Abstract
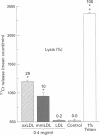
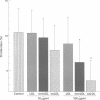
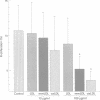
OBJECTIVE--To assess whether the extent of LDL oxidation influences its cytotoxic effects, thus contributing to its atherogenic potential. DESIGN AND SETTING--The effects of native and modified LDL on cultured human coronary artery smooth muscle cells (SMC) and endothelial cells (ECs) were investigated. MAIN OUTCOME MEASURES--Four indices of cytotoxicity were studied: (i) chromium-51 release; (ii) 5-bromo-2'-deoxyuridine (BrDUrd) uptake; (iii) morphological appearance; and (iv) EC migration. RESULTS--(i) Minimally modified (mm) LDL (400 micrograms/ml) causes significant 51Cr release; the cytotoxic effect was significantly greater for copper oxidised (ox) LDL (400 micrograms/ml). Native LDL had no effect. (ii) BrDUrd uptake studies showed significant inhibition of cell proliferation by 100 micrograms/ml of oxLDL and to a lesser extent by mmLDL; native LDL had no effect. (iii) Morphological appearance was not altered by native LDL. Changes in cell morphology were induced by mmLDL (400 micrograms/ml), and were more pronounced with oxLDL in concentrations of > or = 200 micrograms/ml. (iv) EC migration was significantly inhibited by oxLDL (100 micrograms/ml), but not by native or mmLDL. CONCLUSION--The extent of oxidation of LDL determined its cytotoxicity to coronary artery cells. Native LDL had no cytotoxic effect. In contrast, oxLDL and to a lesser extent mmLDL caused cytotoxicity at concentrations to which cells in vivo might be exposed. This may contribute to the atherogenicity of modified LDL by enhancing cellular injury and inflammation, and by inhibiting re-endothelialisation of areas of coronary artery damaged during the atherogenic process.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berliner J. A., Territo M. C., Sevanian A., Ramin S., Kim J. A., Bamshad B., Esterson M., Fogelman A. M. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990 Apr;85(4):1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger C. M., Tanner F. C., Béa M. L., Hahn A. W., Werner A., Lüscher T. F. Oxidized low density lipoproteins induce mRNA expression and release of endothelin from human and porcine endothelium. Circ Res. 1992 Jun;70(6):1191–1197. doi: 10.1161/01.res.70.6.1191. [DOI] [PubMed] [Google Scholar]
- Drake T. A., Hannani K., Fei H. H., Lavi S., Berliner J. A. Minimally oxidized low-density lipoprotein induces tissue factor expression in cultured human endothelial cells. Am J Pathol. 1991 Mar;138(3):601–607. [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Dieber-Rotheneder M., Waeg G., Puhl H., Tatzber F. Endogenous antioxidants and lipoprotein oxidation. Biochem Soc Trans. 1990 Dec;18(6):1059–1061. doi: 10.1042/bst0181059. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Dieber-Rotheneder M., Waeg G., Striegl G., Jürgens G. Biochemical, structural, and functional properties of oxidized low-density lipoprotein. Chem Res Toxicol. 1990 Mar-Apr;3(2):77–92. doi: 10.1021/tx00014a001. [DOI] [PubMed] [Google Scholar]
- Flavahan N. A. Atherosclerosis or lipoprotein-induced endothelial dysfunction. Potential mechanisms underlying reduction in EDRF/nitric oxide activity. Circulation. 1992 May;85(5):1927–1938. doi: 10.1161/01.cir.85.5.1927. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Hooker S., Sanford G. L., Montgomery V., Rivers R., Emmett N. Influence of low density lipoproteins on vascular smooth muscle cell growth and motility: modulation by extracellular matrix. Cell Biol Int Rep. 1992 May;16(5):433–450. doi: 10.1016/s0309-1651(06)80063-9. [DOI] [PubMed] [Google Scholar]
- Hiramatsu K., Rosen H., Heinecke J. W., Wolfbauer G., Chait A. Superoxide initiates oxidation of low density lipoprotein by human monocytes. Arteriosclerosis. 1987 Jan-Feb;7(1):55–60. doi: 10.1161/01.atv.7.1.55. [DOI] [PubMed] [Google Scholar]
- Isner J. M., Kearney M., Bortman S., Passeri J. Apoptosis in human atherosclerosis and restenosis. Circulation. 1995 Jun 1;91(11):2703–2711. doi: 10.1161/01.cir.91.11.2703. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Latron Y., Chautan M., Anfosso F., Alessi M. C., Nalbone G., Lafont H., Juhan-Vague I. Stimulating effect of oxidized low density lipoproteins on plasminogen activator inhibitor-1 synthesis by endothelial cells. Arterioscler Thromb. 1991 Nov-Dec;11(6):1821–1829. doi: 10.1161/01.atv.11.6.1821. [DOI] [PubMed] [Google Scholar]
- Ludwig J. D., Avis K. E. Dry heat inactivation of endotoxin on the surface of glass. J Parenter Sci Technol. 1990 Jan-Feb;44(1):4–12. [PubMed] [Google Scholar]
- Morel D. W., Hessler J. R., Chisolm G. M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983 Aug;24(8):1070–1076. [PubMed] [Google Scholar]
- Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991 Dec;88(6):2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Barnett J., Fong L. G. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1990 May 22;1044(2):275–283. doi: 10.1016/0005-2760(90)90314-n. [DOI] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987 May;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavashisth T. B., Andalibi A., Territo M. C., Berliner J. A., Navab M., Fogelman A. M., Lusis A. J. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990 Mar 15;344(6263):254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- Richardson P. D., Davies M. J., Born G. V. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet. 1989 Oct 21;2(8669):941–944. doi: 10.1016/s0140-6736(89)90953-7. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Simon B. C., Cunningham L. D., Cohen R. A. Oxidized low density lipoproteins cause contraction and inhibit endothelium-dependent relaxation in the pig coronary artery. J Clin Invest. 1990 Jul;86(1):75–79. doi: 10.1172/JCI114718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiko-Rahm A., Hultgårdh-Nilsson A., Regnström J., Hamsten A., Nilsson J. Native and oxidized LDL enhances production of PDGF AA and the surface expression of PDGF receptors in cultured human smooth muscle cells. Arterioscler Thromb. 1992 Sep;12(9):1099–1109. doi: 10.1161/01.atv.12.9.1099. [DOI] [PubMed] [Google Scholar]
- Territo M. C., Berliner J. A., Almada L., Ramirez R., Fogelman A. M. Beta-very low density lipoprotein pretreatment of endothelial monolayers increases monocyte adhesion. Arteriosclerosis. 1989 Nov-Dec;9(6):824–828. doi: 10.1161/01.atv.9.6.824. [DOI] [PubMed] [Google Scholar]
- Wellicome S. M., Thornhill M. H., Pitzalis C., Thomas D. S., Lanchbury J. S., Panayi G. S., Haskard D. O. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1, or lipopolysaccharide. J Immunol. 1990 Apr 1;144(7):2558–2565. [PubMed] [Google Scholar]
- Yin E. T., Galanos C., Kinsky S., Bradshaw R. A., Wessler S., Lüderitz O., Sarmiento M. E. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972 Jan 28;261(1):284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]