Abstract
This project examined the attitudes of sexual and gender minority youth (SGMY) toward guardian permission for a pre-exposure prophylaxis (PrEP) adherence trial and their preparedness to provide informed, rational and voluntary self-consent. Sixty sexually active SGMY (ages 14–17) participated in online survey and asynchronous focus group questions after watching a video describing a PrEP adherence study. Youth responses highlighted guardian permission as a significant barrier to research participation, especially for those not “out” to families. Youth demonstrated understanding of research benefits, medical side effects, confidentiality risks, and random assignment and felt comfortable asking questions and declining participation. Reasoning about participation indicated consideration of health risks and benefits, personal sexual behavior, ability to take pills everyday, logistics, and post-trial access to PrEP. Results demonstrate youth’s ability to self-consent to age- and population- appropriate procedures, and underscore the value of empirical studies for informing IRB protections of SGMY research participants.
Keywords: informed consent by minors, Pre-exposure prophylaxis, adolescent medicine, HIV prevention, ethics, research, sexual orientation, gender identity
There is an urgent need for effective prevention tools for sexual and gender minority youth (SGMY) at risk for the human immunodeficiency virus (HIV). However, HIV prevention research continues to suffer from disproportionately low representation of SGMY between fourteen and seventeen years of age. This inequity persists despite evidence that, starting in mid-adolescence, young men who have sex with men (YMSM), transgender women who have sex with men, and transgender men and young women who have sex with both men and women are at increasingly high risk for HIV (Center for Disease Control, 2012; Lindley & Walsemann, 2015; Santos et al., 2014). For example, based on research with YMSM over 18 years of age, in 2014 the Center for Disease Control recommended pre-exposure prophylaxis (PrEP) for this high HIV risk priority group (Center for Disease Control, 2014). To date however there is no evidence-based PrEP prevention program for YMSM under age eighteen, due in substantial part to the limited research knowledge base and in spite of clear evidence of need (Pettifor et al., 2015). This is troublesome since PrEP is likely to be prescribed off-label to YMSM and other sexual minority youth in this age group and extrapolations of data from PrEP studies involving young adults who have reached the age of majority may not be appropriate given this population’s unique challenges of uptake and adherence and structural barriers facing this population including stigma, discrimination, and family rejection (Fisher & Mustanski, 2014; Pettifor et al., 2015).
A major factor in the paucity of research essential to reducing HIV among SGMY is reluctance of many institutional review boards (IRBs) to apply federal regulations permitting adolescent self-consent when participants have attained their state-defined legal age for independent consent to HIV preventive interventions or when guardian permission is not a reasonable requirement to protect the subjects (Department of Health and Human Services, 2009; Fisher & Mustanski, 2014; Hill, 2012; Mustanski, 2011). Failure of IRBs to approve self-consent and waiver of guardian permission is a significant barrier to SGMY participation in HIV studies because youth fear being stigmatized, punished or in some cases victimized by their families if guardian permission results in disclosure of their sexual orientation or gender identity (D’Amico & Julien, 2012; DiClemente, Sales, & Borek, 2010; Mustanski, 2011). This, in turn, can result in smaller, unrepresentative samples (Jelsma, Burgess, & Henley, 2012) that skew findings in ways that may limit the generalizability of findings to SGMY whose parents are nonaccepting. This, in fact, was the unfortunate situation faced by investigators in the Adolescent Trials Network (ATN 113) when the IRBs at six out of the thirteen testing sites refused to permit youth self-consent in a study on the use of PrEP among 15–17 year old YMSM and transgender women (Gilbert et al., 2015).
Denying SGMY the right to self-consent is antithetical to current ethical discourse on youth’s right to participate in trials that will protect them from receiving developmentally untested, inappropriate and unsafe HIV biomedical preventive treatments (Fisher et al., 2013; Flicker & Guta, 2008; Santelli et al., 2003). The aforementioned PrEP study is one such example. Two inter-related factors are responsible for IRB resistance to approving self-consent for adolescent sexual health protocols. First is inconsistent legal interpretations of the extent to which self-consent to HIV research is covered by state laws permitting minors autonomous access to HIV testing and medical interventions (Fisher & Mustanski, 2014; Gilbert et al., 2015; Hill, 2012; Mustanski, 2011). Second is the unsupported assumption that minors do not have the ability to provide informed, rational and voluntary consent to sexual health research (Steinberg, 2013). Although the first of these problems can only be rectified by state legislatures, the second can be addressed by providing empirical data on SGMY’s ability to provide informed, voluntary and rationale self-consent to HIV prevention trials.
Study purpose and aims
Protecting the rights and welfare of minors requires applying empirical data on participant consent strengths and vulnerabilities to design procedures that reflect a “fit” between participant characteristics and the unique demands of the research context (Fisher, 2015; Masty & Fisher, 2008). Despite empirical data demonstrating that by age 14, most adolescents approach adult understanding of components of informed consent, there is a paucity of research on SGMY’s ability to self-consent to biomedical HIV prevention in general and PrEP studies specifically (Alexander et al., 2015; Corneli et al., 2015; Field & Behrman, 2004; Gilbert et al., 2015; Hosek & Zimet, 2010; Lally et al., 2014; Ott et al., 2013). Drawing on current approaches to increasing PrEP adherence in young adults (Harper & Riplinger, 2013), the purpose of this study was to inform IRB decision-making through an examination of SGMY attitudes toward and ability to self-consent to participation in a hypothetical PrEP adherence trial that compared the effectiveness of standard counseling versus mobile phone texting. Using online asynchronous focus groups, we examined: (1) the effect of requiring guardian permission on participation decisions; (2) attitudes towards and understanding of the study purpose, research risks and benefits, adherence requirements, and random assignment; (3) whether youth felt empowered to ask questions and to consent voluntarily; (4) and their ability to make a reasoned participation choice.
