Abstract
An increasing number of individuals are being recruited to whole genome sequencing (WGS) research. When asked hypothetically, the majority of the public express willingness to participate in this type of research, yet little is known about how many individuals will actually consent to research participation or what they perceive the risks to be. The MedSeq Project is a clinical trial exploring WGS in clinical care. We documented primary reason(s) for declining participation, and reviewed audio-recorded informed consent sessions to identify participants’ concerns. Of 511 individuals recruited, 173 (34%) actively declined, 205 (40%) enrolled, and the remaining 133 (26%) were ineligible or unresponsive. Although the majority of active decliners cited logistical barriers, 40% cited risks related to the ethical, legal, or social implications (ELSI) of WGS research. Participants similarly discussed ELSI-related concerns, but felt the potential benefits of participation outweighed the risks. Findings provide insight into the perspectives of potential WGS research participants and identify potential barriers to participation.
Keywords: whole genome sequencing, informed consent, participant and decliner perspectives, risks of WGS research
In 2016, the National Institutes of Health (NIH) plans to begin recruitment of more than 1 million Americans to the Precision Medicine Initiative (PMI) Cohort Study, “the world’s largest study of how genes influence disease risk and drug response” (Kaiser, 2015). Although the initial plan is to genotype individuals due to budget constraints, eventually the goal is to perform whole genome sequencing (WGS). In preparation for this study, the Foundation for the NIH conducted a survey in which 54% of 2,601 US adults reported that they would participate in a PMI-like study (http://www.nih.gov/precisionmedicine/2015-07-01-workshop-summary.pdf, 2015). Whether participants would have access to their health information and what privacy protections were afforded were cited as the two most important factors influencing whether individuals would participate (http://www.nih.gov/precisionmedicine/2015-07-01-workshop-summary.pdf, 2015). Although there is enthusiasm for a PMI-like study, uncertainty remains about how many individuals will actually consent to research participation or what they perceive the risks to be.
WGS and other next-generation sequencing technologies present significant challenges for obtaining research informed consent because the information is complex and changing rapidly, and public understanding of genetics is poor (Grady, 2015; http://www.bioethics.gov, 2012). Unlike most medical tests that provide limited amounts of information about an individual at specific points in time, genomic data are often stored and used indefinitely, may provide a vast amount of information on an individual (and with health implications for family members), and may provide inaccurate information, be reinterpreted, or change in clinical relevance over time. Adding to the complexity, research studies are increasingly returning genomic results to individuals, and in some cases, the results are included in the participant’s medical record. Current recommendations provided by the NIH National Human Genome Research Institute (NHGRI) include special considerations for informed consent in genomics research and suggest specific language to describe the physical, psychosocial, and security risks to participants and their relatives (http://www.genome.gov/27026588). Yet, little is known about how potential participants perceive these risks and what impact they have on decisions to participate in WGS research.
We present findings from the recruitment and enrollment phases of the MedSeq Project, one of nine NIH Clinical Sequencing Exploratory Research (CSER) projects specifically charged to empirically study the ethical, legal and social implications (ELSI) inherent with genome sequencing technologies, and the first randomized clinical trial of WGS. We describe the perspectives of individuals being recruited to the MedSeq Project regarding the risks of participation and primary reasons for declining participation. These data provide unique insights into the decision making process for potential WGS research participants and identify potential barriers to participation.
Methods
Study design
The MedSeq Project is an ongoing randomized clinical trial examining the impact of integrating WGS into clinical care in two cohorts, primary care and cardiology. Details of the study design have been described in detail elsewhere (Vassy et al., 2014). Briefly, we recruited patients of 9 cardiologists and 11 primary care physicians at a single large urban network of academic hospitals and outpatient practices to be randomized to receive either standard of care (including a review of family history) or standard of care plus WGS. Eligible primary care patients were ostensibly healthy adults aged 40–65. Eligible cardiology patients were adults of any age with hypertrophic or dilated cardiomyopathy diagnoses.
Throughout the study, both physician- and patient-participants completed surveys and interviews at multiple time points, including before and after disclosure of WGS results. Outcomes of interest included the psychological and behavioral impact on patients, attitudes and preferences, and healthcare utilization. The Partners HealthCare Human Research Committee and the Baylor College of Medicine institutional review board approved this study.
Recruitment and informed consent process
The study team developed a multi-step recruitment process that provided potential participants with opportunities to learn about the study and review the informed consent document (ICD) and ask questions before being asked to sign the ICD.
