Abstract
The circadian system serves one of the most fundamental properties present in nearly all organisms: it generates 24-hr rhythms in behavioral and physiological processes and enables anticipating and adapting to daily environmental changes. Recent studies indicate that the circadian system is important in regulating the daily rhythm in glucose metabolism. Disturbance of this circadian control or of its coordination relative to the environmental/behavioral cycle, such as in shift work, eating late or due to genetic changes, results in disturbed glucose control and increased type 2 diabetes risk. Therefore, an in-depth understanding of the mechanisms underlying glucose regulation by the circadian system and its disturbance may help in the development of therapeutic interventions against the deleterious health consequences of circadian disruption.
Keywords: circadian rhythms, sleep, glucose metabolism, type 2 diabetes, melatonin, food timing
I. Lifestyle changes relevant to circadian disturbances and type 2 diabetes risk
Over the past decades, type 2 diabetes (T2D) has reached epidemic proportions worldwide and is projected to reach 439 million by 2030 [1]. The increasing prevalence of T2D can be attributed to dramatic lifestyle changes in response to the industrialization of modern society that may not be limited to changes in our diet and physical activity. It is estimated that 15-20% of the working population is involved in shift work and cross-time-zone travel [2]; more than 80% of the US population is regularly exposed to artificial light at night [3]; 50-70 million US adults have chronic sleep and wakefulness disorders [4]; and 70% of surveyed population in central Europe suffers from social jet lag [5]. In addition, irregular eating patterns and reduced daylight exposure are also common lifestyles changes in the modern 24/7 society [6, 7]. There is convincing evidence that all these lifestyle changes contribute to circadian (see glossary) disturbances [8-10]. Recently, accumulating epidemiological evidence has indicated that circadian disturbances in the forms of shift work [11, 12], late meal timing [6], late chronotype [13, 14], social jet lag [15], and sleep loss [16] are associated with increased risks of T2D. Evidence for a causal role of circadian disruption in the increased risk for T2D is provided by experimental studies showing that circadian disruption leads to impaired glucose control in healthy participants [17]. Moreover, the long-known daily rhythms in glucose control in both healthy humans and rodents have raised the question, what the contribution is of the endogenous circadian system. Therefore, in this review, we discuss the most current state of knowledge regarding the role of the circadian system in glucose control in human and rodent models, the mechanism underlying the association between circadian disruption and T2D, and potential therapeutic strategies to counteract the health hazards caused by circadian disruption.
II. Diurnal and circadian rhythms in glucose metabolism
It has been recognized for nearly five decades that, in normal human subjects, there is a diurnal rhythm in glucose tolerance. Oral glucose, intravenous glucose infusions, and identical meals all results in a significantly higher elevation of plasma glucose (i.e., lower glucose tolerance) in the evening than in the morning [18]. Similar diurnal rhythms have also been observed in rodents, such as in mice and rats. However, because they are nocturnal (i.e., active at night), they have lower glucose tolerance during the daytime (resting phase) than nighttime (active phase) [19]. There are multiple factors that could contribute to the reduced glucose tolerance, including decreased insulin sensitivity, excessive hepatic glucose production (HGP), and decreased beta-cell function. Multiple studies have shown that in healthy humans, both insulin sensitivity and beta-cell responsivity to glucose are lower at dinner than at breakfast [18]. The role of HGP in the diurnal regulation of glucose homeostasis in normoglycemic humans is still not clear. While some studies showed a sleep-associated fall in HGP [20] and an increased HGP at dawn [21], other studies found no diurnal rhythm in 24-hr fasting HGP [22] or a lower pre-meal HGP at breakfast comparing to lunch and dinner [23]. Different meal schedule on the test day in these studies may contribute to the different observation. However, convincing evidence has demonstrated a clear diurnal rhythm of HGP in patients with T2D that contributes to the dawn phenomenon (hyperglycemia in the morning) often observed in the diabetic patients [18, 22]. The elevated morning HGP before breakfast could be due to a prolonged overnight fast and the resultant surge of counterregulatory hormones (e.g., cortisol, growth hormones, norepinephrine) and/or due to a circadian modulation of HGP as suggested by rodent studies [19].
Lessons from human studies
Behavioral factors, such as food intake, fasting duration and activity level, are known to strongly impact glucose metabolism [6, 24, 25]. Therefore, due to the presence of a behavioral cycle (feeding/fasting, sleep/wake cycle, etc.) that occurs concurrent with the endogenous circadian cycle in the above studies, it is not possible to assess the independent effect of the circadian system on the 24-hr diurnal variations in glucose metabolism. There are a number of experimental protocols used in humans to disentangle the influence of the endogenous circadian system from the influences of environmental and behavioral cycles, as well as to look at their interaction (i.e., the effect of circadian misalignment, discussed below). One approach has been to displace the sleep episode by keeping individuals awake during their habitual sleep time and, instead scheduling daytime sleep (Figure 1A) [26]. This protocol takes advantage of the fact that the circadian system is slow to re-entrain to a shifted behavioral/environmental cycle. In healthy participants, using such a protocol in combination with constant glucose infusion and a seated posture, a significant rise in glucose level was observed at a time corresponding to the habitual sleep period, even though the participants were kept awake during that time [26]. As this rise occurred in the absence of sleep and of a fasting/feeding cycle, the result indicated circadian regulation of plasma glucose level in humans.
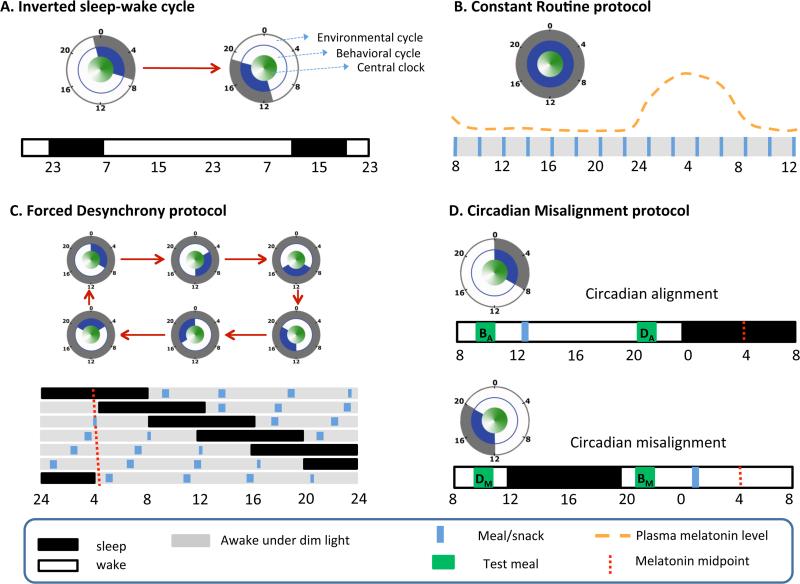
Figure 1. Protocols to assess circadian effects.