Method
Study population, recruitment, and dates
As part of a larger study on ethical issues in HIV research involving SGMY, we report on 60 14–17 year old self-identified sexually active SGMY. Inclusion criteria were sexual experience or romantic interest in male partners (higher HIV risk); negative HIV serostatus; reliable access to a phone and Internet; and U.S. residency. From January to April 2015, participants were recruited nationally through paid Facebook advertisements targeted to youth whose profiles indicated romantic interest in same gender persons and, to increase representation, had Facebook interests reflecting diverse racial/ethnic interests. Clicking on the advertisement directed youth to an online eligibility survey followed by telephone contact to confirm eligibility, provide participants with more study information, assess decisional capacity, and obtain verbal self-consent. The University IRBs approved all procedures including waiver of guardian permission for minimal risk research for which permission was not an appropriate protective mechanism. A DHHS Certificate of Confidentiality was obtained.
Data collection procedures
Following verbal consent, youth received via email a consent form and link to a demographic survey including sexual history, sexual orientation, gender identity, and whether youth were “out” to and accepted by family. Six focus groups were conducted from February to April 2015, using a secure website accessed with a pseudonym and unique password created by the participant. To ensure comfort and representation, four groups were stratified by age (14–15 years; 16–17 years) and gender identity, and two were specifically aimed at youth not “out” to guardians.
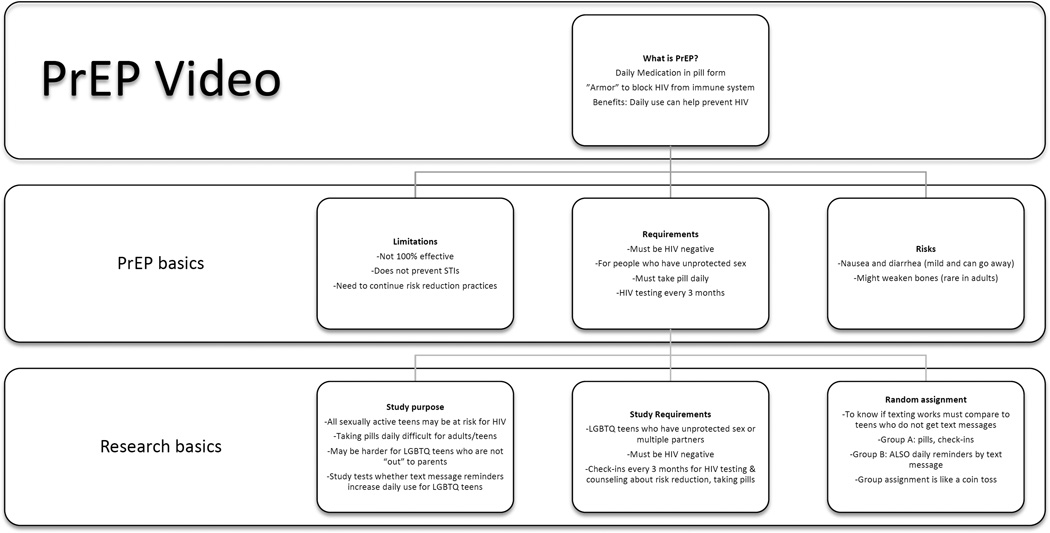
Each focus group was conducted over the course of 3 days. Concepts related to HIV and sexual health were introduced during the first day. The second and third days were focused on discussing PrEP, initiated by participants viewing a 6-minute video describing (at an 8th grade reading level) a 12-month PrEP pill randomized adherence trial comparing youth who received medication plus regular 3-month HIV testing and counseling, to those who also received daily text message reminders (Ragsdale & Rotheram-Borus, 2015). As illustrated in Figure 1, the video began with a description of how PrEP works to prevent HIV, limitations on effectiveness, and potential side effects. It then described the purpose of the study, inclusion criteria, study requirements and random assignment to standard and text messaging conditions. The content, age appropriateness, and population-sensitive language of the video were enhanced by review and feedback from an SGMY advisory group and from an ethicist/scientist expert panel.
Figure 1.
Content of 6-minute video describing a 12-month PrEP pill randomized adherence trial.
After viewing the PrEP video, participants responded to questions posted that day and the next. Participants were permitted to type in answers and replies to other members at their convenience (i.e., asynchronously). Moderators prompted participants who did not respond to a given question. Survey questions embedded within and following the focus group discussions addressed guardian permission, random assignment, privacy concerns, and PrEP medication adherence. Fully engaged participants received a $30 Visa gift card.
Coding and analysis
Participant’s transcripts were imported into a Web-based qualitative/mixed-methods analysis program. We identified initial codebook themes (Braun & Clarke, 2006) followed by selection of excerpts representing key topics. The lead coder then applied the codebook to all excerpts and a second team member independently coded 20% of the excerpts following training. A pooled Kappa of .71 indicated good inter-coder reliability (Landis & Koch, 1977). Thematic differences did not emerge among the 6 focus groups. A\ survey question indicating whether youth would agree to participate in a PrEP trial if guardian permission were required was analyzed using a nonparametric Chi-square test.
Results
As illustrated in Table 1, the majority of respondents self identified as non-Hispanic white and reported that their parents or guardians had at least 1 year of college. There were more females than males among cisgender respondents (meaning, youth who self-identify with the gender assigned to them at birth), and few respondents were transgender. Half the youth identified their sexual orientation as bisexual, a third as gay, and 10% lesbian; 30% reported they had sex without a condom and less than half had been tested for HIV/STI, although 40% had a “gut feeling” they were likely to be infected.
Table 1.