First, each physician provided study staff with a list of patients potentially meeting eligibility criteria (some physicians did this after introducing the study to their patients personally). All identified patients were sent an announcement mailing, which included a study brochure and an informational letter that instructed patients to contact study staff within two weeks if they were not interested in participating (see Supplementary Online Document). Study staff then called patients up to two times to assess eligibility and to formally invite them to participate. This phone screen was guided by a script that was a simplified version of the background and study procedures section of the ICD, including a brief description of the randomization process and what would happen at each study visit (see Supplementary Materials). During the phone screen, study staff answered questions and documented concerns. Patients who expressed interest in participating at the end of the phone screen were scheduled for a baseline visit, sent the ICD to review, and encouraged to call the study staff before their scheduled visit with any additional questions or concerns. The baseline visit began with the research assistant verbally reviewing the ICD with the patient, which was audio-recorded with the patient’s verbal consent for research purposes. After signing the ICD, participants completed an online family history assessment and survey, and underwent a blood draw.
For those who declined participation, study staff asked an open-ended question about the primary reason(s) for decline, which was documented along with some basic demographic information, including their gender, age, race and ethnicity, and education level. Individuals who directly told study staff that they were not willing or able to participate in the MedSeq Project were categorized as having ‘actively declined.’ No demographic information was obtained about individuals who could not be reached for contact or were later unresponsive to scheduling efforts.
Informed consent documents
The ICDs were developed by a multidisciplinary team with expertise in randomized clinical trials, genetics, genetic counseling, and bioethics. The format of the ICDs was based on templates created by the Partners HealthCare Human Research Committee for human subjects research. Content was informed by a thorough review of the literature (Biesecker et al., 2009; Lautenbach, Christensen, Sparks, & Green, 2013; McGuire & Beskow, 2010) and ICDs used by research team investigators for clinical and research genetic testing. ICDs had a reading level of grade 11.7 (Flesch-Kincaid grade level) and were 17 pages in length, including signature pages (see [Abhilasha, please provide URL for Online Supplementary Documents] for consent form).
Potential risks of study participation included in the ICD were the physical risks from the blood draw, and risks specific to WGS included: insurance discrimination, which was followed by an explanation of what is and isn’t protected by the Genetic Information Non-Discrimination Act of 2008; emotional or psychological distress from learning genetic information about oneself or family members, including the potential for learning about non-paternity or a different ethnic background than expected; and loss of privacy. Also noted as a potential risk was the possibility that some results returned may be misclassified or later found to be unreliable (‘not predictive’), which may lead to one feeling unnecessarily upset. In terms of benefits, it was noted that their participation may provide them with information about their risk for developing diseases and about their response to certain medications, but that there may be no direct benefit for some participants.
Data analysis
We calculated descriptive statistics for reasons for study decline and individuals’ characteristics including study cohort and socio-demographic characteristics (self-reported age, gender, race/ethnicity, and education level). We evaluated differences between participants and active decliners’ socio-demographic characteristics using chi-square tests for categorical variables and analysis of variance (ANOVA) for continuous variables. Statistical analyses were conducted using SPSS 22 (IBM Corp., Armonk, NY). All p-values were 2-sided and with statistical significance set at p ≤ 0.05.
Responses to open-ended questions about primary reasons for declining participation were documented and categorized for quantification and comparison. IC sessions were audio-recorded, transcribed, and coded for thematic content analysis (Hsieh & Shannon, 2005). Specifically, analysis focused on coding concerns, comments, and questions participants raised during the IC session. Through an iterative process using inductive and deductive methods (Merriam, 2009), we identified themes that were used to develop and refine a codebook with definitions and examples for each code. To ensure inter-coder reliability, two coders (TC and JOR) used this codebook to independently code a portion of the IC sessions and discrepancies were resolved through a consensus approach.
Results
Recruitment and Sample Characteristics
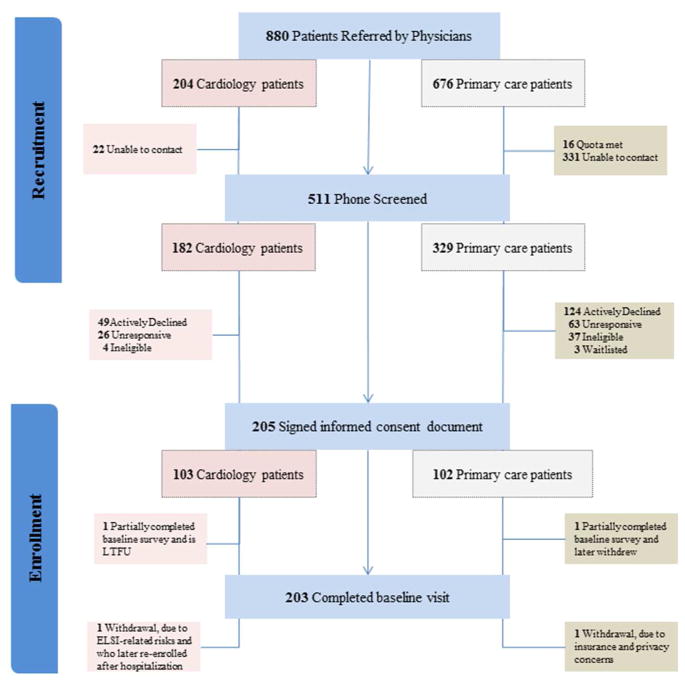
Study recruitment began in December 2012 and ended April 2015. Enrolled physicians provided study staff with information about 880 patients to contact for recruitment. Of these, study staff were able to contact 511 via phone, 41 (8%) were ineligible, 89 (17%) were unresponsive after expressing initial interest, 173 (33%) actively declined, 3 (0.6%) were waitlisted, 211 (41%) attended the baseline visit, and 205 (39%) ultimately signed an ICD (Figure 1). On average, the IC session at the baseline visit lasted 16 minutes with a maximum duration of 41 minutes (median 15 minutes).
