Abstract
The Basic-Helix-Loop-Helix-Orange (bHLH-O) transcription factor Hairy-related 4 (her4) is a downstream effector of Notch-Delta signaling that represses expression of typically pro-neural genes in proliferative domains of the central nervous system. Notch-Delta signaling in the retina has been shown to increase in response to injury and influences neuroprotective properties of Müller glia. In contrast to mammals, teleost fish are able to regenerate retinal neurons in response to injury. In zebrafish, her4 is upregulated in the regenerating neural retina in response to both acute and chronic photoreceptor damage, but the contribution of her4 expressing cells to neurogenesis following acute or chronic retinal damage has remained unexplored. Here we investigate the role of her4 in the regenerating retina in a background of chronic, rod-specific degeneration as well as following acute light damage. We demonstrate that her4 is expressed in the persistently neurogenic ciliary marginal zone (CMZ), as well as in small subsets of slowly proliferating Müller glia in the inner nuclear layer (INL) of the central retina. We generated a transgenic line of zebrafish that expresses the photoconvertible Kaede reporter driven by a her4 promoter and validated that expression of the transgene faithfully recapitulates endogenous her4 expression. Lineage tracing analysis revealed that her4-expressing cells in the INL contribute to the rod lineage, and her4 expressing cells in the CMZ are capable of generating any retinal cell type except rod photoreceptors. Our results indicate that her4 is involved in a replenishing pathway that maintains populations of stem cells in the central retina, and that the magnitude of the her4-associated proliferative response mirrors the extent of retinal damage.
Keywords: Her4, Hes5, zebrafish, retina, regeneration, Müller glia, stem cells
1. Introduction
The vertebrate retina is a highly conserved and specialized extension of the central nervous system that enables vision by transducing photons of light into an electrical signal. It is a complex, multi-layered tissue that consists of three nuclear layers: the outer nuclear layer (ONL), inner nuclear layer (INL), and ganglion cell layer (GCL). In addition, there are two plexiform layers where synaptic transmission between retinal neurons occurs (Fadool and Dowling, 2008). There are six major classes of retinal neurons and one intrinsic glial cell type (Stenkamp, 2007, Fadool and Dowling, 2008). The two general classes of photoreceptor cells in the retina are the rods and cones. Rods are exquisitely sensitive to light, allow for detection of as little as one quanta of photons, and are responsible for scotopic vision. Cones allow for color vision in photopic conditions. Mutations in several photoreceptor specific genes cause chronic damage to the retina, ultimately leading to blinding retinopathic diseases such as retinitis pigmentosa (RP) (Hartong et al., 2006, Morris, 2011). In addition, photoreceptor cells are sensitive to both chronic and acute damage from natural ionizing radiation such as ultraviolet light or simply from exposure to intense white light (Abler et al., 1996).
Although the various retinal cell types as well as the overarching laminar organization are similar in cold- and warm-blooded vertebrates, mammals lack the ability to regenerate neurons of the CNS (Fischer and Bongini, 2010). Whereas both acute and chronic damage to the mammalian retina is generally permanent, the damaged zebrafish retina undergoes a robust regenerative response (Vihtelic and Hyde, 2000, Morris et al., 2005, Goldman, 2014, Gorsuch and Hyde, 2014). Following injury, Müller glia in the central regions of the zebrafish retina de-differentiate and proliferate to produce neural progenitor cells that migrate to the correct location, differentiate into the correct cell type, and functionally integrate into the existing tissue. In contrast, in response to injury or chronic degeneration, the mammalian retina undergoes gliosis, scarring, and various degrees of vision loss (Raymond and Hitchcock, 1997, Fischer and Reh, 2001, Fischer and Reh, 2003, Fischer and Bongini, 2010, Nelson and Hyde, 2012). In the undamaged, but continuously growing zebrafish retina, new retinal neurons arise from proliferating stem cells in the ciliary marginal zone (CMZ) in the peripheral retina (Raymond et al., 2006). Progenitors in the CMZ, however, are not thought to produce rod photoreceptors. Rod photoreceptors are generated from a discrete population of rod progenitor cells located in the ONL, which are themselves seeded from more slowly dividing stem cells associated with radial Müller glia in the INL (Johns and Fernald, 1981, Johns, 1982, Raymond and Rivlin, 1987, Otteson et al., 2001, Raymond et al., 2006, Morris et al., 2008a, Morris et al., 2008b).
It has been observed that the zebrafish retina is capable of matching its regenerative response to the amount of damage detected. For example, the XOPS:mCFP transgenic line of zebrafish exhibits selective degeneration of rod photoreceptor cells without any secondary effects on other retinal neurons (Morris et al., 2005). In contrast to acute damage models, in this background of chronic, rod-specific degeneration and regeneration, there is no reactive gliosis and the number of proliferating Müller glia cells in the INL appears to be unchanged. Rather, rod regeneration in this model is mediated solely by an increase in the number of proliferating rod progenitor cells in the ONL (Morris et al., 2005). A microarray analysis of mRNA from adult wild-type and XOPS:mCFP retinas identified several transcription factors with increased expression in response to the chronic rod damage and regeneration. The present study focuses on one of those transcription factors: Her4, an effector of Notch-Delta signaling (Takke et al., 1999, Morris et al., 2011).
The Notch-Delta pathway is a highly conserved juxtacrine signaling system that regulates lateral inhibition, proliferation, and gliogenesis (Furukawa et al., 2000, Scheer et al., 2001), and components of the Notch pathway are upregulated in the retina following injury (Kassen et al., 2007, Ghai et al., 2010). When a signaling cell expressing a Delta-like ligand comes into physical contact with a signal-receiving cell expressing a Notch receptor, a series of proteolytic cleavage events liberates the Notch intracellular domain (NICD) from the cell membrane. The soluble NICD then diffuses through the cytoplasm and binds to Suppressor of hairless (Su(H)) sites where it interacts with the CBF1/Su(H)/Lag1 (CSL) family of proteins and activates transcription of target genes (Campos-Ortega, 1995, Lai, 2004). Notch activity in the retina has been shown to be necessary for maintenance of pools of stem cells and progenitor cells and mediates lateral inhibition during neural differentiation (Nelson et al., 2007). During retinal development, Notch-Delta signaling functions to prevent the premature depletion of progenitor cells before all of the retinal neurons and glial cells have differentiated in their correct proportions (Bao and Cepko, 1997, Bernardos et al., 2005, Nelson et al., 2007). In the regenerating retina, the Müller glia are directed by various extrinsic signals, including Notch-Delta signaling, to dedifferentiate and re-enter the cell cycle, acquiring progenitor-like phenotypes (Gorsuch and Hyde, 2014, Lenkowski and Raymond, 2014).
Hairy-related (her) genes are the highly evolutionarily conserved zebrafish counterparts of the Hairy and Enhancer-of-split type genes in Drosophila, and of the Hes/Hey genes in mammals (Muller et al., 1996). Her4 is an ortholog of mammalian HES5, and represents one of over 20 members of the hairy/enhancer of split gene superfamily of transcription factors found in the zebrafish genome, not including duplicate variants of an individual gene (Davis and Turner, 2001). The her4 gene is comprised of five tandem duplicate repeats on linkage group 23 of the zebrafish genome. All variants of her4 have nearly identical transcripts with minor sequence polymorphisms in the 3′ untranslated region (UTR) and are translated into identical peptides. Her4 is a basic-helix-loop-helix-orange (bHLH-O) transcriptional repressor that is directly regulated by the Notch-Delta signaling pathway (Takke et al., 1999). Her4 is expressed throughout the developing nervous system and hypoblast where it has been shown to be necessary for primary neuron and hypochord development, as well as maintaining cyclic gene expression during somitogenesis (Takke et al., 1999, Pasini et al., 2004). In the developing CNS, Her4 is required for establishing peripheral outgrowth of subsets of sensory neurons in the trigeminal ganglia as well as regulating the number of neurog1 and deltaB-positive otic neurons during inner ear development (So et al., 2009, Radosevic et al., 2014). In the adult zebrafish CNS, subsets of Her4 and GFAP-GFP positive glia in the optic tectum act as proliferating neural precursors (Jung et al., 2012). In addition, her4-expressing radial glia in the adult zebrafish CNS have been shown to proliferate and generate neuroblasts in response to brain lesions resulting in efficient regeneration of neurons without glial scarring (Kroehne et al., 2011, Skaggs et al., 2014). In previous studies of adult retinal regeneration, her4 has primarily been used as a marker for active Notch-Delta signaling in response to acute damage (Conner et al., 2014). However, her4 has not been studied in the context of chronic damage, and the fate of her4-expressing cells during retinal regeneration has not been determined.
In this study, we investigated the role of her4 during regeneration of photoreceptor cells in a chronic, rod-specific degeneration background and in an acute light damage model. We generated a transgenic zebrafish line that expresses the photoconvertible protein Kaede in her4-positive cells. We showed that the her4:Kaede reporter is expressed in a spatiotemporal pattern that faithfully recapitulates endogenous her4 expression in the retina. Due to the ability of Kaede to be irreversibly photoconverted, it is a useful tool to not only track Kaede expressing cells, but to establish a timeframe for cellular migration. Lineage tracing analysis using adult her4:Kaede; XOPS:mCFP zebrafish revealed that her4 is expressed in subsets of slowly proliferating Müller glia cells in the INL which give rise to progenitor cells that feed into the rod lineage, and that the entire process from her4 expression to rod neurogenesis takes place in under three days. We demonstrated that her4 expressing stem cells in the CMZ contribute to the lineage of Müller glia and all retinal neurons except rod photoreceptors. We established that her4 is also upregulated in response to acute light damage that results in rod and cone photoreceptor degeneration, and that the magnitude of the her4 response in the regenerating retina correlates with the amount of damage. Our results suggest that her4 and Notch-Delta signaling may play a role in a pathway that replenishes depleted progenitor cell populations by maintaining appropriate numbers of retinal stem cells.