A) An inverted sleep-wake cycle protocol includes a period of extended wakefulness where sleep is displaced to the daytime (e.g., by 12 hr in this example). Study participants are sometimes required to have minimal physical activity, constant body posture, and/or constant nutritional state (e.g., constant glucose infusion). B) The Constant Routine (CR) protocol requires participants to remain awake, at rest, in a constant posture, with isocaloric intake distributed at equal intervals and under dim light conditions (to prevent the influence of light on the circadian system). CR protocols last longer than 24 hr allowing assessment of an entire circadian cycle after removal of the first few (transition) hr. C) The Forced Desynchrony (FD) protocol includes non-24-hr behavioral cycles (e.g., 28 or 20 hr, including sleep/wake and fasting/feeding cycles) under dim light conditions. The sleep:wake ratio is typically (although not necessarily) maintained at a 1:2 ratio. D) A misalignment protocol that simulates night work is compared with an alignment protocol simulating day work using a within-participant, cross-over design in randomized order. The independent effects are assessed by i) averaging breakfast (BA and BM) and dinner (DA and DM) values separately (behavioral effect); ii) averaging (BA and DM) and (DA and BM) values separately (circadian phase effect); iii) averaging (BA and DA) and (BM and DM) values within each protocol (circadian misalignment effect). The concentric circles indicate the phase relationship among environmental cycle (grey), behavioral cycle (blue), and the central clock (green) in each protocol. A filled grey/blue circle means constant environment/behaviors.
A similar but more standardized approach is the Constant Routine (CR) protocol, in which participants are kept awake in dim light to minimize the influence of light on the circadian system, and under strict semi-recumbent posture with equally spaced isocaloric snacks. Hereby, the CR protocol removes the periodic influence from light/dark, feeding/fasting, sleep/wake, rest/activity and postural cycles which are known to affect many physiological outputs [27] (Figure 1B). Under CR conditions, plasma glucose levels show a clear but relatively small circadian rhythm with a peak in the circadian night [28], consistent with the results from aforementioned study [26].
One limitation of both the displaced sleep protocol and the CR protocol is the gradually increasing homeostatic sleep pressure across the forced wakefulness, which may influence the outcome variable of interest, in this case plasma glucose control. One protocol designed to minimize such influence is the forced desynchrony (FD) protocol during which the study participants are scheduled to live on a behavioral cycle that is outside the range of entrainment of the central circadian pacemaker, e.g., a 20-hr or 28-hr cycle, under dim light conditions (Figure 1C). Under these conditions, the circadian system will express its internal circadian period. As a result, the scheduled behavioral cycle (including fasting/feeding, sleep/wake, and rest/activity under dime light conditions) is distributed evenly across all circadian phases, allowing the study of the separate effects of the circadian system from those of the behavioral cycles – as well as their interacting effects, i.e., circadian alignment vs. circadian misalignment which will be discussed next [29]. Under such FD conditions, a significant circadian rhythm in plasma glucose with a relatively small peak during the biological night is also observed [29]. This consistent finding across the three aforementioned protocols (including either continuous glucose infusion, small snacks, or meals), of a circadian peak in plasma glucose concentrations in the biological night, indicates that the endogenous circadian rhythm in glucose control in humans is robust.
Decreased glucose tolerance is a risk factor for and diagnostic measure of T2D. In healthy, normoglycemic individuals, glucose tolerance in response to an identical meal is relatively impaired in the evening and night, as compared to the morning [30]. A recent study tested whether this difference in glucose tolerance between morning and evening/night was due to an influence of the circadian system or of the behavioral and environmental cycle (e.g., overnight fasting, sleep, inactivity and darkness preceding the morning and a shorter fast, extended wakefulness, physical activity, and light exposure preceding the evening). The study used a randomized, crossover design to determine glucose tolerance in response to identical mixed meals, using a rapid 12-h shift of the behavioral/environmental cycle. The meals were given in the circadian morning and circadian evening, when the behavioral and environmental cycle was either aligned or misaligned relative to the endogenous circadian system [31] (Figure 1D). A substantially larger contribution from the endogenous circadian system to the morning-evening difference in glucose tolerance was found, as compared to the combined behavioral/environmental differences between morning and evening, suggesting a dominating role of the endogenous circadian system in the morning-evening difference in glucose tolerance.
Limited lessons from animal experimental studies
Few studies have tried to disassociate the circadian effects from behavior cycle effects in in-vivo rodent models, partly because it is difficult to control their behavior and keep them continuously inactive and awake without anesthesia or without causing restraint stress. Feeding regimens, such as evenly distributed food intake over 24 hr, have been used in rats to demonstrate a daily rhythm in glucose control and meal-induced insulin responses independent of the rhythm in food intake [19, 32]. However, because sleep and physical activity were not uniformly distributed across the day and night in those studies, it cannot be excluded that the presence of a sleep/wake and rest/activity cycle contributes to this rhythm in glucose metabolism. Despite the above limitations, animal models have their own advantages, especially when it comes to exploring mechanism (See Box.2).
III. Mechanisms linking circadian disruption and glucose metabolism
Given the clear circadian regulation of glucose metabolism, it is not surprising that disruption of the circadian system exerts adverse effects on glucose metabolism. As the term “circadian disruption” describes various circumstances, different experimental strategies have been used to investigate its metabolic consequences (Figure 2). Generally speaking, circadian disruption is a disturbance of biological timing, which can occur at different organizational levels and/or between different organizational levels, ranging from molecular rhythms (See Box 1) in individual cells to misalignment of behavioral cycles with environmental changes [33]. The Key Figure (Figure 3) presents a schematic depiction of the different types of circadian disruption. When studying the mechanisms of circadian disruption on glucose metabolism, it should be noted that different experimental approaches may lead to different levels of circadian disruption, thus resulting in different metabolic consequences through different pathways.
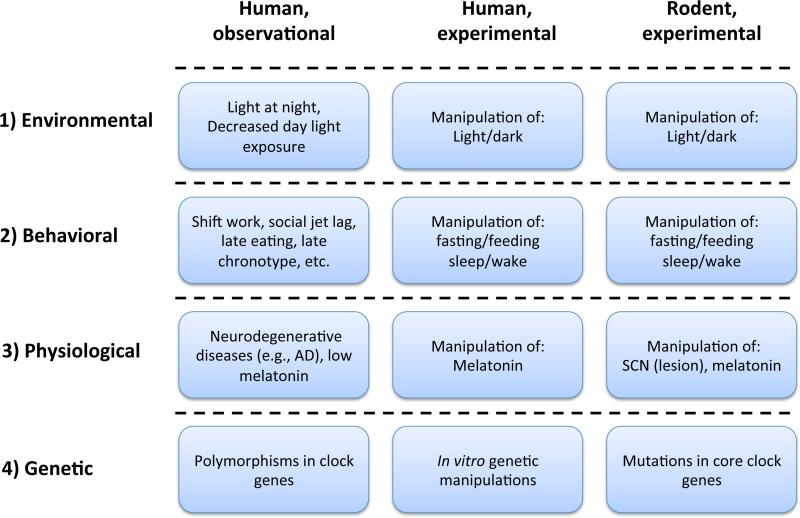
Figure 2. Different strategies to study the adverse effects of circadian disruption in rodents and humans.