Key informant demographics and responses to polls (N = 60)
| Age | N | Percent | Gender Identity | N | Percent |
|---|---|---|---|---|---|
| 14 | 5 | 8.3% | Cisgender Male | 22 | 38% |
| 15 | 20 | 33.3% | Cisgender Female | 33 | 55% |
| 16 | 15 | 25.0% | Transgender | 5 | 8% |
| 17 | 20 | 33.3% | |||
| Sexual orientation | |||||
| Race/Ethnicity | Gay | 19 | 32% | ||
| White | 41 | 68.3% | Lesbian | 6 | 10% |
| Black/African American | 5 | 8.3% | Bisexual | 35 | 58% |
| Asian | 2 | 3.3% | Sex without condom | ||
| American Indian/Alaska Native |
1 | 1.7% | Anal sex | 17 | 28% |
| More than one race | 6 | 10.0% | Penile-vaginal sex | 18 | 30% |
| Other | 4 | 6.7% | |||
| Did not answer | 1 | 1.7% | Perceived risk for HIV | 24 | 40% |
| Hispanic/ Latino | 13 | 21.7% | HIV/STI test past year | 16 | 27% |
|
Parent education ≥ 1 yr college |
37 | 62% |
Number of male sexual partners |
Mean (SD) |
Range |
|
Number of male sexual partners M (SD) Range |
Cisgender Males |
Cisgender Females | Transgender youth |
||
| M = 4.81 SD = 8.51 Range 1 – 40 |
M = 4.76 SD = 5.94 Range 0 – 30 |
M = 3.20 SD = 2.28 Range 1 – 7 |
|||
| Willingness to participate if guardian permission required |
Out to at least 1 parent |
Not out to parents | Total | ||
| No | 5 (18%) | 21 (68%) | 26 (44%) | ||
| Maybe | 13 (46%) | 7 (23%) | 20 (34%) | ||
| Yes | 10 (36%) | 3 (10%) | 13 (22%) | ||
| Total | 28 | 31 | 59 | ||
|
Assignment to messaging or control group |
Random Assignment |
“Based on a random procedure” or “like a coin toss”) |
36 (88%) | ||
| Preventive Misconception |
“they will place me in the group that is best for me” or “let me choose the group” |
5 (12%) | |||
X2(2) = 15.30, p < .01
Is guardian permission a barrier to participation?
Most youth, especially those who were not out to family, would either not participate or were unsure if they would participate if guardian permission were required (see Table 1). As illustrated in Table 2, guardian permission presented a major barrier to participation for the many youth who feared it would “out them” to parents described as “intolerant” of SGM individuals for religious reasons or social prejudice and lead to punishment or removal from the home. Some youth who were “out” and described their family as accepting were comfortable with guardian permission; other “out” youth described their parents as unsupportive and either refusing to discuss the youth’s sexual orientation/gender identity or to describe it as a “phase.”
Table 2.
Exemplar quotes: Attitudes toward guardian permission
Guardian permission requirement – parents as sources of support
|
Guardian permission requirement – concerns about parental resistance or bias
|
Guardian permission requirement – fear of being “outed” or punished
|
Do youth understand research risks and benefits?
As illustrated in Table 3, youth’s discussion of research risks and benefits accurately reflected the information provided in the video, including an appreciation of the potential for directly benefitting from PrEP’s protection against HIV and increased knowledge about their own risk for HIV and safer sex practices. Many commented on the potential benefits of participation to the sexual health of other SGMY. Their assessment of research risks reflected an informed reflection on PrEP side effects and confidentiality risks associated with parents finding out about their daily pill intake. Interestingly, in response to a survey item, the majority of youth (84%) indicated they were only somewhat or not at all concerned with text message privacy, and in focus groups, several noted that they already implemented strategies to protect the confidentiality of their texting.
Table 3.
Exemplar quotes: Understanding key elements of informed consent
Understanding research benefits
|
Understanding research risks:
|
Understanding adherence and 3-month check-up requirements
|
Understanding random assignment
|
Do youth understand adherence requirements?
When asked about requirements for daily adherence, youth reflected on their own successful experiences taking other daily medications or their self-described forgetfulness (see Table 3). The majority of youth understood the importance of daily PrEP intake: In response to a survey question, only 18% expressed high confidence that PrEP would be effective if they did not take it every day. When asked whether they would return for 3-month study checkups if they did not take the pill regularly, most youth wrote they would be embarrassed or feel they were disappointing the researcher if they had been non-adherent. At the same time, these youth said they would attend the check-ups either because they understood that these lapses would be valuable information for the purpose of the study or because they believed the research counselor could help get them back on track. Although no specific questions were posed, focus group comments gave no indication that taking PrEP would lead to behavioral disinhibition. In fact, some youth indicated concern that PrEP did not protect against other sexually transmitted infections.
Do youth understand random assignment?
The majority understood random assignment. For example 88% endorsed the multiple-choice item “They will place me in a group based on a random procedure like a coin toss” (see Table 1). In response to the focus group question “How would you feel about being randomly assigned to one of the 2 groups?” those with a favorable attitude gave rationales indicating an appreciation for the scientific value of random assignment as the best way to ensure youth were assigned to groups fairly. Some youth who expressed negative attitudes preferred to be assigned to a group of their choice or did not want to feel “like guinea pigs” (see Table 3).
Do youth feel empowered to ask questions and dissent to research participation?
Most respondents indicated they would be comfortable asking questions, noting it was their responsibility to make decisions that would affect their health. All but two said they would feel comfortable dissenting and several described the purpose of the consent process as an opportunity to refuse participation (see Table 4). When asked how the consent process could be enhanced, youth focused on: (1) relational factors, e.g., a researcher “who would listen;” (2) confidentiality protections, e.g., “Will my parents find out?” (3) health concerns, e.g., “How common are side effects”; and (4) endorsed the value of having a peer advocate to provide “an unbiased opinion”.