Figure 1.
Study flow diagram for the recruitment and enrollment phase of the MedSeq Project
Three participants consented to study participation, but later withdrew prior to receiving their study results. Two of the withdrawals were primary care participants; one individual, who mentioned insurance and privacy concerns for herself and her children during the IC session, later withdrew for these very reasons, and the other withdrew for reasons not related to the study. The third withdrawal was a cardiology participant who withdrew due to concerns about insurance discrimination and emotional distress about learning genetic information. This individual later re-enrolled in the study following a medical issue that required hospitalization and resulted in the implantation of a heart device (an Automatic Implantable Cardioverter Defibrillator (AICD)). Another participant who provided consent did not complete the baseline survey and was unreachable for further contact.
Participants and active decliners’ socio-demographic characteristics were not significantly different (Table 1). Both groups were middle aged, primarily non-Hispanic whites, and highly educated. Compared to participants, however, a significant majority of active study decliners were from the primary care cohort (72%). Overall, 38% (n=124) of primary care patients declined participation, compared to 27% (n=49) of cardiology patients actively declining
Table 1.
Characteristics of enrolled participants and active study decliners
| No. (%), unless otherwise noted | Enrolled Participants (n=205) | Active Study Decliners (n=173) | p-value |
|---|---|---|---|
| Study cohort | <0.001 | ||
| Cardiology | 103 (50) | 49 (28) | |
| Primary care | 102 (50) | 124 (72) | |
| Mean age, in years (range) | 55 (18–84) | 55 (22–76) | 0.878 |
| Female gender | 101 (49) | 89 (51) | 0.680 |
| Race/Ethnicity | |||
| Non-Hispanic white | 185 (90) | 137 (85) | 0.149 |
| Other | 20 (10) | 24 (15) | |
| Not reported | 12 | ||
| Highest education level | 0.114 | ||
| Did not graduate from college | 40 (20) | 38 (27) | |
| College graduate or higher | 165 (80) | 103 (73) | |
| Not reported | 32 | ||
The most frequently cited reasons for study decline (59% of active decliners, n=102) were time constraints and study logistics, like not wanting to undergo a blood draw. Decliners also cited ELSI-related risks as reasons for declining participation, including fear of insurance discrimination, psychological impact of return of results, and privacy concerns. Participants similarly discussed these risks during the IC sessions (Table 2), but ultimately decided to participate, presumably because they felt the benefits of participation outweighed the risks. Summarized below are participants and decliners’ perspectives on each of these ELSI-related risks of participating in WGS research.
Table 2.
Frequency of ELSI-related risks expressed by participants and study decliners*
| No. (%) | Enrolled Participants (n=205) | Active Study Decliners (n=173) |
|---|---|---|
| Fear of insurance discrimination | 68 (33) | 48 (28) |
| Psychological impact of return of results | 37 (18) | 22 (13) |
| Privacy concerns | 33 (16) | 14 (8) |
Participants and decliners may have cited more than one risk.
Insurance Discrimination
Fear of insurance discrimination was cited as a primary reason for decline by 28% of active decliners, and 33% of participants raised this issue during the IC session. Some decliners noted that they would “sign up in a heartbeat” if the study were totally confidential and that putting the information in the medical record was like a “ticking time bomb.” Other decliners described the results as exploratory or research results and felt that this type of information did not belong in one’s medical record because of the potential impact on insurance.
During the IC session, participants expressed both concerns about insurance discrimination as well as reasons why the risk was an important consideration, but not critical to their decision to participate. For many participants, especially those with cardiomyopathy diagnoses, the concern was often mitigated by their current health and/or insurance status. As one cardiology participant explained,
Well, [that insurance companies can access patients’ medical records] is interesting. I guess I’m not too worried about it, I mean [I] already have the HCM [hypertrophic cardiomyopathy]. So it’s kind of like, you take what you can get. I am who I am. But, yeah I guess it is something to be aware of.