2. Material and methods
2.1 Zebrafish
All zebrafish (Danio rerio) strains were bred, raised, and maintained in accordance with established animal care protocols for zebrafish husbandry (Westerfield, 1995). Adult fish, embryos, and larvae were housed at 28°C, on a 14 hr light: 10 hr dark cycle. Embryos were staged as previously described (Kimmel et al., 1995). The Tg(XRho:gap43-mCFP)q13 transgenic line, hereafter called XOPS:mCFP, has been previously described (Morris et al., 2005, Morris et al., 2011). The Tg(gfap:GFP)mi2001 line, hereafter called gfap:GFP has been previously described (Bernardos and Raymond, 2006) and was obtained from the Zebrafish International Resource Center (ZIRC, Eugene, OR). The Tg(UAS:myc-Notch1a-intra)Kca3 and Tg(hsp70i:Gal4)Kca4 lines were obtained from ZIRC and have been previously described (Scheer and Campos-Ortega, 1999). The Tg(her4:Kaede) line was generated using Tol2-mediated transgenesis (Kwan et al., 2007). Briefly, an expression clone was assembled using site-specific recombination cloning with multisite Gateway technology (Life Technologies, Carlsbad, CA). The construct contains 3.4 kb of the her4.3 promoter cloned upstream of the photoconvertible Kaede reporter, and a separate transgenesis marker consisting of the cardiac myosin light chain promoter (cmlc) cloned upstream of GFP (Ando et al., 2002). The expression clone DNA and Tol2 transposase RNA were injected into 1-cell stage zebrafish embryos at 30 and 25 ng/μl respectively. The injected embryos were screened for GFP expression and raised to adulthood, then outcrossed to identify germline transmitting F0 animals that were used to establish the her4:Kaede colony. All animal procedures were carried out in accordance with guidelines established by the University of Kentucky Institutional Animal Care and Use Committee (IUCAC).
2.2 Whole mount in situ hybridization (WISH), fluorescent in situ hybridization (FISH)
WISH, FISH, and two color FISH were performed as previously described (Forbes-Osborne et al., 2013, Pillai-Kastoori et al., 2014). Antisense RNA probes were prepared by in vitro transcription of linearized plasmids containing a portion of the coding sequence of the gene of interest using T7 polymerase and digoxigenin (DIG) or fluorescein (FITC) labeling mix (Roche Applied Science, Indianapolis, IN). The her4 and kaede containing plasmids were prepared by cloning PCR products into the pGEM-T-easy vector (Promega, Madison, WI). The sequences of all PCR primers used in this study are presented in Table S1. Images were obtained on an inverted fluorescent microscope (Eclipse Ti-U; Nikon Instruments), and were exported into Adobe Photoshop for figure preparation.
2.3 Immunohistochemistry and TUNEL assay
Immunohistochemistry was performed on retinal cryosections as previously described (Fadool, 2003, Pillai-Kastoori et al., 2014, Wen et al., 2014). The following primary antibodies and dilutions were used: 4C12 (mouse, 1:100) generously provided by J. Fadool (Florida State University, Tallahassee, FL), which labels rod photoreceptor cell bodies; 1D1 (mouse, 1:100, J. Fadool, FSU, Tallahassee, FL), which recognizes Rhodopsin; Zpr-1 (mouse, 1:20, ZIRC), which labels red-green double cones; Zrf-1 (mouse, 1:5000, ZIRC), which labels Müller glia; HuC/D (mouse, 1:20, Invitrogen, Grand Island, NY), which recognizes retinal ganglion cells and amacrine cells; PKCα (rabbit, 1:100, Santa Cruz Biotechnology, Santa Cruz, CA), which recognizes bipolar cells, Prox1 (rabbit, 1:2000, Millipore, Billerica, MA), which recognizes horizontal cells; Nr2e3 (rabbit, 1:100), generously provided by J. Nathans (Johns Hopkins, Baltimore, MD), which labels rod photoreceptor progenitor/precursor cells; anti-PCNA (mouse, 1:100, Santa Cruz Biotechnology, Dallas, TX), which labels proliferating cells; anti-BrdU (mouse, 1:500, Sigma, St. Louis, MO), which marks cells in S phase of the cell cycle; and anti-Kaede (rabbit, 1:500, MBL, Nagoya, Japan), which labels Kaede expressing cells. Alexa fluor-conjugated secondary antibodies (Invitrogen, Grand Island, NY) and Cy-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were all used at a dilution of 1:200. Sections were counterstained with DAPI (1:10,000, Sigma, St. Louis, MO) to visualize cell nuclei. Terminal deoxynucleotide transferase (TdT)-mediated dUTP nick end labeling (TUNEL) was performed on retinal cryosections using the ApopTag Fluorescein Direct In Situ Apoptosis Detection Kit (Millipore, Billerica, MA) according to the manufacturer’s instructions.
2.4 BrdU exposure
Adult zebrafish were exposed to 0.5 % BrdU (Sigma, St. Louis, MO) in fish water overnight (16 hours) or continuously for 5 days. BrdU exposure took place in the dark, and the solution was refreshed daily. Eyes of 3 animals were dissected immediately following BrdU exposure for the indicated time, and retinal cryosections were prepared for anti-BrdU immunohistochemistry.
2.5 Real-time quantitative RT-PCR
Total RNA was extracted from the eyes of adult XOPS:mCFP and her4:Kaede zebrafish using TRIzol reagent (Invitrogen, Grand Island, NY) and first-strand cDNA synthesis was performed using the GoScript Reverse Transcriptase System (Promega, Madison, WI). Real time PCR was performed using FastStart Essential DNA Green Master Mix (Roche, Indianapolis, IN) on a Light Cycler 96 Real-Time PCR System (Roche, Indianapolis, IN). For all experiments, three biological replicates and three technical replicates were analyzed and the gene expression change was determined using a relative standard curve quantification method with atp5h expression as the normalization control.
2.6 Photoconversion
Albino her4:Kaede; XOPS:mCFP zebrafish were briefly anesthetized with Tricaine (Sigma, St. Louis, MO) and mounted on a coverslip in a pool of dilute Tricaine in fish water. The right eye of each animal was exposed to intense UV light for one minute using the DAPI filter on an inverted fluorescent microscope (Eclipse Ti-U; Nikon Instruments) with the neutral density filter at the lowest setting and at 100x total magnification. The left eye of each animal served as non-UV irradiated control. Following photoconversion, the fish were placed in fresh fish water for recovery and the eyes of three animals were dissected for cryosectioning at 24 hour increments from 0–7 days post photoconversion. A minimum of three animals were photoconverted and imaged for each time point.
2.7 Acute light damage
Acute light damage was performed as described previously (Vihtelic and Hyde, 2000). Adult albino her4:Kaede fish were dark adapted for 14 days in complete darkness, then immediately exposed to 18,000 lux light from four 250 W halogen bulbs centered around the tank. A bubbler, cooling fan, and water recirculation system were used to keep the temperature under 32°C. Eyes were dissected and retinal cryosections prepared at the indicated time points during and after light treatment and were processed for IHC. Eyes from three fish were analyzed for each time point.
2.8 Heat shock and DAPT treatment
24 hpf Tg(hsp70i:Gal4)Kca4 and Tg(UAS:myc-Notch1a-intra)Kca3 (hereafter called hsp70:Gal4 and UAS:NICD, respectively) compound heterozygote embryos and single transgenic controls were heat shocked twice for 30 minutes at 39°C and processed for WISH at 48 hpf. Pharmacological inhibition of Notch-Delta signaling in embryonic zebrafish was performed using N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT), a γ-secretase inhibitor that prevents proteolytic cleavage of the Notch intracellular domain from the lumen of the cell membrane (Yang et al., 2008). Wild type embryos were dechorionated and transferred to pre-warmed embryo medium containing 100 μM DAPT in 1% DMSO. Time-matched siblings in embryo medium with 1% DMSO served as carrier controls. Embryos were continuously treated from 10 until 48 hpf and then processed for WISH.
2.9 Dual luciferase assays
HEK293 cells were transfected with varying amounts of the pcDNA3.1 mammalian expression vector (Invitrogen) containing the zebrafish notch1a intracellular domain (NICD) cDNA or her4 cDNA, the pGL3 Firefly Luciferase reporter vector (Promega) containing a 3.4 kb her4 promoter fragment driving expression of the luciferase gene, the pRL-TK vector (Promega) containing the Renilla luciferase gene driven by a ubiquitous tyrosine kinase promoter (to control for transfection efficiency), and balancing levels of pGEM3Z (Invitrogen) using Fugene 6 (Promega) transfecting reagent according to the manufacturer’s instructions. The total mass of DNA and molar ratios of pGL3 and pRL-TK were held constant across transfections, which were repeated a minimum of 3 times. Dose response curves were generated using NICD at 0:100, 1:20, 1:10, and 1:5 molar ratios to the her4 reporter. Firefly and Renilla luciferase activity were measured at 24–36 hours post transfection using the DualGlo Luciferase Assay System (Promega). Data was analyzed as follows: Firefly luciferase (FFluc) was baselined against untransfected control (UTC) samples (= FFLuc – UTC) and normalized using the Renilla luciferase (RLuc). The Relative Luciferase Activity (RLA) was calculated as (FFLuc-UTC)/(RLuc).
2.10 Quantification and statistical analysis
Statistical analysis was performed using a Student’s t-test with Microsoft Excel or GraphPad Prism software. For comparing the number of her4-positive sections and the total number of her4-expressing cells in the CMZ and INL, optic-nerve containing and optic-nerve adjacent sections of 6 wild type and 6 XOPS:mCFP animals were examined. For all graphs, data are represented as the mean ± the standard deviation (s.d.).