Table 4.
Exemplar quotes: The voluntary nature of self-consent and reasons for and against
Voluntary self-consent
|
Reasoned participation decision
|
Obligation for post-trial PrEP access or referral
|
Can youth make a reasoned participation choice?
Beyond understanding the purpose and nature of research and voluntary participation, self-consent requires the ability to take these factors into account and arrive at a reasonable participation choice (Appelbaum & Roth, 1982). Youth’s explanations for their participation decisions indicated mature reflections on the personal risks and benefits of participation. Their rationales included their current health status, whether they had engaged in or planned to engage in high-risk sexual activity, the ability to take the pill every day, and the logistics of traveling to the 3-month checkups (see Table 4). When asked, all youth thought post-trial access to PrEP was important, especially if parents were unsupportive. Although some thought the investigator should be responsible for providing post-trial medication, most recognized the difficulty of such a requirement and instead stressed the investigator’s responsibility to provide information and referrals for continued access.
Discussion
A major goal of the National HIV/AIDS Strategy for the United States (White House Office of National Aids Policy, 2015) is that by 2020, new HIV infections will be rare and access to medical care unfettered and free from stigma and discrimination. Without evidence-based HIV preventive strategies for SGMY, this national vision will not be fulfilled (Holtgrave, 2015). The purpose of this study was to provide empirical data on SGMY self-consent that can assist investigators and IRBs in strategies to increase their research participation in ways that best protect their rights and welfare. Our method was grounded in the premise that integrating SGMY perspectives into the fabric of ethical planning is critical to enhancing the responsible conduct of research and for reducing IRB barriers to HIV prevention research (Fisher, 1999, 2004, 2015). SGMY responses provide a preliminary empirical basis for approving self-consent for PrEP prevention trials, and the method we used provides groundwork for gathering similar data for other biomedical and behavioral HIV prevention approaches as they continue to develop.
Guardian Permission
The first question we examined was whether failure to waive guardian permission for research participation is a barrier to acquiring representative samples of SGMY in PrEP trials. Whether or not youth under 18 years of age are considered adults for the purposes of HIV testing and treatment, IRBs are permitted to approve a waiver of guardian permission if the waiver is “not a reasonable requirement to protect the subjects (for example, neglected or abused children)” (Department of Health and Human Services, 2009). Participant responses supported prior studies demonstrating that most SGMY will be reluctant to participate in studies that require guardian permission because they fear being “outed,” stigmatized, or punished by their families (D’Amico & Julien, 2012; DiClemente et al., 2010; Mustanski, 2011). Our data also reveal that simply being “out” to parents cannot be applied as a criterion for assuming guardian permission is acceptable, since many respondents who were “out” described parents as unsupportive of their SGM identity.
These findings support recommendations from scientific organizations for waiver of guardian permission for research involving SGMY based on the credible probability that serious physical, social, or psychological harm may result if guardians are informed about the reason for the study (Field & Behrman, 2004; Fisher et al., 2013; Santelli et al., 2003). At the same time, youth in our study whose parents were supportive of their SGM identity were more likely to see parental involvement as a protective factor. For these youth, family connectedness is important (Garofalo, Mustanski, & Donenberg, 2008). Thus while we strongly support the ethical urgency of self-consent for SGMY HIV prevention research, we recommend consent procedures offering youth an opportunity to consult with their parents about participation if they desire, including tools to support teen consultation with their parents.
Youth’s ability to give informed and voluntary self-consent
Youth’s responses provided support for the premise that SGMY are prepared to provide informed and voluntary self-consent to studies involving HIV prevention when information is provided in an age-appropriate and youth-friendly format (Calderon et al., 2013; Merchant, Clark, Santelices, Liu, & Cortes, 2015). Throughout the focus groups, youth indicated an understanding of the health-related benefits, side effects, and limitations of PrEP for preventing HIV and STIs. Although we did not directly address this question, consistent with other studies (Grov, Whitfield, Rendina, Ventuneac, & Parsons, 2015; Schenk et al., 2014), there was no indication that youth believed taking PrEP would lead to an increase in unsafe sexual behaviors (i.e., risk disinhibition; Eaton & Kalichman, 2007). In fact, many described how participation would provide them information to help them better protect their sexual health (Dellar et al., 2014; Protogerou & Johnson, 2014). While some were concerned about privacy risks associated with text messaging, many described precautions they already instituted to protect online privacy. Many suggested informed consent would be enhanced through discussion of confidentiality protections and risks related to parents finding out they were participating in the study.
Youth responses also indicated an understanding that the purpose of the study was to address concerns regarding the ability of SGMY to adhere to a PrEP daily regimen. Many mentioned how their participation could help improve PrEP practices for other SGMY. In addition, their attitudes toward random assignment indicated an appreciation of the probability of being assigned to either the standard HIV testing/counseling condition or the mobile texting addon. Many youth thought random assignment was a fair way of ensuring no one received preferential treatment. Interestingly, several respondents preferred not to be in the group getting the daily text reminders, considering it either a privacy concern or a burden. The consent competencies reported in this study are consistent with those recently reported for youth’s understanding of HIV vaccine trials (Alexander et al., 2015; Ott et al., 2013). However, our sample appeared to understand random assignment at somewhat higher levels. One reason may be that randomization to the two “treatment” groups described in our PrEP adherence study is not as susceptible to preventive misconception as assignment to either a vaccine or placebo condition.
Most respondents were confident that despite initial shyness or embarrassment, they would ask questions during the informed consent process and would be willing to dissent if they believed participation was too burdensome or a risk to their health. They did emphasize that research team members should be patient and nonjudgmental, encourage questions, give time to consider options, and engender trust (Kadivar et al., 2014) and responded positively to the idea of a peer advocate who could present their options in an unbiased manner.
