues obtained at necropsy for each animal, including the female reproductive tract, draining lymph nodes, gastrointestinal tract, distal lymph nodes, tonsil, spleen, bone marrow, thymus, lung, liver, and central nervous system.
We observed low levels of viral RNA in at least one tissue outside the female reproductive tract in 83% (5 of 6) animals necropsied on day 1 following challenge (Figure 1, Supplementary Data S1A). Viral RNA in the female reproductive tract presumably reflected the inoculum challenge virus. Low levels of viral RNA were also variably detected in duodenum, jejunum, ileum, colon, cecum, and rectum, as well as occasionally in genital/pelvic lymph nodes, spleen, and bone marrow of animals necropsied at this timepoint. Although most distal tissues remained viral RNA negative on day 1, the fraction of gastrointestinal tissues that were positive for viral RNA on day 1 following SIVmac251 inoculation was significantly greater than the very low background false positive rate observed in SIV-naïve control animals (P=0.006, Fisher’s exact test; Figure 2), demonstrating that virus had disseminated from the mucosal site of inoculation to distal anatomic sites within the first 24 hours.
Figure 1. Viral RNA in tissues.
Tissue viral RNA (log RNA copies / 108 cell equivalents) across multiple tissues at necropsy in animals on days 1, 3, 7, or 10 following inoculation with SIVmac251. Colors on each plot reflect individual animals. Values plotted below the horizontal line indicate samples in which viral RNA was not measured above the threshold of detection, which varied depending on the amount of each tissue analyzed. See also Supplementary Figure S1 and Supplementary Data S1.
Figure 2. Viral dissemination to distal tissues.
(A) Mean log RNA copies / 108 cell equivalents and probability of each tissue positive for virus is plotted versus the day of necropsy. Decline in levels of viral RNA in female reproductive tract tissues from day 1 to day 7 likely reflects clearance of residual inoculum challenge virus, with the subsequent increase from day 7 to day 10 likely reflecting productive infection. (B) Statistical comparisons of the tissue viral loads and the percent of each tissue positive for virus in animals necropsied on days 1, 3, 7, and 10 following SIVmac251 inoculation as compared with uninoculated controls (Naïve) by linear mixed models and Fisher’s exact tests.
In monkeys necropsied on day 3, 89% (8 of 9) animals had detectable levels of viral RNA in at least one distal tissue, including the gastrointestinal tract, draining and distal lymph nodes, tonsil, spleen, bone marrow, thymus, lung, liver, and central nervous system (Figure 1). Wide variations in levels of viral RNA in multiple tissues were observed in monkeys necropsied on day 7, and high levels of viral RNA were observed in the majority of animals necropsied on day 10, particularly in the gastrointestinal tract and lymphoid tissues, consistent with previous descriptions of acute infection (Brenchley et al., 2004; Li et al., 2005; Mattapallil et al., 2005). Viral DNA was also detected in a progressively greater proportion of tissues in animals necropsied on days 3, 7, and 10 (Supplementary Data S1B).
Immunohistochemistry and in situ hybridization for viral RNA were also performed in multiple tissues in each animal. We did not observe foci of viral replication in the female reproductive tract or in distal tissues at the early timepoints (data not shown), possibly because of the limited sensitivity of these assays and the fact that the levels of viral RNA in tissues in this study were lower than those observed in previously published experiments (Li et al., 2009a; Miller et al., 2005; Zhang et al., 1999).
Initial T Lymphocyte Responses by Day 7 in Mucosal Tissues and Bone Marrow
We evaluated SIV Gag-specific CD8+ and CD4+ T lymphocyte responses in 23 distinct tissues in each monkey by multiparameter flow cytometry (Supplementary Data S1C) (Baden et al., 2015; Li et al., 2011). SIV Gag-specific T lymphocyte responses were first observed on day 7 in a subset of animals in female reproductive tract mucosa, gastrointestinal mucosa, and bone marrow, but not in lymph nodes or peripheral blood (Figure 3, Supplementary Data S1D). On day 10, all monkeys exhibited high frequency Gag-specific T lymphocyte responses in these tissues, but no responses were observed in peripheral blood at this timepoint. These data demonstrate that antiviral cellular immune responses initially develop in tissue effector sites during acute infection prior to the emergence of responses in the periphery, but this occurs “too little and too late” (Haase, 2010; Reynolds et al., 2005), after the virus has already widely disseminated (Figure 1). Humoral immune responses were not detected at these early timepoints (data not shown).
Figure 3. SIV Gag-specific T lymphocyte responses in tissues.