1) Light is the strongest Zeitgeber for the central clock. Light/dark cycles are widely used in both human and rodent experimental studies to modulate and disturb the circadian system. Environmental light conditions are often considered in human observational studies. 2) In experimental studies, behavioral misalignment with the central clock is achieved by manipulation of specific behavioral cycles. Lifestyles related to circadian disruption (as shown) are often assessed in human observational studies. 3) Considering the importance of melatonin in the circadian system, melatonin administration and suppression of endogenous melatonin (e.g. bright light treatment, pinealectomy) can be used as experimental approaches to alter the circadian system. Low nocturnal melatonin levels have also been found in T2D patients [99]. In rodent, SCN lesioning has been used as a circadian disruption model. In human postmortem studies, changes in the anatomy of the SCN has been found in Alzheimer's patients who are also known to display circadian deficits. 4) Rodent models with genetic mutations in core clock genes have been used to study the role of molecular clock. Studying the function of clock genes in human is difficult. One approach is to study polymorphisms in those genes. Another is by manipulating clock gene expression in human isolated tissues.
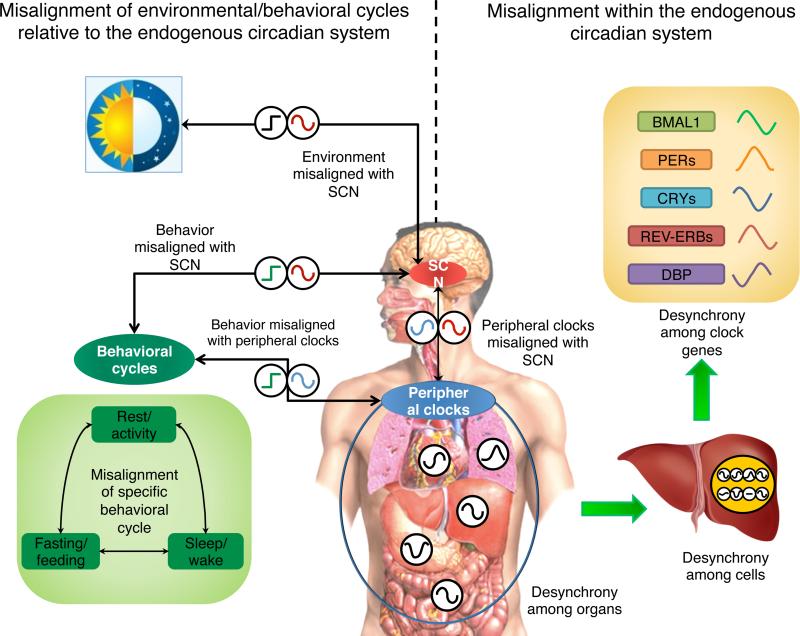
Figure 3. Circadian disruption at different levels.
First, at a systemic level, circadian disruption occurs when environmental cycles (black square wave, e.g., light/dark cycle; which we refer to as “environmental misalignment”) and/or behavioral cycles (green square wave, e.g., sleep/wake, fasting/feeding, rest/activity cycle; “behavioral misalignment”) are misaligned relative to the central clock in the SCN (red cosine). Alternatively, exposure to light at night can shift the central clock, which can cause misalignment of the central clock with the behavioral cycle if the behavioral cycle doesn't shift, which may occur in an intensive care unit. Second, at an organismal level, circadian disruption can be caused by internal misalignment (also called “internal desynchrony”) between the central clock and peripheral clocks (blue cosine), which can be induced by, e.g., misaligned eating (although direct evidence in humans is missing). It also refers to misalignment among peripheral clocks in different organs, where peripheral clocks are in abnormal phase relationships with each other. At a tissue level, circadian disruption can be caused by desynchronization among cells within a tissue (clocks in each individual organs and/or cells are represented as black cosine) Finally, at cellular level, expression of clock genes should also follow particular phase relationships that can be disturbed. Note that the illustrated phases of the cosine/square wave curves in the Figure does not necessarily convey the time of the highest levels, but conveys a conceptual illustration of alignment (when the acrophases, or timing of peaks, occur at an optimal phase relationship) versus misalignment (when the relationships between acrophases are abnormal).
One common tool used here is the genetic animal model with loss-of-function mutations in circadian genes. Global circadian mutants are commonly used to elucidate the importance of the molecular clock to glucose metabolism [34]. However, because glucose regulation is a tightly controlled process involving multiple organs, these studies cannot distinguish the effects of different central/peripheral clocks. Therefore, tissue-specific knockout studies are needed to delineate how the circadian clock in each peripheral tissue contributes to the observed metabolic phenotypes and whether these effects are independent of changes in circadian rhythms in locomotor activity (that are primarily driven by the master pacemaker).
Impact of BMAL1 tissue-specific deletion in rodents
BMAL1 is a transcription factor and core clock gene that drives rhythmic gene expression and regulates biological functions under circadian control. Liver-specific Bmal1 knockout (L-Bmal1KO) mice were first shown to exhibit hypoglycemia during the fasting phase, and increased glucose tolerance [34], which might be masked by reduced gluconeogenic gene expression when fed a chow diet. When challenged with high-fat diet, the L-Bmal1KO mice developed hepatic insulin resistance due to the accumulation of oxidative stress caused by dysfunctional mitochondria in the liver [35].
Deletion of Bmal1 in pancreatic beta-cells seems to have a pronounced effect on glucose tolerance, since these mice have diminished glucose-stimulated insulin secretion due to defective insulin exocytosis [36], and elevated oxidative stress [37]. When challenged with a high-fat diet, the normal adaptive beta-cell expansion is absent in these mice due to diminished cell proliferation and increased apoptosis [38].
In muscle-specific Bmal1 knockout mice, neither glucose tolerance nor insulin tolerance tests show any significant differences under chow-diet [39]. However, a closer look revealed defective muscle glucose uptake in-vitro [39], and reduced expression of circadian genes involved in glucose utilization [40].
Adipocyte-specific Bmal1 knockout in mice does not result in changes in insulin sensitivity or downstream insulin signaling in the adipose tissue, even on a high-fat diet and with significantly more weight gain [41].