Some who had a clinical diagnosis on record felt that they were already at risk for insurance discrimination and did not believe that genomic testing would substantially increase that risk. For example, the cardiology participant who initially withdrew due to concerns about insurance discrimination and later re-enrolled in the study noted,
Once I had that [AICD implanted], and once it was clear that I was at risk for [HCM], then I figured there was no reason for me not to be in the genome study. Because if I was already obviously at risk and known to be, how much worse would it be if [insurance companies] knew what my genotype was?
Often the decision to participate in the study involved a trade-off between the perceived risks and benefits. For some, even though the risk of insurance discrimination was a concern, it was outweighed by the perceived benefits of study participation. For example, after the research assistant noted during the IC session that storing WGS information in the participant’s medical record may influence their eligibility and premiums, a participant responded,
I would say that if there is any concern this is probably the only one.....Yes, I’ve thought about it, but frankly I think the possibility of being selected for the study outweighs that. Because I think that it will be beneficial for me to know. So yes, I have thought about it, so it’s just a risk you take.
Both decliners and participants expressed concerns about the impact on specific types of insurance. Summarizing these concerns and the protections of GINA, one participant said,
Am I concerned about future eligibility? Yes. I’m not concerned about health insurance or employment specifically because of GINA. Life insurance with an established diagnosis makes it hard enough if not impossible already, so that’s not an issue. The question mark on my mind is long-term care insurance, which I don’t have any and I have not looked into it, I haven’t thought about it, I probably should; we don’t know how it’s going to affect it…so I’m weighing an enormous unknown.
Interestingly, individuals not only considered the insurance implications of participating for themselves, but also for their family members. For example, the primary care participant who withdrew due to insurance and privacy concerns was particularly concerned about the impact of this information on her children’s future insurability. During the baseline visit she asked,
Ok, so, if an insurance company has access to [my study results], on something that may be genetic or family related, theoretically in the future could that affect my children’s ability to be insured or that type of thing?
Other participants were hopeful that having the genetic information in their medical records could be a potential benefit for their family members. A cardiology participant said,
Because it’s part of my medical record, in 50 years from now, [if] my daughter needed to know something, because I’m assuming down the road genetics is going to become a bigger and bigger concern, this would still be available to her based on the idea that it’s part of my record?
Psychological impact of receiving results
Another risk frequently mentioned by individuals being recruited into this study was the anticipated psychological impact of receiving WGS results, including concerns about receiving unwanted information. Thirteen percent of decliners cited the psychological impact of receiving WGS results as a primary reason for declining and 18% of participants raised this issue in the IC session. Reasons for why this risk was a barrier to participation were sometimes described by individuals as “ignorance is bliss” or knowing one’s capacity to handle bad news. For example, one primary care decliner described herself as a “worrywart” and noted that it would be better for her, mentally, to not know her genomic information. Some decliners also specifically stated that they would not want to participate in a study in which they might learn about conditions that cannot be prevented or treated because of the emotional burden and inability to act upon the information.
Contrary to decliners, participants expressed a willingness to risk possible emotional distress for what they viewed to be the benefit of more knowledge. As one primary care participant explained,
You just can’t possibly know, right? And so how can you prepare yourself for it? You just don’t know what you’re going to get and it could be difficult, on the one hand, and on the other hand it’s just a way cool study! And you could learn some really interesting and valuable stuff, and so, I’m prepared to take the risk.
While some cardiology decliners expressed feeling overwhelmed by their current cardiomyopathy condition and unable to handle the potential of learning about another condition, many cardiology participants described feeling like they could handle any new medical information because of their condition. A cardiology participant said,
It’s interesting how worried you are about what people will think. I guess some people can’t respond to bad news well. I always tell people: I should be dead by now so why not know everything you could possibly know, you know what I mean?
Although they were not given a choice about which types of results they would receive in this study, some participants were explicit about the types of results they would prefer not to receive. Often these related to conditions that could not be prevented or treated, like Alzheimer’s disease. Other participants felt differently about receiving results that would not be considered medically actionable, noting how knowledge of the condition would itself be beneficial and outweighed the risk of the potential psychological burden. A primary care participant stated,
The psychological thing, I’m sure it’s disturbing if you figure out that in 20 years you get ALS or something, but it is what it is, and maybe it’ll give me a better appreciation of the way things are now.
The potential psychological impact of receiving WGS results was not only a personal consideration; individuals also considered the impact this information could have on their family members. For participants, this concern did not outweigh the desire to participate, but it was a serious consideration. A primary care participant said,
Obviously I have concerns about what’s going to be in the genome report… I could find out things that I may not want to find out, quite honestly. I’m also going to be a grandfather in a couple months, so I have downstream concerns, as you might expect.
