Abstract
Neuropathic pain is often associated with behavioral depression. Intraplantar formalin produces sustained, neuropathy-associated depression of intracranial self-stimulation (ICSS) in rats. This study evaluated pharmacological modulation of formalin-induced ICSS depression. Rats with intracranial electrodes targeting the medial forebrain bundle responded for electrical brain stimulation in an ICSS procedure. Bilateral intraplantar formalin administration depressed ICSS for 14 days. Morphine (0.32–3.2 mg/kg), ketoprofen (0.1–10 mg/kg), bupropion (3.2–32 mg/kg), and Δ9-tetrahydrocannabinol (THC; 0.32–3.2 mg/kg) were evaluated for their effectiveness to reverse formalin-induced depression of ICSS. Drug effects on formalin-induced mechanical allodynia were evaluated for comparison. Morphine and bupropion reversed both formalin-induced ICSS depression and mechanical allodynia, and effects on ICSS were sustained during repeated treatment. Ketoprofen failed to reverse either formalin effect. THC blocked mechanical allodynia, but decreased ICSS in control rats and exacerbated formalin-induced depression of ICSS. The failure of ketoprofen to alter formalin effects suggests that formalin effects result from neuropathy rather than inflammation. The effectiveness of morphine and bupropion to reverse formalin effects agrees with other evidence that these drugs block pain-depressed behavior in rats and relieve neuropathic pain in humans. The effects of THC suggest general behavioral suppression and do not support the use of THC to treat neuropathic pain.
Keywords: neuropathic pain, formalin, morphine, bupropion, ketoprofen, Δ9-tetrahydrocannabinol, intracranial self-stimulation, pain-depressed behavior, rat
INTRODUCTION
Clinical pain is often associated with functional impairment and depression of behavior, and alleviation of pain-related depression of behavior is a common goal of treatment (Cleeland and Ryan, 1994; Dworkin et al., 2005). Intracranial self-stimulation (ICSS) is a preclinical procedure in which operant behavior is maintained by delivery of electrical stimulation to brain reward areas, and pain-related depression of ICSS has served as one experimental tool for research on expression and treatment of pain-related depression of behavior in rats (Negus, 2013; Negus and Miller, 2014). ICSS in rats can be depressed by relatively transient pain stimuli including intraperitoneal injection of dilute acid (Negus, 2013; Negus and Altarifi, 2013) and hindpaw incision (Ewan and Martin, 2014). Moreover, acid-induced depression of ICSS can be alleviated by treatment with clinically effective analgesics such as mu opioid receptor agonists and nonsteroidal anti-inflammatory drugs (NSAIDs), but not by treatment with other drug classes (e.g. centrally acting kappa opioid receptor agonists) that do not function as effective analgesics in humans despite producing apparent antinociception in many conventional preclinical pain assays (Negus, 2013; Negus and Altarifi, 2013). One implication of these findings is that preclinical assays of pain-related depression of ICSS or other behaviors may contribute to improved preclinical-to-clinical translation of results for candidate analgesics.
Although a need persists for safer and more effective analgesics to treat acute pain, there is a more pressing need to develop improved treatments for chronic pain in general and chronic neuropathic pain in particular (Gilron et al., 2015; Institute of Medicine, 2011; Kerstman et al., 2013). For example, current treatments are considered to be largely symptomatic, and one recent meta-analysis found that currently available pharmacotherapies are effective in only a fraction of the patients treated (Finnerup et al., 2015). A common approach to modeling neuropathic pain in rodents involves strategies to injure sensory nerves innervating the rear paw to produce hypersensitive withdrawal responses to mechanical or thermal stimuli; however, a spinal nerve ligation injury sufficient to produce mechanical hypersensitivity failed to decrease ICSS in rats (Ewan and Martin, 2014), and this finding is consistent with other evidence to suggest the absence or relatively subtle expression of pain-related behavioral depression in common nerve injury models (Benbouzid et al., 2008; Hu et al., 2009; Urban et al., 2011). As an alternative to nerve injury models, formalin is an aqueous formulation of formaldehyde that cross-links proteins to produce cell death, including neuropathy (Fu et al., 2000; 2001; Vierck et al., 2008), and we recently reported that bilateral intraplantar administration of dilute formalin produced not only mechanical hypersensitivity, but also a sustained pain-related depression of ICSS in rats (Leitl et al., 2014b). These results suggested that formalin-induced depression of ICSS in rats may serve as a useful procedure to evaluate drug effects on behavioral depression associated with sustained neuropathic pain.
Accordingly, the present study represents an initial step to examine pharmacological modulation of formalin-induced depression of ICSS, and there were two goals. First, we reported previously that acute morphine treatment produced a dose-dependent reversal of formalin-induced depression of ICSS (Leitl et al., 2014b). Sensitivity to an opioid analgesic supports the inference that formalin-induced depression of ICSS may be related to pain; however, opioids and other pharmacotherapies for neuropathic pain are typically administered chronically, and changes in drug effects (e.g. tolerance to analgesic effects or to undesirable side effects) can influence drug effectiveness and safety (Morgan and Christie, 2011). Accordingly, one goal of this study was to assess potential changes in morphine effects during repeated treatment with an effective dose. Based on previous studies that evaluated effects of repeated morphine on acute acid-induced depression of ICSS (Altarifi and Negus, 2015; Miller et al., 2015a), we hypothesized that repeated morphine would retain its antinociceptive efficacy, and that tolerance would develop to undesirable rate-decreasing effects. Second, we compared effects of repeated morphine to effects of repeated treatment with three other drugs that have been tested previously in the assay of acute acid-induced depression of ICSS. Ketoprofen is an NSAID analgesic and cyclooxygenase inhibitor that blocks acid-induced depression of ICSS (McQuay and Moore, 2006; Negus et al., 2012), and it was tested here to evaluate the potential role of inflammation and associated prostaglandin synthesis in formalin-induced depression of ICSS. NSAIDs are generally considered to have poor effectiveness to treat neuropathic pain (Vo et al., 2009), and we predicted that ketoprofen would not be effective to reverse formalin-induced depression of ICSS. Bupropion is a dopamine/norepinephrine uptake inhibitor used clinically as an antidepressant and smoking-cessation treatment (Foley et al., 2006; Moreira, 2011). We have reported previously that pain-related depression of ICSS may be mediated by a depression of mesolimbic DA signaling in nucleus accumbens, and monoamine uptake inhibitors like bupropion can block acute acid-induced depression of both ICSS and nucleus accumbens DA levels (Miller et al., 2015b; Rosenberg et al., 2013). Chronic pain may also involve depressed mesolimbic dopaminergic function (Wood, 2008), and bupropion has been reported to alleviate neuropathic pain clinically (Semenchuk et al., 2001). Accordingly, we hypothesized that bupropion would display sustained effectiveness to reverse formalin-induced depression of ICSS. Δ9-Tetrahydrocannabinol (THC), a cannabinoid receptor agonist and the principal psychoactive constituent of marijuana, failed to block acid-induced depression of ICSS in rats (Kwilasz and Negus, 2012), a finding consistent with the poor effectiveness of THC to treat acute pain in humans (Raft et al., 1977). However, some studies have suggested effectiveness of THC to treat some forms of neuropathic pain (Boychuk et al., 2015; Lynch and Ware, 2015; McQuay, 2010; Phillips et al., 2010), and we hypothesized that THC might also reverse formalin-induced depression of ICSS. For comparison with drug effects on formalin-induced depression of ICSS, effects of all drugs were also examined on the more conventional measure of mechanical hypersensitivity.
METHODS
Subjects
Studies were conducted in male Sprague-Dawley rats (Harlan, Frederick MD) with initial weights of 285 to 350 g. Rats were individually housed and maintained on a 12-h light/dark cycle with lights on from 06:00 to 18:00 h. Food and water were continuously available in the home cage. Animal-use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee and complied with the National Research Council (2011) Guide for the Care and Use of Laboratory Animals.
Noxious Stimulus and Drugs
Formalin was obtained from Fisher Scientific (Waltham, MA; Catalog #305-510) and diluted in saline to obtain a 5% final concentration. Rats were lightly restrained in a soft cloth for 100µl bilateral injections administered into the plantar aspect of the left and right hind paws using a 27 gauge needle. Morphine sulfate (NIDA Drug Supply Program, Bethesda, MD) and bupropion HCl (Sigma Chemical, St. Louis, MO) were dissolved in sterile saline. Ketoprofen (Spectrum Chemical Co., New Brunswick, NJ) and Δ9-tetrahydrocannabinol (THC; NIDA Drug Supply Program) were prepared in a vehicle of ethanol, Emulphor EL-630 (Rhone-Poulenc; Princeton, NJ), and sterile saline in a ratio of 1:1:18, respectively. For all drugs, doses are expressed as the drug forms named above.
Assay of Intracranial Self-Stimulation (ICSS)
Surgery
A total of 61 rats were anesthetized with isoflurane (2.5–3% in oxygen; Webster Veterinary, Phoenix, AZ) for implantation of stainless-steel electrodes (Plastics One, Roanoke, VA). One pole (the cathode) of each bipolar electrode was 0.25 mm in diameter and covered with polyamide insulation except at the flattened tip, and the other pole (the anode) was 0.125 mm in diameter and uninsulated. The cathode was implanted in the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral from the midsagittal suture, and 8.8 mm below the skull). The anode was wrapped around one of the three skull screws to serve as the ground, and the skull screws and electrode assembly were secured to the skull with orthodontic resin. The animals were allowed to recover for at least 7 days before commencing ICSS training.