These tissue-specific studies show that ablation of Bmal1 in various tissues causes different effects on glucose homeostasis. Bmal1 is widely used in tissue-specific studies as the only core circadian gene which can confer arrhythmicity with a single knockout. However, it should be pointed out that since Bmal1 also has non-circadian functions, the phenotypes observed in Bmal1 knockout animals may not be totally attributed to disruption of the molecular clock [42]. For example, opposite to L-Bmal1KO mice, RNAi-mediated knockdown of Cry1 and Cry2, both essential for maintenance of circadian rhythms, in the liver in-vivo, increased circulating glucose level and glucagon-stimulated hepatic glucose production [43]. In addition, clock genes also can play a role in development [44]. Therefore, studies on inducible tissue-specific double knockout of other clock genes are needed to verify these findings. Alternatively, constitutive expression of clock gene models could unravel whether the expression of clock genes or their rhythmicity is vital to glucose homeostasis.
Environmental and behavioral circadian misalignment
Besides genetic models, environmental/behavioral protocols to induce circadian misalignment are often used in both animal and human studies to explore the physiological/pathological consequences of circadian disruption. It should be pointed out that circadian misalignment protocols involve a collection of behavioral cycles [29, 31, 45] (e.g., sleep/wake, fasting/feeding, rest/activity, and posture cycles), as well as a light/dark cycle [46-51] that can be misaligned separately or in concert relative to the central circadian clock. Most protocols induce circadian misalignment by uncoupling overall behavioral cycles (the collection of all behavioral cycles) and/or light/dark cycles from the central circadian clock. As people become more aware of the different contributions of specific environmental/behavioral cycles to the metabolic consequences of misalignment, specific circadian misalignment protocols aiming to dissect the differential effects and mechanisms will be important in determining effective behavioral interventions for maladaptation to circadian misalignment. In human studies, for example, in the aforementioned 10-day FD protocol [29], circadian misalignment induced glucose intolerance quickly (within three days) in previously normoglycemic participants, seemingly due to a reduction in insulin sensitivity and insufficient beta-cell compensation. FD plus sleep restriction for 3 weeks led to more severe metabolic outcomes, as both increased postprandial glucose level and reduced insulin response were observed [52]. Because circadian misalignment in the ‘real world’ doesn't happen in the form of 28-hr days under dim light, Morris et al. recently conducted a simulated night shift protocol to simulate more realistic conditions. In this study, a rapid 12-hr inversion of the behavioral and environmental (light/dark) cycles decreased postprandial glucose tolerance, possibly by decreasing insulin sensitivity [31], and these adverse metabolic effects were also observed in chronic shift workers who underwent a similar protocol [53]. The above studies, using statistical methods, suggested that the effects of circadian misalignment were at least in part independent of the sleep loss (which is itself a consequence of circadian misalignment) [29, 31, 53]. By keeping the sleep duration identical in both circadian alignment and misalignment groups (by restricting it to the same amount), Leproult et al. could show experimentally that circadian misalignment reduced insulin sensitivity independently of sleep loss [45]. These results together indicate that circadian misalignment influences metabolism above and beyond the effects of circadian misalignment-induced sleep loss.
Among all the components of behavioral misalignment, disrupted sleep/wake and feeding/fasting cycles are two major aspects of circadian misalignment, which have received a lot of attention recently. Therefore, in the next part, we discuss the differential effects of sleep/wake cycle, food timing, and circadian system on metabolism in addition to the mechanisms mediating those effects.
Consequences of disrupted sleep/wake cycle and food timing
Without explicit circadian misalignment (although sleep disturbances may cause them, for example due to changes in the light/dark cycle), sleep disturbances per se are known to result in impaired glucose tolerance and increased diabetes risks [16]. The mechanisms linking sleep disturbances with glucose metabolism are still under investigation. One study suggested that 4 nights of sleep restriction in healthy lean human participants reduced peripheral insulin sensitivity in part by altering the insulin signaling pathways in the white adipose tissues [54]. However, how sleep loss influences fat cell biology is still unknown. Another possible explanation is elevated plasma cortisol levels and activation of the sympathetic nervous system that has been shown by studies including sleep restriction [55], since both factors can promote insulin resistance and abnormalities in glucose metabolism [56, 57]. In the long run, sleep restriction may also contribute to obesity - a major risk factor for insulin resistance, since it appears to increase caloric consumption in excess of changes in daily energy expenditure [59] and without increases in physical activity [58]. Sleep disturbances impair glucose tolerance even without restriction of sleep duration, as both sleep fragmentation and selective suppression of slow-wave sleep without change in total sleep duration have been shown to reduce glucose tolerance in health human participants [55, 60]. It is known that sleep is regulated by 2 primary biological processes: sleep homeostasis (increasing sleep drive with increasing duration of wakefulness) and the circadian system [61]. Based on this circadian influence, sleep at an abnormal circadian time results in reduced sleep duration and altered sleep architecture [62, 63]. Thus, sleep disturbances may partially (but not fully, see above) mediate the relationship between circadian misalignment and abnormal glucose metabolism. On the other hand, sleep disturbances may lead to circadian misalignment [64-67], which raises the possibility that circadian misalignment and disrupted sleep/wake form a vicious circle that contributes to the metabolic dysfunctions through both shared and differential pathways.
A late eating pattern is another modern life style that may lead to a certain degree of circadian misalignment. Emerging evidence indicates that a late eating pattern can exert negative impacts on glucose control. On the one hand, late meal timing may cause insulin resistance indirectly by increasing body weight [68-70]. On the other hand, even after adjusted for body mass index (BMI), a habit of late-night-dinner eating is still associated with hyperglycemia [71]. In addition, in-laboratory studies show that experimentally delaying meals in healthy individuals decreases glucose tolerance [72, 73]. Because the circadian system causes glucose tolerance to decrease as the day progresses [31], the observed decrease in postprandial glucose tolerance when eating late may be due to a later time of food intake relative to the circadian system. Interestingly, in rodents it has been demonstrated that feeding can entrain peripheral clocks (especially those in the digestive system) without altering the SCN rhythm [74]. In this way, misaligned feeding can lead to internal misalignment between the central and peripheral clocks, so called internal desynchrony (Figure 3). Future studies are required to test whether food timing can also induce internal desynchrony in humans. Such internal misalignment may compromise glucose tolerance by disturbing the time-coordinated tissue functions. Furthermore, a number of studies proposed that feeding-related hormones, such as insulin [75], glucagon-like peptide-1 (GLP-1) [76], and oxyntomodulin [77], can act as entrainment signals for peripheral clocks. However, it is not known how these feeding-related entrainment signals interact with those controlled by central clock (body temperature, glucocorticoids, etc.), and how these mixed signals affect the molecular clock and metabolic functions of each peripheral tissue.