SIV Gag-specific CD8+ and CD4+ T lymphocyte responses analyzed by IFN-γ intracellular cytokine staining assay across multiple tissues at necropsy in animals on days 7 and 10 following inoculation with SIVmac251. See also Supplementary Data S1.
Systemic Transcriptional Changes Occur by Day 1 Following SIV Infection and Highlight a Potent Inflammatory Response
We next performed transcriptional profiling in multiple tissues in each animal at necropsy to identify the earliest evidence of host sensing of virus by analyzing gene expression changes following SIVmac251 inoculation. cDNA synthesized from RNA extracted from each tissue was hybridized to HT-12 V4 BeadChips and quantified using an Illumina iScan System (Gentleman et al., 2004). This approach provided >50% coverage of the rhesus transcriptome (Fukazawa et al., 2012). Multidimensional scaling plots revealed that the largest transcriptomic variance between specimens was driven by tissue type (Supplementary Data S1E). To correct for any potential tissue-specific bias, we pooled tissues into groups that exhibited similar transcriptomic profiles: female reproductive tract (FRT), gastrointestinal tissue (GI), lymph nodes (LN), and peripheral blood. These groupings were based on Euclidian distances of the transcriptomic signals and are shown in a dendrogram based on hierarchical clustering (Supplementary Data S1E). The time of necropsy accounted for the second largest transcriptomic variance.
Gene expression profile changes indicative of activation of innate immune responses were evident by day 1 following SIVmac251 inoculation as compared with uninoculated controls in all tissues analyzed (FRT, GI, LN, blood) (Supplementary Figure S2). These changes included substantial numbers of differentially expressed genes as well as activation of innate immune pathways. We observed particularly striking differences in transcriptomic profiles in viral RNA positive versus viral RNA negative gastrointestinal and lymph node tissues obtained from the same animals at the same time on day 1 and day 3 (Supplementary Figure S3). This finding allowed a focused evaluation of the nature of the earliest host innate immune responses to initial disseminated virus specifically in viral RNA positive tissues.
Early Activation of the Inflammasome in Viral RNA Positive Tissues
We analyzed transcriptomic profiles specifically in viral RNA positive tissues following SIVmac251 challenge to gain mechanistic insight into the initial host responses to the virus. As early as day 1 following infection, pathway analysis confirmed the induction of interferon signaling in viral RNA positive tissues, although it is unclear if these initial transcriptomic changes required viral replication. Analysis of antiviral interferon-stimulated genes (ISGs) in viral RNA positive tissues showed that several ISGs (CCR2, CCR6, CXCR4) were upregulated on day 1 and day 3, but importantly these did not include antiviral restriction factors such as ISG15, IRF7, APOBEC, MX2, and TRIM5 until peak viral replication on day 10 (P = 0.02; Figure 4A; Supplementary Figure S4). Components of the inflammasome were strongly upregulated on day 1 in the female reproductive tract as compared with uninoculated controls (P = 4.1 × 10−4; Figure 4B), as well as on day 1 in viral RNA positive as compared with viral RNA negative gastrointestinal tissues from the same animals (P = 3.2 × 10−3; Figure 4B, Supplementary Figure S4). We compared female reproductive tract tissues from day 1 with uninoculated controls rather than day 1 viral RNA negative tissues since nearly all female reproductive tract tissues were viral RNA positive on day 1, presumably reflecting the inoculum challenge virus. This day 1 proinflammatory transcriptomic signature was observed in all viral RNA positive tissues analyzed (FRT, GI, LN, blood) but was not seen in similar tissues from uninfected animals (Supplementary Figure S5).
Figure 4. Proinflammatory transcriptomic signature in viral RNA positive tissues.
(A) Classic antiviral restriction factors (ISG15, IRF7, APOBEC, MX2, TRIM5) are not observed in gastrointestinal (GI) tissues until day 10. F-test heatmap depicts viral RNA positive GI tissues over time by ANOVA with an antiviral gene filter (51 samples from 5 monkeys). (B) Heatmaps reveal an inflammasome signature in female reproductive tract (FRT) on day 1 as compared with uninoculated controls (17 samples from 5 monkeys) as well as in viral RNA positive as compared with viral RNA negative GI tissues (20 samples from 4 monkeys) on day 1. Gene expression is represented as a gene-wise standardized expression (Z-score) with P < 0.05. Red and blue correspond to up- and down-regulated genes respectively. (C) Gene Mania network representing co-expression of upregulated genes, including NLRX1 (arrow), on day 1 in viral RNA positive tissues reveals a proinflammatory signature. (D) Correlation between expression of the day 1 common gene signature and expression of the inflammasome signature (red) and antiviral signature (blue). (E) Correlation between expression of NLRX1 and expression of IRF7. P-values reflect Spearman correlation tests. See also Supplementary Figures S2-S5 and and Supplementary Data S1.