Apparatus
Experiments were conducted in sound-attenuating boxes that contained modular acrylic test chambers (29.2 × 30.5 × 24.1 cm) equipped with a response lever (4.5 cm wide, extended 2 cm through the center of one wall, 3 cm off the floor), stimulus lights (three lights colored red, yellow, and green; positioned 7.6 cm directly above the response lever), a 2-W house light, and an ICSS stimulator (MED Associates, St. Albans, VT). Electrodes were connected to the stimulator via bipolar cables routed through a swivel connector (model SL2C; Plastics One). The stimulator was controlled by computer software that also controlled programming parameters and data collection (MED Associates).
Training
After initial shaping of lever press responding, rats were trained under a fixed-ratio 1 (FR 1) schedule of brain stimulation using procedures identical to those described previously (Leitl et al., 2014b). During sessions lasting 30 to 60 minutes, each lever press resulted in the delivery of a 0.5-s train of square wave cathodal pulses (0.1-ms pulse duration), and stimulation was accompanied by illumination of the stimulus lights over the lever. Responses during the 0.5-s stimulation period did not earn an additional stimulation. Initially, the frequency of stimulation was held constant at 158 Hz, and the stimulation intensity for each rat was adjusted gradually to the lowest value that would sustain a high rate of reinforcement (> 30 stimulations/minute). This intensity (120–300 µA across rats) was then held constant for the remainder of the study, and frequency manipulations were introduced. Sessions involving frequency manipulations consisted of sequential 10-min components. During each component, a descending series of 10 current frequencies (158–56 Hz in 0.05-log increments) was presented, with each frequency available during sequential 1-min frequency trials. Each frequency trial began with a 10-s timeout, during which responding had no scheduled consequences. During the last 5 seconds of this timeout, five non-contingent “priming” stimulations were delivered once per second at the frequency available during that trial, and the lever lights were illuminated during each stimulation. This non-contingent stimulation was then followed by a 50-s “response” period, during which responding produced electrical stimulation under the FR 1 schedule. Training continued with presentation of three components per day until responding stabilized. Stability was defined as three consecutive days during which the total number of stimulations per component on each day varied by less than 15% from the mean for those three days with no upward or downward trends, and data from these sessions were used to establish a “Pretreatment Baseline” measure of ICSS (see Data Analysis). Training was completed in all rats in ≤6 weeks.
Testing
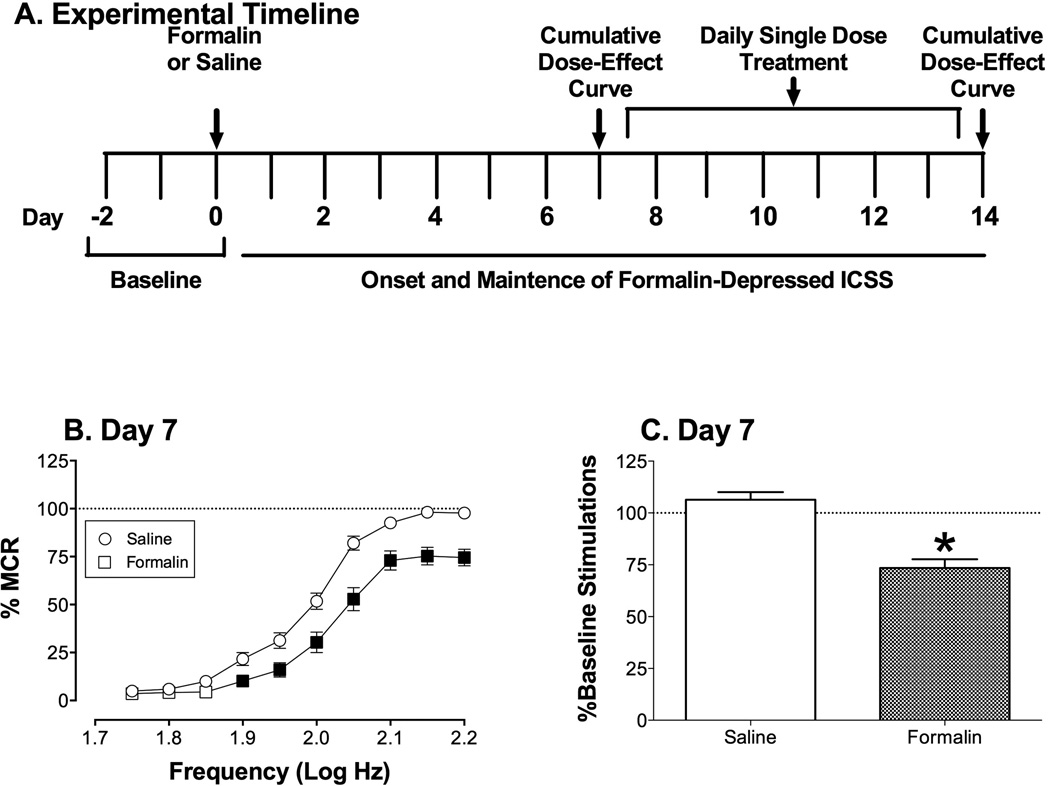
We reported previously that bilateral intraplantar injections of 100 µl/paw 5% formalin produced a stable, pain-related depression of ICSS from 7–14 days after formalin treatment (Leitl et al., 2014b). Accordingly, once stable ICSS was established, studies with each drug were conducted over a period of 14 days as illustrated in Figure 1. On Day 0, rats received bilateral intraplantar injections of 5% formalin or saline. On Days 1–6, no treatments were administered, and ICSS was evaluated daily during three-component sessions to monitor onset of formalin-induced depression of ICSS. On Days 7–13, drugs were administered and ICSS testing continued in three phases. First, on Day 7, a dose-ranging experiment was conducted using a cumulative dosing procedure. On these days, experimental sessions consisted of three daily-baseline components followed by three 60-min test periods. During each test period, rats were removed from the experimental chambers, placed into their home cages, and administered a dose of drug 10 min after the start of the test period. Thirty min later, rats were returned to the experimental chambers, and ICSS was evaluated during two ICSS test components. Each sequential dose increased the total cumulative dose by 0.5 or 1.0 log units, and dose ranges for each drug were as follows: morphine (0.32–3.2 mg/kg, s.c.), ketoprofen (0.1–10 mg/kg, i.p.), bupropion (3.2–32 mg/kg, i.p.), or Δ9-tetrahydrocannabinol (THC; 0.32–3.2, i.p.). Doses, routes of administration, and pretreatment times were based on previous ICSS studies with morphine, ketoprofen, bupropion and THC (Altarifi et al., 2015; Kwilasz and Negus, 2012; Leitl et al., 2014b; Rosenberg et al., 2013). Next, on Days 8–13, the effects of repeated daily dosing with a single drug dose were examined. Experimental sessions consisted of three daily-baseline components followed by administration of a single dose of test drug and then 30 min later by two ICSS test components. The dose of drug administered on Days 8–13 was selected on the basis of the results of the Day 7 dose-ranging study (see Results). Finally, on Day 14, the cumulative dose-effect curve was re-determined using procedures identical to those on Day 7 to assess changes in drug effects associated with repeated treatment. Studies were completed in a total of 48 rats (6 treated with saline and 6 with formalin, for each of the four test drugs). The remaining 13 rats either failed to meet training criteria or failed to complete all phases of testing.
Figure 1.
Effects of bilateral intraplantar treatment with saline or 5% formalin on ICSS for all rats used in ICSS studies. (A) Panel A shows the experimental timeline for behavioral testing, intraplantar treatments with formalin or saline, and drug treatments. (B) Panel B shows full frequency-rate curves determined on day 7 after saline or formalin treatment and before initiation of drug treatments. Horizontal axis: frequency of electrical brain stimulation (Log Hz). Vertical axis: ICSS rate expressed as percent maximum control rate (%MCR). Two-way ANOVA indicated significant main effects of treatment [F(1,23)=27.42; p<0.001] and frequency [F(9,207)=307.1; p<0.001)], and a significant interaction [F(9,207)=5.879; p<0.001)]. Filled points indicate a significant between-group effect of treatment at a given brain-stimulation frequency (Holm-Sidak post hoc test, p<0.05). (C) Panel B shows summary data for the total number of stimulations delivered across all brain-stimulation frequencies on day 7 after saline or formalin treatment. Horizontal axis: Intraplantar treatment. Vertical axis: total number of stimulations per component, expressed as a percentage of the pretreatment baseline. The asterisk indicates a significant difference between groups as determine by t-test (t(46)=5.91, p<0.001). All points and bars show mean ± SEM from 24 rats.
Data analysis
The primary dependent measure for ICSS experiments was the total number of stimulations delivered across all 10 frequency trials of each component. The first ICSS component each day was considered to be a warm-up component, and data were discarded. A “Pretreatment Baseline” measure of ICSS in each subject was determined by averaging the number of stimulations per component during the second and third components across the three baseline days before intraplantar formalin/saline treatments (6 components total; see Figure 1). Daily-baseline data and drug-test data collected after intraplantar injections for each subject were then normalized to the Pretreatment Baseline using an equation to calculate % Pretreatment Baseline Stimulations per Component. For daily-baseline components, data from the first component were discarded, and data from the second and third components on each day were first expressed as (Stimulations per Daily-Baseline Component/Pretreatment Baseline) × 100, and then averaged across components. For drug-test components, data for each of the two test components after each drug dose were expressed as (Stimulations per Drug-Test Component/Pretreatment Baseline) × 100, then averaged across components.