3. Results
3.1 Her4 expression in the adult zebrafish retina
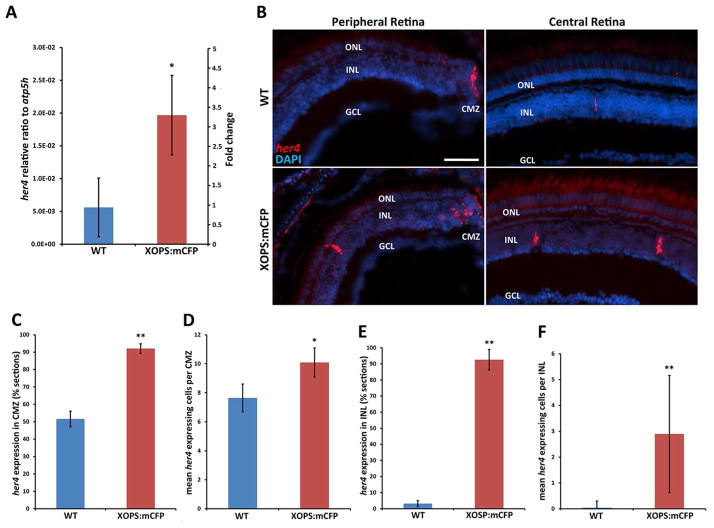
Previously, we found by microarray analysis that her4 was upregulated in the adult XOPS:mCFP retina, which undergoes chronic rod-specific degeneration and regeneration (Morris et al., 2011). To validate the microarray data, we performed quantitative RT-PCR (qRT-PCR) on mRNA prepared from wild type and XOPS:mCFP retinas using primers designed to amplify all five her4 tandem duplicate repeat variants (her4.1-her4.5) (Table S1). We found that her4 was upregulated over 3.5 fold in the XOPS:mCFP retina compared to wild type (3.5±1.08, p<.05) (Fig. 1A), confirming our previous results. To further investigate the possible roles of her4, we determined the expression pattern of her4 in wild type and XOPS:mCFP retinas by fluorescent in situ hybridization (FISH) with antisense probes that were designed to recognize all her4 variants. To validate our her4 in situ probes we performed FISH in the developing retina and found that the resulting expression pattern confirmed previous studies (Fig. S1A). From 48 hours post fertilization (hpf) to 5 days post fertilization (dpf), her4 expression shifted from the central retina to the ciliary marginal zone (CMZ) where expression persists into adulthood (Fig. 1B). Treatment with the Notch signaling inhibitor DAPT caused a decrease in her4 expression (Fig. S1B), whereas overexpression of the Notch intracellular domain (NICD) resulted in an increase in her4 expression (Fig. S1C), confirming that her4 is a target of Notch signaling in the zebrafish retina.
Figure 1. Her4 is expressed in proliferative regions of the adult retina and is upregulated in the XOPS:mCFP background.
A) qRT-PCR revealed a ~3.5 fold increase in her4 expression in the XOPS:mCFP retina. B) Fluorescent in situ hybridization (FISH) for her4 expression in the adult wild type and XOPS:mCFP central and peripheral retina demonstrated increased her4 expression in the XOPS:mCFP CMZ and INL. C) Percentage of retinal sections that contained her4-expressing cells at the CMZ (n=6 animals per group, >200 sections analyzed). D) Mean number of her4-expressing cells per CMZ. E) Percentage of sections that contained at least 1 her4-expressing cell in the INL. F) Mean her4-expressing cells per INL (n=6 animals per group, >200 INL sections analyzed). L, lens; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; CMZ, ciliary marginal zone; hpf, hours post fertilization; dpf, days post fertilization; scale bar, 50 μm; * P<0.05; ** P<0.001.
In the adult wild type retina, her4 was primarily expressed in the peripheral most areas of the CMZ (51.5±4.5% of all sections observed) (Fig. 1B, C). Occasionally, her4 expressing cells were also detected in the inner nuclear layer (INL) of the central wild type retina (3.1±1.8% of all INL sections observed) (Fig. 1B, E). In the wild type background, not only were the majority of sections negative for her4 expression in the INL, but the mean number of her4 positive cells per her4 expressing INL was low (1.37±0.66). In contrast, in a background of chronic, rod-specific degeneration and regeneration (XOPS:mCFP) her4 expression was significantly elevated. The percentage of XOPS:mCFP retinal sections containing her4-positive cells in the CMZ was nearly double that of the wild type retina (92.05±2.7%), and the total number of her4-expressing cells in the CMZ was also increased in the XOPS:mCFP background (WT retina: mean 7.64±0.95; XOPS:mCFP retina 10.08±1.0) (Fig. 1C, D). These results were unexpected because only rod photoreceptors are degenerating/regenerating in the XOPS:mCFP retina, and the CMZ is not thought to produce new rods. In addition to an increase in her4 expressing cells in the CMZ, we also observed an increase in her4-positive cells in the INL of XOPS:mCFP adult retinas compared to wild type (92.6±6.4% vs. 3.19±1.8% of sections analyzed) (Fig. 1E). The her4 positive cells in the INL of the XOPS:mCFP retinas were widely scattered across the central retina, and the total number of her4 expressing cells per section was highly variable, ranging from zero to as many as eleven positive cells per section (Fig. 1F). Interestingly, her4 expression was never detected in the outer nuclear layer (ONL) of the central retina, where rod progenitor cells reside in either wild type or XOPS:mCFP adults. Taken together, these results show that expression of her4 is significantly increased in both the INL of the central retina and the peripheral CMZ in response to selective rod photoreceptor degeneration.
3.2 Her4 is expressed in slowly proliferating subsets of Müller glia in the INL and more rapidly proliferating cells in the CMZ
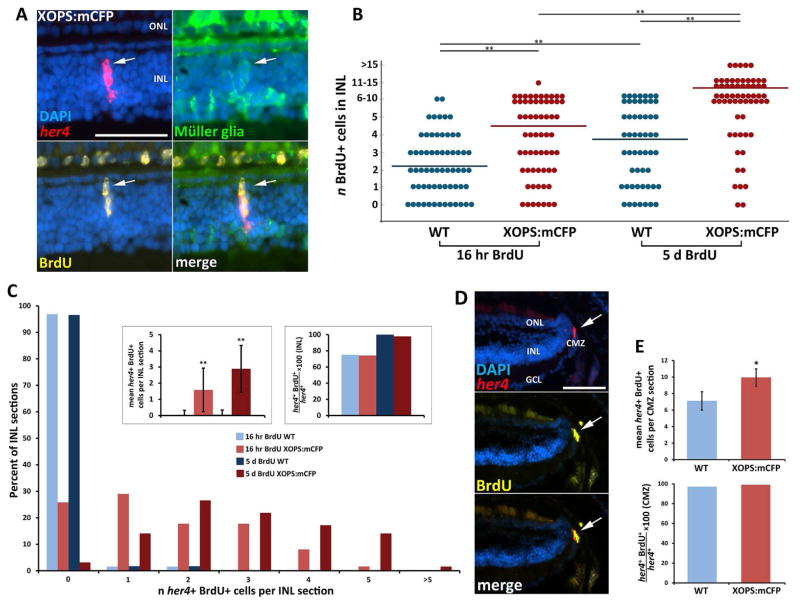
Previous studies have shown that Notch-Delta signaling regulates the proliferative response of Müller glia to acute retinal damage. Therefore, we investigated whether the her4 expressing cells in the INL of the XOPS:mCFP retinas were proliferating Müller glia. We performed FISH and immunolabeling experiments on adult gfap:GFP; XOPS:mCFP and gfap:GFP siblings that were exposed to BrdU for either 16 hours or 5 days. The gfap:GFP line expresses the GFP reporter specifically in glial cells of the zebrafish nervous system, including Müller glia in the retina (Bernardos and Raymond, 2006). In the INL of both the single transgenic gfap:GFP and double transgenic gfap:GFP; XOPS:mCFP retina, all her4 FISH signal co-localized with the GFP reporter, indicating that her4 expression in the INL is restricted to BrdU-positive Müller cells (Fig. 2A). In previous studies, we found that there is an increased number of proliferating rod progenitor cells in the ONL of the XOPS:mCFP when compared to the wild type retina (Morris et al., 2008b), but we did not detect a significant increase in BrdU positive cells in the INL of XOPS:mCFP retinas at 15 dpf, nor did we detect an increase in PCNA positive cells in the INL of XOPS:mCFP adults (Morris et al., 2005, Morris et al., 2008b). However, when we repeated these experiments with a greater number of samples we found a small but significant increase in the number of BrdU positive cells in the INL of the XOPS:mCFP central retina, and this increase over wild type was even larger when fish were exposed to BrdU for five days (Fig. 2B). To determine the proportion of BrdU-positive cells in the INL that also expressed her4, we performed FISH for her4 combined with immunohistochemistry for BrdU. After 16 hours of BrdU exposure, approximately 75% of her4 expressing cells in the INL of both the wild type and XOPS:mCFP retina had incorporated the BrdU label, whereas after 5 days of BrdU exposure, this proportion reached nearly 100%. This suggests that her4 is expressed in slowly proliferating subsets of Müller glia in the INL. Interestingly, after both short and long exposures to BrdU, the total number of her4/BrdU positive cells was significantly higher in the XOPS:mCFP INL than the wild type INL (Fig. 2C). This result suggests that chronic rod photoreceptor degeneration induces a small but significant increase in the number of slowly proliferating Müller glia in the inner retina.
Figure 2. Her4 is expressed in slowly proliferating subsets of Müller glia in the INL and rapidly proliferating cells in the CMZ.
A)Her4 FISH with anti-GFP and anti-BrdU immunohistochemistry (IHC) in retinal sections from XOPS:mCFP; gfap:GFP zebrafish revealed that her4 is expressed in proliferating Müller cells in the INL. B) Total number of BrdU-positive cells in the INL following either 16 hour or 5 day BrdU exposure in wild type and XOPS:mCFP zebrafish. There was a significant increase in the number of BrdU-positive cells in the INL with the longer BrdU exposure, and an increase of BrdU-positive cells in the XOPS:mCFP INL compared to WT (n=3 animals per group, >60 INL sections per group observed). C) Percentage of sections that contained n her4+ BrdU+ cells in the INL; insets show mean number of her4+ BrdU+ cells per INL section (left) and the percentage of her4+ cells that were also BrdU+ (right). D) Her4 FISH with anti-GFP and anti-BrdU IHC in retinal sections from XOPS:mCFP; gfap:GFP. Nearly all her4-expressing cells in the CMZ were BrdU+. E) There was a small but significant increase in the number of her4+ BrdU+ cells in the CMZ of the XOPS:mCFP retina (top). All of the her4+ cells in the CMZ were also BrdU+ after 16 hours of BrdU exposure in both WT and XOPS:mCFP retinas (bottom; n=6 animals, >120 sections analyzed). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; CMZ, ciliary marginal zone; scale bar, 50 μm; * P<0.05; ** P<0.001.