Integrating the different tissue signatures generated a common day 1 signature in viral RNA positive tissues. These data show a robust day 1 proinflammatory gene expression signature in both local tissues and viral RNA positive distal tissues within 24 hours following intravaginal SIVmac251 inoculation (P = 5 × 10−8; Figure 4C). This common signature revealed upregulation of genes of the NFκB pathway, pro-inflammatory chemokines (CCL3), and genes expressed by monocytes/macrophages (CD14) and NK cells (TYROBP). The expression of this day 1 proinflammatory signature was also accompanied by the upregulation of several NLRs, including anti-inflammatory NLRs (NLRX1), and several genes of the anti-inflammatory TGF-β pathway (MAP4K4, TAB2, PPP1R15A). Importantly, expression of the day 1 signature correlated inversely with the antiviral ISG signature (P = 3 × 10−6; Figure 4D) and correlated directly with the inflammasome signature (P = 7 × 10−7; Figure 4D) across all animals in this study. Consistent with these findings, expression of NLRX1 also correlated inversely with expression of IRF7, a key antiviral ISG (P = 0.02; Figure 4E).
NLRX1 Expression Correlates with Early Virus Replication
NLRX1 is a negative regulator of the inflammasome pathway that inhibits expression of antiviral ISGs, IRF7, RIG-I, and TLRs (Allen et al., 2011; Moore et al., 2008) and was a component of the day 1 transcriptomic signature (arrow, Figure 4C). NLRX1 expression also correlated inversely with antiviral ISG expression at the site of inoculation (P = 0.02; Figure 5A) and correlated directly with the magnitude of viral RNA in gastrointestinal tissues at multiple timepoints (P = 2.6 × 10−3, Pearson correlation test; Figure 5B-C). Moreover, the subset of animals with the highest NLRX1 expression also demonstrated the highest levels of viral RNA (P = 4.3 × 10−3; Wilcoxon rank sum test, Figure 5D). Multiple additional NLRs (NLRP5, NLRP6, NLRP8, NLRC4) were also upregulated in addition to NLRX1 (Figure 5B, Supplementary Figure S4). NLRX1 gene upregulation was confirmed by Western blots for NLRX1 protein expression (P=0.016) and demonstrated an inverse correlation with IRF7 protein expression (P=0.008) (Supplementary Figure S6A-B), consistent with the transcriptomics data (Figure 4E).
Figure 5. NLRX1 expression is correlated inversely with antiviral gene expression and is correlated directly with viral RNA.
(A) Early NLRX1 expression is inversely correlated with antiviral restriction factors in FRT. F-test heatmap depicts viral RNA positive FRT tissues for NLRX1 or with an antiviral gene filter (20 samples from 8 monkeys). (B) Linear regression analysis of viral RNA levels and gene expression in viral RNA positive gastrointestinal tissues. Heatmap represents the top positively correlated proinflammatory genes, including NLRX1, which were part of the full regression signature. (C) Correlation analysis between NLRX1 expression and viral RNA levels in viral RNA positive gastrointestinal tissues. (D) Bee-swarm plot shows that samples with the highest NLRX1 expression had the highest viral RNA levels. P-values reflect Wilcoxon rank sum tests. See also Supplementary Figures S4 and S6.
NLRX1 exerts its anti-inflammatory activity by inhibiting expression of NFκB. We observed that NLRX1 expression correlated directly with expression of NFKBIA (P = 1.2 × 10−3), the inhibitor of NFκB, and correlated inversely with expression of NFKB1 (P = 1.7 × 10−7; Figure 4C, Supplementary Figure S6C-D). These data suggest that the initial host response to virus, both at the local site of inoculation and at sites of early distal virus spread, was characterized by a potent proinflammatory response and a negative feedback loop highlighted by the induction of NLRX1, which may have suppressed expression of antiviral restriction factors and thereby facilitated viral replication.