An additional dependent measure was the reinforcement rate in stimulations per trial during each of the 10 frequency trials of each component. To normalize these data, raw reinforcement rates from each trial in each rat were converted to percentage of maximum control rate (%MCR) for that rat, with the maximum control rate defined as the mean of the maximal rates observed during any frequency trial of the second and third baseline components across the three Pretreatment Baseline days. Thus, %MCR values for each trial were calculated as (response rate during a frequency trial ÷ maximum control rate) × 100.
ICSS data were averaged across rats in each experimental condition and compared by one or two-way ANOVA, as appropriate. A significant ANOVA was followed by either a Dunnett’s post-hoc test (one-way ANOVA) or a Holm-Sidak post-hoc test (two-way ANOVA), and the criterion for significance was set a priori at p < 0.05.
Assay of Mechanical Sensitivity
Behavioral procedure
To provide a comparison with drug effects on formalin-induced depression of ICSS, separate groups of rats were used to assess drug effects on formalin-induced mechanical allodynia. Specifically, the von Frey filament test was used to measure sensitivity to a punctate pressure stimulus, as previously described (Leitl et al., 2014b). Briefly, rats were placed on an elevated mesh galvanized steel platform in individual chambers with a hinged lid and allowed to acclimate for at least 20 min. Subsequently, von Frey filaments (0.4 – 15 g in approximate 0.25 log increments; North Coast Medical, Morgan Hill, CA) were applied to the plantar aspect of the left hind paw using the “up-down” method to determine log median withdrawal threshold (Chaplan et al., 1994). Thresholds were determined before intraplantar injection of formalin or saline and again 7 days after intraplantar treatments. On day 7, thresholds were determined five times, once before any further treatment, once after treatment with drug vehicle, and three additional times after treatment with each of three cumulative doses of the test drug. The dose ranges and dose intervals for cumulative dosing were identical to that used on day 7 of ICSS studies.
Data analysis
The primary dependent measure for von Frey experiments was log median withdrawal threshold (Chaplan et al., 1994), and these values were averaged across rats for each drug dose, and data for each drug dose were compared to the respective vehicle using one-way ANOVA, followed by Dunnett post hoc test comparing the dose to the respective drug vehicle (p<0.05).
RESULTS
Formalin-induced depression of ICSS
For all rats used in the study, the mean±SEM Pretreatment Baseline number of stimulations per component was 319.5±9.7, and the mean±SEM Maximum Control Rate (MCR) was 52.9±0.3 stimulations per trial. Prior to intraplantar treatment, pretreatment baseline rates of ICSS did not differ between groups that subsequently received either saline or formalin (data not shown). Figure 1 shows that bilateral injection of intraplantar formalin depressed ICSS by day 7 relative to ICSS in saline-treated rats. Specifically, saline treatment had no effect on ICSS, whereas formalin produced a rightward and downward shift in the ICSS frequency-rate curve (Figure 1B) and a decrease in the total numbers of stimulations per component delivered across all brain-stimulation frequencies (Figure 1C). Moreover, baseline levels of ICSS were similar across groups on Day 7 after saline treatment (c.f. Day 7 Baseline data in Figures 2A, 3A, 4A and 5A; [F(3,20)=0.99, NS]), and formalin-induced depression of ICSS was also similar across groups on Day 7 after formalin treatment (c.f. Day 7 Baseline data in Figures 2D, 3D, 4D and 5D; [F(3,20)=1.40, NS]. This formalin-induced depression of ICSS served an example of sustained pain-related depression of behavior, and drugs were evaluated for their effectiveness to reverse formalin effects.
Figure 2.
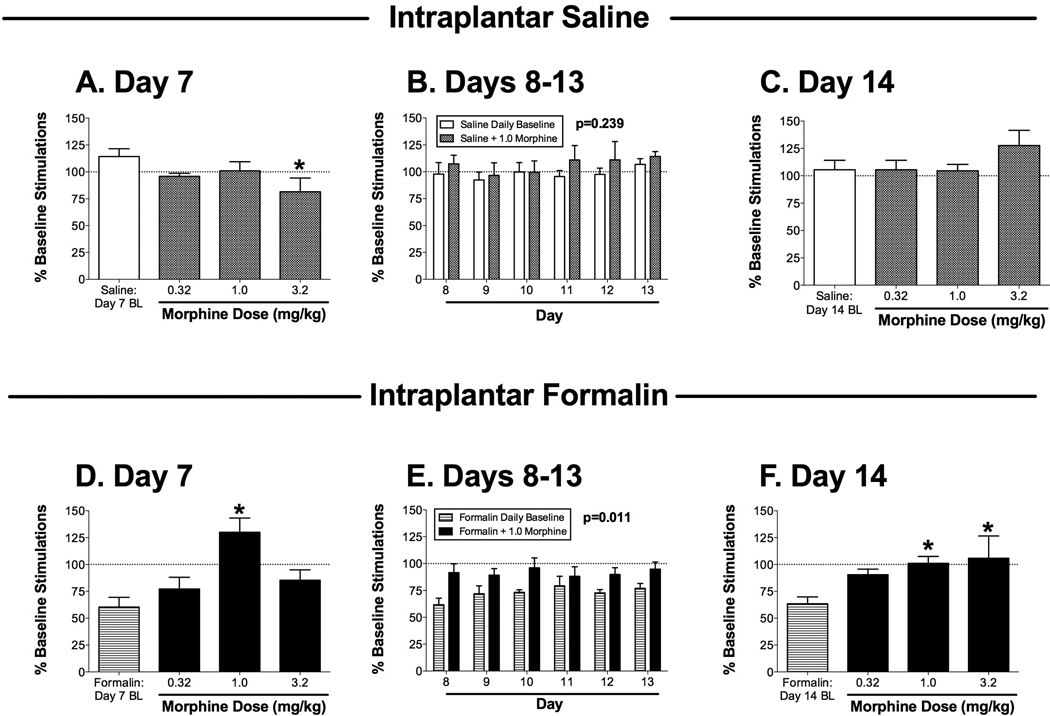
Effects of the mu opioid agonist morphine on ICSS 7–14 days after bilateral intraplantar saline (A–C; one group of 6 rats) or 5% formalin (D–F; a second group of 6 rats). Panels A, C, D and F show effects of cumulative morphine (0.32–3.2 mg/kg) administered on day 7 (A,D) or day 14 (C,F) after intraplantar treatment. Horizontal axes: dose of morphine in mg/kg. Baseline (BL) ICSS determined before morphine treatment on each day is also shown in each panel. Vertical axes: total number of stimulations per component, expressed as a percentage of pretreatment baseline. Asterisks indicate significantly different from the daily baseline as determined by a significant one-way ANOVA followed by the Dunnett post hoc test (p<0.05; see ANOVA results below). Panels B and E show effects of 1.0 mg/kg morphine administered on days 8–13 after intraplantar treatment. Horizontal axes: day after intraplantar treatment. Vertical axes: total number of stimulations per component, expressed as a percentage of pretreatment baseline. For each day, data are shown for ICSS before morphine administration (Daily Baseline) and after morphine administration (+1.0 morphine). The p value for the main effect of morphine treatment is shown in each panel (see below for full 2-way ANOVA results). ANOVA results for each panel were as follows: (A) significant effect of dose [F(3,15)=5.0; p<0.02]; (B) no significant main effect of morphine [F(1,5)=1.79; NS] or day [F(5,25)=1.436; NS)], and no significant interaction [F(5,25)=0.31; NS]; (C) no significant effect of dose [F(3,15)=1.78; NS]; (D) significant effect of dose [F(3,15)=9.99; p<0.001]; (E) significant main effect of morphine [F(1,5)=15.56; p<0.02] but not day [F(5,25)=1.11; NS], and no significant interaction [F(5,25)=0.58; NS]; (F) significant effect of dose [F(3,15)=4.45; p<0.02]. All points show mean + SEM from 6 rats.
Figure 3.