As described above, we detected the expression of her4 in the CMZ of wild type adult retinas and this expression was significantly increased in the CMZ of the XOPS:mCFP line. In both the wild type and XOPS:mCFP retinas, 99% of her4-expressing cells in the CMZ had incorporated BrdU after the 16-hr exposure (Fig. 2D, E). This result suggests that the population of cells at the her4-expressing cells in the CMZ proliferate more rapidly than the her4-expressing cells in the INL.
3.3 Generation of the her4:Kaede transgenic zebrafish line
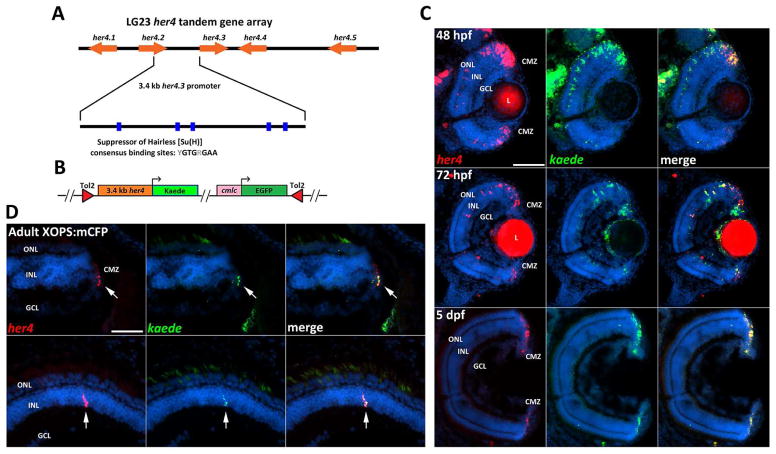
The identification of a subset of proliferating her4 expressing Müller glia in the XOPS:mCFP retina suggests that these cells may function to replenish the pool of more rapidly dividing rod progenitor cells in the ONL. To determine whether her4 expressing cells contribute to the rod photoreceptor lineage, we generated a transgenic line that expresses the photoconvertible Kaede reporter driven by a her4 promoter. A 3.4 kb region containing a segment of the 3′ UTR of her4.2 and the presumptive promoter of her4.3 (Fig. 3A) has been used previously to generate reporter lines indicating active Notch-Delta signaling (Yeo et al., 2007). This fragment contains five Suppressor of Hairless [Su(H)] consensus binding sites, 64 E-box and 2 N-box sequences. To verify that this her4 promoter segment is responsive to Notch signaling, we carried out in vitro reporter assays using Notch1a intracellular domain (NICD) cDNA and a luciferase reporter driven by the 3.4 kb her4 promoter. Co-transfection of the NICD expression vector and the luciferase reporter into HEK293 cells significantly increased luciferase activity, demonstrating that the 3.4 kb her4 promoter segment is sufficient to respond to Notch signaling (Fig. S2A). When a her4 expression vector was co-transfected with the her4 luciferase reporter, there was a dose-dependent decrease in luciferase activity, suggesting that her4 negatively regulates its own expression (Fig S2B).
Figure 3. Her4:Kaede zebrafish express the kaede transgene in a spatiotemporal pattern that recapitulates endogenous her4 expression.
A) Schematic representation of the her4 locus. The 3.4 kb fragment between her4.2 and her4.3 contains 5 Suppressor of Hairless [Su(H)] consensus binding sites that are necessary for Notch-Delta signaling. B) Schematic of the her4:Kaede expression construct. C) Two-color FISH shows co-localization of kaede and endogenous her4 transcript during development (red fluorescence in the lens is non-specific). D) Two-color FISH in the adult XOPS:mCFP retina shows co-localization of kaede and her4 transcripts in the CMZ (top row) and INL of the central retina (bottom row). L, lens; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; CMZ, ciliary marginal zone; hpf, hours post fertilization; dpf, days post fertilization; scale bar, 50 μm.
An expression construct was assembled containing the 3.4 kb her4 promoter upstream of the Kaede fluorescent reporter (Fig. 3B). The her4:Kaede line was generated using Tol2-mediated transgenesis (Kwan et al., 2007). To determine if the kaede transgene demonstrated the same spatiotemporal expression pattern in the retina as endogenous her4, two-color FISH was performed using DIG-labeled and FITC-labeled riboprobes to detect her4 and kaede mRNA respectively. In the developing retina of the her4:Kaede embryo, expression of the transgene overlapped with expression of endogenous her4 at 48, 72, and 96 hpf, and both transcripts could only be detected in the CMZ by 5 dpf (Fig. 3C). In adult her4:Kaede; XOPS:mCFP retinas, expression of the kaede transgene co-localized with endogenous her4 in all her4-positive cells in the INL and the majority of her4-positive cells in the CMZ (Fig. 3D). These data demonstrate that expression of the her4:Kaede transgene faithfully recapitulates expression of endogenous her4 in both the developing and regenerating retina.
The Kaede reporter is a highly stable 116 kDa homotetrameric protein that has a relatively long biological half-life compared to other fluorescent reporters (Chudakov et al., 2010). Following irradiation with ultraviolet light, the green fluorescing chromophore undergoes a β-elimination reaction that results in an irreversible photoconversion to red fluorescence (Ando et al., 2002). We tested the feasibility of photoconverting Kaede in living tissues to establish a UV treatment paradigm and to confirm that the her4:Kaede transgenic zebrafish would be useful for lineage tracing studies. Her4:Kaede embryos were briefly anesthetized at 48 hpf and exposed to low intensity UV light for 1 minute. Before photoconversion, green-emitting-Kaede (gKaede) could be observed in the retina and throughout the CNS in the GFP channel only (Fig. S3A, B). Following the short UV pulse, photoconversion into red-emitting-Kaede (rKaede) could readily be detected along with a reduction of green-emitting-Kaede (gKaede) (Fig S3C, D). The animals were allowed to recover and were observed intermittently for 3 weeks following the photoconversion procedure. Fluorescence of both gKaede and rKaede persisted for the entire observation period (data not shown). We then tested the photoconversion paradigm on juvenile (4–6 week old) zebrafish eyes to determine if rKaede fluorescence could be detected in the mature retina for a minimum of 1 week post photoconversion. We found that the rKaede signal was easily detectable in the central retina, and that within the 7 day window, a significant amount of new gKaede expression originating from the CMZ could be observed (Fig. S3E). These data not only indicate that the Kaede reporter is suitable for lineage tracing studies, but also confirm that her4-expressing cells in the CMZ proliferate to generate new retinal cells in the growing eye.
3.4 Her4-expressing Müller cells in the INL of the XOPS:mCFP retina contribute to the rod photoreceptor cell lineage
To determine the fate of her4-expressing Müller glia in the XOPS:mCFP retina, we conducted lineage tracing analyses. The entire right eye of albino XOPS:mCFP; her4:Kaede adults was briefly exposed to intense UV light, with the left eye serving as a non-UV treated control. Eyes were dissected at various time points post photoconversion, and retinal sections were processed for immunohistochemistry. Lineage tracing analysis was restricted to the central retina to avoid Kaede-expressing cells derived from the CMZ. Because the XOPS:mCFP reporter is expressed in rods prior to degeneration, it was not possible to distinguish green fluorescing Kaede (gKaede) from mCFP fluorescence in the ONL in the non-photoconverted eye. We therefore immunolabeled retinal sections with a rod-specific antibody to distinguish gKaede-expressing non-rod cells from mCFP-expressing rods.
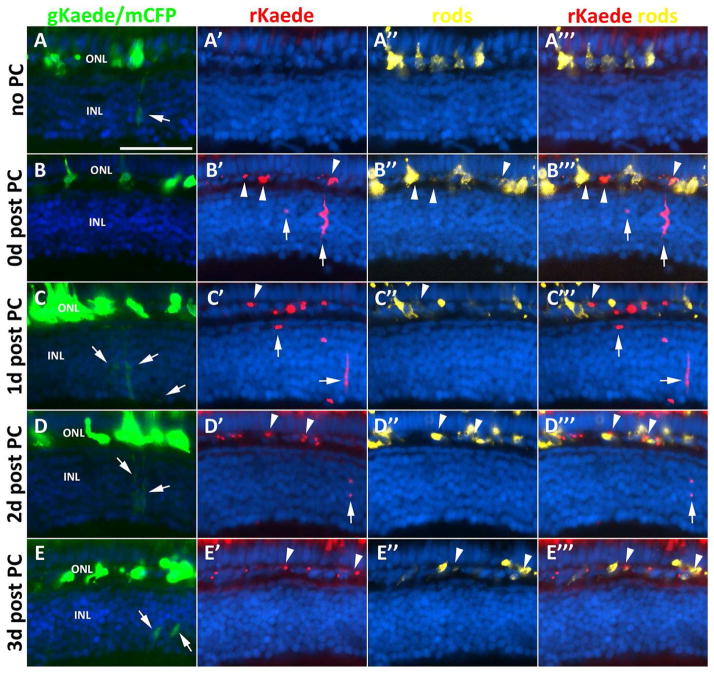
In the non-UV treated control eyes, all her4 expressing cells in the retina displayed green fluorescence, with no detectable red signal (Fig. 4A). In contrast, when observed immediately after UV treatment, the UV-exposed eye displayed complete green to red photoconversion of all Kaede expressing cells in the retina (Fig. 4B). At 0 days post photoconversion (0d post PC), cells in the ONL that expressed the photoconverted rKaede reporter co-localized with markers for rod photoreceptors, indicating that cells that once expressed her4 contribute to the rod lineage (Fig. 4B‴). In the INL, rKaede expressing cells with morphological characteristics of Müller glia were observed, as well as smaller round cells located in the vicinity of the presumptive rKaede-positive Müller glia. This pattern suggests that at the time of UV treatment, the lineage of her4 expressing cells was comprised of various cells in different stages of the rod replenishing pathway.