Early Transcriptomic Changes Are Observed in NK Cells, Monocytes, and T Cells
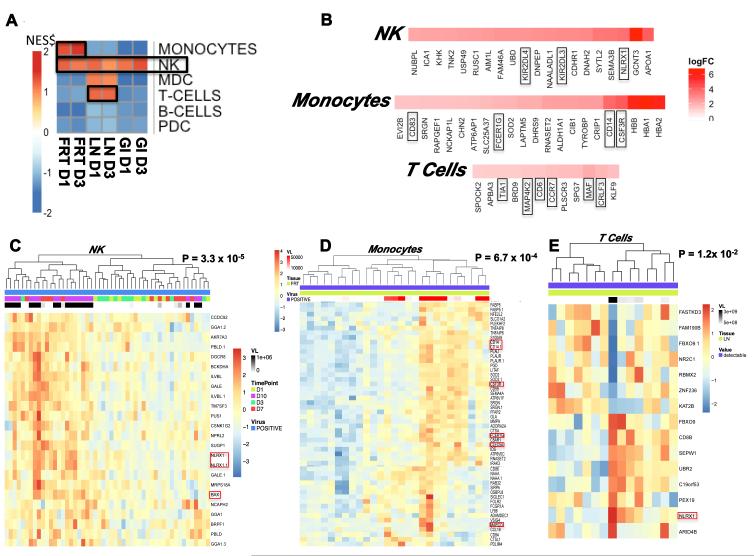
Gene set enrichment analysis (GSEA) using Nakaya modules (Nakaya et al., 2011; Subramanian et al., 2005) was performed to assess the contribution of various cell subsets within the analyzed mixed cell populations in each tissue to the observed early transcriptomic changes. Interestingly, NK cell-specific genes were upregulated in all analyzed tissues on day 1 and day 3 following SIVmac251 inoculation (Figure 6A) and included NLRX1, KIR2DL4, and KIR2DL3 (Figure 6B), suggesting that NK cells contributed substantially to the early innate response to the virus. We defined a common NK-specific gene expression signature that was upregulated in all tissues on days 1 and 3 (Supplementary Figure S7A-B). Monocyte-specific genes (CD14, CD83, FCER1G, CSF3R) were also upregulated in the female reproductive tract, and T cell-specific genes (MAF, CD6, CCR7) were upregulated in lymph nodes at these early timepoints (Figure 6A-B). Moreover, we observed a significant correlation between expression of these genes in NK cells, monocytes, and T cells and viral RNA levels in tissues (Figure 6C-E). These results suggest that SIVmac251 infection triggered proinflammatory transcriptomic profiles, particularly in these cell subsets, which likely facilitated virus replication.
Figure 6. Subset analysis reveals activation of NK cells in all SIV infected tissues on day 1 and day 3 following inoculation.
(A) Gene Set Enrichment Analysis (GSEA) using Nakaya modules and pathway heatmap representing enrichment of genes characteristic of specific cell subsets in each tissue type on day 1 and day 3. Red and blue squares represent a positive or negative enrichment of genes characteristic of a subset, respectively. (B) Checkerboard figures reflecting NK, monocyte, and T cell subset leading edge analysis on day 1. Gene members (5% FDR) contributing to enrichment are shown, with magnitude represented as log2 FC. Linear regression analysis of positive viral RNA with (C) NK, (D) monocyte, and (E) T cell specific gene expression in viral RNA positive gastrointestinal tissues. Gene expression is represented as a gene-wise standardized expression (Z-score) with P < 0.05. Red and blue correspond to up- and down-regulated genes respectively. See also Supplementary Figure S7.
The TGF-β Pathway Negatively Regulates Adaptive T Lymphocyte Responses
We next defined transcriptomic signatures associated with early virus-specific CD8+ and CD4+ T lymphocyte responses in gastrointestinal tissues. Since virus-specific T lymphocyte responses were first detected on day 7 (Figure 2), we first addressed this question utilizing tissues from day 7 and day 10 following SIVmac251 challenge. By GSEA, the pathways that showed the strongest direct correlation with SIV-specific CD8+ T lymphocyte responses in tissues were metabolic pathways involved in T cell activation, including oxidative phosphorylation, adipogenesis, fatty acid metabolism, and xenobiotic metabolism (Figure 7A). In contrast, immune regulation pathways, including TGF-β and IL-2/STAT5 signaling, correlated inversely with antigen-specific T lymphocyte responses (Figure 7A). Upregulation of genes downstream of the anti-inflammatory TGF-β signaling pathway (Chen et al., 2005) was observed not only in viral RNA positive tissues on day 7 and day 10, but also in viral RNA positive tissues as early as day 1 (Figure 7B-C), suggesting that triggering of the TGF-β pathway was an early event. The early TGF-β signaling genes that were upregulated by day 1 included MAP3K7, which regulates TGF-β signaling, and FNTA (farnesyl transferase–α), which is an effector anti-inflammatory gene (Figure 7B-C, Supplementary Figure S7C). These data suggest that upregulation of TGF-β signaling occurred rapidly in response to virus and preceded the generation of detectable Gag-specific T lymphocyte responses.
Figure 7. Transcriptomic signatures in tissues with cellular immune responses.