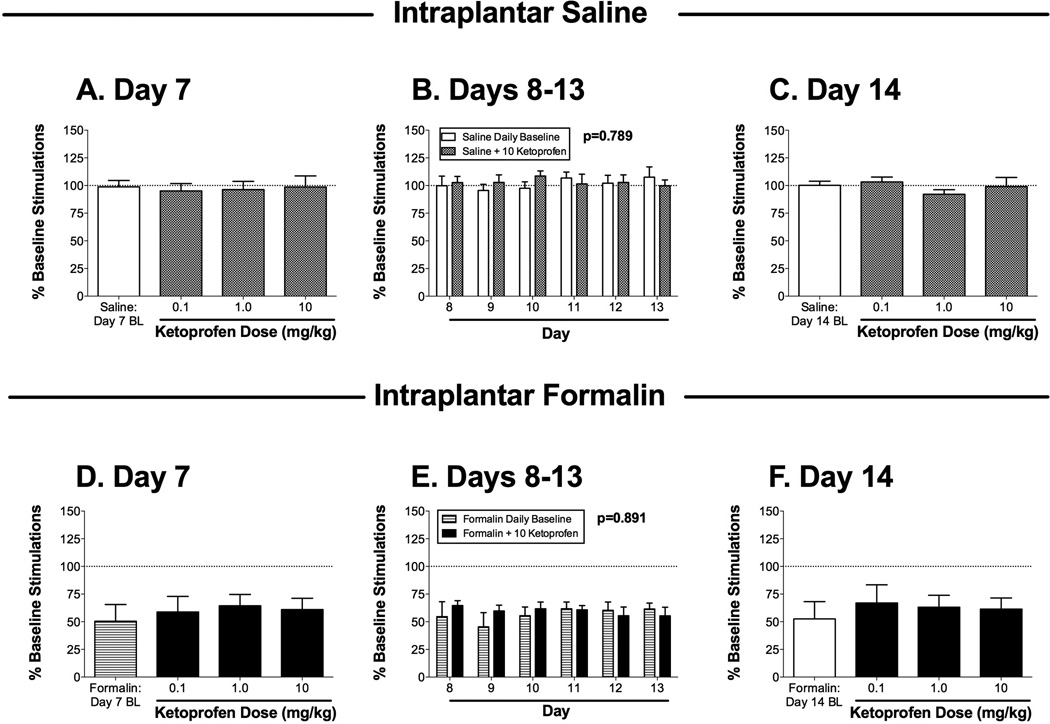
Effects of the nonsteroidal anti-inflammatory drug ketoprofen on ICSS 7–14 days after bilateral intraplantar saline (A–C; one group of 6 rats) or 5% formalin (D–F; a second group of 6 rats). Panels A, C, D and F show effects of cumulative ketoprofen (0.1–10 mg/kg) administered on day 7 (A,D) or day 14 (C,F) after intraplantar treatment. Horizontal axes: dose of ketoprofen in mg/kg. Baseline (BL) ICSS determined before ketoprofen treatment is also shown in each panel. Vertical axes: total number of stimulations per component, expressed as a percentage of pretreatment baseline. Panels B and E show effects of 10 mg/kg ketoprofen administered on days 8–13 after intraplantar treatment. Horizontal axes: day after intraplantar treatment. Vertical axes: total number of stimulations per component, expressed as a percentage of pretreatment baseline. For each day, data are shown for ICSS before ketoprofen administration (Daily Baseline) and after ketoprofen administration (+10.0 ketoprofen). The p value for the main effect of ketoprofen treatment is shown in each panel. ANOVA results for each panel were as follows: (A) no significant effect of dose [F(3,15)=0.14; NS]; (B) no significant main effect of ketoprofen [F(1,5)=0.08; NS] or day [F(5,25)=0.25; NS], and no significant interaction [F(5,25)=0.98; NS]; (C) no significant effect of dose [F(3,15)=0.43; NS]; (D) no significant effect of dose [F(3,15)=0.16; NS]; (E) no significant main effect of ketoprofen [F(1,5)=0.31; NS] or day [F(5,25)=0.33; NS], and no significant interaction [F(5,25)=0.50; NS]; (F) no significant effect of dose [F(3,15)=1.82; NS]. All points show mean + SEM from 6 rats.
Figure 4.
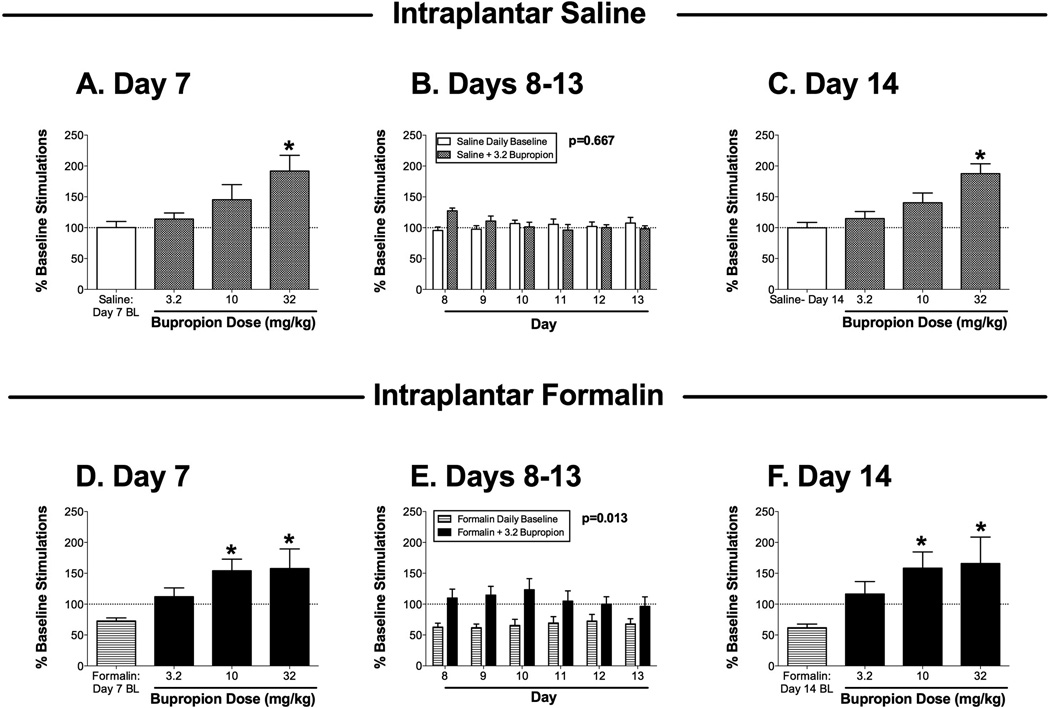
Effects of the dopamine uptake inhibitor bupropion on ICSS 7–14 days after bilateral intraplantar saline (A–C; one group of 6 rats) or 5% formalin (D–F; a second group of 6 rats). Panels A, C, D and F show effects of cumulative bupropion (3.2–32 mg/kg) administered on day 7 (A,D) or day 14 (C,F) after intraplantar treatment. Horizontal axes: dose of bupropion in mg/kg. Baseline (BL) ICSS determined before bupropion treatment is also shown in each panel. Vertical axes: total number of stimulations per component, expressed as a percentage of pretreatment baseline. Asterisks indicate significantly different from the daily baseline as determined by a significant one-way ANOVA followed by the Dunnett post hoc test (p<0.05; see ANOVA results below). Panels B and E show effects of 3.2 mg/kg bupropion administered on days 8–13 after intraplantar treatment. Horizontal axes: day after intraplantar treatment. Vertical axes: total number of stimulations per component, expressed as a percentage of pretreatment baseline. For each day, data are shown for ICSS before bupriopion administration (Daily Baseline) and after bupropion administration (+3.2 bupropion). The p value for the main effect of bupropion treatment is shown in each panel. ANOVA results for each panel were as follows: (A) significant effect of dose [F(3,15)=6.1; p<0.01]; (B) no significant main effect of bupropion [F(1,5)=0.21; NS] or day [F(5,25)=0.84; NS], but a significant interaction [F(5,25)=5.45; p<0.002]; (C) significant effect of dose [F(3,15)=9.31; p<0.001]; (D) significant effect of dose [F(3,15)=7.15; p<0.005], (E) significant main effect of bupropion [F(1,5)=14.36; p<0.02] but not day [F(5,25)=0.24; NS], and no significant interaction [F(5,25)=1.77; NS]; (F) significant effect of dose [F(3,15)=0.0029; p<0.005]. All points show mean + SEM from 6 rats.
Figure 5.
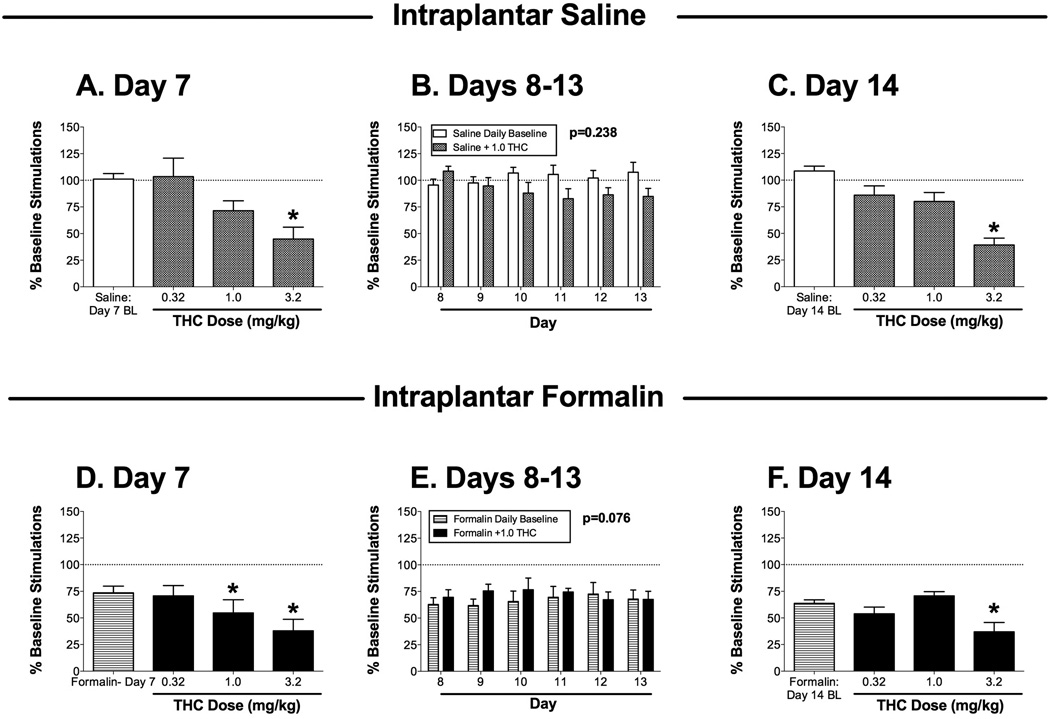
Effects of the cannabinoid receptor agonist THC on ICSS 7–14 days after bilateral intraplantar saline (A–C; one group of 6 rats) or 5% formalin (D–F; a second group of 6 rats). Panels A, C, D and F show effects of cumulative THC (0.32–3.2 mg/kg) administered on day 7 (A,D) or day 14 (C,F) after intraplantar treatment. Horizontal axes: dose of THC in mg/kg. Baseline (BL) ICSS determined before THC treatment is also shown in each panel. Vertical axes: total number of stimulations per component, expressed as a percentage of pretreatment baseline. Asterisks indicate significantly different from the daily baseline as determined by a significant one-way ANOVA followed by the Dunnett post hoc test (p<0.05; see ANOVA results below). Panels B and E show effects of 1.0 mg/kg THC administered on days 8–13 after intraplantar treatment. Horizontal axes: day after intraplantar treatment. Vertical axes: total number of stimulations per component, expressed as a percentage of pretreatment baseline. For each day, data are shown for ICSS before THC administration (Daily Baseline) and after THC administration (+1.0 THC). The p value for the main effect of THC treatment is shown in each panel. ANOVA results for each panel were as follows: (A) significant effect of dose [F(3,15)=8.21; p<0.002]; (B) no main effect of THC [F(1,5)=1.80; NS] or day [F(5,25)=0.59; NS], but there was a significant interaction [F(5,25)=5.16; p<0.002]; (C) significant effect of dose [F(3,15)=20.16; p<0.001]; (D) significant effect of dose [F(3,15)=29.30; p<0.001], (E) no significant main effect of THC [F(1,5)=4.97; NS] or day [F(5,25)=0.23; NS], and no significant interaction [F(5,25)=1.50; NS]; (F) significant effect of dose [F(3,15)=6.57; p<.005]. All points show mean + SEM from 6 rats.