IV. Clinical implication; prevention and treatment
Given the increasing prevalence of circadian disruption and its deleterious health consequences, it is important to prevent and minimize circadian disruptions in our daily life and their detrimental effects. Currently, a growing number of researchers are looking for interventions ranging from modulating environmental cues to manipulation of molecular machinery. First, at the environmental level, recommendations include increasing daytime light exposure while minimizing artificial light at night. For those already suffering from circadian disorders, bright light treatment can be considered [78]. Second, at the behavioral level, we need to increase public awareness about healthy timing of sleep and food intake (although knowledge on the best timing of the latter is still very much in development). When shift work is unavoidable, shift work schedules with gradual phase delay instead of abrupt schedule inversion, and personalized shift (and sleep) schedules based on chronotype may be also beneficial [79]. Regarding food timing, animal studies have suggested that time-restricted feeding (TRF) beneficially effects metabolism. In these studies, restricting feeding to the active phase can limit body weight gain without decreased caloric intake in rodents with simulated night work schedules [80] or exposed to light at night [81]. Future studies are required to determine how to best use meal timing as an intervention against circadian disruption in humans, and what the relative importance is of timing of food intake as opposed to lengthening the duration of fasting that is typical in most TRF protocols [6]. Third, at the physiological level, administration of circadian-related hormones might be a therapeutic strategy. For example, melatonin is a hormone produced in the pineal gland at night, acutely inhibited by light exposure, tightly controlled by the SCN and can act as an entrainment signal for the circadian system [33]. The relatively recent discovery of the strong association between MTNR1B (gene for melatonin type 2 receptor, expressed in various tissues including pancreatic beta-cells) variants and T2D risks [82-84], has raised the question whether supplementing melatonin can be used in the treatment or prevention for T2D. However, as existing studies have showed opposite effects of in vivo/in vitro [85, 86], acute/chronic [87, 88] and diurnal human/nocturnal rodent [89] melatonin administration, future studies aimed to clarify the exact action and functional impact of melatonin need to take these aspects into consideration. Last but not least, small molecules that can modulate the circadian clock are also emerging as putative therapeutic agents. As these small molecules have the potential to change the period, increase the amplitude, or shift the phase of the clock machinery [90, 91], they may be useful to minimize the duration of individual's exposure to an unhealthy, misaligned state.
V. Concluding Remarks
In recent years, the role of the circadian system in the control of glucose metabolism gained clinical interest based on epidemiological data linking lifestyles related to circadian disruption to increased risks of T2D and obesity. A role of the circadian system in the daily glucose control was further indicated by human in-laboratory studies demonstrating a circadian rhythm in glucose control, independent of the behavioral influences. Animal studies have shown that circadian regulation of glucose metabolism can happen at the level of the central clock within the SCN and at the level of peripheral clocks. The SCN exerts a direct influence on glucose metabolism through ANS and hormonal outputs. Peripheral clocks influence glucose metabolism by governing the circadian expression of genes involved in cellular metabolic pathways. It is still not clear how these different levels of circadian regulation are coordinated with each other to achieve optimal glucose control (see Outstanding Questions). An emerging area of research is trying to address this question with systems biology approaches (e.g. transcriptomics, epigenomics, proteomics, metabolomics and network analysis). On the other side, glucose homeostasis is a physiological process governed by multiple organs, including liver, pancreas, muscle, adipose tissues, and gut. Rodent studies with tissue-specific Bmal1 knockout have suggested varying impacts of different tissues. For this reason, future human studies are needed to conduct in-depth metabolic phenotyping to unravel tissue-specific contributions (e.g., stable isotope methods) to locate the sites of defect during circadian disruption which will help in the development of therapeutics. Another way to counter the detrimental effects of circadian disruption is through behavioral/environmental interventions. Proof-of-concept studies in rodents examining the feasibility of behavioral interventions to optimize circadian function in the prevention or treatment of diabetes are just emerging. Future studies in human are needed to determine the separate and interacting influences of specific behavioral/environmental cycles and circadian rhythms on glucose metabolism as well as the underlying mechanism. This will help us design recommendations for the timing of behavioral and environmental factors such as meals and lighting to optimize glucose control in both shift workers and the general population.
Text Box 1: The organization of the mammal circadian system.
Since the beginning of life on earth, some 3.5 billion years ago, life has been exposed to the cycle of day and night. Most life has adapted to this predictable daily rhythm and has developed an internal circadian timing system to anticipate these changes. One of the proposed main purposes of having a coherent circadian system is to generate daily rhythms in biological and physiological processes so that the individual can be better prepared for the rhythmic changes in the environment. In mammals, this is achieved by a hierarchy of multiple circadian oscillators, consisting of the central clock located in the hypothalamic suprachiasmatic nucleus (SCN) and peripheral oscillators in nearly every other tissue and cell type. The SCN can be synchronized with the 24-hr light-dark cycle through direct photic inputs received from the retina and transmitted via the retinohypothalamic tract. To maintain the peripheral oscillators in proper phase relationships with each other, the SCN conveys its temporal information to them through multiple pathways, including direct neural projections and hormonal signals, or indirectly by modulating body temperature and behavior cycles [92]. It is now known that besides the temporal cues given by the SCN, peripheral oscillators can also be directly entrained by various other stimuli, with feeding being the dominant entrainment signal for many [74].
Even under constant environmental conditions, both the SCN and peripheral oscillators are still able to maintain their rhythmicity. This is mainly attributed to the cell-autonomous intracellular molecular clock composed of highly-conserved transcriptional-translational feedback loops. Lying at the heart of this molecular clock, the transcriptional factors CLOCK and BMAL1 form a heterodimeric complex which activates transcription of period (PER) genes and cryptochrome (CRY) genes through a circadian E-box regulatory element. Once synthesized, PER and CRY negatively feedback to suppress the CLOCK:BMAL1-mediated transcription, thus inhibiting their own expression [92]. Post-translational modification of these core clock genes together with the presence of other interlocking feedback loops, such as the ROR–REV–ERB-associated loop and the D-site of albumin promoter-binding protein (DBP)–E4 promoter-binding protein 4 (E4BP4)-associated loop, fine-tunes the core feedback loop to a period close to 24 hr [93]. This clockwork circuitry can directly drive the circadian expression of various output genes related to cellular functions in a tissue-specific manner, thus translating the molecular oscillation into the circadian rhythm of diverse physiological and metabolic processes [94].
Text Box 2: Mechanisms of circadian regulation of glucose metabolism at the level of the SCN and peripheral clocks.
The SCN has multi-synaptic projections to many glucose-metabolism-related organs, including liver, adipose tissues, and pancreas. Studies have shown a clear functional relevance of the autonomic projections to the liver [95]. Future studies are needed to clarify such relevance for other tissues involved in glucose control. Various glucose-metabolism-related hormones are driven by the circadian system [33]. Glucocorticoid levels rise during sleep and peak at the start of the active phase, both in nocturnal and diurnal mammals. Epinephrine, an important counterregulatory hormone, displays a strong and consistent endogenous circadian oscillation in humans, with a broad peak during the middle of the biological day [96]. However, its role towards the decreasing glucose tolerance across the biological day is unclear. The role of melatonin will be discussed in section IV.