(A) Checkerboard figure shows gene expression pathways positively (red) and negatively (blue) associated with Gag-specific CD8+ T lymphocyte responses in gastrointestinal tissues. Pathways (MSigDB Hallmark database) are plotted on the y-axis and leading edge analysis (gene members contributing most to enrichment) are plotted along the x-axis. (B) TGF-β signature genes upregulated on Day 1-7 and Day 7-10. F-test heatmap depicts viral RNA positive gastrointestinal tissue over time by ANOVA with a TGF-β filter (51 samples from 5 monkeys). (C) Heatmap reveals a TGF-β signature in viral RNA positive gastrointestinal tissue on day 1 as compared with day 10. Linear regression analyses of viral RNA positive (D) gastrointestinal and (E) lymph node tissues reveal TGF-β specific genes that are correlated directly with the magnitude of the Gag-specific CD8+ and CD4+ T lymphocyte responses in these tissues. CD8+ T lymphocyte responses in tissues are (F) correlated inversely with TGIF1 expression and (G) correlated directly with SMAD7 expression. P values reflect Spearman correlation tests. See also Supplementary Figure S7.
The negative impact of TGF-β expression on CD8+ and CD4+ T lymphocyte responses was confirmed by linear regression analyses showing that TGIF1, a master positive regulator of the TGF-β pathway, as well as other TGF-β response genes correlated inversely with the magnitude of CD8+ and CD4+ T lymphocyte responses on day 7 and day 10 in gastrointestinal tissues (P = 5.4 × 10−5 and 6.3 × 10−5, respectively; Figure 7D). In contrast, SMAD7, a key negative regulator of TGF-β responses (Monteleone et al., 2008; Monteleone et al., 2001), correlated directly with CD8+ and CD4+ T lymphocyte responses (P = 4.0 × 10−4 and 7.0 × 10−6, respectively; Figure 7E). Moreover, the magnitude of Gag-specific CD8+ T lymphocyte responses across all tissues correlated inversely with TGIF1 expression (R = −0.62, P = 1.8 × 10−4; Figure 7F) and correlated directly with SMAD7 expression (R = 0.75, P = 1.3 × 10−6; Figure 7G). These data show that the TGF-β pathway was triggered rapidly in viral RNA positive tissues and correlated with lower levels of adaptive T lymphocyte responses and higher levels of viral replication. Taken together, these findings show that the early proinflammatory host responses in viral RNA positive tissues correlated with suppressed innate and adaptive immunity, likely resulting in increased viral replication.
DISCUSSION
In this study, we describe a comprehensive picture of early mucosal SIV infection of rhesus monkeys at a substantially greater level of detail than previously reported. Integrated virologic, immunologic, and transcriptomic data from multiple tissues from the first few days following intravaginal SIV challenge demonstrated the rapid kinetics of virus dissemination from the site of inoculation to distal tissues, the initial development of virus-specific T lymphocyte responses in tissues, and the proinflammatory early innate host response to virus. These findings support a model of early SIV pathogenesis in which at least a limited amount of virus can rapidly disseminate to distal tissues by 24 hours following inoculation and induce robust expression of components of the inflammasome as well as NLRX1, which suppresses antiviral restriction factors (Allen et al., 2011; Moore et al., 2008), both at the mucosal portal of entry and in viral RNA positive distal tissues. Classic antiviral restriction factors were not observed until day 10 in the context of overwhelming peak viral replication. The TGF-β pathway, which suppresses adaptive immune responses (Chen et al., 2005), was also induced early in viral RNA positive tissues and correlated with reduced T lymphocyte responses. Taken together, these data suggest that the virus triggers host responses in the first few days following infection and that these responses may suppress the generation of antiviral innate and adaptive immunity, thus facilitating its own replication.
Previous studies have suggested the importance of chemokines such as MIP-3α for recruiting CD4+ T lymphocyte target cells to the site of inoculation, and glycerol monolaurate, which inhibits MIP-3α and other cytokines, was reported to protect against intravaginal SIV infection (Li et al., 2009a). Inflammatory cytokines such as IL-1 have also been reported in the gastrointestinal tract within 3 days of SIV infection (Hirao et al., 2014). The present study extends these prior reports by comprehensively assessing the early host innate responses in viral RNA positive tissues in the first few days following intravaginal SIV infection.