Morphine
Figure 2 shows effects of morphine on ICSS in saline-treated rats (Figure 2A–C) and formalin-treated rats (Figure 2D–F). On day 7 after intraplantar saline treatment, cumulative doses of 0.32 and 1.0 mg/kg morphine did not alter ICSS, and 3.2 mg/kg morphine significantly depressed ICSS (Figure 2A). A dose of 1.0 mg/kg morphine was selected for daily treatments on days 8–13 after intraplantar saline (see below for rationale), and this dose did not significantly alter ICSS on any day (Figure 2B). On day 14 after intraplantar saline, cumulative doses of 0.32–3.2 mg/kg morphine had no significant effect on ICSS (Figure 2C), indicating tolerance to the initial rate-decreasing effects of 3.2 mg/kg morphine observed on day 7.
On day 7 after intraplantar formalin treatment, baseline ICSS was depressed, and cumulative doses of morphine reversed this formalin-induced depression of ICSS with an inverted-U shaped dose-effect curve (Figure 2D). Significant reversal was obtained with 1.0 mg/kg morphine, so this dose was used for repeated daily treatments on days 8–13 after intraplantar formalin. Two-way ANOVA during this treatment period indicated a significant main effect of morphine to alleviate formalin-induced depression of ICSS (Figure 2E). On day 14 after intraplantar formalin, cumulative doses of morphine produced a dose-dependent reversal of formalin-induced depression of ICSS, with significant effects produced by 1.0 and 3.2 mg/kg morphine (Figure 2F).
Ketoprofen
Figure 3 shows effects of ketoprofen on ICSS in saline-treated rats (Figure 3A–C) and formalin-treated rats (Figure 3B–F). On day 7 after intraplantar saline treatment, cumulative doses of 0.1, 1.0, and 10 mg/kg ketoprofen did not alter ICSS. A dose of 10 mg/kg ketoprofen was selected for daily treatments on days 8–13 after intraplantar saline (see below for rationale), and this dose did not alter ICSS on any day (Figure 3B). On day 14 after intraplantar saline, cumulative doses of 0.1, 1.0, and 10 mg/kg ketoprofen did not alter ICSS (Figure 3C).
On day 7 after intraplantar formalin treatment, baseline ICSS was depressed, and cumulative doses of ketoprofen doses up to 10 mg/kg did not reverse this formalin-induced depression of ICSS (Figure 3D). Because no ketoprofen dose altered ICSS, and higher doses may produce gastrointestinal toxicity in rodents (Alarcon de la Lastra et al., 2002; Lamon et al., 2008), the dose of 10 mg/kg was selected for repeated daily treatments on days 8–13 after intraplantar formalin, and two-way ANOVA during this treatment period did not indicate a significant main effect of ketoprofen to alleviate formalin-induced depression of ICSS (Figure 3E). On day 14 after intraplantar formalin, cumulative doses of 0.1, 1.0, and 10 mg/kg ketoprofen did not alter ICSS (Figure 3F).
Bupropion
Figure 4 shows effects of bupropion on ICSS in saline-treated rats (Figure 4A–C) and formalin-treated rats (Figure 4D–F). On day 7 after intraplantar saline treatment, cumulative doses of 3.2 and 10 mg/kg bupropion did not significantly alter ICSS, and 32 mg/kg bupropion significantly facilitated ICSS (Figure 4A). A dose of 3.2 mg/kg bupropion was selected for daily treatments on days 8–13 after intraplantar saline (see below for rationale), and this dose did not significantly alter ICSS (Figure 4B). On day 14 after intraplantar saline, cumulative doses of 3.2 and 10 mg/kg bupropion had no significant effect on ICSS, but 32 mg/kg bupropion again significantly facilitated ICSS (Figure 4 C), indicating a lack of sensitization or tolerance to the initial effects of bupropion observed on day 7.
On day 7 after intraplantar formalin treatment, baseline ICSS was depressed, and cumulative doses of bupropion reversed this formalin-induced depression of ICSS in a dose-dependent manner (Figure 4D). Significant reversal was obtained with 10 and 32 mg/kg bupropion, but 3.2 mg/kg bupropion increased mean ICSS levels back to approximately 100% of the pre-formalin baseline, and the lack of statistical significance was due in part to high variability in effects of 32 mg/kg bupropion (e.g. 3.2 mg/kg bupropion did significantly increase ICSS relative to the daily baseline when evaluated by t-test, p<0.05). Accordingly, 3.2 mg/kg bupropion was used for repeated daily treatments on days 8–13 after intraplantar formalin, and two-way ANOVA during this treatment period indicated a significant main effect of bupropion to alleviate formalin-induced depression of ICSS (Figure 4E). On day 14 after intraplantar formalin, cumulative doses of bupropion produced a dose-dependent reversal of formalin-induced depression of ICSS, with significant effects again produced by 10 and 32 mg/kg bupropion (Figure 4F). As on day 7, effects of 3.2 mg/kg bupropion were not statistically significant, but mean ICSS levels were restored to approximate baseline levels.
THC
Figure 5 shows effects of THC on ICSS in saline-treated rats (Figure 5 A–C) and formalin-treated rats (Figure 5D–F). On day 7 after intraplantar saline treatment, cumulative doses of 0.32 and 1.0 mg/kg THC did not alter ICSS, and 3.2 mg/kg THC significantly depressed ICSS (Figure 5A). A dose of 1.0 mg/kg THC was selected for daily treatments on days 8–13 after intraplantar saline (see below for rationale), and this dose did not significantly alter ICSS during repeated treatment (Figure 5B). On day 14 after intraplantar saline, cumulative doses of 0.32, and 1.0 mg/kg THC did not alter ICSS, and 3.2 mg/kg again significantly depressed ICSS (Figure 5C), indicating a lack of tolerance to the initial rate-decreasing effects of 3.2 mg/kg THC observed on day 7.
On day 7 after intraplantar formalin treatment, baseline ICSS was depressed, and cumulative doses of THC produced a dose-dependent exacerbation of formalin-induced depression of ICSS (Figure 5D). Because none of the doses of THC tested alleviated formalin effects, an intermediate dose of 1.0 mg/kg THC was evaluated on days 8–13 to assess the potential for repeated treatment to produce tolerance to its rate-decreasing effects and unmask a reversal of formalin-induced depression of ICSS. However, two-way ANOVA during this treatment period did not indicate a significant main effect of THC (Figure 5E), and on day 14, cumulative THC again only exacerbated formalin-induced depression of ICSS (Figure 5F). A dose of 1.0 mg/kg THC, which significantly decreased ICSS on day 7 in the formalin-treated rats, did not significantly alter ICSS on Day 14, suggesting tolerance to the rate-decreasing effects of this THC dose.
Drug effects on mechanical hypersensitivity
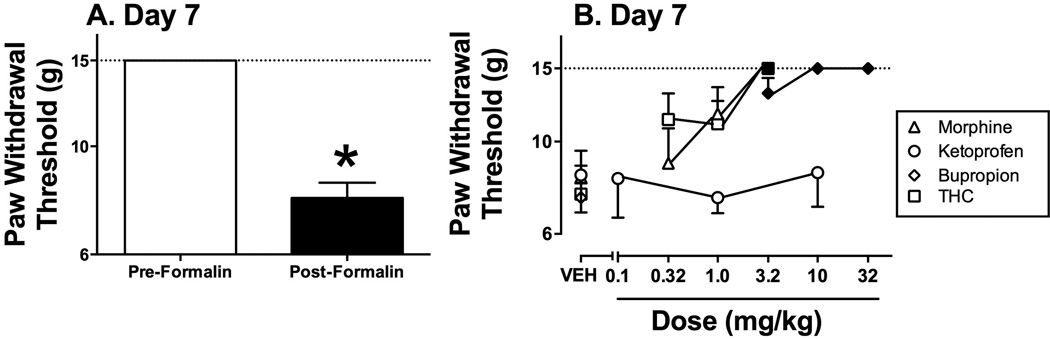
Figure 6 shows that paw-withdrawal thresholds to mechanical stimulation were significantly reduced 7 days after formalin injection relative to pre-formalin values (Figure 6A). Morphine, bupropion and THC produced dose-dependent reversal of formalin-induced mechanical hypersensitivity, whereas ketoprofen was ineffective (Figure 6B).