Youth’s ability to make a reasoned participation choice
Respondents’ explanations for how they would make a participation choice indicated mature and reasoned reflections on study risks and benefits. Some youth who had prior medical problems such as immune deficiencies or “weak bones” considered these conditions as important reasons not to participate. Other youth felt that the low risk of bone density reduction and regular 3-month check-ups would be adequate protection against these risks. Youth also referred to their experience taking birth control pills or other daily medication as reasons for or against participation. This type of reasoning reflects an appreciation of past medical history as a key element for making a rational participation choice. Referring back to the video, a number of youth also considered the extent to which their current sexual behaviors placed them in an at-risk category meeting PrEP study requirements. For example, some indicated they would not participate if they were abstinent or in a sexually exclusive relationship with a single partner.
Other reasons for choosing not to participate included concerns about confidentiality, the logistics of taking pills daily, and transportation to quarterly check-ups without parental knowledge. Youth’s concerns regarding cost, ability to take medications daily, and potential for long term side effects expressed in this study are consistent with those of MSM 18–73 years of age (King et al., 2014), providing additional support for the conclusion that the reasoned research participation of SGM minors are similar to those of adults. Finally, youth were aware of the limitations on investigators to provide post-trial access to PrEP and suggested that informed consent should clearly state whether such referrals would be provided.
Best Practices: Protecting the rights and welfare of sexual minority youth
Current interpretations of federal regulations that refuse to permit youth self-consent deprive SGMY their right to evidence-based interventions essential to their health and wellbeing (Fisher & Mustanski, 2014; Flicker & Guta, 2008; Gilbert et al., 2015; Mustanski, 2011). CDC recommendations for providing PrEP to HIV-risk populations underscore the urgent need for age- and population-targeted research to avoid the use of treatments tested in adult populations that may be ill-suited for SGMY (Center for Disease Control, 2014; Rudy et al., 2010). Instead of classifying SGMY as a vulnerable population based solely on age or social characteristics viewed as disadvantageous (DiClemente et al., 2010; Fisher, 1999, 2015; Masty & Fisher, 2008; Ott, 2014; Steinberg, 2013), we hope the data from this study encourages IRBs to approve self-consent procedures that draw on empirical data to build on youth consent strengths, to ensure SGMY have opportunities to participate in research critical to their health.
Limitations and Recommendations for a Research Agenda
The limitations of this study suggest fruitful avenues for future research. First, although geographically and economically diverse, participants represented youth who were on Facebook and who were interested in finding out more about an SGMY-related link, were willing to be contacted by phone, had continued access to the Internet, and were comfortable responding in narrative form. Additional in-person research is needed to determine the extent to which our participants’ views reflect those of youth who may not feel connected to an online SGM community, who may not have access to the Internet, who may hold telephone and Internet privacy concerns (Curtis, 2014), or may not feel comfortable expressing their views in written form.
Second, the majority of respondents identified as non-Hispanic white and few identified as transgender; ethnic minority and transgender youth deserve additional attention to illuminate their distinct research attitudes and participation needs. Although these youth may still face barriers to sexual health care rooted in social prejudices regarding sexual orientation, such barriers are compounded by institutional and structural biases facing SGM racial/ethnic minority youth and by unique challenges related to transitioning, gender discrimination, and transphobia facing transgender youth (Ceballos et al., 2014; Pettifor et al., 2015; Quinn et al., 2012; Traube, Kerkorian, Cederbaum, Bhupali, & McKay, 2013). Our team has begun to explore this issue in focus groups involving racial/ethnic minority transgender youth. One preliminary finding is that recruitment materials designed to attract a wide range of sexual minority youth may not be appropriate for transgender or non-binary youth who do not always feel part of a community defined in terms of sexual orientation rather than gender identity. In addition, once a study is begun, commonly used questions regarding gender assigned at birth, gender identity, and sexual orientation may be confusing or discomforting to transgender youth, resulting in study withdrawal.
Third, our study examines youth’s attitudes toward a hypothetical PrEP adherence study and future research is needed to determine whether their responses fully reflect actual barriers and willingness to participation (Buchbinder S. P., Metch B., & Holte S. E., 2004). Finally, as with all focus group research, the need to keep group membership small to facilitate discussion, the unique community history of participants, and the interactive nature of focus group designs means that analyses do not lend themselves to generalization beyond the particular discussants; rather this study provides an analysis of youth perspectives that can inform current ways of thinking about SGMY self-consent and point to new directions of scientific inquiry (Fisher & Wallace, 2000).
Educational Implications
HIV prevention research continues to suffer from disproportionately low representation of SGMY younger 18 years of age, despite evidence of risk. Many investigators have formally or informally expressed reluctance to conduct research with SGMY minors because of anticipated or actual experiences with difficulties obtaining IRB approval (Department of Health and Human Services, 2009; Fisher & Mustanski, 2014; Hill, 2012; Mustanski, 2011). IRB reluctance to approve sexual health research involving adolescents in particular and SGMY specifically is due in large part to the lack of empirical data that can inform IRB estimations of the magnitude and probability of potential harms and benefits of youth involvement in HIV prevention research, application of regulations permitting a waiver of guardian permission, and the ability of underage SGMY to independently consent to research participation. To reduce barriers to SGMY participation in research necessary to ensure their health and wellbeing requires equipping investigators with the knowledge and skills to conduct empirical research on these critical research ethics questions. To date, although there is an increasing number of U.S. and international research ethics programs (Glass, 2013; Matar, Garner, Millum, Sina, & Silverman, 2014), most doctoral and postdoctoral programs in public health and the social and medical sciences do not provide specific training in the investigative skills needed to understand the attitudes and perspectives of marginalized youth toward the goals and methods of research practices that may not be readily discerned simply through professional logic or inference (Fisher, 2014, 2015; Fisher & Yuko, in press). We hope this research encourages health science training programs to include instruction in generating systematic and generalizable knowledge on key ethical and regulatory issues that can assist investigators and IRBs in constructing age-appropriate human subjects protections for HIV prevention research. In doing so, we will help advance the rights of sexual and gender minority youth to share in the fair and equitable distribution of research risks and potential benefits and create a just path toward the development and implementation of effective prevention policies to reduce and ameliorate HIV/AIDS acquisition and transmission.