In particular, our data highlight the role of the inflammasome in early SIVmac251 pathogenesis as early as 1 day following infection. We speculate that NLRX1 is part of a negative feedback loop triggered by the proinflammatory milieu in viral RNA positive tissues (Moore et al., 2008; Parvatiyar and Cheng, 2011). NLRX1 expression correlated inversely with expression of antiviral restriction factors (Figure 4) and correlated directly with levels of viral RNA (Figure 5) and likely was expressed by tissue NK cells (Figure 6). Moreover, the early induction of the TGF-β pathway correlated inversely with Gag-specific CD8+ T cell responses (Figure 7), suggesting that the virus may have hindered the induction of effective adaptive cellular immune responses at sites of early virus replication. This possibility is supported by the observation that expression of SMAD7, which is involved in downregulating the TGF-β pathway, was associated with higher Gag-specific CD8+ T cell responses (Figure 7). These findings suggest that the virus activates negative feedback pathways that impede host antiviral innate and adaptive immune responses in the first few days following infection.
The early virus dissemination observed in the present study extends prior reports (Miller et al., 2005; Zhang et al., 1999) by showing that low levels of virus are detectable in at least some distal tissues in nearly all animals within 24 hours following intravaginal SIVmac251 challenge. These low levels of virus were sufficient to trigger profound transcriptomic changes in these viral RNA positive tissues, resulting in a local milieu that favors viral replication. These data also suggest that the “window of vulnerability” to contain or to eliminate the virus at the mucosal portal of entry is more limited than previously appreciated (Haase, 2005, 2010). HIV-1 vaccination and other prevention strategies should take into account the ability of the virus to disseminate rapidly to distal tissues and to suppress host antiviral innate and adaptive immune responses.
Significant differences exist between this experimental model of SIV infection in rhesus monkeys and HIV-1 infection in humans. In particular, we utilized a virus dose that infected 100% of monkeys with a single inoculation, which is substantially higher than typical human HIV-1 exposures. However, this dose was not overwhelming, as it still resulted in a substantial genetic bottleneck of transmitted/founder viruses. Moreover, this dose of virus resulted in kinetics and magnitudes of acute viremia that were comparable to lower limiting doses of this challenge virus (Liu et al., 2010).
Our findings have important implications for our understanding of HIV-1 pathogenesis and for the development of HIV-1 prevention strategies. The detailed picture of the kinetics of early virus spread and the nature of the early host innate immune responses suggest that the eclipse period is far more complex and dynamic than previously recognized. Moreover, the virus appears to trigger host responses in the first 24 hours that may inhibit the generation of antiviral innate and adaptive immunity and thus may facilitate its own replication. Our data also provide a detailed picture of acute SIV infection in rhesus monkeys that may help guide future studies to assess the protective efficacy of vaccines, antibodies, drugs, and other preventative interventions.
EXPERIMENTAL PROCEDURES
Animals
44 outbred, Indian-origin, adult or juvenile female rhesus monkeys (Macaca mulatta) were genotyped and selected as negative for the protective MHC class I alleles Mamu-A*01, Mamu-B*08, and Mamu-B*17. TRIM5 polymorphisms were balanced equally among groups, and animals were otherwise randomly allocated. All monkeys were housed at Bioqual, Rockville, MD. Animals were infected with 5×104 TCID50 of our SIVmac251 challenge stock (Barouch et al., 2012; Liu et al., 2010; Liu et al., 2009) by the intravaginal route and were necropsied on day 0, 1, 3, 7, or 10 following infection. At necropsy, multiple samples of 23-37 tissues were obtained for virology, immunology, histopathology, and gene expression profiling. All animal studies were approved by the appropriate Institutional Animal Care and Use Committee (IACUC).
Viral RNA and DNA
Plasma SIV RNA levels were measured using a gag targeted quantitative real-time RT-PCR assay, and tissue levels of SIV RNA and DNA were measured using gag targeted, nested quantitative hybrid real time/digital RT-PCR and PCR assays, essentially as described (Hansen et al., 2013).
Cellular immune assays
SIV-specific cellular immune responses in blood and tissues were assessed by multiparameter ICS assays essentially as previously described (Baden et al., 2015; Barouch et al., 2012; Li et al., 2011). 9-color ICS assays were performed with predetermined titers of mAbs against CD3 (SP34; Alexa700), CD4 (L200; AmCyan), CD8 (SK1; allophycocyanin-cyanine 7 [APC-Cy7]), CD28 (L293; peridinin chlorophyll-A-cyanine 5.5 [PerCP-Cy5.5]), CD95 (DX2; phycoerythrin [PE]), CD69 (TP1.55.3; phycoerythrin-Texas Red [energy coupled dye; ECD]; Beckman Coulter), IFN-γ (B27; phycoerythrin-cyanine 7 [PE-Cy7]), IL-2 (MQ1-17H12; allophycocyanin [APC]) and TNF-α (Mab11; fluorescein isothiocya [FITC]). IFN-γ backgrounds were consistently <0.01% in PBMC and <0.05% in tissues.