Figure 6.
Effects of morphine, ketoprofen, bupropion and THC on formalin-induced mechanical hypersensitivity (or allodynia) 7 days after formalin-treatment. Panel A shows magnitude of formalin-induced decrease in paw-withdrawal thresholds for all rats in the study. Horizontal axis: Pre- or 7-day Post-formalin treatment conditions. Vertical axis: paw withdrawal threshold from von Frey filaments in grams (log scale). Formalin significantly decreased thresholds (t(29)=13.83; p<0.001). All bars show mean ± SEM for 24 rats. Panel B shows test drug effects on formalin-induced mechanical hypersensitivity. Horizontal axis: dose of drug in mg/kg (log scale). Vertical axis: paw withdrawal threshold from von Frey filaments in grams (log scale). Filled symbols indicate significantly different from vehicle (VEH) as determined by a significant one-way ANOVA followed by the Dunnett post hoc test (p<0.05). ANOVA results were as follows: morphine: [F(3,15)=6.84; p<0.005]; ketoprofen [F(3,15)=0.27; NS]; bupropion [F(3,15)=40.13; p<0.001]; THC [F(3,15)=7.93; p<0.005]. All points show mean ± SEM from 6 rats.
DISCUSSION
This study evaluated effects of repeated treatment with morphine, ketoprofen, bupropion, and THC on neuropathic pain-related depression of ICSS produced by intraplantar formalin injection. For comparison, acute drug effects were also examined on the more conventional endpoint of formalin-induced mechanical allodynia using the von Frey assay. There were three main findings. First, in agreement with previous studies, intraplantar formalin produced both mechanical allodynia and sustained depression of ICSS. Second, morphine produced a dose-dependent reversal of both formalin-induced stimulation of mechanical allodynia and formalin-induced depression of ICSS, and morphine antinociception in the assay of formalin-depressed ICSS was sustained during repeated treatment. Third, the DA/NE uptake inhibitor bupropion also blocked formalin-induced mechanical allodynia and produced a sustained reversal of formalin-induced depression of ICSS; however, ketoprofen was not effective to reverse either formalin effect, and THC was effective to reverse formalin-induced mechanical allodynia but not formalin-induced depression of ICSS. These results illustrate a range of potential effect profiles and provide further evidence to suggest that evaluation of drug effects on pain-related depression of ICSS may both (a) differ from drug effects on more conventional endpoints in preclinical pain assays, and (b) contribute new insights to preclinical evaluation of candidate analgesic drugs.
Morphine effects
The magnitude and duration of formalin-induced stimulation of mechanical allodynia and depression of ICSS observed here are consistent with previous studies (Fu et al., 2000; Leitl et al., 2014b). Morphine is a clinically effective analgesic and an agonist at mu opioid receptors (Yaksh and Wallace, 2011). The effectiveness of morphine to reverse both formalin-induced mechanical allodynia and depression of ICSS on Day 7 after formalin treatment agrees with previous findings that morphine blocks or reverses other formalin-stimulated behaviors (e.g. flinching), mechanical allodynia induced by other neuropathy manipulations (e.g. spinal nerve ligation), and formalin-induced depression of ICSS (Bian et al., 1995; Gallantine and Meert, 2005; Leitl et al., 2014b). This study expanded upon these previous results in finding that morphine retained its effectiveness to reverse formalin-induced depression of ICSS during repeated treatment for eight consecutive days. Tolerance is often observed to morphine antinociception in conventional assays of pain-stimulated behavior (Cicero and Meyer, 1973; Morgan and Christie, 2011; Way et al., 1969; Yu et al., 2014); however, the absence of antinociceptive tolerance in the present study agrees with previous studies reporting both minimal tolerance to low-dose morphine antinociception in assays of neuropathy-induced allodynia (Narita et al., 2013; Neil et al., 1990; Sounvoravong et al., 2004; Suzuki et al., 1992) and resistance to morphine tolerance in assays of pain-related ICSS depression (Altarifi and Negus, 2015; Miller et al., 2015a). Moreover, these results agree with evidence for the clinical effectiveness of morphine and other opioids to treat neuropathic pain (Finnerup et al., 2015) and with evidence for sustained analgesic effects of mu opioids during chronic opioid treatment in many clinical contexts (Foley, 1995; Harden et al., 2010; Morgan and Christie, 2011; Rosenblum et al., 2008). Although tolerance did not develop to morphine antinociception in the present study, tolerance did develop to the rate-decreasing effects of morphine. This agrees with previous studies to show that repeated morphine treatment in rats produces tolerance to ICSS rate-decreasing effects and enhanced expression of rate-increasing effects of morphine (Altarifi and Negus, 2011; Miller et al., 2015a), and these findings may also be related to evidence for tolerance to some undesirable side effects, such as sedation, during repeated opioid administration for treatment of pain (Benyamin et al., 2008; Labianca et al., 2012).
Ketoprofen effects
Ketoprofen is an NSAID that inhibits prostaglandin synthesis and associated pain by blocking the cyclo-oxygenase enzymes necessary to produce prostaglandins during inflammation (McQuay and Moore, 2006). Consistent with the clinical effectiveness of ketoprofen and other NSAIDs to treat inflammatory pain, ketoprofen and other NSAIDs block inflammation-associated pain-stimulated and pain-depressed behaviors in rats and mice (Cobos et al., 2012; Girard et al., 2008; Leitl et al., 2014a; Negus et al., 2015). The clinical effectiveness of NSAIDs to treat neuropathic pain is more variable, and NSAIDs are generally considered to have low and/or unreliable efficacy for treatment of neuropathic pain (Vo et al., 2009). Preclinical studies have also reported mixed results for NSAIDs in alleviating mechanical allodynia in various neuropathy models; however, ketoprofen is generally ineffective to reverse neuropathy-associated mechanical allodynia in rodents (Benbouzid et al., 2008; Ossipov et al., 2000), and other NSAIDs were effective when administered within 24 hr but not at later times after nerve injury (Takahashi et al., 2004; Zhao et al., 2000). Accordingly, the ineffectiveness of ketoprofen to reverse mechanical allodynia 7 days after formalin administration is consistent with these previous findings, and the present results extend on these earlier findings by showing that neither initial nor repeated ketoprofen treatment reversed pain-related depression of behavior by formalin. The poor effectiveness of ketoprofen cannot be attributed to inadequate dosing, because ketoprofen was tested here at doses 10 times higher than the dose shown previously to block pain-stimulated and pain-depressed behaviors elicited by intraperitoneal acid administration in rats (Kwilasz and Negus, 2012; Negus et al., 2012), and higher ketoprofen doses produce gastrointestinal toxicity in rats (Alarcon de la Lastra et al., 2002; Lamon et al., 2008). Overall, the effectiveness of morphine but not ketoprofen to reverse formalin-induced mechanical allodynia and depression of ICSS in the present study supports the conclusion that these formalin effects are related to neuropathic pain but not inflammatory pain.
Bupropion effects
Bupropion blocks dopamine and norepinephrine transporters, and it is used clinically as an antidepressant and smoking-cessation medication (Foley et al., 2006; Moreira, 2011). Bupropion was also shown to relieve neuropathic pain in the one clinical study that has been conducted (Semenchuk et al., 2001). Other monoamine transporter inhibitors have also shown relatively good effectiveness to treat neuropathic pain, although the compounds usually used to treat neuropathic pain (e.g. amitryptiline) are more selective to block transporters of norepinephrine and/or serotonin than dopamine (Finnerup et al., 2015; Watson et al., 2006). In preclinical studies, bupropion alleviates both inflammation- and neuropathy-associated pain-stimulated behaviors, including mechanical allodynia in nerve injury models (Hajhashemi and Khanjani, 2014; Hoshino et al., 2015; Naderi et al., 2014; Pedersen et al., 2005), and bupropion also blocked depression of ICSS produced by intraperitoneal acid administration (Rosenberg et al., 2013). The present results confirm and extend this evidence for preclinical antinociceptive effects of bupropion, in particular by showing that bupropion produces a dose-dependent and sustained reversal of neuropathy-associated pain-depressed ICSS in rats. Although the precise mechanisms of bupropion effects remain to be determined, pain is often associated with decreased dopaminergic signaling (Wood, 2008). In support of a connection between pain behaviors and reduced dopamine signaling, we recently reported that an acute noxious stimulus sufficient to depress ICSS (intraperitoneal administration of dilute acid) also depressed mesolimbic dopamine release in the nucleus accumbens of rats. Moreover, pain-related depression of both ICSS and dopamine release could be blocked not only by opioid and NSAID analgesics, but also by the novel “triple” uptake inhibitor amitifadine (Leitl et al., 2014a; Miller et al., 2015b). The present results with bupropion are consistent with the working hypothesis that chronic pain-related depression of behavior produced by a neuropathy manipulation may also be associated with a decrease in dopamine signaling and may be responsive to treatment with drugs that block dopamine transporters.