Acknowledgments
We thank Alan Ashbeck for assistance with participant recruitment, retention and feedback on the questionnaire and Zenaida Rivera for her work on the PrEP video and with moderating the focus groups. We would also like to thank our Scientific Advisory Council and the IMPACT Youth Advisory Council for their feedback on all aspects of the study procedures and our participants, who generously gave us their time and from whom we learned so much.
Funding
During the preparation of this article the authors were supported on a grant from the National Institute of Minority Health Disparities of the National Institutes of Health under award number R01MD009561 (PIs C. B. Fisher & B. Mustanski). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biographies
Celia B. Fisher is the director of the Fordham University HIV and Drug Abuse Prevention Research Ethics Training Institute, the Marie Ward Doty Endowed University Chair, founding director of the Fordham University Center for Ethics Education, and Professor of Psychology. Her current projects include sexual health research ethics, health disparities, and research ethics training. She is a Principal Investigator on this research project and is responsible for the content and writing of the article, including conception and design, analysis, and interpretation of data.
Miriam R. Arbeit is a Postdoctoral Research Fellow at the Center for Ethics Education at Fordham University. Her research is on adolescent sexuality development and sexual health, with particular attention to issues of gender justice and the experiences of sexual and gender minority youth. She served as the Program Administrator for this research project and conducted the data analysis for the present study.
Melissa S. Dumont is a doctoral student in the Psychology Department at Fordham University. Her research interests include the health and development of sexual and gender minority youth. She served as a graduate assistant at the Center for Ethics Education at Fordham University and assisted with the data analysis for the present study.
Kathryn Macapagal is a Research Assistant Professor of Medical Social Sciences, and Psychiatry and Behavioral Sciences at Northwestern University. Her primary interests intersect sexual health, HIV, and the health of sexual and gender minority individuals. She is an Investigator on this research project and conducted the data collection for the present study.
Brian Mustanski is an Associate Professor of Medical Social Sciences, Psychiatry and Behavioral Sciences, and Psychology at Northwestern University; Director of the Northwestern Institute for Sexual and Gender Minority Health and Wellbeing; and Co-Director of the Third Coast Center for AIDS Research. His research focuses on the mental and behavioral health of sexual and gender minority individuals, with a particular focus on youth. He is a Principal Investigator on this research project and supervised the present study in collaboration with Celia B. Fisher.
Footnotes
Conflicts of interest
The authors report no conflicts of interest
References
- Alexander AB, Ott MA, Lally MA, Sniecinski K, Baker A, Zimet GD. Adolescent decision making about participation in a hypothetical HIV vaccine trial. Vaccine. 2015;33:1331–1337. doi: 10.1016/j.vaccine.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum PS, Roth LH. Competency to consent to research: A psychiatric overview. Archives of General Psychiatry. 1982;39(8):951–958. doi: 10.1001/archpsyc.1982.04290080061009. [DOI] [PubMed] [Google Scholar]
- Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;3:77–102. [Google Scholar]
- Buchbinder SP, Metch B, Holte SE. Determinants of enrollment in a preventive HIV vaccine trial: Hypothetical versus actual willingness and barriers to participation. Journal of Acquired Immune Deficiency Syndromes. 2004;36:604–612. doi: 10.1097/00126334-200405010-00009. [DOI] [PubMed] [Google Scholar]
- Calderon Y, Cowan E, Leu CS, Brusalis C, Rhee JY, Nickerson J, Bauman LJ. A human immunodeficiency virus posttest video to increase condom use among adolescent emergency department patients. Journal of Adolescent Health. 2013;53(1):79–84. doi: 10.1016/j.jadohealth.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos RM, Knerr S, Scott MA, Hohl SD, Malen RC, Vilchis H, Thompson B. Latino beliefs about biomedical research participation: A qualitative study on the U.S.-Mexico border. Journal of Empirical Research on Human Research Ethics. 2014;9(4):10–21. doi: 10.1177/1556264614544454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control. HIV Surveillance in Adolescents and Young Adults. 2012 Retrieved from http://www.cdc.gov/hiv/pdf/statistics_surveillance_Adolescents.pdf.
- Center for Disease Control. Preexposure prophylaxis for the prevention of HIV infection in the United States – 2014. 2014 Retrieved from http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf.