Transcriptomic analysis
Cells were isolated from tissues and lysed for RNA extraction (Qiagen, Valencia, CA, USA). Reverse transcription reactions were performed to obtain cDNAs, which were hybridized to the Illumina Human HT-12 version 4 Expression BeadChip according to the manufacturer’s instructions and quantified using an Illumina iScan System. Data were collected with Illumina GenomeStudio software. Analysis of the genome array output data was conducted using the R statistical language and the Limma statistical package from Bioconductor (Gentleman et al., 2004). First, arrays displaying unusually low median intensity, low variability, or low correlation relative to the bulk of the arrays were tagged as outliers and were discarded from the rest of the analysis. Quantile normalization followed by a log2 transformation using the Bioconductor package LIMMA was applied to process microarrays. The LIMMA package was used to fit a linear model to each probe and to perform a moderated Student’s t-test on various differences of interest between tissue types and time points. For data mining and functional analyses, genes that satisfied a P-value < 0.05 were selected. Probes that did not map to annotated RefSeq genes and control probes were removed. When indicated, the expected proportions of false positives (FDR) were estimated from the unadjusted P-value. 697 total samples from 34 monkeys were analyzed. Transcriptomic data and correlations with virologic and immunologic data were analyzed by heatmaps, F-tests, gene set enrichment analyses (GSEA) (Subramanian et al., 2005), Gene Mania networks (Mostafavi and Morris, 2010; Mostafavi et al., 2008), and module analyses (Merico et al., 2010).
Western blots
Lymph node mononuclear cells from uninfected monkeys as well as monkeys identified as NLRX1 high or NLRX1 low by transcriptomics were washed twice in PBS. Mitochondrial and soluble nuclear extracts were prepared using Subcellular Protein Fractionation Kit for Cultured Cells (Thermo Scientific), and protein concentrations were determined by Bradford assays. 25 ug of the mitochondrial extracts and 15 ug of the nuclear extracts were resolved by 10% SDS-PAGE (Mini Protean TGX precast gels, Biorad) and transferred onto methanol activated PVDF membrane for 1 h at a constant voltage of 100V. The membranes were blocked with 5% BSA and incubated with specific mAbs overnight. Mitochondrial lysates were probed with NLRX1 (Aviva Systems Biology, 1:100) and VDAC (Cell Signalling, 1:1000). Soluble nuclear extracts were probed for phosphorylated IRF7 and TATA binding protein (TBP) (Cell Signaling, 1:1000 for both). Detection of the target proteins utilized Pierce ECL Plus Western Blotting Substrate (Thermo Scientific) followed by autoradiography (USA Scientific). For densitometric scanning, band intensities for the experimental samples were normalized with the corresponding loading controls (VDAC for NLRX1, TBP for IRF7) and expressed as percent arbitrary unit (% AU).
Statistics
To estimate differences in mean tissue viral RNA of each experimental group in comparison to the naïve group, we applied linear mixed effects models. Observations from the same animal were treated as a cluster and a random intercept was prescribed for each animal in the model. P-values for the differences were estimated for each tissue group. Mean log10 levels of viral RNA were calculated and plotted by day and tissue group. We utilized logistic regression models to estimate the probabilities that each tissue type was positive for viral RNA. Observations from the same experimental animal were treated as an independent cluster. The probabilities were estimated and plotted by day and tissue group. To assess whether the proportions of each experimental group differed from those of the naïve group, we used Fisher's exact tests. Tests were carried separately for each tissue group.
Supplementary Material
ACKNOWLEDGEMENTS
We thank A. Schultz, Q. Dang, J. Warren, L. Picker, P. Johnson, A. Haase, B. Keele, J. Bess, R. Fast, K. Oswald, R. Shoemaker, T. Hooper, C. Shaver, J. Yalley-Ogunro, I. Koralnik, L. Parenteau, S. Blackmore, E. Balandya, J. Jimenez, K. Stanley, S. Mathews, J. Smith, A. McNally, and F. Stephens for generous advice, assistance, and reagents. The SIVmac239 peptides were obtained from the NIH AIDS Research and Reference Reagent Program. We acknowledge support from the National Institutes of Health (AI060354, AI078526, AI084794, AI095985, AI096040, AI100645, HHSN261200800001E) and the Ragon Institute of MGH, MIT, and Harvard.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
D.H.B., K.G., J.D.L., and R.P.S. designed the study and wrote the manuscript. C.G., C.B., W.W., and M.G.L. led the necropsies. J.L., K.S., E.B., C.C., L.P., A.B., M.S., and H.L. led the sample processing and immunologic analyses. W.J.B., Y.L., B.B., M.H., J.D.L, and M.P. led the virologic analyses. K.D., S.B., M.C., and R.P.S. led the transcriptomic analyses. K.G., A.C., H.Y.K., and W.L. performed the data and statistical analyses.