Peripheral clocks contribute to circadian glucose control through generating tissue-specific rhythmic gene expression. Early microarray studies showed that ~10% of the transcriptome exhibits daily oscillation with only ~1-3% overlap shared among different organs [97]. These ubiquitous rhythmic transcripts are enriched with core clock genes, whereas genes oscillating in a tissue-dependent manner are largely tied to tissue-specific functions. While microarray studies demonstrate diurnal rhythms in transcription, ChIP-seq studies show direct circadian transcriptional regulation through clock-related transcription factors binding (e.g., CLOCK:BMAL1 to E-box, REV-ERBs to RORE, and DBP to DBPE). Recent studies revealed that circadian transcriptional regulation also occurs through epigenetic modification and chromatin remodeling. This mechanism is tightly linked to cellular metabolic status and allows for large-scale transcriptional regulation by altering nearby DNA accessibility [98]. In addition, circadian control of poly(A) tail-length can contribute to the circadian mRNA rhythms through regulation of mRNA stability [97]. Surprisingly, hepatic proteomic analysis shows an even higher percentage of soluble protein oscillation with half of the cycling protein lacking a corresponding cycling transcript [97], suggesting a prominent contribution of post-transcriptional/translational regulation to circadian protein expression. Studies focusing on post-transcriptional/translational regulation of clock targets are just emerging. Future work is needed to elucidate underlying mechanisms and their role in circadian metabolism.
Trends Box.
- Many aspects of the modern life style, including shift work, social jet lag, disturbed/short sleep, light at night, and late eating have been associated with increased risk for adverse metabolic consequences, including T2D.
- Misalignment of (specific) behavioral/environmental cycles relative to the circadian system results in adverse metabolic consequences and thus may provide a mechanism underlying the aforementioned associations.
- Recent human studies suggest a dominating role for the circadian system in the daily variation in glucose tolerance, independent of behaviors.
- Determining the relative role and optimal use of different behavioral/environmental interventions (e.g., timing of food, sleep, activity, and light) will be important in developing approaches in the prevention and treatment of circadian disruption and its adverse metabolic consequences.
ACKNOWLEDGMENTS
J.Q. and F.A.J.L.S. were supported in part by NHLBI Grant R01 HL118601, NIDDK Grant R01 DK099512, and NIDDK Grant R01 DK102696 to F.A.J.L.S.
Glossary
Chronotype: A measure of preferred timing of sleep and activity. This is typically assessed by questionnaires, such as the Horne-Ostberg Questionnaire or the Munich Chronotype Questionnaire.
Circadian misalignment: A circadian rhythm being in abnormal phase relationship with other cycles (e.g., with the environmental light/dark cycle or other circadian rhythms).
Circadian phase: The timing of a consistent point in the circadian cycle, such as the peak or trough. The circadian phase of the central pacemaker in humans is usually determined by circadian markers such as dim-light, melatonin onset, or the time of the core body temperature minimum, assessed under a circadian protocol (such as constant routine or forced desynchrony protocol, see text).
Circadian rhythm: An endogenous biological rhythm with a ~24-h period that is persistent under constant environmental conditions. It can be synchronized to the environmental cycle by the light/dark cycle. In order to determine whether rhythms are driven by the endogenous circadian system, and not a secondary “masking” consequence of behaviors, such as sleep, activity, and food intake (often driven by non-circadian mechanisms, especially in humans), it is necessary to assess endogenous circadian rhythms in the absence of 24-h rhythms in behavior (i.e., constant routine protocol), or when behaviors are uniformly distributed across the circadian cycle (i.e., forced desynchrony protocol).
Diurnal rhythm: Daily changes in physiology or behavior across the 24-h light/dark cycle. In conditions in which environmental and behavioral changes are present (e.g., light–dark cycle), it is impossible to tell whether and to what degree a diurnal rhythm is endogenously generated, and/or a consequence of changes in behaviors or the environment.
Melatonin: A hormone mainly synthesized and secreted by the pineal gland in a circadian manner: high circulating concentrations at night and near-undetectable concentrations during the day in both diurnal and nocturnal mammals. It is well-known as a phase marker of the timing of the central clock, for its central role in the entrainment of the circadian system and for its soporific properties.
Peripheral oscillators/clocks: Circadian oscillators/clocks located outside of the SCN and in virtually all cells and organs, such as in other brain regions, liver, heart, pancreas, kidneys, lungs, intestines, skin and lymphocytes. Depending on the context, the term peripheral clock typically refers to the molecular machinery generating the circadian rhythm (e.g., transcription-translation feedback loop[s]) and peripheral oscillator typically refers to the peripheral cell(s) able to generate cell-autonomous circadian rhythms.
Slow wave sleep (SWS): Also called Stage 3 sleep, characterized by a larger amount of synchronized slow-wave EEG (brainwave activity) than in other stages. It is considered the deepest Non-REM sleep as it is the hardest stage from which to awaken.
Social jetlag: The misalignment between the circadian system and the sleep/wake cycle and the accompanying symptoms brought on by the shift in sleep schedule between workdays and days off. These are similar to those experienced after traveling rapidly across different time zones (i.e., jet lag).
Zeitgeber: Literally time-giver, a time cue that can phase shift a circadian rhythm.
Outstanding Questions
How do the SCN and peripheral oscillators talk to each other to achieve optimal glucose control? What is the relative role of the autonomic nervous system, humoral factors and peripheral clocks in the regulation of glucose metabolism? System biology approaches integrating ‘omics’ techniques followed by targeted experimental approaches are needed to unravel potential mechanistic pathways.
What is the relative contribution of different organs to disturbed glucose control during circadian disruption? In-depth glucose metabolic phenotyping, such as rate of gluconeogenesis, rate of insulin extraction/secretion, glucose disposal, and peripheral/hepatic insulin sensitivity, is required for targeting the primary sites impacted by circadian disruption.
What are the separate and interacting influences of the sleep/wake cycle, fasting/feeding cycle, dark/light cycle, central clock and peripheral clocks on glucose metabolism? What are the mechanisms mediating those effects?
How can we prevent or minimize the adverse physiological consequences of circadian disruption caused by lifestyles such as night shift work? What are the underlying mechanisms? How can we best design general and personalized behavioral and environmental recommendations (e.g., eating and lighting schedules)? Can we use melatonin to optimize glucose control?