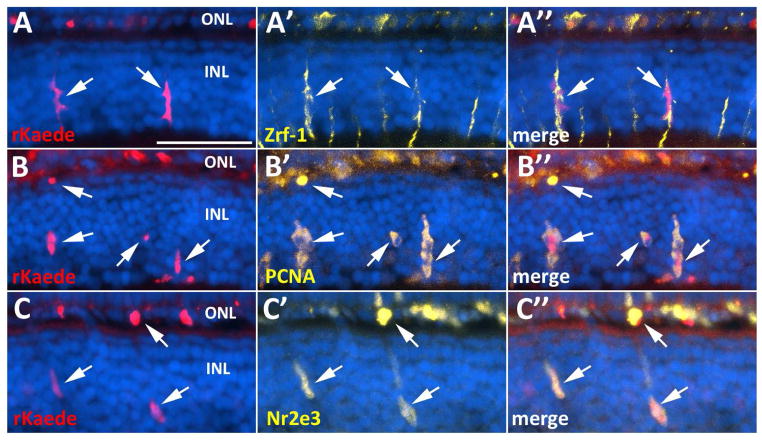
Figure 4. Her4-expressing cells in the INL contribute to the rod photoreceptor lineage.
Column 1, non-photoconverted Kaede (gKaede) and mCFP fluorescence; column 2, photoconverted Kaede (rKaede); column 3, immunolabeling for rod photoreceptors in the ONL with 4C12 antibody; column 4, merge of rKaede and 4C12. A–A‴) The non-photoconverted retina (no PC) shows gKaede fluorescence in the INL, and no rKaede fluorescence. B–B‴) The photoconverted retina immediately after UV treatment (0d post PC) shows only rKaede fluorescence in the INL (arrows), and co-localization of rKaede and 4C12 in the ONL (arrowheads). C–C‴) At 1d post PC, new gKaede expressing cells can already be detected in the INL (arrows) along with persistent rKaede in the INL and ONL. D–D‴) At 2d post PC, very little rKaede remains in the INL, but in the ONL rKaede persists in rod precursors. E–E‴) By 3d post PC, only gKaede can be detected in the INL (arrows). 3 animals and 30 sections were observed per time point. INL, inner nuclear layer; ONL, outer nuclear layer; CMZ, ciliary marginal zone; scale bar, 50 μm.
By 1d post PC, a large reduction in the number of rKaede-positive, elongated cells was observed in the INL, with an increase in the number of the smaller rounded cells in both the INL and ONL that retained rKaede signal. This same pattern was observed in the UV-treated eyes of at least three animals analyzed and in all sections examined. In addition, at 1d post PC, gKaede signal could already be detected in the INL of the same sections, indicating that her4 continued to be expressed in these cells after the UV treatment (Fig. 4C). At 2d post PC, the majority of sections observed contained no rKaede reporter expression in the INL. The few INL sections that were rKaede positive contained no elongated rKaede positive cells (Fig. 4D). By 3d post PC, only gKaede positive cells were found in the INL. However, numerous small rKaede positive cells were observed in the ONL (Fig. 4E). These data suggest that the cycle of her4 expression to rod neurogenesis is completed in under 3 days.
In order to determine the identity of her4 expressing cells in the INL of the XOPS:mCFP central retina, we performed immunohistochemistry with cell-type specific antibodies on retinal sections from XOPS:mCFP; her4:Kaede adults. Elongated rKaede-positive cells in the INL co-localized with markers for Müller glia, although only a small subset of Müller glial cells expressed the Kaede reporter (Fig. 5A). The elongated cells in the INL as well as the smaller rounded cells in the INL and ONL were also PCNA positive, indicating that subsets of Müller cells as well as their descendants were proliferating (Fig. 5B). Furthermore, some of the small, rounded rKaede-positive cells in the INL and all of the cells in the ONL that retained rKaede fluorescence co-localized with Nr2e3, a marker for rod progenitor cells and post-mitotic rod precursors (Fig. 5C). These data confirm that subpopulations of her4 expressing Müller glia cells proliferate in the INL and give rise to rod progenitor cells, which migrate to the ONL and differentiate into rods.
Figure 5. The her4-expressing lineage in the XOPS:mCFP retina includes proliferating Müller glia and photoreceptor progenitors.
A–A″) Photoconverted Kaede (rKaede) co-localizes with Zrf-1, a marker for Müller glia. B–B″) rKaede+ elongated cells and small rounded cells co-localize with PCNA. C–C′) Subsets of Nr2e3-positive cells in the INL and ONL of XOPS:mCFP retinas co-localize with the rKaede reporter. INL, inner nuclear layer; ONL, outer nuclear layer; scale bar, 50 μm.
3.5 Her4 expressing cells in the CMZ differentiate into retinal neurons and Müller glia, but not rods
To determine the fate of the her4 expressing cells in the CMZ, immunolabeling experiments were performed on her4:Kaede and her4:Kaede; XOPS:mCFP adult retinal sections. To rule out confounding mCFP fluorescence, double labeling was performed with anti-Kaede antibodies as well as markers for the various retinal cell types. Following the double labeling experiments, only the peripheral most areas of the central retina adjacent to the CMZ were analyzed to ensure that the Kaede positive cells we observed were derived from the CMZ.
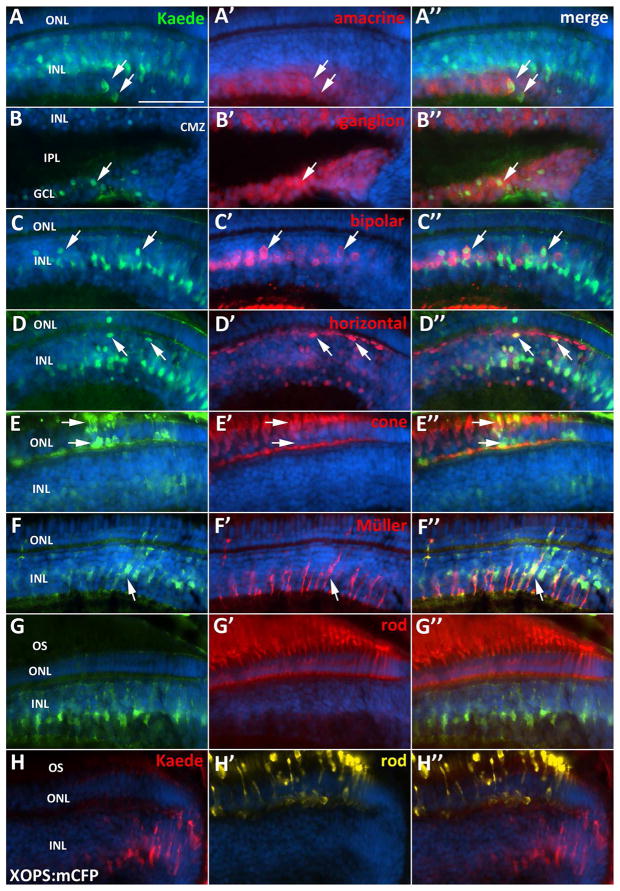
In the peripheral retina adjacent to the CMZ, we observed co-localization of the Kaede reporter with markers for Müller glia, amacrine, bipolar, and horizontal cells in the INL, as well as ganglion cells in the GCL (Fig. 6). Interestingly, although Kaede expression was detected in each of these cell types, not all of the cells in the peripheral retina were Kaede-positive. In addition, the intensity of Kaede fluorescence decreased with increasing distance from the CMZ. Markers for cone photoreceptors in the ONL also co-localized with Kaede, but the number of these double labeled cells detected in the ONL was small compared to the number of Kaede-positive INL and GCL neurons, suggesting that perhaps the turnover rate of Kaede is higher in cones, which are highly metabolically active (Fig. 6E).
Figure 6. Her4 expressing cells in the CMZ generate any retinal cell type except rod photoreceptors.
A–A″) Kaede and amacrine cell marker (HuC/D) co-localization in the INL. B–B″) Kaede and ganglion cell marker (HuC/D) co-localization in the GCL. C–C″) Kaede and bipolar cell marker (PKCα) co-localization. D–D″) Kaede and horizontal cell marker (Prox-1) co-localization. E–E″) Kaede and red-green cone photoreceptor marker (Zpr-1) co-localization. F–F″) Kaede and Müller glia marker (Zrf-1) co-localization. G–H″) Kaede does not co-localize with a marker for rod photoreceptors (4C12) in the WT retina (G–G″) or XOPS:mCFP retina (H–H″). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; OS, photoreceptor outer segments; CMZ, ciliary marginal zone; scale bar, 50 μm.
Previous studies have suggested that rod photoreceptors are not generated from the CMZ of teleost fish (Johns and Fernald, 1981, Johns, 1982, Otteson et al., 2001). However, as described above, we observed a significant increase in the number of her4-expressing cells in the CMZ of the XOPS:mCFP retina, in which only rod photoreceptors degenerate. We therefore asked whether her4-positive cells in the CMZ could also produce rod photoreceptors. We immunolabeled retinal sections with a rod-specific antibody and searched for rods in the peripheral retina that co-localized with the CMZ-derived her4:Kaede reporter. In agreement with previous studies, we did not detect Kaede expression in rods adjacent to the CMZ in any wild type sections (Fig. 6G–H). In addition, in XOPS:mCFP retinas we only detected one Kaede-positive rod adjacent to the CMZ in a single section (out of 160 sections examined). Although we cannot unequivocally conclude that rods are not generated from the CMZ, our data are in agreement with previous results, and suggest that the her4-positive cells at the peripheral most CMZ have the capacity to generate any retinal cell type except rods, even in the XOPS:mCFP background where the her4-expressing cell population in the CMZ is expanded.