Other participants were not at all concerned about the psychological impact of receiving WGS results. Importantly, many individuals being recruited into the study seemed to have a good understanding of the personal health implications of genomic information, including its probabilistic nature, and this may have impacted how concerned they were about the potential psychological burden. These participants viewed the return of WGS results as being beneficial for their family members’ healthcare despite the potential psychological burden. In response to reviewing the risk of emotional distress, a cardiology participant who was adopted explained,
It doesn’t mean I’m going to get whatever you find, it just means I’m predisposed to it.....My history is unknown; I got little kids so I’d sort of like to protect myself and others.
Participants raised few questions about variants of unknown significance (VUSes), and no individuals cited the potential receipt of VUSes as a reason for decline. Some participants who mentioned VUSes seemed unconcerned about this information and to understand that there were limits to scientists’ current understanding of WGS information, while others sought clarity about the need for this “disclaimer” and worried about the psychological impact of returning this uncertain information. One participant referred to the line in the ICD describing VUSes and asked,
Were you saying that there might be information on there that could just scare a regular patient? There may be information you don’t fully understand, and it may say you have the propensity to something, it could just be nothing, but some people could take it as really- as hypochondriacs, I could have this, I could have that?
Privacy concerns
Discussions about privacy concerns primarily centered on issues like potential privacy breaches, how the WGS data would be stored and shared and who could access and use the data, and the identifiability of the information. Eight percent of active decliners cited privacy concerns as a primary reason for decline, and 16% of participants discussed this issue during the IC session. Decliners noted not being comfortable with their genomic data being put into databases that could be used by other researchers, as well as concerns about cybersecurity and “hackers” having access to this information. Participants expressed similar privacy concerns, but seemed to be more comfortable with and trusting in the security safeguards of the study. For example, one participant noted,
It says your genome sequence may be shared with other researchers within and outside [the study’s institution]. I’m trusting that if you guys are sharing research information with other researchers you’re making sure that they have similar privacy protections in place.
Other participants wondered about the information being publicly available given the level of identifiability of the information. Some used familiar analogous news stories of online security breaches when discussing their concerns about identifiability and risks to privacy, like Netflix or WikiLeaks. A primary care participant asked,
The only thing that would identify me is my study ID, and there’s no way that no one knows who these genome sequencing and the other data belongs to?.....Can you guarantee that? I won’t find it on WikiLeaks website or something?
Ultimately, for participants, the benefits of participation and study safeguards seemed to outweigh their privacy concerns. As one cardiology participant summarized,
I think I am concerned a little bit about who will be the data gatekeepers of information, but I’m ok. I kind of thought through everything about the positive applications of test[ing], so I decided I would want to participate.
Discussion
We identified three main themes related to the risks of WGS that individuals considering participation in our study discussed during recruitment and enrollment: fear of insurance discrimination, the psychological impact of receiving results, and privacy concerns. Although the majority of individuals who actively declined did so primarily because of time constraints and concerns about the study logistics, a substantial minority of decliners also cited these ELSI-related risks as reasons to decline. Ultimately, participants felt that the perceived study benefits outweighed these risks. Our findings provide unique insight into the perspectives of potential WGS research participants, including ostensibly healthy adults, and identify potential barriers to WGS research participation.
About half of the individuals recruited to the MedSeq study declined participation. This finding supports studies examining uptake of clinical genome sequencing, which have found that even individuals from similar backgrounds and clinical contexts may vary in their enthusiasm for and concerns about this technology (Kimball, Nowakowski, Maschke, & McCormick, 2014; McGowan, Glinka, Highland, Asaad, & Sharp, 2013). Our finding that significantly more primary care patients declined participation than cardiology patients is consistent with a previous study reporting that participants’ interest in genomic testing was lower when individuals did not have any current medical issues(McGowan, et al., 2013).
We found that fear of insurance discrimination was the risk most often discussed by active decliners and participants. Fear of insurance discrimination may have been brought up frequently in our study because of the attention drawn to the potential risk for insurance discrimination in the ICD. Since WGS results are placed in the patient’s medical record, the ICD specifically described what the US Genetic Information Nondiscrimination Act (GINA) does and does not cover. Given that both decliners and participants expressed concerns about specific types of insurance, those considering enrollment into our study might have had a good understanding for what the law covered and this may have heightened awareness about this risk. Even with this heightened awareness, however, many of our participants were willing to assume this risk. In fact, some MedSeq participants saw storage of WGS results in the medical record as a benefit because it would be accessible for future use.