- Corneli AL, McKenna K, Perry B, Ahmed K, Agot K, Malamatsho F, VanDamme L. The science of being a study participant: FEM-PrEP participants’ explanations for overreporting adherence to the study pills and for the whereabouts of unused pills. Journal of Acquired Immune Deficiency Syndromes. 2015;68(5):578–584. doi: 10.1097/QAI.0000000000000525. [DOI] [PubMed] [Google Scholar]
- Curtis BL. Social networking and online recruiting for HIV research: Ethical challenges. Journal of Empirical Research on Human Research Ethics. 2014;9(1):58–70. doi: 10.1525/jer.2014.9.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico E, Julien D. Disclosure of sexual orientation and gay, lesbian, and bisexual youths’ adjustment: Associations with past and current parental acceptance and rejection. Journal of GLBT Family Studies. 2012;8(3):215–242. [Google Scholar]
- Dellar RC, Abdool Karim QA, Mansoor LE, Grobler A, Humphries H, Werner L, Abdool Karim SS. The preventive misconception: Experiences from CAPRISA 004. AIDS and Behavior. 2014;18:1746–1752. doi: 10.1007/s10461-014-0771-6. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services. Title 45 Public Welfare, Part 46, Code of Federal Regulations, Protection of Human Subjects. [Press release] 2009 Retrieved from http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html. [PubMed]
- DiClemente RJ, Sales JM, Borek N. Barriers to adolescents’ participation in HIV biomedical prevention research. Journal of Acquired Immune Deficiency Syndromes. 2010;54(Suppl 1):S12–S17. doi: 10.1097/QAI.0b013e3181e1e2c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton LA, Kalichman SC. Risk compensation in HIV prevention: Implications for vaccines, microbicides, and other biomedical HIV prevention technologies. Current HIV/AIDS Reports. 2007;4(4):165–172. doi: 10.1007/s11904-007-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, Behrman RE. Ethical Conduct of Clinical Research Involving Children. Washington DC: Institute of Medicine; 2004. [PubMed] [Google Scholar]
- Fisher CB. Reports on Research Involving Persons with Mental Disorders that May Affect Decision-Making Capacity. Vol. 2. Commissioned Papers by the National Bioethics Advisory Committee; 1999. Relational ethics and research with vulnerable populations; pp. 29–49. August 21, 2015, from http://www.bioethics.gov/reports/past_commissions/nbac_mental2.pdf. [Google Scholar]
- Fisher CB. Ethics in drug abuse and related HIV risk research. Applied Developmental Science. 2004;8(2):90–102. [Google Scholar]
- Fisher CB. HIV prevention research ethics: An introduction to the special issue. Journal of Empirical Research on Human Research Ethics. 2014;9(1):1–5. doi: 10.1525/jer.2014.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CB. Enhancing the responsible conduct of sexual health prevention research across global and local contexts: Training for evidence-based research ethics. Ethics and Behavior. 2015;25(2):1–10. doi: 10.1080/10508422.2014.948956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CB, Brunquell DJ, Hughes DL, Liben LS, Maholmes V, Plattner S, Sussman EJ. Preserving and enhancing the responsible conduct of research involving children and youth: A response to proposed changes in federal regulations. Social Policy Report. 2013;27(1):3–15. [Google Scholar]
- Fisher CB, Mustanski B. Reducing the Health Disparities and Enhancing the Responsible Conduct of Research Involving LGBT Youth. The Hastings Center Report. 2014;44(Suppl 4):S28–S31. doi: 10.1002/hast.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CB, Wallace SA. Through the community looking glass: reevaluating the ethical and policy implications of research on adolescent risk and sociopathology. Ethics and Behavior. 2000;10(2):99–118. doi: 10.1207/S15327019EB1002_01. [DOI] [PubMed] [Google Scholar]
- Fisher CB, Yuko E. The HIV and Drug Abuse Prevention Research Ethics Training Institute: Training early-career scientists to conduct research on research ethics. Journal of Empirical Research on Human Research Ethics. doi: 10.1177/1556264615614937. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicker S, Guta A. Ethical approaches to adolescent participation in sexual health research. Journal of Adolescent Health. 2008;42(1):3–10. doi: 10.1016/j.jadohealth.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Garofalo R, Mustanski B, Donenberg G. Parents know and parents matter; is it time to develop family-based HIV prevention programs for young men who have sex with men? Journal of Adolescent Health. 2008;43(2):201–204. doi: 10.1016/j.jadohealth.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert AL, Knopf AS, Fortenberry JD, Hosek SG, Kapogiannis BG, Zimet GD. Adolescent self-consent for biomedical human immunodeficiency virus prevention research. Journal of Adolescent Health. 2015;57(1):113–119. doi: 10.1016/j.jadohealth.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R. International research ethics education. Journal of Empirical Research on Human Research Ethics. 2013;8(5):1–2. doi: 10.1525/jer.2013.8.5.1. [DOI] [PubMed] [Google Scholar]
- Grov C, Whitfield TH, Rendina HJ, Ventuneac A, Parsons JT. Willingness to take PrEP and potential for risk compensation among highly sexually active gay and bisexual men. AIDS and Behavior. 2015;19(12):2234–2244. doi: 10.1007/s10461-015-1030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper GW, Riplinger AJ. HIV prevention interventions for adolescents and young adults: What about the needs of gay and bisexual males? AIDS and Behavior. 2013;17(3) doi: 10.1007/s10461-012-0178-1. [DOI] [PubMed] [Google Scholar]
- Hill J. Medical decision making by and on behalf of adolescents: Reconsidering first principles. Health Care Law and Policy. 2012;15(1):37–73. [Google Scholar]
- Holtgrave DR. Achieving and advancing the goals of the National HIV/AIDS Strategy for the United States. AIDS and Behavior. 2015;19(2):211–213. doi: 10.1007/s10461-014-0903-z. [DOI] [PubMed] [Google Scholar]