3.6 Her4 is upregulated in the retina following acute light damage
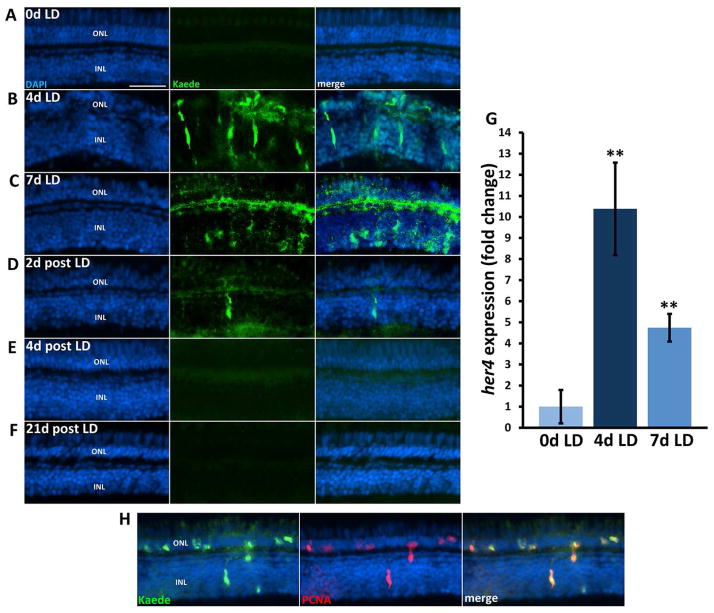
Because there was a small but significant increase of her4-expressing Müller glia in a background of chronic rod degeneration, we wanted to determine whether more significant damage would result in more her4 expression. Therefore, we examined her4:Kaede expression following acute light damage, which results in both rod and cone photoreceptor degeneration (Vihtelic and Hyde, 2000). In contrast to the slow, chronic rod degeneration observed in the XOPS:mCFP retina, intense light damage causes synchronized photoreceptor cell death, reactive gliosis, and proliferation followed by migration and differentiation into new photoreceptor cells. Adult her4:Kaede albino zebrafish were dark adapted for 14 days and then exposed to 18,000 lux light for 7 days. Immunohistochemistry was performed on retinal sections to confirm that the light damage resulted in rod and cone degeneration (Fig. S4). TUNEL and PCNA labeling confirmed that the light treatment resulted in photoreceptor apoptosis and an ensuing proliferative response (data not shown). An antibody to the Kaede reporter was used to characterize the extent and timing of her4 expression. Before the onset of light damage the Kaede reporter was not detected in the central retina (Fig. 7A). In contrast, after 4 and 7 days of light damage (4d and 7d LD), numerous Kaede positive cells were observed in the INL and ONL of the central retina (Fig. 7B, C). Double labeling experiments with PCNA revealed that the Kaede positive cells were proliferating (Fig. 7H). After 2 days of recovery post light damage (2d PLD), there were very few Kaede-positive cells in the INL and only a diffuse Kaede signal in the ONL (Fig. 7D). At 4 and 21d PLD, Kaede was not detected anywhere in the central retina (Fig 7E, F). QRT-PCR confirmed that her4 was upregulated over 10-fold at 4d LD (Fig. 4G). Interestingly, by 7d LD, the magnitude of her4 expression change decreased to 5-fold over 0d LD, and this correlated with an increase in rod and cone labeling in the ONL, even though the light treatment was ongoing (Fig. S4). These results suggest that after four days of intense light exposure, the zebrafish retina may adapt such that regeneration can proceed and further light damage is minimized. Taken together, these results demonstrate that the extent of her4 expression in the retina depends upon the amount of damage the retina receives.
Figure 7. Her4 expression is upregulated in the light damaged retina.
A) Kaede signal is not detected in the central retina before light damage onset (0d LD). B) Kaede signal is abundant in the central retina after 4 days of light damage (4d LD) and the morphology of the ONL is highly disrupted. C) At 7d LD Kaede signal is still highly expressed, the localization is more diffuse in the ONL. D) Few Kaede positive cells were observed at 2 days post light damage (2d post LD). E–F″) No Kaede fluorescence was observed in the central retina at 4d or 21d post LD. G. qRT-PCR confirms that her4 is upregulated ~11 fold at 4d LD and decreases to ~5 fold over 0d LD at 7d LD. H) Kaede and PCNA double labeling shows that Kaede expressing cells are proliferating in the acute light damaged retina. INL, inner nuclear layer; ONL, outer nuclear layer; scale bar, 50 μm; * P<0.05; ** P< .001.
4. Discussion
Due to its Notch-dependent expression in most tissues, the bHLH-O transcriptional regulator her4 has been studied in the context of neurogenesis and differentiation of various components of the CNS during development (Takke et al., 1999, So et al., 2009, Forbes-Osborne et al., 2013, Radosevic et al., 2014). In addition, her4 expression has been used as an indicator of active Notch-Delta signaling during regeneration of CNS tissues (Conner et al., 2014, Skaggs et al., 2014). Previous microarray data indicated that her4 was upregulated in the XOPS:mCFP retina, in addition to other components of the Notch pathway, including notch1a and deltaB (Morris et al., 2011). We therefore hypothesized that her4 plays a role in the regeneration of rod photoreceptors downstream of Notch-Delta signaling. In this study, we provide support for this hypothesis by characterizing and tracking the lineage of her4-expressing cells in the XOPS:mCFP retina. Although we showed that expression of her4 decreases when Notch signaling is knocked down, and increases when components of the pathway are upregulated, it is unclear if Notch signaling activates all variants of her4, or what variants of the gene are being expressed in the retina. It is possible that only one or a few of the her4 variants are expressed in the retina downstream of Notch and other duplicates could be expressed in non-retinal tissues downstream of other pathways.
In the undamaged adult retina, her4 was primarily expressed in proliferating cells in the peripheral CMZ. In contrast, in the XOPS:mCFP retina, we observed her4 expression in a small number of slowly proliferating Müller glia cells in the INL. This result is consistent with previous work showing that in response to acute retinal damage, Müller cells in the INL proliferate to produce neuronal progenitors (Bernardos et al., 2007, Thummel et al., 2008, Montgomery et al., 2010, Gorsuch and Hyde, 2014). However, whereas acute damage induces proliferation of a large number of Müller glia, in the XOPS:mCFP line only a very small subset of Müller cells scattered throughout the INL expressed her4 and were BrdU-positive. Significantly more of these her4-expressing INL cells incorporated BrdU after exposure to BrdU for five days (compared to an overnight exposure), demonstrating that her4 is expressed in slowly proliferating Müller glia as opposed to the more rapidly proliferating rod progenitor cells at the base of the ONL. Furthermore, even though the rods are continually degenerating in the XOPS:mCFP line and there is a significant increase of rod progenitor proliferation at the base of the ONL, we did not detect expression of her4 in the rod progenitor cells. Taken together, these data suggest that the role of the her4-positive proliferating Müller glia may be to replenish the rod progenitor pool as it gets depleted by continual rod photoreceptor regeneration.
Lineage tracing analysis using our her4:Kaede transgenic line crossed onto the XOPS:mCFP background revealed that the slowly proliferating her4-positive Müller cells in the INL give rise to Nr2e3-expressing progenitor cells. These progenitor cells then migrate to the base of the ONL where they differentiate into rod precursor cells and then rods. In contrast to the more rapid response resulting in neurogenesis following acute damage (Thummel et al., 2008, Thomas et al., 2012), we found the cycle of her4 expression to rod neurogenesis can take up to three days. As early as one day post-photoconversion, we observed new non-photoconverted Kaede in presumptive Müller glia in INL, demonstrating that the chronic rod degeneration and regeneration experienced in the XOPS-mCFP retina elicits a continual, albeit low-level, response in the INL. These data support the hypothesis that her4 upregulation in small subsets of Müller cells is associated with the need to restock the rod progenitor pool as those progenitors are continually depleted. It is possible that ONL rod progenitors have an intrinsic limit with regard to how many times they can divide to produce rod precursors, and therefore INL derived stem cells must replenish ONL progenitor numbers. The her4:Kaede transgenic line was also used to compare the her4 response in the XOPS:mCFP chronic damage model to the acute light damaged retina. We found that her4 was upregulated over 10 fold after 4 days of intense light treatment. Similarly, IHC experiments revealed that Müller cells in the INL that expressed the Kaede reporter were responsible for contributing to the proliferative response. These experiments show that the magnitude of the her4 response is correlated with the amount of damage to the retina.
In addition to upregulation of her4 in the INL of the XOPS:mCFP retina, her4 was also upregulated in the CMZ. This result was surprising because unlike other retinal neurons, rod photoreceptors are not derived from the CMZ but from populations of stem cells that reside in the INL of the central retina (Raymond et al., 2006, Morris et al., 2008a, Morris et al., 2008b). Increased mitotic activity, however, has been reported in the CMZ of the retina following acute damage, even though the cells generated do not directly contribute to the population of regenerated neurons (Negishi et al., 1982, Hitchcock and Raymond, 1992). We used the her4:Kaede transgenic line to determine what cell types her4-expressing stem cells in the CMZ have the capacity to generate. We found that cells in the peripheral retina that retained the Kaede reporter included all retinal cell types except for rod photoreceptors. Although we cannot rule out the possibility that small numbers of rods are generated from the CMZ, our results are consistent with previous observations. Why we observe a significantly expanded number of her4-expressing cells in the CMZ of the XOPS:mCFP retina even though they do not contribute to rod neurogenesis remains unclear. It has been shown that acute photoreceptor damage results in release of cytokines such as ciliary neurotrophic factor (CNTF) and tumor necrosis factor alpha (TNFα), resulting in increased expression of stat3 and ascl1a and Müller glia proliferation in the INL of the central retina (Kassen et al., 2007, Kassen et al., 2009, Nelson et al., 2012, Nelson et al., 2013). It is possible that soluble factors released by apoptotic rods in the ONL diffuse to the CMZ as well as the INL of the central retina, and the stem cell niche of the CMZ is stimulated to increase her4 expression, but is still restricted as to what cell types it can produce. In addition, the specific signals that stimulate the small number of Müller glia in the INL of the XOPS:mCFP retina to express her4 and contribute to the rod lineage remain to be elucidated. Possible mechanisms include juxtacrine signaling that stimulates Notch activity, gradients of extrinsic factors generated by dying photoreceptors, intrinsic cues that sensitize subsets of Müller cells to rod degeneration, or some combination of the above.