AUTHOR INFORMATION
The authors declare no competing financial interests. Correspondence and requests for materials should be addressed to D.H.B. (dbarouch@bidmc.harvard.edu).
REFERENCES
- Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, Gris D, Roney KE, Zimmermann AG, Bowzard JB, et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011;34:854–865. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden LR, Liu J, Li H, Johnson JA, Walsh SR, Kleinjan JA, Engelson BA, Peter L, Abbink P, Milner DA, Jr., et al. Induction of HIV-1-specific mucosal immune responses following intramuscular recombinant adenovirus serotype 26 HIV-1 vaccination of humans. J Infect Dis. 2015;211:518–528. doi: 10.1093/infdis/jiu485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y, Park H, Cameron MJ, Lefebvre F, Lum R, Coombes N, Mahyari E, Hagen SI, Bae JY, Reyes MD, 3rd, et al. Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med. 2012;18:1673–1681. doi: 10.1038/nm.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome biology. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- Hansen SG, Piatak M, Jr., Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao LA, Grishina I, Bourry O, Hu WK, Somrit M, Sankaran-Walters S, Gaulke CA, Fenton AN, Li JA, Crawford RW, et al. Early mucosal sensing of SIV infection by paneth cells induces IL-1beta production and initiates gut epithelial disruption. PLoS Pathog. 2014;10:e1004311. doi: 10.1371/journal.ppat.1004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen MR, Olagnier D, Hiscott J. Innate immune sensing of HIV-1 infection. Curr Opin HIV AIDS. 2015;10:96–102. doi: 10.1097/COH.0000000000000129. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu J, Carville A, Mansfield KG, Lynch D, Barouch DH. Durable mucosal simian immunodeficiency virus-specific effector memory T lymphocyte responses elicited by recombinant adenovirus vectors in rhesus monkeys. J Virol. 2011;85:11007–11015. doi: 10.1128/JVI.05346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009a;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Skinner PJ, Ha SJ, Duan L, Mattila TL, Hage A, White C, Barber DL, O'Mara L, Southern PJ, et al. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009b;323:1726–1729. doi: 10.1126/science.1168676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Keele BF, Li H, Keating S, Norris PJ, Carville A, Mansfield KG, Tomaras GD, Haynes BF, Kolodkin-Gal D, et al. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol. 2010;84:10406–10412. doi: 10.1128/JVI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens JR, Gurung P, Shaw PJ, Barr MJ, Zaki MH, Brown SA, Vogel P, Chi H, Kanneganti TD. The NLRP12 Sensor Negatively Regulates Autoinflammatory Disease by Modulating Interleukin-4 Production in T Cells. Immunity. 2015;42:654–664. doi: 10.1016/j.immuni.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS ONE. 2010;5:e13984. doi: 10.1371/journal.pone.0013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone G, Boirivant M, Pallone F, MacDonald TT. TGF-beta1 and Smad7 in the regulation of IBD. Mucosal immunology. 2008;(1 Suppl 1):S50–53. doi: 10.1038/mi.2008.55. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:601–609. doi: 10.1172/JCI12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L, Ye Z, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- Mostafavi S, Morris Q. Fast integration of heterogeneous data sources for predicting gene function with limited annotation. Bioinformatics. (2010);26:1759–1765. doi: 10.1093/bioinformatics/btq262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi S, Ray D, Warde-Farley D, Grouios C, Morris Q. GeneMANIA: a real-time multiple association network integration algorithm for predicting gene function. Genome biology. 2008;(9 Suppl 1):S4. doi: 10.1186/gb-2008-9-s1-s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvatiyar K, Cheng G. NOD so fast: NLRX1 puts the brake on inflammation. Immunity. 2011;34:821–822. doi: 10.1016/j.immuni.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MR, Rakasz E, Skinner PJ, White C, Abel K, Ma ZM, Compton L, Napoe G, Wilson N, Miller CJ, et al. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J Virol. 2005;79:9228–9235. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Dos Santos P, Rancez M, Pretet JL, Michel-Salzat A, Messent V, Bogdanova A, Couedel-Courteille A, Souil E, Cheynier R, Butor C. Rapid dissemination of SIV follows multisite entry after rectal inoculation. PLoS ONE. 2011;(6):e19493. doi: 10.1371/journal.pone.0019493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss CA, Elinav E. NF-kappaB Regulation by NLRs: T Cells Join the Club. Immunity. 2015;42:595–597. doi: 10.1016/j.immuni.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.