In this study, we did not give participants a choice about what types of WGS results they would receive. Therefore, for individuals who did not want to learn their genomic information, declining participation was the only option. Although individuals did not express frustration with the lack of choice, some declined participation due to concerns about the potential psychological impact of the information they might receive. This is consistent with the findings of a similar study of clinical sequencing-based multiplex testing for cancer, which reported that many who declined worried that testing beyond which was relevant to their medical decision making might be overwhelming or something they were not mentally prepared for (Bradbury et al., 2015). That study also reported that one third of individuals declined participation because they were concerned about the uncertainty of the information they might receive (Bradbury, 2015), however few individuals considering participation in the MedSeq Project raised concerns about the uncertainty of WGS information, or about the potential to receive a VUS. This may be due in part to the high education level of our participants and decliners, most of whom seemed to understand that the source of uncertainty was centered on the nascence of the field.
The fact that most (all but 6) individuals who actively declined participation in the MedSeq Project did so prior to attending the IC session at the baseline visit may be a reflection of a well-designed recruitment process. We developed a multi-step recruitment process that provided potential participants several opportunities to ask study staff questions about their concerns related to participation. Potential participants often developed a good rapport with study staff, which may have helped them to be more open about their concerns. Although this was a labor-intensive approach, it benefited individuals who might have otherwise made an unnecessary trip to the clinic and it provided those considering enrollment more time and resources to help them think through and carefully consider the risks in the context of their own individual life and health situations.
There are several limitations to this study. Participants are highly educated, recruited from their trusted physician into a study run by a well-respected local research institution. Those less trusting of research and medical doctors and who are less knowledgeable and enthusiastic about WGS might respond differently. Additionally, we were unable to obtain demographic information on those being recruited into the study who we were unable to contact, which represents a large portion of those referred (especially in the primary care cohort). To minimize burdens on participants and because the analyses presented here were considered secondary, we did not assess knowledge before consent was administered and cannot comment on how our informed consent process may have improved understandings. Also, the ICD developed for this study was at the reading level of a high school graduate, which is the high end of the range of reading levels among 9 CSER study ICDs examined by Henderson et al. (2014). The authors argue that this high reading level could add to the complexity of information and cause confusion. Therefore, although the recruitment process and ICDs may have been well-designed for this study population, this protocol and materials might need to be adapted before being used in study populations that have more diverse backgrounds and who have less familiarity with genomics.
Despite these limitations, findings from our research can help investigators anticipate and prepare for the concerns that may affect participation in the PMI Cohort Study and other similar initiatives. The benefits of linking genomic data with electronic health records must be weighed against potential participants’ concerns with insurance discrimination. And decisions about how much control to give participants in deciding which results to receive will need to weigh the challenges of tracking individual preferences and facilitating informed decisions with the desire to maximize participation. Special attention should also be given to the informed consent process to ensure that participants understand and can carefully consider the risks and benefits of study participation.
Best Practices
Investigators conducting genomic research will need to consider their study design and anticipate the study staff and other resources needed to conduct a multi-step recruitment process as described here. If possible, the recruitment process should provide patients multiple opportunities to learn about the study’s purpose, risks, and benefits and should ensure that patients are given plenty of time to understand the implications of this information in the context of their own life and health situations. To assess the long-term effectiveness of our study’s recruitment approach and informed consent process, our future work will use longitudinal survey data to explore whether participants express decisional regret (Brehaut et al., 2003) and satisfaction over time about their decision to participate.
Additionally, our findings suggest that the importance placed on impact of this information on family members should not be underestimated. Participants in this study often made comments and asked questions about the implications that learning their own genetic information would have for their family members, especially as that information would be added to their electronic medical record. With initiatives dedicated to linking electronic medical records to enable large-scale research, future studies will need to further explore individuals’ attitudes and perspectives about implications for family members, and how to handle informed consent to account for these implications. Some family-centric initiatives and dynamic consent tools have been proposed for obtaining consent in personal genome research (Minari, Teare, Mitchell, Kaye, & Kato, 2014). Many advanced technological interfaces like dynamic consent have been proposed to handle informed consent in the genomic era (Kaye et al., 2015). As these advanced technologies are implemented more research will be needed to assess the feasibility of using these types of tools, especially with those who do not have access to or are not comfortable using them.
Research Agenda
While this study provides needed empirical data on the perspectives of those being recruited into WGS research, more studies are needed. We were unable to obtain demographic data on a large portion of individuals referred to the study and it could be that these individuals had more diverse demographic backgrounds than those who provided this information to study staff. Groups who have historically displayed low trust in medical researchers are often from minority backgrounds and may feel differently about the risks of WGS research. Future research should expand to populations with more diverse racial, educational, and regional and cultural backgrounds, and those less familiar with genomic sequencing. More studies are also needed in varied clinical settings, as it has been shown that individuals make informed consent decisions that are primarily driven by trust in their doctors or deference to authority than information provided about the study (Grady, 2015). Furthermore, how well individuals understand the risks and long-term and downstream (familial) implications of their participation needs to be explored.