In summary, our results demonstrate that her4 is an effector of Notch-Delta signaling that is expressed in slowly proliferating subsets of Müller cells in the INL of the central retina as well as in proliferating cells of the peripheral CMZ. Previous studies concluded that there was no observable difference in the number of Müller glia in the chronic rod degeneration line (Morris et al., 2008b), and that there must be a large amount of synchronized rod cell death to initiate Müller glia proliferation in the INL (Montgomery et al., 2010). Here, we demonstrate that there is a small but significant increase in the number of slowly proliferating subsets of Müller cells in the INL of the chronic rod degenerating XOPS:mCFP retina, as well as an increase in her4 expression in the CMZ. Why her4 expression was increased in the XOPS:mCFP CMZ even though the CMZ did not generate rods, as well as why this proliferative zone is restricted from rod neurogenesis remains unclear. In addition, the her4:Kaede transgenic zebrafish proved to be a useful tool for lineage tracing analyses due to temporal labeling via photoconversion and the robust nature of the Kaede reporter. In the future, the her4:Kaede line could be used to not only track descendants of proliferating glia in the retina, but throughout the CNS.
Supplementary Material
A) Fluorescent in situ hybridization (FISH) for her4 expression in the developing retina at 48 hpf, 72 hpf, and 5 dpf. B) Whole mount in situ hybridization (WISH) performed on 48 hpf embryos show a decrease in her4 expression in embryos treated with the γ-secretase inhibitor DAPT compared to vehicle controls (1% DMSO). C) Single transgenic hsp70:Gal4 and double transgenic hsp70:Gal4; UAS:NICD 48 hpf embryos were heat shocked at 24 hpf and processed for WISH at 48 hpf. The double transgenic embryos displayed higher levels of her4 expression upon ectopic expression of the NICD. A, anterior; P, posterior; V, ventral; D, dorsal; L, lens; scale bar, 50 μm.
A) Dual luciferase assays were performed in HEK293 cells transfected with the pGL3-her4 firefly luciferase reporter, the pRL-TK Renilla luciferase transfection control reporter, and varying amounts of the pCDNA3-NICD expression vector with balancing amounts of pGEM3Z. There is a dose dependent increase in luciferase activity with increasing amounts of NICD. B) Dual luciferase assays in HEK293 cells using pGL3-her4 firefly luciferase reporter, the pRL-TK Renilla luciferase transfection control reporter, and varying amounts of the pCDNA3-her4 expression vector show that with increasing amounts of her4 expression there is a dosage dependent decrease in relative luciferase activity. * P<0.05, ** P<0.001.
A) Non-photoconverted (gKaede) fluorescence in the brain and retina (arrowheads) and cmlc-EGFP transgenesis reporter in the heart (arrow) in the GFP channel. B) No photoconverted Kaede (rKaede) can be detected in the red channel before UV treatment. C–D) Following brief UV exposure, there is a decrease of gKaede (C) and an increase of rKaede fluorescence (D). E) Cryosection of a mature retina 1 week after UV treatment reveals rKaede fluorescence in the central retina and the contribution of new gKaede-positive cells from the CMZ. L, lens; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; CMZ, ciliary marginal zone; hpf, hours post fertilization; scale bar, 50 μm.
Immunohistochemistry on retinal cryosections of light damaged her4:Kaede albino zebrafish confirm degeneration of rod and cone photoreceptors at 4 and 7 days post light onset (LD). OS, outer segments; ONL, outer nuclear layer; INL, inner nuclear layer; scale bar, 50 μm.
Supplemental Table 1. DNA sequence of primers and probes used in this study.
We characterize the expression of her4 in the regenerating zebrafish retina in a background of chronic, rod photoreceptor specific degeneration as well as following acute light damage
We describe a novel transgenic her4:Kaede reporter line for tracing the lineage of her4-expressing cells
We demonstrate that her4-expressing cells in the inner nuclear layer of the retina contribute to the rod photoreceptor lineage
We show that the magnitude of her4 expression during regeneration correlates with the extent of damage to the retina
Acknowledgments
Funding
This work was supported by a Grant from the National Institutes of Health (RO1EY021769, A.C.M.), the Pew Biomedical Scholar Program (A.C.M.) and Gertrude Flora Ribble Endowment summer Grants from the University of Kentucky Department of Biology. Some fish lines and antisera were obtained from Zebrafish International Resource Center (supported by NIH-NCRR grant P40 RR012546).
The authors would like to thank Sara Perkins for care and maintenance of zebrafish stocks, and the laboratory of Dr. Jakub Famulski for technical assistance.
Footnotes
Author contributions
A.C.M. and S.G.W. conceived and designed experiments, S.G.W., W.W., and L.P.K. performed the experiments. A.C.M. and S.G.W. analyzed the data, and A.C.M. and S.G.W wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abler AS, Chang CJ, Ful J, Tso MO, Lam TT. Photic injury triggers apoptosis of photoreceptor cells. Res Commun Mol Pathol Pharmacol. 1996;92:177–189. [PubMed] [Google Scholar]
- Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proceedings of the National Academy of Sciences. 2002;99:12651–12656. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao ZZ, Cepko CL. The expression and function of Notch pathway genes in the developing rat eye. J Neurosci. 1997;17:1425–1434. doi: 10.1523/JNEUROSCI.17-04-01425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardos RL, Lentz SI, Wolfe MS, Raymond PA. Notch-Delta signaling is required for spatial patterning and Müller glia differentiation in the zebrafish retina. Developmental Biology. 2005;278:381–395. doi: 10.1016/j.ydbio.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene expression patterns: GEP. 2006;6:1007–1013. doi: 10.1016/j.modgep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA. Genetic mechanisms of early neurogenesis in Drosophila melanogaster. Mol Neurobiol. 1995;10:75–89. doi: 10.1007/BF02740668. [DOI] [PubMed] [Google Scholar]
- Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA. Fluorescent Proteins and Their Applications in Imaging Living Cells and Tissues. 2010. [DOI] [PubMed] [Google Scholar]
- Conner C, Ackerman KM, Lahne M, Hobgood JS, Hyde DR. Repressing notch signaling and expressing TNFalpha are sufficient to mimic retinal regeneration by inducing Muller glial proliferation to generate committed progenitor cells. J Neurosci. 2014;34:14403–14419. doi: 10.1523/JNEUROSCI.0498-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Turner DL. Vertebrate hairy and Enhancer of split related proteins: transcriptional repressors regulating cellular differentiation and embryonic patterning. Oncogene. 2001;20:8342–8357. doi: 10.1038/sj.onc.1205094. [DOI] [PubMed] [Google Scholar]
- Fadool JM. Development of a rod photoreceptor mosaic revealed in transgenic zebrafish. Dev Biol. 2003;258:277–290. doi: 10.1016/s0012-1606(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Fadool JM, Dowling JE. Zebrafish: a model system for the study of eye genetics. Prog Retin Eye Res. 2008;27:89–110. doi: 10.1016/j.preteyeres.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Bongini R. Turning Müller Glia into Neural Progenitors in the Retina. Molecular Neurobiology. 2010;42:199–209. doi: 10.1007/s12035-010-8152-2. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Potential of Müller glia to become neurogenic retinal progenitor cells. Glia. 2003;43:70–76. doi: 10.1002/glia.10218. [DOI] [PubMed] [Google Scholar]
- Forbes-Osborne MA, Wilson SG, Morris AC. Insulinoma-associated 1a (Insm1a) is required for photoreceptor differentiation in the zebrafish retina. Dev Biol. 2013;380:157–171. doi: 10.1016/j.ydbio.2013.05.021. doi:110.1016/j.ydbio.2013.1005.1021. Epub 2013 Jun 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Mukherjee S, Bao Z-Z, Morrow EM, Cepko CL. rax, Hes1, and notch1 Promote the Formation of Müller Glia by Postnatal Retinal Progenitor Cells. Neuron. 2000;26:383–394. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- Ghai K, Zelinka C, Fischer AJ. Notch signaling influences neuroprotective and proliferative properties of mature Muller glia. J Neurosci. 2010;30:3101–3112. doi: 10.1523/JNEUROSCI.4919-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. Muller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15:431–442. doi: 10.1038/nrn3723. doi:410.1038/nrn3723. Epub 2014 Jun 1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorsuch RA, Hyde DR. Regulation of Muller glial dependent neuronal regeneration in the damaged adult zebrafish retina. Experimental eye research. 2014;123:131–140. doi: 10.1016/j.exer.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Hitchcock PF, Raymond PA. Retinal regeneration. Trends Neurosci. 1992;15:103–108. doi: 10.1016/0166-2236(92)90020-9. [DOI] [PubMed] [Google Scholar]
- Johns PR. Formation of photoreceptors in larval and adult goldfish. J Neurosci. 1982;2:178–198. doi: 10.1523/JNEUROSCI.02-02-00178.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns PR, Fernald RD. Genesis of rods in teleost fish retina. Nature. 1981;293:141–142. doi: 10.1038/293141a0. [DOI] [PubMed] [Google Scholar]
- Jung SH, Kim HS, Ryu JH, Gwak JW, Bae YK, Kim CH, Yeo SY. Her4-positive population in the tectum opticum is proliferating neural precursors in the adult zebrafish brain. Mol Cells. 2012;33:627–632. doi: 10.1007/s10059-012-0091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassen SC, Ramanan V, Montgomery JE, CTB, Liu CG, Vihtelic TS, Hyde DR. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev Neurobiol. 2007;67:1009–1031. doi: 10.1002/dneu.20362. [DOI] [PubMed] [Google Scholar]