THC effects
THC is the primary psychoactive constituent of marijuana, and it functions as a low-efficacy agonist at cannabinoid 1 and cannabinoid 2 receptors (Pertwee, 2008). THC is not effective to treat acute forms of pain, such as post-surgical pain (Raft et al., 1977), but it has been extensively evaluated for more chronic forms of pain including neuropathic pain. Although some studies have reported modest effectiveness of THC or marijuana preparations to alleviate neuropathic pain (Boychuk et al., 2015; Lynch and Ware, 2015; McQuay, 2010; Phillips et al., 2010), other studies failed to find significant effects, and overall, the use of THC or marijuana preparations for treatment of neuropathic pain remains controversial because of both its limited and unreliable effectiveness and its undesirable effects. For example, one recent meta-analysis of treatments for neuropathic pain recommended against use of THC (Finnerup et al., 2015). In contrast to the relatively poor effectiveness of THC to treat acute or chronic clinical pain, preclinical studies using conventional assays of pain-stimulated behavior have consistently reported high antinociceptive efficacy of THC (Martin and Lichtman, 1998), and the present effectiveness of THC to reverse formalin-induced mechanical allodynia is consistent with this previous literature. Thus, THC and other cannabinoids represent a class of drugs that have produce stronger evidence for analgesic effectiveness in preclinical than clinical studies. This apparent discrepancy may reflect a nearly exclusive reliance on pain-stimulated behaviors to assess THC effects in preclinical studies. For example, although THC and the higher efficacy cannabinoid agoinst CP55940 blocked acute i.p. acid-stimulated stretching in rats, THC and CP55940 doses that produced these effects also produced signs of general behavioral suppression, and both THC and CP55940 failed to block acid-induced depression of either ICSS or feeding in rats (Kwilasz and Negus, 2012). Thus, neither THC nor CP55940 were effective to produce antinoiception in assays of acute pain-depressed behavior. The present results extend this finding to a model of sustained pain-related depression of behavior. THC doses that reduced mechanical allodynia also reduced ICSS (suggestive of general behavioral supression), and THC also failed to reverse formalin-induced depression of ICSS. Overall, these results suggest that THC and other cannabinoid agonists are not effective to block signs of pain-related behavioral depression in rats, and effectiveness of these compounds to block signs of pain-stimulated behaviors may reflect nonselective behavioral suppression rather than decreased sensitivity to the noxious stimulus.
Interpretation of drug effects in assays of pain-stimulated and pain-depressed behavior
This study compared drug effects in assays of a pain-stimulated behavior (paw-withdrawal responses from a mechanical stimulus) and a pain-depressed behavior (ICSS) induced by intraplantar formalin. In both types of procedure, behavioral effects of a noxious stimulus can be alleviated not only by treatments that reduce sensitivity to the noxious stimulus (i.e. true analgesia), but also by treatments that produce non-selective effects on behavior (Negus, 2013). Specifically, in assays of pain-stimulated behavior, the noxious stimulus increases expression of the target behavior (e.g. hypersensitive paw-withdrawal responses), and this behavior can be reduced not only by analgesics, but also by drugs that produce non-selective behavioral impairment. Conversely, in assays of pain-depressed behavior, the noxious stimulus decreases expression of the target behavior (e.g. depression of ICSS), and pain-related behavioral depression can be blocked or reversed not only by analgesics, but also by drugs that produce non-selective behavioral stimulation. One advantage of using complementary assays of pain-stimulated and pain-depressed behaviors elicited by the same noxious stimulus is that analgesics are expected to be effective in both types of procedure, whereas drugs that produce non-selective behavioral impairment or stimulation would be expected to produce analgesia-like effects in only one type of the two types of procedure (Negus, 2013). Accordingly, drugs most likely to function as true analgesics will produce antinociception in both types of procedure, and in the present study, both morphine and bupropion met this criterion. Bupropion also produced robust stimulation of ICSS in the absence of the noxious stimulus. This finding is consistent with previous studies that examined effects of bupropion and other dopamine/norepinephrine uptake inhibitors on ICSS (Negus et al., 2012; Rosenberg et al., 2013), and this raises the possibility that bupropion reversal of formalin-induced ICSS depression may reflect wholly or in part a non-selective increase in ICSS independent of pain state. However, two findings suggest that non-selective behavioral stimulation is not sufficient to explain bupropion effects. First, bupropion was more potent to reverse formalin-induced ICSS depression than to facilitate control ICSS in the absence of the noxious stimulus. Second, the same bupropion doses that were effective to treat a formalin-depressed behavior (ICSS) also were effective to treat a formalin-stimulated behavior (mechanical allodynia).
In contrast to morphine and bupropion, THC was only effective in the assay of formalin-stimulated mechanical allodynia but not in the assay of formalin-depressed ICSS, and THC doses that reversed mechanical allodynia also decreased ICSS in the absence of a noxious stimulus. As discussed above, the most parsimonious interpretation of these data is that THC does not block sensitivity to the noxious stimulus, and apparent antinociception by THC in the assay of mechanical allodynia results from non-selective behavioral suppression. An alternative possibility is that THC might produce both analgesia and general behavioral suppression, and the latter effect could obscure expression of antinociception in assays of pain-depressed behavior. However, clinical evidence for weak effectiveness of THC to treat acute or neuropathic pain clinically would seem to argue against this possibility, and even if this possibility were to hold true, the present results still suggest poor effectiveness of THC to alleviate pain-related behavioral depression.
ACKNOWLEDGEMENTS
No additional acknowledgements
Funding: NIH grant R01 NS070715
Footnotes
Conflicts of Interest: None declared
REFERENCES
- Alarcon de la Lastra C, Nieto A, Martin MJ, Cabre F, Herrerias JM, Motilva V. Gastric toxicity of racemic ketoprofen and its enantiomers in rat: oxygen radical generation and COX-expression. Inflamm Res. 2002;51:51–57. doi: 10.1007/BF02683999. [DOI] [PubMed] [Google Scholar]
- Altarifi AA, Negus SS. Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behav Pharmacol. 2011;22:663–673. doi: 10.1097/FBP.0b013e32834aff54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Negus SS. Differential tolerance to morphine antinociception in assays of pain-stimulated vs. pain-depressed behavior in rats. Eur J Pharmacol. 2015;748:76–82. doi: 10.1016/j.ejphar.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS. Effects of mu-opioid receptor agonists in assays of acute pain-stimulated and pain-depressed behavior in male rats: role of mu-agonist efficacy and noxious stimulus intensity. J Pharmacol Exp Ther. 2015;352:208–217. doi: 10.1124/jpet.114.219873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbouzid M, Pallage V, Rajalu M, Waltisperger E, Doridot S, Poisbeau P, Freund-Mercier MJ, Barrot M. Sciatic nerve cuffing in mice: a model of sustained neuropathic pain. European journal of pain (London, England) 2008;12:591–599. doi: 10.1016/j.ejpain.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- Bian D, Nichols ML, Ossipov MH, Lai J, Porreca F. Characterization of the antiallodynic efficacy of morphine in a model of neuropathic pain in rats. Neuroreport. 1995;6:1981–1984. doi: 10.1097/00001756-199510010-00007. [DOI] [PubMed] [Google Scholar]
- Boychuk DG, Goddard G, Mauro G, Orellana MF. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29:7–14. doi: 10.11607/ofph.1274. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Meyer ER. Morphine pellet implantation in rats: quantitative assessment of tolerance and dependence. J Pharmacol Exp Ther. 1973;184:404–408. [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ. Differential suppression of intracranial self-stimulation, food-maintained operant responding, and open field activity by paw incision and spinal nerve ligation in rats. Anesth Analg. 2014;118:854–862. doi: 10.1213/ANE.0000000000000119. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley KF, DeSanty KP, Kast RE. Bupropion: pharmacology and therapeutic applications. Expert review of neurotherapeutics. 2006;6:1249–1265. doi: 10.1586/14737175.6.9.1249. [DOI] [PubMed] [Google Scholar]
- Foley KM. Misconceptions and controversies regarding the use of opioids in cancer pain. Anticancer Drugs. 1995;6(Suppl 3):4–13. doi: 10.1097/00001813-199504003-00002. [DOI] [PubMed] [Google Scholar]
- Fu KY, Light AR, Maixner W. Relationship between nociceptor activity, peripheral edema, spinal microglial activation and long-term hyperalgesia induced by formalin. Neuroscience. 2000;101:1127–1135. doi: 10.1016/s0306-4522(00)00376-6. [DOI] [PubMed] [Google Scholar]
- Fu KY, Light AR, Maixner W. Long-lasting inflammation and long-term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. J Pain. 2001;2:2–11. doi: 10.1054/jpai.2001.9804. [DOI] [PubMed] [Google Scholar]
- Gallantine EL, Meert TF. A comparison of the antinociceptive and adverse effects of the mu-opioid agonist morphine and the delta-opioid agonist SNC80. Basic & clinical pharmacology & toxicology. 2005;97:39–51. doi: 10.1111/j.1742-7843.2005.pto_97107.x. [DOI] [PubMed] [Google Scholar]
- Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 2015;90:532–545. doi: 10.1016/j.mayocp.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Girard P, Verniers D, Coppe MC, Pansart Y, Gillardin JM. Nefopam and ketoprofen synergy in rodent models of antinociception. Eur J Pharmacol. 2008;584:263–271. doi: 10.1016/j.ejphar.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Hajhashemi V, Khanjani P. Analgesic and anti-inflammatory activities of bupropion in animal models. Res Pharm Sci. 2014;9:251–257. [PMC free article] [PubMed] [Google Scholar]