Educational Implications
Given that we found that the potential for insurance discrimination and privacy were often cited ELSI-related risks by both participants and active decliners, and despite laws designed to address these issues, lack of public knowledge surrounding these laws remains a problem. Although both GINA and the Affordable Care Act provide protection against health insurance discrimination, not all types of insurance are protected, and it is unclear how effective any of these laws will be for individuals participating in WGS research (Green, Lautenbach, & McGuire, 2015). Excluding results from EMRs and obtaining certificates of confidentiality may be ways to mitigate potential participants’ concerns, but researchers conducting genome sequencing studies will need to determine what impact doing so would have on the research. Finally, policy makers will need to assess and address any gaps in these laws and, while investigators continue to assess what information individuals need to understand in order to provide valid informed consent, more efforts to engage the public in general genomics education are warranted.
Acknowledgments
Sources of support: This work was supported by the US National Institutes of Health (U01-HG006500, F32-HG006993).
Funding
This study is a Clinical Sequencing Exploratory Research (CSER) program project supported by NHGRI U01HG006500.
The authors thank the members and participants of the MedSeq Project for their important contributions. The authors thank 5AM Solutions, Inc. (Rockville, MD, USA), for their help in customizing the workflow of the “My Family Health Portrait” web tool for this study.
Abbreviations
- ELSI
ethical, legal, and social implications
- NIH
National Institutes of Health
- PMI
Precision Medicine Initiative
- WGS
whole genome sequencing
- NHGRI
National Human Genome Research Institute
- CSER
Clinical Sequencing Exploratory Research
- ICD
informed consent documents
- IC
informed consent
- HCM
hypertrophic cardiomyopathy
- GINA
Genetic Information Nondiscrimination Act of 2008
- VUS
Variant of uncertain significance
- AICD
Automatic Implantable Cardioverter Defibrillator
Biographies
Jill Oliver Robinson is a Senior Project Manager with the Center for Medical Ethics and Health Policy at Baylor College of Medicine. She conducted the primary data analysis for this manuscript, drafted the initial manuscript, and edited and approved the final version.
Tom Carroll is an undergraduate at Rice University. Mr. Carroll is a Health, Humanism, and Society Scholar working with the Center for Medical Ethics and Health Policy at Baylor College of Medicine. He conducted the primary data analysis for this manuscript, and edited and approved the final version.
Lindsay Feuerman is the Operations Manager of the Center for Medical Ethics and Health Policy at Baylor College of Medicine. She participated in data collection and analysis for this manuscript and edited and approved the final version.
Denise L. Perry was the Senior Project Manager and Genetic Counselor for the MedSeq Project with the G2P Research Program in Translational Genomics and Health Outcomes a Brigham and Women’s Hospital and played a key role in the development of the informed consent process for the MedSeq Project.
Lily Hoffman-Andrews is a Senior Research Assistant for the G2P Research Program in Translational Genomics and Health Outcomes at Brigham and Women’s Hospital and Harvard Medical School. She participated in data collection and analysis, edited this manuscript, and approved the final version.
Rebecca Walsh is a Senior Research Assistant for the G2P Research Program in Translational Genomics and Health Outcomes at Brigham and Women’s Hospital and Harvard Medical School. She participated in data collection and analysis, edited this manuscript, and approved the final version.
Kurt D. Christensen is a postdoctoral research fellow at Brigham and Women’s Hospital and Harvard Medical School. His research focuses on the behavioral and economic impact of emerging genomic technologies in clinical settings, including the use of whole genome and whole exome sequencing in the care of adults and newborns. He participated in study design and data analysis, edited this manuscript, and approved the final version.
Robert C. Green is a medical geneticist and physician-scientist who directs the G2P Research Program in Translational Genomics and Health Outcomes (genomes2people.org) at Brigham and Women’s Hospital and Harvard Medical School. He is the PI of a National Human Genome Research Institute funded study, the MedSeq Project (Green U01HG006500), which supported the work described in this article. He conceptualized and supervised the research project, participated in data collection and analysis, edited this manuscript, and approved the final version.
Amy L. McGuire is the Leon Jaworski Professor in Biomedical Ethics and Director of the Center for Medical Ethics and Health Policy at Baylor College of Medicine. Dr. McGuire is the Co-PI of National Institutes of Health grant/National Human Genome Research Institute funded study, the MedSeq Project (Green U01HG006500), which supported the work described in this article. She participated in study design and data analysis, edited this manuscript, and approved the final version.
Footnotes
Clinical Trials Registration: ClinicalTrials.gov # NCT01736566.