- Hosek SG, Zimet GD. Behavioral considerations for engaging youth in HIV clinical research. Journal of Acquired Immune Deficiency Syndromes. 2010;54(1):S25–S30. doi: 10.1097/QAI.0b013e3181e15c22. [DOI] [PubMed] [Google Scholar]
- Jelsma J, Burgess T, Henley L. Does the requirement of getting active consent from parents in school-based research result in a biased sample? An empirical study. Journal of Empirical Research on Human Research Ethics. 2012;7(5):56–62. doi: 10.1525/jer.2012.7.5.56. [DOI] [PubMed] [Google Scholar]
- Kadivar H, Thompson L, Wegman M, Chisholm T, Khan M, Eddleton K, Shenkman E. Adolescent views on comprehensive health risk assessment and counseling: Assessing gender differences. Journal of Adolescent Health. 2014;55(1):24–32. doi: 10.1016/j.jadohealth.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HL, Keller SB, Giancola MA, Rodriguez DA, Chau JJ, Young JA, Smith DM. Cost and threshold analysis of positive charge, a multi-site linkage to HIV care program in the United States. AIDS and Behavior. 2014;19(10) doi: 10.1007/s10461-015-1124-9. [DOI] [PubMed] [Google Scholar]
- Lally M, Goldsworthy R, Sarr M, Kahn J, Brown L, Peralta L, Zimet G. Evaluation of an intervention among adolescents to reduce preventive misconception in HIV vaccine clinical trials. Journal of Adolescent Health. 2014;55(2):254–259. doi: 10.1016/j.jadohealth.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- Lindley L, Walsemann K. Sexual orientation and risk of pregnancy among New York City high-school students. American Journal of Public Health. 2015;105(7) doi: 10.2105/AJPH.2015.302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masty J, Fisher CB. A goodness-of-fit approach to informed consent for pediatric intervention research. Ethics and Behavior. 2008;18(2–3):139–160. [Google Scholar]
- Matar A, Garner S, Millum J, Sina B, Silverman H. Curricular aspects of the Fogarty bioethics international training programs. Journal of Empirical Research on Human Research Ethics. 2014;9(2):12–23. doi: 10.1525/jer.2014.9.2.12. [DOI] [PubMed] [Google Scholar]
- Merchant RC, Clark MA, Santelices CA, Liu T, Cortes DE. Efficacy of an HIV/AIDS and HIV testing video for Spanish-speaking Latinos in healthcare and non-healthcare settings. AIDS and Behavior. 2015;19(3):523–535. doi: 10.1007/s10461-014-0889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustanski B. Ethical and regulatory issues with conducting sexuality research with LGBT adolescents: A call to action for a scientifically informed approach. Archives of Sexual Behavior. 2011;40(4):673–686. doi: 10.1007/s10508-011-9745-1. [DOI] [PubMed] [Google Scholar]
- Ott MA. Vulnerability in HIV prevention research with adolescents, reconsidered. Journal of Adolescent Health. 2014;54(6):629–630. doi: 10.1016/j.jadohealth.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Ott MA, Alexander AB, Lally M, Steever JB, Zimet GD HIV/AIDS, t. A. M. T. N. f. Preventive misconception and adolescents’ knowledge about HIV vaccine trials. Journal of Medical Ethics. 2013;39(12):765–771. doi: 10.1136/medethics-2012-100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettifor A, Nguyen NL, Celum C, Cowan FM, Go V, Hightow-Weidman L. Tailored combination prevention packages and PrEP for young key populations. Journal of the International AIDS Society. 2015;18(2 Suppl 1):19434. doi: 10.7448/IAS.18.2.19434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protogerou C, Johnson BT. Factors underlying the success of behavioral HIV-prevention interventions for adolescents: A meta-review. AIDS and Behavior. 2014;18(10):1847–1863. doi: 10.1007/s10461-014-0807-y. [DOI] [PubMed] [Google Scholar]
- Quinn SC, Garza MA, Butler J, Fryer CS, Casper ET, Thomas SB, Kim KH. Improving informed consent with minority participants: Results from researcher and community surveys. Journal of Empirical Research on Human Research Ethics. 2012;7(5):44–55. doi: 10.1525/jer.2012.7.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale A, Rotheram-Borus MJ. Re-shaping HIV interventions with technology. AIDS and Behavior. 2015;19(Suppl 2):77–80. doi: 10.1007/s10461-015-1086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy BJ, Kapogiannis BG, Lally MA, Gray GE, Bekker LG, Krogstad P, McGowan I. Youth-specific considerations in the development of preexposure prophylaxis, microbicide, and vaccine research trials. Journal of Acquired Immune Deficiency Syndromes. 2010;54(Suppl 1):S31–S42. doi: 10.1097/QAI.0b013e3181e3a922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelli JS, Smith Rogers A, Rosenfeld WD, DuRant RH, Dubler N, Morreale M M., S. f. A. Guidelines for adolescent health research. A position paper of the Society for Adolescent Medicine. Journal of Adolescent Health. 2003;33(5):396–409. [PubMed] [Google Scholar]
- Santos GM, Wilson EC, Rapues J, Macias O, Packer T, Raymond HF. HIV treatment cascade among transgender women in a San Francisco respondent driven sampling study. Sexually Transmitted Infections. 2014;90(5):430–433. doi: 10.1136/sextrans-2013-051342. [DOI] [PubMed] [Google Scholar]
- Schenk KD, Friedland BA, Chau M, Stoner M, Plagianos MG, Skoler-Karpoff S, Ngcozela N. Enrollment of adolescents aged 16–17 years old in microbicide trials: an evidence-based approach. Journal of Adolescent Health. 2014;54(6):654–662. doi: 10.1016/j.jadohealth.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Does recent research on adolescent brain development inform the mature minor doctrine? Journal of Medicine and Philosophy. 2013;38(3):256–267. doi: 10.1093/jmp/jht017. [DOI] [PubMed] [Google Scholar]
- Traube DE, Kerkorian D, Cederbaum JA, Bhupali C, McKay MM. African American children’s perceptions of HIV-focused community-based participatory research. Journal of Empirical Research on Human Research Ethics. 2013;8(1):79–90. doi: 10.1525/jer.2013.8.1.79. [DOI] [PubMed] [Google Scholar]
- White House Office of National Aids Policy. National HIV/AIDS Strategy for the United States: Updated to 2020. 2015 Retrieved from https://www.whitehouse.gov/administration/eop/onap/nhas/