- Harden RN, Gagnon CM, Graciosa J, Gould EM. Negligible analgesic tolerance seen with extended release oxymorphone: a post hoc analysis of open-label longitudinal data. Pain medicine (Malden, Mass. 2010;11:1198–1208. doi: 10.1111/j.1526-4637.2010.00898.x. [DOI] [PubMed] [Google Scholar]
- Hoshino H, Obata H, Nakajima K, Mieda R, Saito S. The antihyperalgesic effects of intrathecal bupropion, a dopamine and noradrenaline reuptake inhibitor, in a rat model of neuropathic pain. Anesth Analg. 2015;120:460–466. doi: 10.1213/ANE.0000000000000540. [DOI] [PubMed] [Google Scholar]
- Hu B, Doods H, Treede RD, Ceci A. Depression-like behaviour in rats with mononeuropathy is reduced by the CB2-selective agonist GW405833. Pain. 2009;143:206–212. doi: 10.1016/j.pain.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Kerstman E, Ahn S, Battu S, Tariq S, Grabois M. Neuropathic pain. Handb Clin Neurol. 2013;110:175–187. doi: 10.1016/B978-0-444-52901-5.00015-0. [DOI] [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS. Dissociable Effects of the Cannabinoid Receptor Agonists {Delta}9-Tetrahydrocannabinol and CP55940 on Pain-Stimulated Versus Pain-Depressed Behavior in Rats. J Pharmacol Exp Ther. 2012;343:389–400. doi: 10.1124/jpet.112.197780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labianca R, Sarzi-Puttini P, Zuccaro SM, Cherubino P, Vellucci R, Fornasari D. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin Drug Investig. 2012;32(Suppl 1):53–63. doi: 10.2165/11630080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lamon TK, Browder EJ, Sohrabji F, Ihrig M. Adverse effects of incorporating ketoprofen into established rodent studies. Journal of the American Association for Laboratory Animal Science : JAALAS. 2008;47:20–24. [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA, Jr, Banks ML, Negus SS. Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous kappa-opioids. Neuropsychopharmacology. 2014a;39:614–624. doi: 10.1038/npp.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Potter DN, Cheng K, Rice KC, Carlezon WA, Jr, Negus SS. Sustained pain-related depression of behavior: effects of intraplantar formalin and complete freund's adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Mol Pain. 2014b;10:62. doi: 10.1186/1744-8069-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch ME, Ware MA. Cannabinoids for the Treatment of Chronic Non-Cancer Pain: An Updated Systematic Review of Randomized Controlled Trials. J Neuroimmune Pharmacol. 2015;10:293–301. doi: 10.1007/s11481-015-9600-6. [DOI] [PubMed] [Google Scholar]
- Martin BR, Lichtman AH. Cannabinoid transmission and pain perception. Neurobiol Dis. 1998;5:447–461. doi: 10.1006/nbdi.1998.0218. [DOI] [PubMed] [Google Scholar]
- McQuay HJ. More evidence cannabis can help in neuropathic pain. CMAJ. 2010;182:1494–1495. doi: 10.1503/cmaj.100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuay HJ, Moore A. NSAIDs and coxibs: clinical use. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack's Textbook of Pain. 5th Edition. London: Elsevier; 2006. pp. 471–480. [Google Scholar]
- Miller LL, Altarifi AA, Negus SS. Effects of repeated morphine on intracranial self-stimulation in male rats in the absence or presence of a noxious pain stimulus. Exp Clin Psychopharmacol. 2015a;23:405–414. doi: 10.1037/pha0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LL, Leitl MD, Banks ML, Blough BE, Negus SS. Effects of the triple monoamine uptake inhibitor amitifadine on pain-related depression of behavior and mesolimbic dopamine release in rats. Pain. 2015b;156:175–184. doi: 10.1016/j.pain.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira R. The efficacy and tolerability of bupropion in the treatment of major depressive disorder. Clin Drug Investig. 2011;31(Suppl 1):5–17. doi: 10.2165/1159616-S0-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol. 2011;164:1322–1334. doi: 10.1111/j.1476-5381.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi S, Ghaderi Pakdel F, Ashrafi Osalou M, Cankurt U. Acute systemic infusion of bupropion decrease formalin induced pain behavior in rat. Korean J Pain. 2014;27:118–124. doi: 10.3344/kjp.2014.27.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Imai S, Nakamura A, Ozeki A, Asato M, Rahmadi M, Sudo Y, Hojo M, Uezono Y, Devi LA, Kuzumaki N, Suzuki T. Possible involvement of prolonging spinal micro-opioid receptor desensitization in the development of antihyperalgesic tolerance to micro-opioids under a neuropathic pain-like state. Addiction biology. 2013;18:614–622. doi: 10.1111/j.1369-1600.2011.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Expression and treatment of pain-related behavioral depression. Lab animal. 2013;42:292–300. doi: 10.1038/laban.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Altarifi A. Mu, delta and kappa opioid agonist effects in novel assays of pain-depressed behavior. In: Ko H, Husbands SM, editors. Research and Development of Opioid Releated Ligands. Washington, DC: American Chemical Society; 2013. pp. 163–176. [Google Scholar]
- Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev. 2014;66:869–917. doi: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, O'Connell R, Morrissey E, Cheng K, Rice KC. Effects of peripherally restricted kappa opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther. 2012;340:501–509. doi: 10.1124/jpet.111.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL. Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain. 2015;156:1153–1160. doi: 10.1097/j.pain.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil A, Kayser V, Chen YL, Guilbaud G. Repeated low doses of morphine do not induce tolerance but increase the opioid antinociceptive effect in rats with a peripheral neuropathy. Brain Res. 1990;522:140–143. doi: 10.1016/0006-8993(90)91589-9. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Jerussi TP, Ren K, Sun H, Porreca F. Differential effects of spinal (R)-ketoprofen and (S)-ketoprofen against signs of neuropathic pain and tonic nociception: evidence for a novel mechanism of action of (R)-ketoprofen against tactile allodynia. Pain. 2000;87:193–199. doi: 10.1016/S0304-3959(00)00280-3. [DOI] [PubMed] [Google Scholar]
- Pedersen LH, Nielsen AN, Blackburn-Munro G. Anti-nociception is selectively enhanced by parallel inhibition of multiple subtypes of monoamine transporters in rat models of persistent and neuropathic pain. Psychopharmacology (Berl) 2005;182:551–561. doi: 10.1007/s00213-005-0120-6. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addiction biology. 2008;13:147–159. doi: 10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Cherry CL, Cox S, Marshall SJ, Rice AS. Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomised controlled trials. PLoS One. 2010;5:e14433. doi: 10.1371/journal.pone.0014433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft D, Gregg J, Ghia J, Harris L. Effects of intravenous tetrahydrocannabinol on experimental and surgical pain. Psychological correlates of the analgesic response. Clin Pharmacol Ther. 1977;21:26–33. doi: 10.1002/cpt197721126. [DOI] [PubMed] [Google Scholar]
- Rosenberg M, Carroll FI, Negus SS. Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. J Pain. 2013 doi: 10.1016/j.jpain.2012.11.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16:405–416. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenchuk MR, Sherman S, Davis B. Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology. 2001;57:1583–1588. doi: 10.1212/wnl.57.9.1583. [DOI] [PubMed] [Google Scholar]
- Sounvoravong S, Takahashi M, Nakashima MN, Nakashima K. Disability of development of tolerance to morphine and U-50,488H, a selective kappa-opioid receptor agonist, in neuropathic pain model mice. Journal of pharmacological sciences. 2004;94:305–312. doi: 10.1254/jphs.94.305. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Shiozaki Y, Masukawa Y, Misawa M, Nagase H. The role of mu- and kappa-opioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol. 1992;58:435–442. doi: 10.1254/jjp.58.435. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kawaguchi M, Shimada K, Konishi N, Furuya H, Nakashima T. Peri-sciatic administration of indomethacin early after nerve injury can attenuate the development of tactile allodynia in a rat model of L5 single spinal nerve injury. Neurosci Lett. 2004;356:37–40. doi: 10.1016/j.neulet.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain. 2011;152:990–1000. doi: 10.1016/j.pain.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ, Yezierski RP, Light AR. Long-lasting hyperalgesia and sympathetic dysregulation after formalin injection into the rat hind paw. Neuroscience. 2008;153:501–506. doi: 10.1016/j.neuroscience.2008.02.027. [DOI] [PubMed] [Google Scholar]
- Vo T, Rice AS, Dworkin RH. Non-steroidal anti-inflammatory drugs for neuropathic pain: how do we explain continued widespread use? Pain. 2009;143:169–171. doi: 10.1016/j.pain.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Watson CPN, Chipman ML, Monks RC. Antidepressant analgesics: a systematic review and comparative study. In: McMahon LR, Koltzenburg M, editors. Wall and Melzack's Textbook of Pain. 5th Ed. London: Elsevier; 2006. pp. 481–497. [Google Scholar]
- Way EL, Loh HH, Shen FH. Simultaneous quantitative assessment of morphine tolerance and physical dependence. J Pharmacol Exp Ther. 1969;167:1–8. [PubMed] [Google Scholar]
- Wood PB. Role of central dopamine in pain and analgesia. Expert review of neurotherapeutics. 2008;8:781–797. doi: 10.1586/14737175.8.5.781. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Wallace MS. Opioids, analgesia, and pain management. In: Brunton L, Chabner B, Knollman B, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 12th edition. New York: McGraw-Hill; 2011. pp. 481–526. [Google Scholar]
- Yu G, Zhang FQ, Tang SE, Lai MJ, Su RB, Gong ZH. Continuous infusion versus intermittent bolus dosing of morphine: a comparison of analgesia, tolerance, and subsequent voluntary morphine intake. J Psychiatr Res. 2014;59:161–166. doi: 10.1016/j.jpsychires.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Chen SR, Eisenach JC, Busija DW, Pan HL. Spinal cyclooxygenase-2 is involved in development of allodynia after nerve injury in rats. Neuroscience. 2000;97:743–748. doi: 10.1016/s0306-4522(00)00052-x. [DOI] [PubMed] [Google Scholar]