Declaration of Conflicting Interests
The authors declare that they have no competing interests.
Contributor Information
Thomas M. Carroll, Email: tmc6@rice.edu.
Lindsay Z. Feuerman, Email: Lindsay.feuerman@bcm.edu.
Denise L. Perry, Email: dperry@illumina.com.
Lily Hoffman-Andrews, Email: lhoffmanandrews@genetics.med.harvard.edu.
Rebecca C. Walsh, Email: rwalsh@genetics.med.harvard.edu.
Kurt D. Christensen, Email: kchristensen@genetics.med.harvard.edu.
Robert C. Green, Email: rcgreen@genetics.med.harvard.edu.
Amy L. McGuire, Email: amcguire@bcm.edu.
References
- Biesecker LG, Mullikin JC, Facio FM, Turner C, Cherukuri PF, Blakesley RW, Green ED. The ClinSeq Project: piloting large-scale genome sequencing for research in genomic medicine. Genome research. 2009;19(9):1665–1674. doi: 10.1101/gr.092841.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury AR, Patrick-Miller LJ, Egleston BL, DiGiovanni L, Brower J, Harris D, Domchek SM. Patient feedback and early outcome data with a novel tiered-binned model for multiplex breast cancer susceptibility testing. Genetics in Medicine. 2015 doi: 10.1038/gim.2015.19. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- Brehaut JC, O’Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, Feldman-Stewart D. Validation of a decision regret scale. Medical decision making. 2003;23(4):281–292. doi: 10.1177/0272989X03256005. [DOI] [PubMed] [Google Scholar]
- Grady C. Enduring and emerging challenges of informed consent. [Review] The New England journal of medicine. 2015;372(9):855–862. doi: 10.1056/NEJMra1411250. [DOI] [PubMed] [Google Scholar]
- Green RC, Lautenbach D, McGuire AL. GINA, genetic discrimination, and genomic medicine. New England Journal of Medicine. 2015;372(5):397–399. doi: 10.1056/NEJMp1404776. [DOI] [PubMed] [Google Scholar]
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- P. C. f. t. S. o. B. Issues, editor. Privacy and progress in whole genome sequencing. Washington, DC: 2012. http://www.bioethics.gov. [Google Scholar]
- http://www.genome.gov/27026588. Informed consent for genomics research, from http://www.genome.gov/27026588
- http://www.nih.gov/precisionmedicine/2015-07-01-workshop-summary.pdf. (2015). A Workshop of the Precision Medicine Initiative Working Group of the Advisory Committee to the NIH Director.
- Kaiser J. BIOMEDICINE. NIH opens precision medicine study to nation. Science. 2015;349(6255):1433. doi: 10.1126/science.349.6255.1433. [DOI] [PubMed] [Google Scholar]
- Kaye J, Whitley EA, Lund D, Morrison M, Teare H, Melham K. Dynamic consent: a patient interface for twenty-first century research networks. European Journal of Human Genetics. 2015;23(2):141–146. doi: 10.1038/ejhg.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball BC, Nowakowski KE, Maschke KJ, McCormick JB. Genomic data in the electronic medical record: perspectives from a biobank community advisory board. Journal of empirical research on human research ethics : JERHRE. 2014;9(5):16–24. doi: 10.1177/1556264614553922. [DOI] [PubMed] [Google Scholar]
- Lautenbach DM, Christensen KD, Sparks JA, Green RC. Communicating genetic risk information for common disorders in the era of genomic medicine. Annual Review of Genomics and Human Genetics. 2013;14:491–513. doi: 10.1146/annurev-genom-092010-110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan ML, Glinka A, Highland J, Asaad G, Sharp RR. Genetics patients’ perspectives on clinical genomic testing. Personalized medicine. 2013;10(4):339–347. doi: 10.2217/pme.13.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AL, Beskow LM. Informed consent in genomics and genetic research. Annual review of genomics and human genetics. 2010;11:361–381. doi: 10.1146/annurev-genom-082509-141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam SB. Qualitative Research: A Guide to Design and Implementation. San Francisco, CA: John Wiley & Sons; 2009. [Google Scholar]
- Minari J, Teare H, Mitchell C, Kaye J, Kato K. The emerging need for family-centric initiatives for obtaining consent in personal genome research. Genome Medicine. 2014;6(12):118. doi: 10.1186/s13073-014-0118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassy JL, Lautenbach DM, McLaughlin HM, Kong SW, Christensen KD, Krier J, MedSeq P. The MedSeq Project: a randomized trial of integrating whole genome sequencing into clinical medicine. Trials. 2014;15:85. doi: 10.1186/1745-6215-15-85. [DOI] [PMC free article] [PubMed] [Google Scholar]