Reference
- 1.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nature reviews. Endocrinology. 2012;8(4):228–36. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 2.Haus E, Smolensky M. Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer causes & control : CCC. 2006;17(4):489–500. doi: 10.1007/s10552-005-9015-4. [DOI] [PubMed] [Google Scholar]
- 3.Navara KJ, Nelson RJ. The dark side of light at night: physiological, epidemiological, and ecological consequences. J Pineal Res. 2007;43(3):215–24. doi: 10.1111/j.1600-079X.2007.00473.x. [DOI] [PubMed] [Google Scholar]
- 4.CDC Unhealthy Sleep-Related Behaviors — 12 States, 2009. MMWR. 2011;60:233–266. [PubMed] [Google Scholar]
- 5.Roenneberg T, et al. Social jetlag and obesity. Curr Biol. 2012;22(10):939–43. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 6.Mattson MP, et al. Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A. 2014;111(47):16647–53. doi: 10.1073/pnas.1413965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smolensky MH, Sackett-Lundeen LL, Portaluppi F. Nocturnal light pollution and underexposure to daytime sunlight: Complementary mechanisms of circadian disruption and related diseases. Chronobiol Int. 2015;32(8):1029–48. doi: 10.3109/07420528.2015.1072002. [DOI] [PubMed] [Google Scholar]
- 8.Wong PM, et al. Social Jetlag, Chronotype, and Cardiometabolic Risk. J Clin Endocrinol Metab. 2015;100(12):4612–20. doi: 10.1210/jc.2015-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonken LK, Nelson RJ. The effects of light at night on circadian clocks and metabolism. Endocr Rev. 2014;35(4):648–70. doi: 10.1210/er.2013-1051. [DOI] [PubMed] [Google Scholar]
- 10.Wang XS, et al. Shift work and chronic disease: the epidemiological evidence. Occupational medicine. 2011;61(2):78–89. doi: 10.1093/occmed/kqr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suwazono Y, et al. Shiftwork and impaired glucose metabolism: a 14-year cohort study on 7104 male workers. Chronobiology international. 2009;26(5):926–41. doi: 10.1080/07420520903044422. [DOI] [PubMed] [Google Scholar]
- 12.Pan A, et al. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8(12):e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merikanto I, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiology international. 2013;30(4):470–7. doi: 10.3109/07420528.2012.741171. [DOI] [PubMed] [Google Scholar]
- 14.Reutrakul S, et al. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiology international. 2014;31(1):64–71. doi: 10.3109/07420528.2013.821614. [DOI] [PubMed] [Google Scholar]
- 15.Parsons MJ, et al. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes (Lond) 2015;39(5):842–8. doi: 10.1038/ijo.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nedeltcheva AV, Scheer FA. Metabolic effects of sleep disruption, links to obesity and diabetes. Current opinion in endocrinology, diabetes, and obesity. 2014;21(4):293–8. doi: 10.1097/MED.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris CJ, Yang JN, Scheer FA. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res. 2012;199:337–58. doi: 10.1016/B978-0-444-59427-3.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18(5):716–38. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 19.la Fleur SE, et al. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes. 2001;50(6):1237–43. doi: 10.2337/diabetes.50.6.1237. [DOI] [PubMed] [Google Scholar]
- 20.Clore JN, Nestler JE, Blackard WG. leep-associated fall in glucose disposal and hepatic glucose output in normal humans. Putative signaling mechanism linking peripheral and hepatic events. Diabetes. 1989;38(3):285–90. doi: 10.2337/diab.38.3.285. [DOI] [PubMed] [Google Scholar]
- 21.Bolli GB, et al. Demonstration of a dawn phenomenon in normal human volunteers. Diabetes. 1984;33(12):1150–3. doi: 10.2337/diab.33.12.1150. [DOI] [PubMed] [Google Scholar]
- 22.Radziuk J, Pye S. Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes. Suprachiasmatic deficit or limit cycle behaviour? Diabetologia. 2006;49(7):1619–28. doi: 10.1007/s00125-006-0273-9. [DOI] [PubMed] [Google Scholar]
- 23.Saad A, et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes. 2012;61(11):2691–700. doi: 10.2337/db11-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheard NF, et al. Dietary carbohydrate (amount and type) in the prevention and management of diabetes: a statement by the american diabetes association. Diabetes care. 2004;27(9):2266–71. doi: 10.2337/diacare.27.9.2266. [DOI] [PubMed] [Google Scholar]
- 25.Colberg SR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes care. 2010;33(12):2692–6. doi: 10.2337/dc10-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Cauter E, et al. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88(3):934–42. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? Journal of biological rhythms. 2002;17(1):4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- 28.Shea SA, et al. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90(5):2537–44. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheer FA, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Cauter E, et al. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992;262(4 Pt 1):E467–75. doi: 10.1152/ajpendo.1992.262.4.E467. [DOI] [PubMed] [Google Scholar]
- 31.Morris CJ, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(17):E2225–34. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalsbeek A, Strubbe JH. Circadian control of insulin secretion is independent of the temporal distribution of feeding. Physiol Behav. 1998;63(4):553–8. doi: 10.1016/s0031-9384(97)00493-9. [DOI] [PubMed] [Google Scholar]
- 33.Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012;349(1):91–104. doi: 10.1016/j.mce.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105(39):15172–7. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobi D, et al. Hepatic Bmal1 Regulates Rhythmic Mitochondrial Dynamics and Promotes Metabolic Fitness. Cell Metab. 2015;22(4):709–20. doi: 10.1016/j.cmet.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcheva B, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466(7306):627–31. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, et al. Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in beta-cells. Islets. 2011;3(6):381–8. doi: 10.4161/isl.3.6.18157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rakshit K, Hsu TW, Matveyenko AV. Bmal1 is required for beta cell compensatory expansion, survival and metabolic adaptation to diet-induced obesity in mice. Diabetologia. 2016 doi: 10.1007/s00125-015-3859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dyar KA, et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab. 2014;3(1):29–41. doi: 10.1016/j.molmet.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodge BA, et al. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skeletal muscle. 2015;5:17. doi: 10.1186/s13395-015-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paschos GK, et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012;18(12):1768–77. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang G, et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Science translational medicine. 2016;8(324):324ra16. doi: 10.1126/scitranslmed.aad3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang EE, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16(10):1152–6. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seron-Ferre M, et al. Circadian rhythms in the fetus. Molecular and cellular endocrinology. 2012;349(1):68–75. doi: 10.1016/j.mce.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 45.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63(6):1860–9. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fonken LK, et al. Light at night increases body mass by shifting the time of food intake. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(43):18664–9. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qian J, et al. Circadian disruption and diet-induced obesity synergize to promote development of beta cell failure and diabetes in male rats. Endocrinology. 2015:en20151516. doi: 10.1210/en.2015-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian J, et al. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes. 2013;62(10):3469–78. doi: 10.2337/db12-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gale JE, et al. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J Biol Rhythms. 2011;26(5):423–33. doi: 10.1177/0748730411416341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coomans CP, et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013;27(4):1721–32. doi: 10.1096/fj.12-210898. [DOI] [PubMed] [Google Scholar]