- Kassen SC, Thummel R, Campochiaro LA, Harding MJ, Bennett NA, Hyde DR. CNTF induces photoreceptor neuroprotection and Muller glial cell proliferation through two different signaling pathways in the adult zebrafish retina. Experimental eye research. 2009;88:1051–1064. doi: 10.1016/j.exer.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Developmental dynamics: an official publication of the American Association of Anatomists. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development. 2011;138:4831–4841. doi: 10.1242/dev.072587. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien C-B. The Tol2kit: A multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Developmental Dynamics. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- Lenkowski JR, Raymond PA. Muller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res. 2014;40:94–123. doi: 10.1016/j.preteyeres.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JE, Parsons MJ, Hyde DR. A novel model of retinal ablation demonstrates that the extent of rod cell death regulates the origin of the regenerated zebrafish rod photoreceptors. J Comp Neurol. 2010;518:800–814. doi: 10.1002/cne.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AC. The genetics of ocular disorders: Insights from the zebrafish. Birth Defects Research Part C: Embryo Today: Reviews. 2011;93:215–228. doi: 10.1002/bdrc.20211. [DOI] [PubMed] [Google Scholar]
- Morris AC, Forbes-Osborne MA, Pillai LS, Fadool JM. Microarray analysis of XOPS-mCFP zebrafish retina identifies genes associated with rod photoreceptor degeneration and regeneration. Invest Ophthalmol Vis Sci. 2011;52:2255–2266. doi: 10.1167/iovs.10-6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AC, Scholz T, Fadool JM. Rod progenitor cells in the mature zebrafish retina. Adv Exp Med Biol. 2008a;613:361–368. doi: 10.1007/978-0-387-74904-4_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AC, Scholz TL, Brockerhoff SE, Fadool JM. Genetic dissection reveals two separate pathways for rod and cone regeneration in the teleost retina. Developmental Neurobiology. 2008b;68:605–619. doi: 10.1002/dneu.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AC, Schroeter EH, Bilotta J, Wong ROL, Fadool JM. Cone Survival Despite Rod Degeneration in XOPS-mCFP Transgenic Zebrafish. Investigative Ophthalmology & Visual Science. 2005;46:4762–4771. doi: 10.1167/iovs.05-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, v Weizsacker E, Campos-Ortega JA. Expression domains of a zebrafish homologue of the Drosophila pair-rule gene hairy correspond to primordia of alternating somites. Development. 1996;122:2071–2078. doi: 10.1242/dev.122.7.2071. [DOI] [PubMed] [Google Scholar]
- Negishi K, Teranishi T, Kato S. Growth zone of the juvenile goldfish retina revealed by fluorescent flat mounts. Journal of neuroscience research. 1982;7:321–330. doi: 10.1002/jnr.490070310. [DOI] [PubMed] [Google Scholar]
- Nelson BR, Hartman BH, Georgi SA, Lan MS, Reh TA. Transient inactivation of Notch signaling synchronizes differentiation of neural progenitor cells. Developmental Biology. 2007;304:479–498. doi: 10.1016/j.ydbio.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Ackerman KM, O’Hayer P, Bailey TJ, Gorsuch RA, Hyde DR. Tumor necrosis factor-alpha is produced by dying retinal neurons and is required for Muller glia proliferation during zebrafish retinal regeneration. J Neurosci. 2013;33:6524–6539. doi: 10.1523/JNEUROSCI.3838-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Gorsuch RA, Bailey TJ, Ackerman KM, Kassen SC, Hyde DR. Stat3 defines three populations of Muller glia and is required for initiating maximal muller glia proliferation in the regenerating zebrafish retina. J Comp Neurol. 2012;520:4294–4311. doi: 10.1002/cne.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Hyde DR. Muller glia as a source of neuronal progenitor cells to regenerate the damaged zebrafish retina. Adv Exp Med Biol. 2012;723:425–430. doi: 10.1007/978-1-4614-0631-0_54. [DOI] [PubMed] [Google Scholar]
- Otteson DC, D’Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232:62–76. doi: 10.1006/dbio.2001.0163. [DOI] [PubMed] [Google Scholar]
- Pasini A, Jiang YJ, Wilkinson DG. Two zebrafish Notch-dependent hairy/Enhancer-of-split-related genes, her6 and her4, are required to maintain the coordination of cyclic gene expression in the presomitic mesoderm. Development. 2004;131:1529–1541. doi: 10.1242/dev.01031. [DOI] [PubMed] [Google Scholar]
- Pillai-Kastoori L, Wen W, Wilson SG, Strachan E, Lo-Castro A, Fichera M, Musumeci SA, Lehmann OJ, Morris AC, Forbes-Osborne MA. Sox11 is required to maintain proper levels of Hedgehog signaling during vertebrate ocular morphogenesis. PLoS Genet. 2014;10:e1004491. doi: 10.1371/journal.pgen.1004491. doi:1004410.1001371/journal.pgen.1004491. eCollection 1002014 Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radosevic M, Fargas L, Alsina B. The role of her4 in inner ear development and its relationship with proneural genes and Notch signalling. PLoS One. 2014;9:e109860. doi: 10.1371/journal.pone.0109860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Hitchcock PF. Retinal regeneration: common principles but a diversity of mechanisms. Adv Neurol. 1997;72:171–184. [PubMed] [Google Scholar]
- Raymond PA, Rivlin PK. Germinal cells in the goldfish retina that produce rod photoreceptors. Dev Biol. 1987;122:120–138. doi: 10.1016/0012-1606(87)90338-1. [DOI] [PubMed] [Google Scholar]
- Scheer N, Campos-Ortega JA. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mechanisms of Development. 1999;80:153–158. doi: 10.1016/s0925-4773(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Scheer N, Groth A, Hans S, Campos-Ortega JA. An instructive function for Notch in promoting gliogenesis in the zebrafish retina. Development. 2001;128:1099–1107. doi: 10.1242/dev.128.7.1099. [DOI] [PubMed] [Google Scholar]
- Skaggs K, Goldman D, Parent JM. Excitotoxic brain injury in adult zebrafish stimulates neurogenesis and long-distance neuronal integration. Glia. 2014;62:2061–2079. doi: 10.1002/glia.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So JH, Chun HS, Bae YK, Kim HS, Park YM, Huh TL, Chitnis AB, Kim CH, Yeo SY. Her4 is necessary for establishing peripheral projections of the trigeminal ganglia in zebrafish. Biochem Biophys Res Commun. 2009;379:22–26. doi: 10.1016/j.bbrc.2008.11.149. [DOI] [PubMed] [Google Scholar]
- Stenkamp DL. Neurogenesis in the Fish Retina. In: Kwang WJ, editor. International Review of Cytology. Vol. 259. Academic Press; 2007. pp. 173–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takke C, Dornseifer P, v Weizsacker E, Campos-Ortega JA. her4, a zebrafish homologue of the Drosophila neurogenic gene E(spl), is a target of NOTCH signalling. Development. 1999;126:1811–1821. doi: 10.1242/dev.126.9.1811. [DOI] [PubMed] [Google Scholar]
- Thomas JL, Nelson CM, Luo X, Hyde DR, Thummel R. Characterization of multiple light damage paradigms reveals regional differences in photoreceptor loss. Experimental eye research. 2012;97:105–116. doi: 10.1016/j.exer.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Enright JM, Nelson CM, Montgomery JE, Hyde DR. Characterization of Muller glia and neuronal progenitors during adult zebrafish retinal regeneration. Experimental eye research. 2008;87:433–444. doi: 10.1016/j.exer.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000;44:289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Wen W, Pillai-Kastoori L, Wilson SG, Morris AC. Sox4 regulates choroid fissure closure by limiting Hedgehog signaling during ocular morphogenesis. Dev Biol. 2014;31:00667–00668. doi: 10.1016/j.ydbio.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A guide for the Laboratory Use of Zebrafish (brachydanio rerio) University of Oregon Press; Eugene, OR: 1995. [Google Scholar]
- Yang T, Arslanova D, Gu Y, Augelli-Szafran C, Xia W. Quantification of gamma-secretase modulation differentiates inhibitor compound selectivity between two substrates Notch and amyloid precursor protein. Molecular Brain. 2008;1:15. doi: 10.1186/1756-6606-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo SY, Kim M, Kim HS, Huh TL, Chitnis AB. Fluorescent protein expression driven by her4 regulatory elements reveals the spatiotemporal pattern of Notch signaling in the nervous system of zebrafish embryos. Dev Biol. 2007;301:555–567. doi: 10.1016/j.ydbio.2006.10.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Fluorescent in situ hybridization (FISH) for her4 expression in the developing retina at 48 hpf, 72 hpf, and 5 dpf. B) Whole mount in situ hybridization (WISH) performed on 48 hpf embryos show a decrease in her4 expression in embryos treated with the γ-secretase inhibitor DAPT compared to vehicle controls (1% DMSO). C) Single transgenic hsp70:Gal4 and double transgenic hsp70:Gal4; UAS:NICD 48 hpf embryos were heat shocked at 24 hpf and processed for WISH at 48 hpf. The double transgenic embryos displayed higher levels of her4 expression upon ectopic expression of the NICD. A, anterior; P, posterior; V, ventral; D, dorsal; L, lens; scale bar, 50 μm.
A) Dual luciferase assays were performed in HEK293 cells transfected with the pGL3-her4 firefly luciferase reporter, the pRL-TK Renilla luciferase transfection control reporter, and varying amounts of the pCDNA3-NICD expression vector with balancing amounts of pGEM3Z. There is a dose dependent increase in luciferase activity with increasing amounts of NICD. B) Dual luciferase assays in HEK293 cells using pGL3-her4 firefly luciferase reporter, the pRL-TK Renilla luciferase transfection control reporter, and varying amounts of the pCDNA3-her4 expression vector show that with increasing amounts of her4 expression there is a dosage dependent decrease in relative luciferase activity. * P<0.05, ** P<0.001.
A) Non-photoconverted (gKaede) fluorescence in the brain and retina (arrowheads) and cmlc-EGFP transgenesis reporter in the heart (arrow) in the GFP channel. B) No photoconverted Kaede (rKaede) can be detected in the red channel before UV treatment. C–D) Following brief UV exposure, there is a decrease of gKaede (C) and an increase of rKaede fluorescence (D). E) Cryosection of a mature retina 1 week after UV treatment reveals rKaede fluorescence in the central retina and the contribution of new gKaede-positive cells from the CMZ. L, lens; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; CMZ, ciliary marginal zone; hpf, hours post fertilization; scale bar, 50 μm.
Immunohistochemistry on retinal cryosections of light damaged her4:Kaede albino zebrafish confirm degeneration of rod and cone photoreceptors at 4 and 7 days post light onset (LD). OS, outer segments; ONL, outer nuclear layer; INL, inner nuclear layer; scale bar, 50 μm.
Supplemental Table 1. DNA sequence of primers and probes used in this study.