- 51.Gil-Lozano M, et al. Short-term sleep deprivation with nocturnal light exposure alters time- dependent glucagon-like peptide-1 and insulin secretion in male volunteers. Am J Physiol Endocrinol Metab. 2016;310(1):E41–50. doi: 10.1152/ajpendo.00298.2015. [DOI] [PubMed] [Google Scholar]
- 52.Buxton OM, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4(129):129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris CJ, et al. Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J Clin Endocrinol Metab. 2016:jc20153924. doi: 10.1210/jc.2015-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Broussard JL, et al. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Annals of internal medicine. 2012;157(8):549–57. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137(1):95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43(5):533–49. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- 57.Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab. 1982;54(1):131–8. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- 58.Calvin AD, et al. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest. 2013;144(1):79–86. doi: 10.1378/chest.12-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Markwald RR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110(14):5695–700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tasali E, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borbely AA, et al. The two-process model of sleep regulation: a reappraisal. Journal of sleep research. 2016 doi: 10.1111/jsr.12371. [DOI] [PubMed] [Google Scholar]
- 62.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neuroscience letters. 1994;166(1):63–8. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 63.Gonnissen HK, et al. Sleep architecture when sleeping at an unusual circadian time and associations with insulin sensitivity. PLoS One. 2013;8(8):e72877. doi: 10.1371/journal.pone.0072877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Curie T, et al. In Vivo Imaging of the Central and Peripheral Effects of Sleep Deprivation and Suprachiasmatic Nuclei Lesion on PERIOD-2 Protein in Mice. Sleep. 2015;38(9):1381–94. doi: 10.5665/sleep.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mongrain V, et al. Sleep loss reduces the DNA-binding of BMAL1, CLOCK, and NPAS2 to specific clock genes in the mouse cerebral cortex. PLoS One. 2011;6(10):e26622. doi: 10.1371/journal.pone.0026622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eckel RH, et al. Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Curr Biol. 2015;25(22):3004–10. doi: 10.1016/j.cub.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 67.Moller-Levet CS, et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci U S A. 2013;110(12):E1132–41. doi: 10.1073/pnas.1217154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang JB, et al. Timing of energy intake during the day is associated with the risk of obesity in adults. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2014;27(Suppl 2):255–62. doi: 10.1111/jhn.12141. [DOI] [PubMed] [Google Scholar]
- 69.Garaulet M, et al. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013;37(4):604–11. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arble DM, et al. Circadian timing of food intake contributes to weight gain. Obesity. 2009;17(11):2100–2. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakajima K, Suwa K. Association of hyperglycemia in a general Japanese population with late-night-dinner eating alone, but not breakfast skipping alone. Journal of diabetes and metabolic disorders. 2015;14:16. doi: 10.1186/s40200-015-0147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morgan LM, et al. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br J Nutr. 2012;108(7):1286–91. doi: 10.1017/S0007114511006507. [DOI] [PubMed] [Google Scholar]
- 73.Bandin C, et al. Meal timing affects glucose tolerance, substrate oxidation and circadian- related variables: A randomized, crossover trial. Int J Obes (Lond) 2015;39(5):828–33. doi: 10.1038/ijo.2014.182. [DOI] [PubMed] [Google Scholar]
- 74.Damiola F, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–61. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato M, et al. The role of the endocrine system in feeding-induced tissue-specific circadian entrainment. Cell reports. 2014;8(2):393–401. doi: 10.1016/j.celrep.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 76.Ando H, Ushijima K, Fujimura A. Indirect effects of glucagon-like peptide-1 receptor agonist exendin-4 on the peripheral circadian clocks in mice. PLoS One. 2013;8(11):e81119. doi: 10.1371/journal.pone.0081119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Landgraf D, et al. Oxyntomodulin regulates resetting of the liver circadian clock by food. Elife. 2015;4:e06253. doi: 10.7554/eLife.06253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boivin DB, James FO. Light treatment and circadian adaptation to shift work. Industrial health. 2005;43(1):34–48. doi: 10.2486/indhealth.43.34. [DOI] [PubMed] [Google Scholar]
- 79.Vetter C, et al. Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr Biol. 2015;25(7):907–11. doi: 10.1016/j.cub.2015.01.064. [DOI] [PubMed] [Google Scholar]
- 80.Salgado-Delgado R, et al. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology. 2010;151(3):1019–29. doi: 10.1210/en.2009-0864. [DOI] [PubMed] [Google Scholar]
- 81.Fonken LK, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A. 2010;107(43):18664–9. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lyssenko V, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82–8. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bonnefond A, et al. Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet. 2012;44(3):297–301. doi: 10.1038/ng.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gaulton KJ, et al. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat Genet. 2015;47(12):1415–25. doi: 10.1038/ng.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Costes S, et al. Activation of Melatonin Signaling Promotes beta-Cell Survival and Function. Mol Endocrinol. 2015;29(5):682–92. doi: 10.1210/me.2014-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rubio-Sastre P, et al. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep. 2014;37(10):1715–9. doi: 10.5665/sleep.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sartori C, et al. Melatonin improves glucose homeostasis and endothelial vascular function in high-fat diet-fed insulin-resistant mice. Endocrinology. 2009;150(12):5311–7. doi: 10.1210/en.2009-0425. [DOI] [PubMed] [Google Scholar]
- 88.She M, et al. Piromelatine, a novel melatonin receptor agonist, stabilizes metabolic profiles and ameliorates insulin resistance in chronic sleep restricted rats. Eur J Pharmacol. 2014;727:60–5. doi: 10.1016/j.ejphar.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 89.Ramracheya RD, et al. Function and expression of melatonin receptors on human pancreatic islets. J Pineal Res. 2008;44(3):273–9. doi: 10.1111/j.1600-079X.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 90.Chen Z, Yoo SH, Takahashi JS. Small molecule modifiers of circadian clocks. Cellular and molecular life sciences : CMLS. 2013;70(16):2985–98. doi: 10.1007/s00018-012-1207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jagannath A, et al. The CRTC1-SIK1 pathway regulates entrainment of the circadian clock. Cell. 2013;154(5):1100–11. doi: 10.1016/j.cell.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–62. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nature reviews. Molecular cell biology. 2007;8(2):139–48. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- 94.Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–41. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruiter M, Buijs RM, Kalsbeek A. Hormones and the autonomic nervous system are involved in suprachiasmatic nucleus modulation of glucose homeostasis. Curr Diabetes Rev. 2006;2(2):213–26. doi: 10.2174/157339906776818596. [DOI] [PubMed] [Google Scholar]
- 96.Morris CJ, et al. Circadian misalignment increases cardiovascular disease risk factors in humans. Proceedings of the National Academy of Sciences of the United States of America. 2016 doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24(2):90–9. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koike N, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349–54. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peschke E, et al. Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. Journal of pineal research. 2006;40(2):135–43. doi: 10.1111/j.1600-079X.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 100.Hu K, et al. Noninvasive fractal biomarker of clock neurotransmitter disturbance in humans with dementia. Scientific reports. 2013;3:2229. doi: 10.1038/srep02229. [DOI] [PMC free article] [PubMed] [Google Scholar]